Occurrence of Toxic Biogenic Amines in Various Types of Soft and Hard Cheeses and Their Control by Bacillus polymyxa D05-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cheese Samples
2.2. Chemical Analysis of Cheese
2.3. Determination of BAs
2.4. Activation of B. polymyxa D05-1
2.5. Investigation of the Ability of B. polymyxa D05-1 to Reduce the Experimentally Added BAs into Tallaga Cheese
2.5.1. Tallaga Cheese Manufacturing
2.5.2. Microbiological Analysis of the Investigated Cheese Samples
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis of Cheese Samples
3.2. Occurrence and Levels of BAs in the Collected Cheese Samples
3.3. Effect of Adding B. polymyxa D05-1 Culture on the Levels of Histamine and Tyramine Experimentally Added to Processed Tallaga Cheese
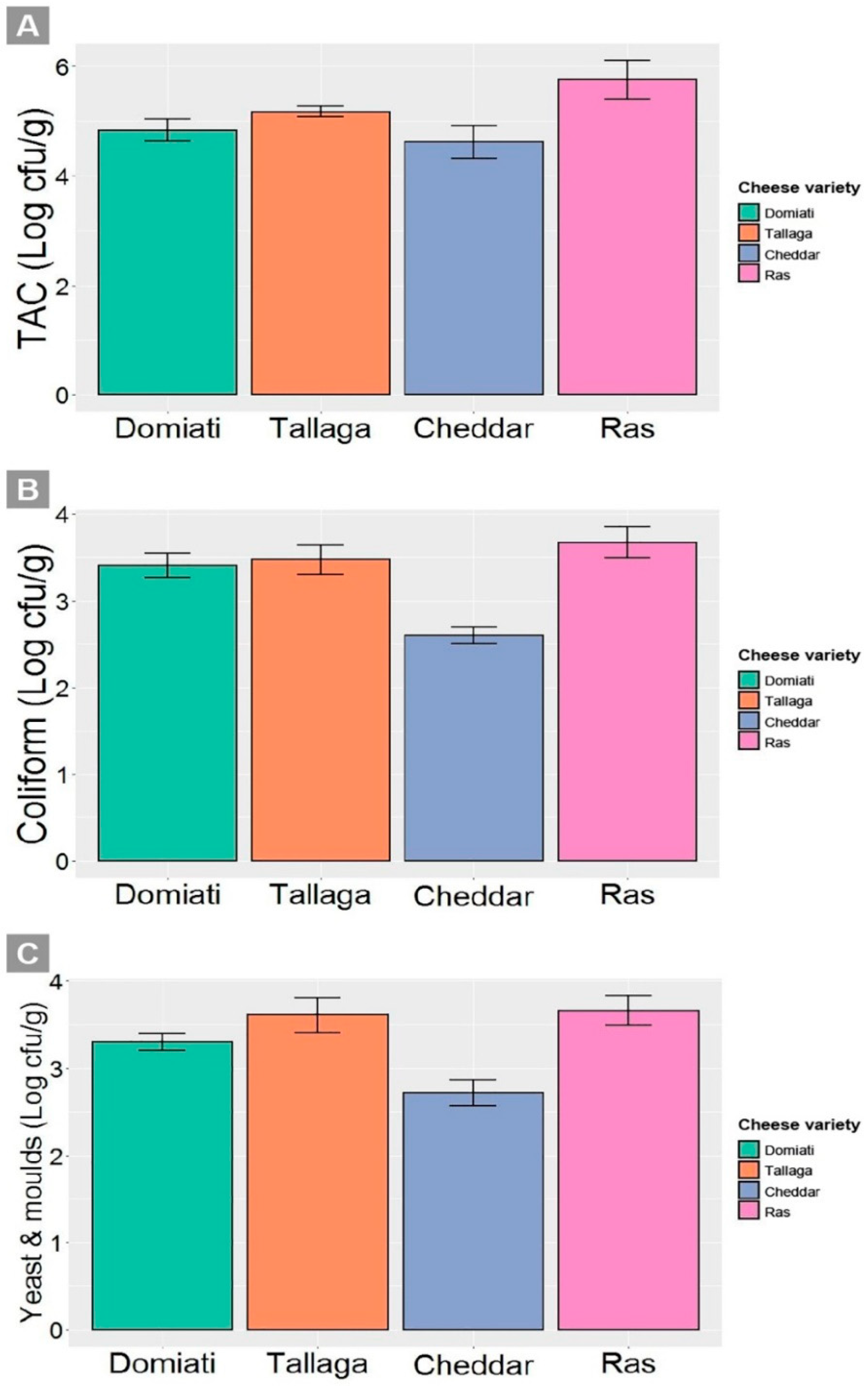
3.4. Microbiological Quality of Cheese Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tabanelli, G. Biogenic amines and food quality: Emerging challenges and public health concerns. Foods 2020, 9, 859. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Herrero, A.M. Review: Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hungerford, J.M. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzada, J.; del Olmo, A.; Picon, A.; Gaya, P.; Nuñez, M. Effect of high-pressure processing on the microbiology, proteolysis, biogenic amines and flavour of cheese made from unpasteurized milk. Food Bioproc. Technol. 2015, 8, 319–332. [Google Scholar] [CrossRef]
- Linares, D.M.; Martín, M.; Ladero, V.; Alvarez, M.A.; Fernández, M. Biogenic amines in dairy products. Crit. Rev. Food Sci. Nutr. 2011, 51, 691–703. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Jimenez-Colenero, F. Biogenic amines in meat and meat products. Crit. Rev. Food Sci. Nutr. 2004, 44, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Shalaby, A.R.; Anwar, M.M.; Sallam, E.M.; Emam, W.H. Quality and safety of irradiated food regarding biogenic amines: Ras cheese. Int. J. Food Sci. Technol. 2016, 51, 1048–1054. [Google Scholar] [CrossRef]
- Ekici, K.; Omer, A. Biogenic amines formation and their importance in fermented foods. Bio. Web. Conf. 2020, 17, 00232. [Google Scholar] [CrossRef] [Green Version]
- Bartkiene, E.; Gruzauskas, R.; Ruzauskas, M.; Zokaityte, E.; Starkute, V.; Klupsaite, D.; Vadopalas, L.; Badaras, S.; Özogul, F. Changes in the microbial community and biogenic amine content in rapeseed meal during fermentation with an antimicrobial combination of Lactic acid bacteria strains. Fermentation 2022, 8, 136. [Google Scholar] [CrossRef]
- Rokka, M.; Eerola, S.; Smolander, M.; Alakomi, H.-L.; Ahvenainen, R. Monitoring of the quality of modified atmosphere packaged broiler chicken cuts stored in different temperature conditions: B. Biogenic amines as quality-indicating metabolites. Food Cont. 2004, 15, 601–607. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Carballo, J.; Jiménez Colmenero, F. Biogenic amines in pressurized vacuum-packaged cooked sliced ham under different chilled storage conditions. Meat Sci. 2007, 75, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Ekici, K.; Okut, H.; Isleyici, O.; Sancak, Y.C.; Tuncay, R.M. The Determination of some microbiological and chemical features in herby cheese. Foods 2019, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleiwa, N.Z.; Lamada, H.M.; Nassif, M.Z. Occurrence of biogenic amines in different types of marketed cheese in Gharbia governorate. J. Vet. Med. Res. 2013, 22, 130–135. [Google Scholar] [CrossRef]
- Guarcello, R.; Diviccaro, A.; Barbera, M.; Giancippoli, E.; Settanni, L.; Minervini, F.; Moschetti, G.; Gobbetti, M. A survey of the main technology, biochemical and microbiological features influencing the concentration of biogenic amines of twenty Apulian and Sicilian (Southern Italy) cheeses. Int. Dairy J. 2015, 43, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Maijala, R.; Eerola, S. Biogenic Amines; Veterinary and Food Research Institute: Helsinki, Finland, 2002. [Google Scholar]
- Vinci, G.; Antonelli, M.L. Biogenic amines: Quality index of freshness in red and white meat. Food Cont. 2002, 13, 519–524. [Google Scholar] [CrossRef]
- Egyptian Organization for Standardization and Quality Control. Detection of Poisons and Control, Report; Egyptian Organization for Standardization and Quality Control: Cairo, Egypt, 1996; p. 1796. [Google Scholar]
- FDA “Food and Drug Administration”. Hazards and Controls Guidance, 3rd ed.; Center of Food Safety and Nutrition: Washington, DC, USA, 2001.
- Latorre-Moratalla, M.L.; Bover-Cid, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Control of biogenic amines in fermented sausages: Role of starter cultures. Front. Microbiol. 2012, 3, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katare, P.; Chaudhari, K.; Mohan, M. Bacillus polymyxa: A Potential Probiotic Species. Elect. J. Biol. 2020, 16, 128–133. [Google Scholar]
- Lee, Y.C.; Lin, C.S.; Liu, F.L.; Huang, T.C.; Tsai, Y.H. Degradation of histamine by Bacillus polymyxa isolated from salted fish products. J. Food Drug Anal. 2015, 23, 836–844. [Google Scholar] [CrossRef] [Green Version]
- Shaghayegh, M.; Marjaneh, S. Nargess. Influence of Bacillus polymyxa starter on chemical and microbial properties of Mahyaveh fermented sauce. J. Appl. Microbiol. Foods Ind. 2019, 5, 1–16. [Google Scholar]
- AOAC “Association of Official Analytical Chemists”. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MA, USA, 2006. [Google Scholar]
- Hwang, C.C.; Kung, H.F.; Lin, C.S. Bacteriological quality and histamine-forming bacteria associated with fish meats and environments in HACCP and non-HACCP fish processing factories. Food Cont. 2011, 22, 1657–1662. [Google Scholar] [CrossRef]
- Eom, J.S.; Seo, B.Y.; Choi, H.S. Biogenic amine degradation by Bacillus species isolated from traditional fermented soybean food and detection of decarboxylase-related genes. J. Microbiol. Biotechnol. 2015, 25, 1519–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kholy, W.; El-Khalek, A.B.A.; Mohamed, S.H.S.; Fouad, M.T.; Kassem, J.M. Tallaga cheese as a new functional dairy product. American J. Food Technol. 2016, 11, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Çetinkaya, A.; Öz, F. Composition and microbiological analysis for quality evaluation of Kars Gravyer cheese: Influence of ripening period. Food Sci. Technol. 2019, 39, 1052–1058. [Google Scholar] [CrossRef] [Green Version]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological factors affecting biogenic amine content in foods: A review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójciak, K.M.; Solska, E. Evolution of free amino acids, biogenic amines and N-nitrosoamines throughout ageing in organic fermented beef. Acta Sci. Pol. Technol. Aliment. 2016, 15, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Kanotor, A.; Kacaniova, M.; Pachlova, V. Biogenic amines content in different wine samples. J. Micro. Biotech. Food Sci. 2015, 4, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, Z.A. Incidence of mycobiota in some dairy products and its public health hazards. Master’s Thesis, Faculty of Veterinary Medicine, Alexandria University, Rasheed, Egypt, 2016. [Google Scholar]
- Lund, P.A.; De Biase, D.; Liran, O.; Scheler, O.; Mira, N.P.; Cetecioglu, Z.; Fernández, E.N.; Bover-Cid, S.; Hall, R.; Sauer, M.; et al. Understanding how microorganisms respond to acid pH is central to their control and successful exploitation. Front. Microbiol. 2020, 11, 556140. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Kirbag, S. Microbial quality and presence of moulds in Kuflu cheese. Int. J. Food Microbiol. 2002, 115, 376–380. [Google Scholar] [CrossRef]
- Farag, I.S.A. Amino acids profile and biogenic amines levels during Egyptian Ras cheese ripening. Masters’s Thesis, Department of Food Hygiene, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt, 2018. [Google Scholar]
- Salama, E.M.; Saad, A.H.; Enan, G.A.; Suzan, I.Y. Incidence and biocontrol of Staphylococcus aureus in some milk products. In 2nd Conference of Food Safety; Faculty of Veterinary Medicine, Suez Canal University: Ismailia, Egypt, 2015; Volume 1, pp. 29–35. [Google Scholar]
- Chong, C.Y.; Abu Bakar, F.; Russly, A.R.; Jamilah, B.; Mahyudin, N.A. The effects of food processing on biogenic amines formation. Int. Food Res. J. 2011, 18, 867–876. [Google Scholar]
- Marcobal, A.; De Las Rivas, B.; Muñoz, R. First genetic characterization of a bacterial β-phenylethylamine biosynthetic enzyme in Enterococcus faecium RM58. FEMS Microbiol. Let. 2006, 258, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Marino, M.; Maifreni, M.; Rondinini, G. Microbiological characterization of artisanal Montasio cheese: Analysis of its indigenous lactic acid bacteria. FEMS Microbiol. Let. 2003, 229, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Durak-Dados, A.; Michalski, M.; Osek, J. Histamine and other biogenic amines in food. J. Vet. Res. 2020, 64, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food—Existing and emerging approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linares, D.M.; del Río, B.; Ladero, V.; Martínez, N.; Fernández, M.; Martín, M.C. Factors influencing biogenic amines accumulation in dairy products. Front. Microbiol. 2012, 3, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.C.; Kung, H.F.; Huang, C.Y.; Huang, T.C.; Tsai, Y.H. Reduction of histamine and biogenic amines during salted fish fermentation by Bacillus polymyxa as a starter culture. J. Food Drug Anal. 2016, 24, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Hassan, G.M.; Gomaa, S.M. Microbiological quality of soft cheese marketed in Cairo and Giza Governorates. Alexandria J. Vet. Sci. 2016, 50, 18–23. [Google Scholar] [CrossRef]
- APHA “American Public Health Association”. Standard Methods for the Examination of Dairy Products.INC, 16th ed.; American Public Health Association: New York, NY, USA, 2004. [Google Scholar]
- Darwish, W.S.; Eldin, W.F.S.; Eldesoky, K.I. Prevalence, molecular characterization and antibiotic susceptibility of Escherichia Coli isolated from Duck Meat and Giblets. J. Food Saf. 2015, 35, 410–415. [Google Scholar] [CrossRef]
- Montel, M.C.; Masson, F.; Talon, R. Comparison of biogenic amine content in traditional and industrial French dry sausages. Sci. Alim. 1999, 19, 247–254. [Google Scholar]
- Sharaf, O.M.; Ibrahim, G.A.; Tawfek, N.F.; Effat, B.A.M.; El Shafei, K.; El-Din, H.M.F.; Salem, M.M.A. Prevalence of some pathogenic microorganisms in factories Domiati, Feta cheeses and UHT milk in relation to public health sold under market conditions in Cairo. Int. J. Chem. Technol. Res. 2014, 6, 2807–2814. [Google Scholar]
- El-Badry, S.; Raslan, A.A. Mould contamination of some Egyptian cheese. Benha Vet. Med. J. 2016, 30, 28–33. [Google Scholar] [CrossRef]
- Özogul, F.; Özogul, Y. Biogenic amine content and biogenic amine quality indices of sardines (Sardina pilchardus) stored in modified atmosphere packaging and vacuum packaging. Food Chem. 2006, 99, 574–578. [Google Scholar] [CrossRef]
- Al Bulushi, I.; Poole, S.; Deeth, H.C.; Dykes, G.A. Biogenic amines in fish: Roles in intoxication, spoilage, and nitrosamine formation, A review. Crit. Rev. Food Sci. Nutrit. 2009, 49, 369–377. [Google Scholar] [CrossRef] [PubMed]



| Cheese Variety | Positive Samples | Min. | Max. | Mean ± S.E * | |
|---|---|---|---|---|---|
| No. | % | ||||
| Histamine | |||||
| Domiati | 11 | 73.33 | 2.1 | 28.9 | 11.72 ± 1.48 a |
| Tallaga | 10 | 66.67 | 2.7 | 26.4 | 9.69 ± 1.15 b |
| Cheddar | 8 | 53.33 | 2.1 | 22.8 | 5.14 ± 0.67 c |
| Ras | 7 | 46.67 | 2.3 | 21.0 | 5.46 ± 0.81 c |
| Tyramine | |||||
| Domiati | 15 | 100 | 3.8 | 29.5 | 19.01 ± 2.17 a |
| Tallaga | 13 | 86.67 | 2.9 | 33.2 | 13.85 ± 1.92 b |
| Cheddar | 12 | 80 | 2.6 | 20 | 11.30 ± 1.26 c |
| Ras | 12 | 80 | 1.3 | 25.7 | 9.57 ± 1.04 d |
| Putrescine | |||||
| Domiati | 9 | 60 | 1.6 | 23.1 | 7.56 ± 0.83 a |
| Tallaga | 9 | 60 | 1.2 | 21.8 | 6.29 ± 0.65 ab |
| Cheddar | 8 | 53.33 | 1.0 | 20.1 | 4.57 ± 0.49 bc |
| Ras | 6 | 40 | 1.0 | 16.7 | 3.88 ± 0.51 cd |
| Cadaverine | |||||
| Domiati | 8 | 100 | 1.3 | 20.6 | 5.18 ± 0.79 a |
| Tallaga | 6 | 40 | 1.1 | 17.9 | 3.37 ± 0.58 ab |
| Cheddar | 6 | 40 | 1.0 | 12.5 | 2.98 ± 0.44 b |
| Ras | 4 | 26.67 | 1.0 | 9.4 | 2.35 ± 0.41 b |
| Tryptamine | |||||
| Domiati | 4 | 26.67 | 1.0 | 2.9 | 1.87 ± 0.27 a |
| Tallaga | 3 | 20 | 1.0 | 1.8 | 1.30 ± 0.19 a |
| Cheddar | 2 | 13.33 | 1.0 | 1.2 | 1.10 ± 0.15 a |
| Ras | 0 | 0 | 0 | 0 | 0 |
| Storage Time | T1 | HI Reduction % | T2 | TY Reduction % |
|---|---|---|---|---|
| Zero time | 40 | − | 40 | − |
| 12 h | 13.1 | 67.3 | 16.8 | 58.0 |
| 24 h | 6.6 | 83.5 | 11.3 | 71.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad, M.A.; Abd-Rabou, H.S.; Elkhtab, E.; Rayan, A.M.; Abdeen, A.; Abdelkader, A.; Ibrahim, S.F.; Hussien, H. Occurrence of Toxic Biogenic Amines in Various Types of Soft and Hard Cheeses and Their Control by Bacillus polymyxa D05-1. Fermentation 2022, 8, 327. https://doi.org/10.3390/fermentation8070327
Saad MA, Abd-Rabou HS, Elkhtab E, Rayan AM, Abdeen A, Abdelkader A, Ibrahim SF, Hussien H. Occurrence of Toxic Biogenic Amines in Various Types of Soft and Hard Cheeses and Their Control by Bacillus polymyxa D05-1. Fermentation. 2022; 8(7):327. https://doi.org/10.3390/fermentation8070327
Chicago/Turabian StyleSaad, Marwa A., Hagar S. Abd-Rabou, Ebrahim Elkhtab, Ahmed M. Rayan, Ahmed Abdeen, Afaf Abdelkader, Samah F. Ibrahim, and Heba Hussien. 2022. "Occurrence of Toxic Biogenic Amines in Various Types of Soft and Hard Cheeses and Their Control by Bacillus polymyxa D05-1" Fermentation 8, no. 7: 327. https://doi.org/10.3390/fermentation8070327
APA StyleSaad, M. A., Abd-Rabou, H. S., Elkhtab, E., Rayan, A. M., Abdeen, A., Abdelkader, A., Ibrahim, S. F., & Hussien, H. (2022). Occurrence of Toxic Biogenic Amines in Various Types of Soft and Hard Cheeses and Their Control by Bacillus polymyxa D05-1. Fermentation, 8(7), 327. https://doi.org/10.3390/fermentation8070327








