Isolation and Characterization of Flavonoids from Fermented Dandelion (Taraxacum mongolicum Hand.-Mazz.), and Assessment of Its Antioxidant Actions In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of FD Extract
2.3. Purification of Flavonoids from FD Extract by Macroporous Resin
2.3.1. Pretreatment of Macroporous Resins
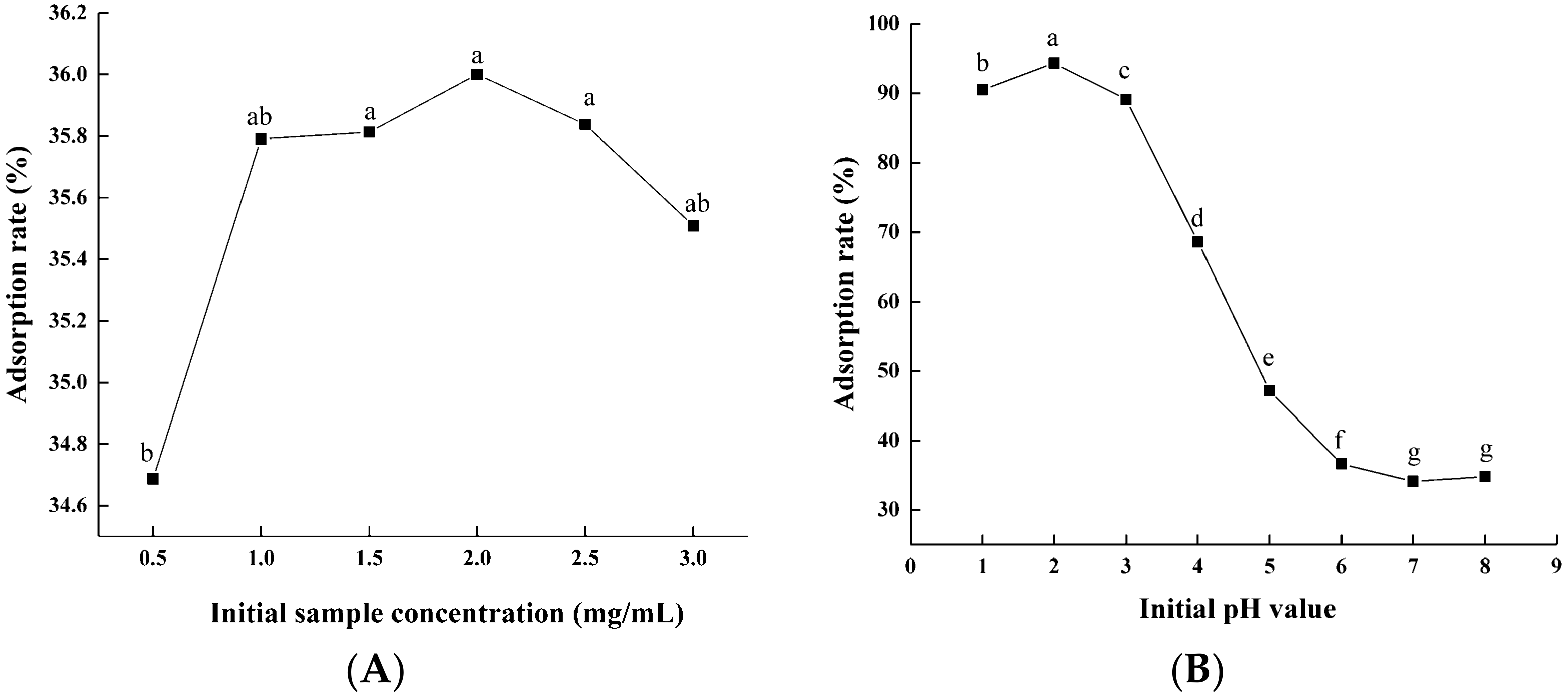
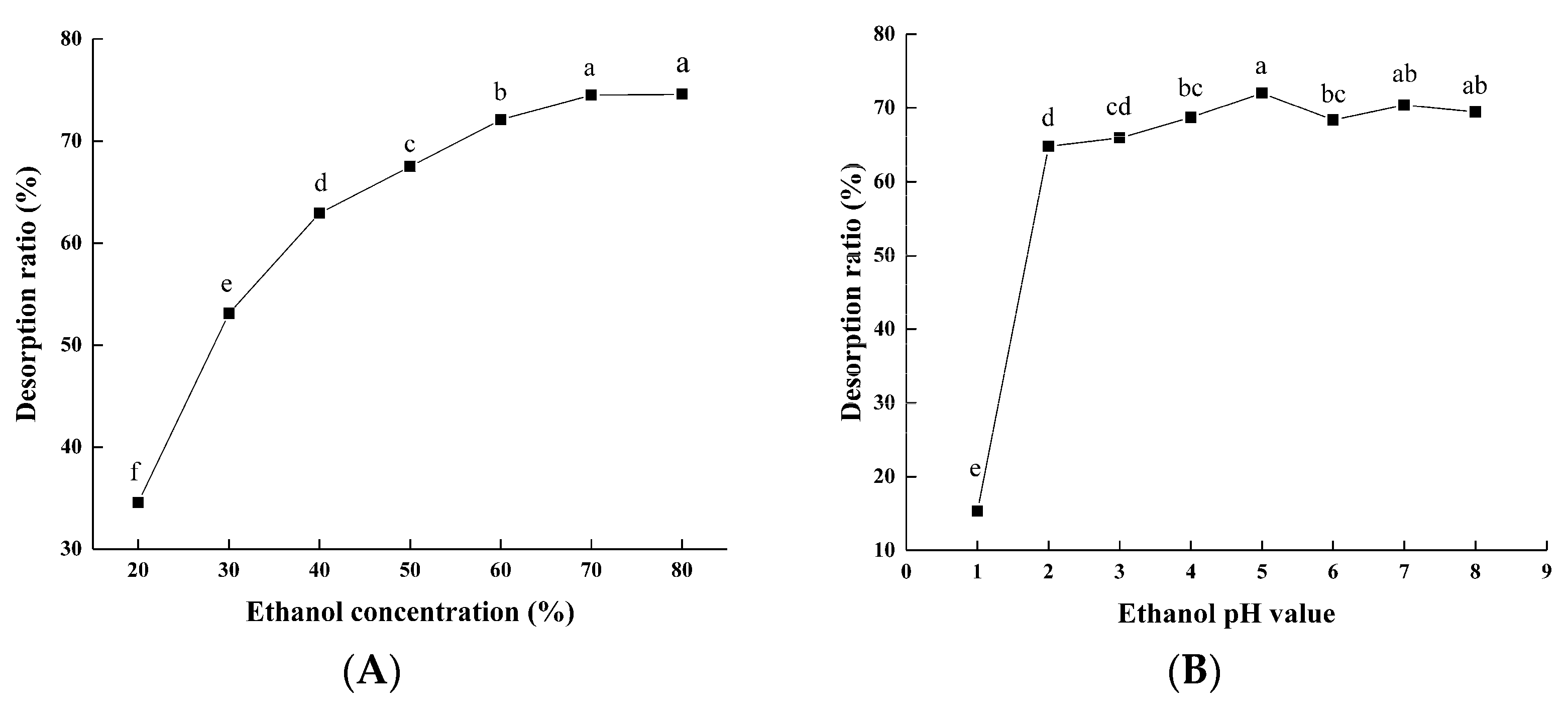
2.3.2. Static Adsorption and Desorption Tests
2.3.3. Dynamic Adsorption and Desorption Tests
2.4. Determination of Total Flavonoid Content
2.5. Determination PFDF Composition
2.6. In Vitro Antioxidant Activity of PFDF
2.6.1. Reducing Power
2.6.2. DPPH Radical Scavenging Activity
2.6.3. Hydroxyl Radical Scavenging Activity
2.7. Zebrafish Embryo Antioxidant Activity of PFDF
2.7.1. Maintenance of Parental Zebrafish
2.7.2. Exposure of Zebrafish Embryos to PFDF
2.7.3. Intracellular ROS Production, Cell Death and Lipid Peroxidation Measurements and Image Analysis
2.7.4. Determination of CAT, SOD, GSH-Px and MDA in Zebrafish Embryos
2.8. Cellular Antioxidant Activity of PFDF
2.8.1. Cell Culture and Treatments
2.8.2. Cell Proliferative Activity Measurements
2.8.3. Determination of CAT, SOD, GSH-Px, GSH and MDA in IPEC-J2 Cells
2.9. Statistical Analysis
3. Results and Discussion
3.1. Purification of Flavonoids FD Extract by AB-8 Macroporous Resin
3.1.1. Static Adsorption and Desorption
3.1.2. Dynamic Adsorption and Desorption
3.2. Composition of PFDF
3.3. In Vitro Antioxidant Activity of PFDF
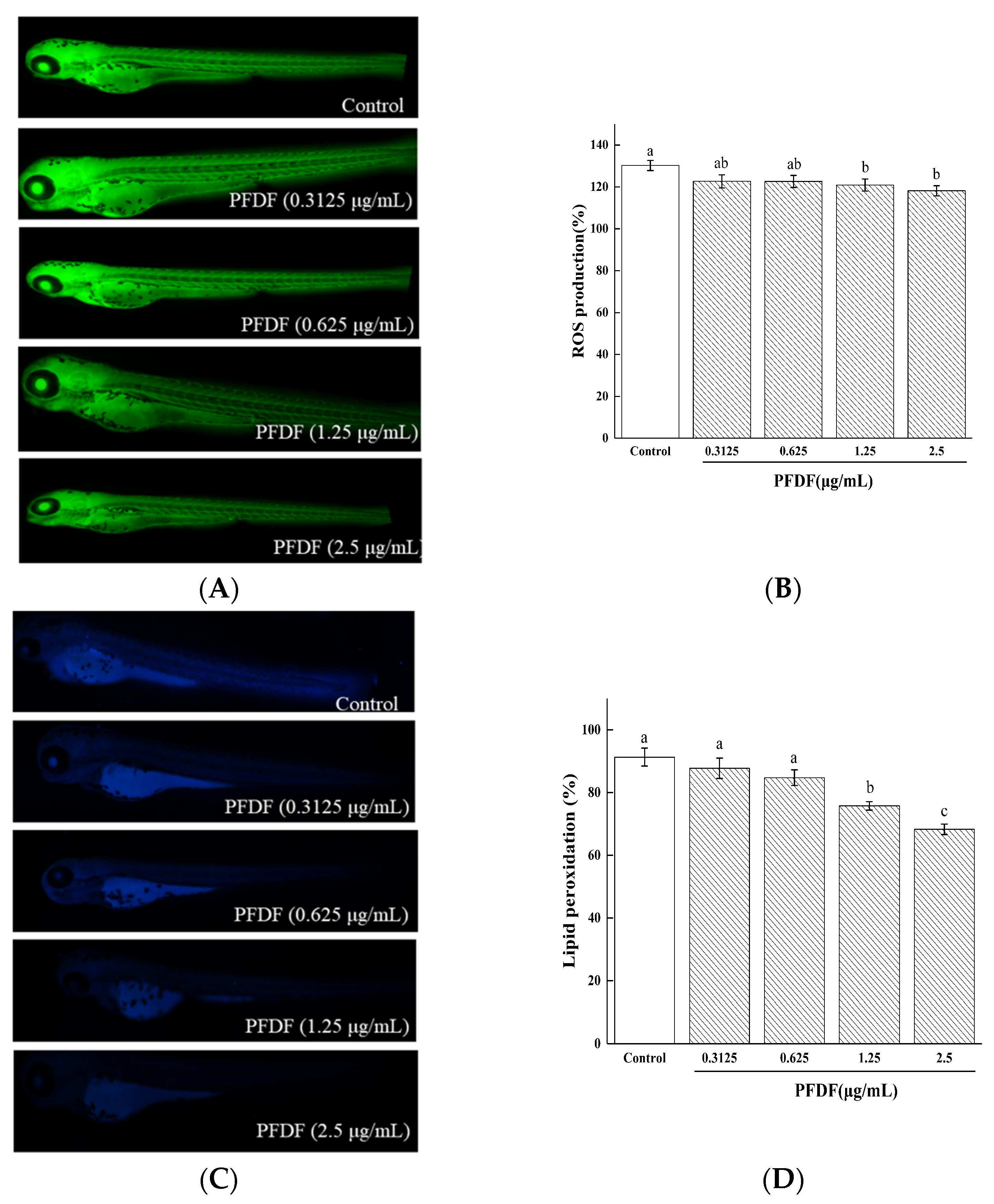
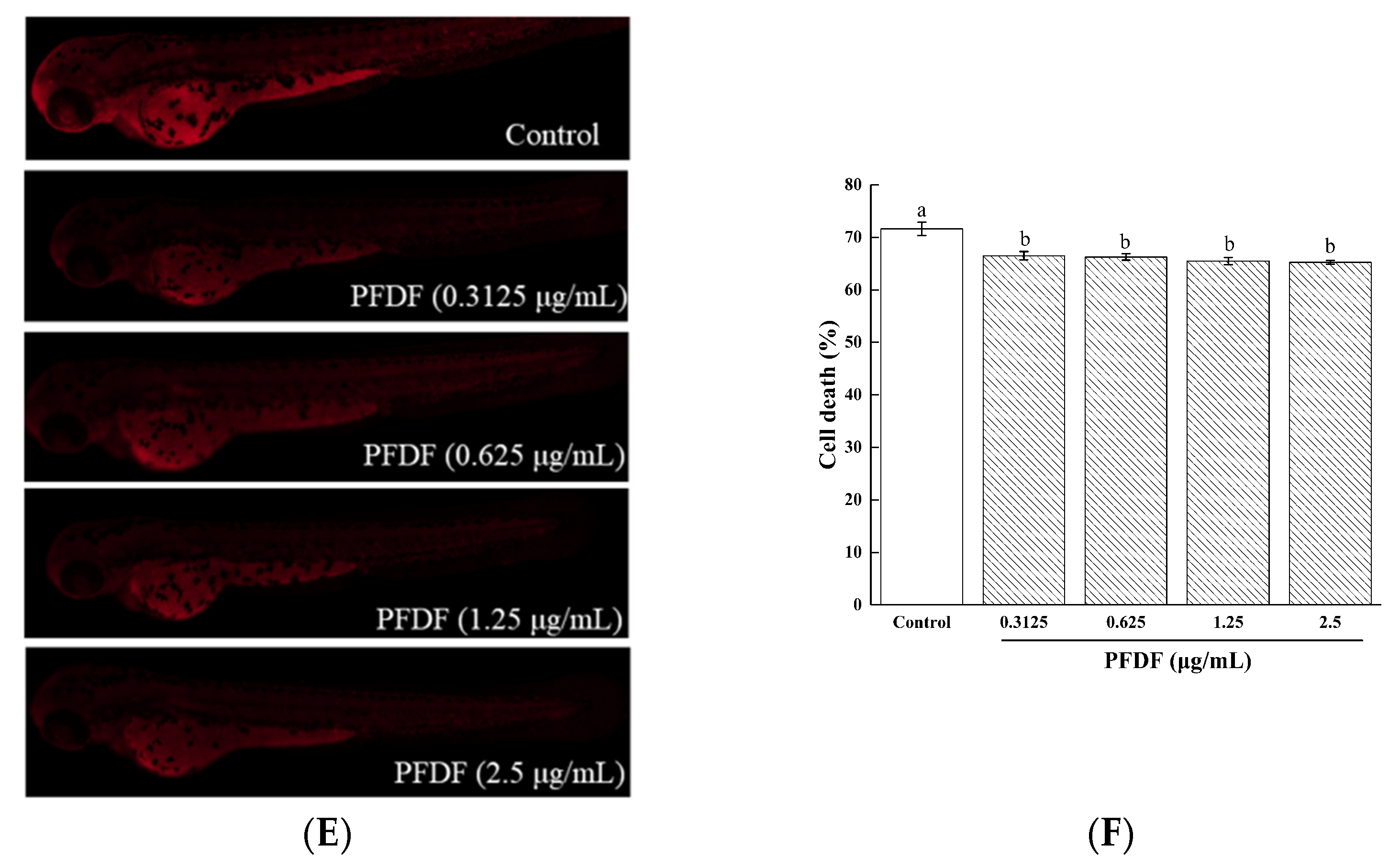
3.4. Intracellular ROS Production, Cell Death and Lipid Peroxidation of Zebrafish Embryo Treated with PFDF
3.5. Cell Proliferative Activity and MDA Content of IPEC-J2 Cells Treated with PFDF
3.6. Antioxidant Enzyme Activities and GSH Content of Zebrafish Embryo and IPEC-J2 Cells Treated with PFDF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huyut, Z.; Beydemir, Ş.; Gülçin, I. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef] [PubMed]
- Oztaskin, N.; Taslimi, P.; Maraş, A.; Gülcin, I.; Göksu, S. Novel antioxidant bromophenols with acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase inhibitory actions. Bioorganic Chem. 2017, 74, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.; Williams, C. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Nagao, A.; Seki, M.; Kobayashi, H. Inhibition of Xanthine Oxidase by Flavonoids. Biosci. Biotechnol. Biochem. 1999, 63, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Hagymasi, K.; Blazovics, A.; Feher, J.; Lugasi, A.; Kristo, S.T.; Kery, A. The in vitro effect of dandelions antioxidants on microsomal lipid peroxidation. Phytother. Res. 2000, 14, 43–44. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine 2005, 12, 588–597. [Google Scholar] [CrossRef]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef]
- Hussain, A.; Bose, S.; Wang, J.-H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Liu, N.; Song, M.; Wang, N.; Wang, Y.; Wang, R.; An, X.; Qi, J. The effects of solid-state fermentation on the content, composition and in vitro antioxidant activity of flavonoids from dandelion. PLoS ONE 2020, 15, e0239076. [Google Scholar] [CrossRef]
- Zhang, G.; He, L.; Hu, M. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innov. Food Sci. Emerg. Technol. 2011, 12, 18–25. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, Y.; Chen, Z.; Zhao, G.; Liu, J.; Wang, X.; Gao, S.; Zhang, D.; Jia, L. Metabolomics analysis of the soapberry (Sapindus mukorossi Gaertn.) pericarp during fruit development and ripening based on UHPLC-HRMS. Sci. Rep. 2021, 11, 11657. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-J.; Schultz, A.W.; Wang, J.; Johnson, C.; Yannone, S.M.; Patti, G.J.; Siuzdak, G. Liquid chromatography quadrupole time-of-flight mass spectrometry characterization of metabolites guided by the METLIN database. Nat. Protoc. 2013, 8, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-Discovery Approach for Sample Matching of a Nerve-Agent Precursor Using Liquid Chromatography−Mass Spectrometry, XCMS, and Chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef]
- Yıldırım, A.; Mavi, A.; Kara, A.A. Determination of Antioxidant and Antimicrobial Activities of Rumex crispus L. Extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef] [PubMed]
- Musa, K.H.; Abdullah, A.; Al-Haiqi, A. Determination of DPPH free radical scavenging activity: Application of artificial neural networks. Food Chem. 2016, 194, 705–711. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Gannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweedhimanthalia elongatafrom western coast of ireland. J. Food Biochem. 2012, 37, 322–335. [Google Scholar] [CrossRef]
- Ko, J.-Y.; Kim, E.-A.; Lee, J.-H.; Kang, M.-C.; Lee, J.-S.; Kim, J.-S.; Jung, W.-K.; Jeon, Y.-J. Protective effect of aquacultured flounder fish-derived peptide against oxidative stress in zebrafish. Fish Shellfish Immunol. 2013, 36, 320–323. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, E.-A.; Kim, Y.-S.; Yu, S.-K.; Choi, C.; Lee, J.-S.; Kim, Y.-T.; Nah, J.-W.; Jeon, Y.-J. Protective effects of polysaccharides from Psidium guajava leaves against oxidative stresses. Int. J. Biol. Macromol. 2016, 91, 804–811. [Google Scholar] [CrossRef]
- Katsube, T.; Imawaka, N.; Kawano, Y.; Yamazaki, Y.; Shiwaku, K.; Yamane, Y. Antioxidant flavonol glycosides in mulberry (Morus alba L.) leaves isolated based on LDL antioxidant activity. Food Chem. 2006, 97, 25–31. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.-J.; Huang, J.-H.; Lv, B.-D.; Huang, X.-J.; Hu, Q.; Fu, J.; Huang, W.-J.; Tao, T.-T. Protective Effects of Total Flavonoids from Lysimachia christinae on Calcium Oxalate-Induced Oxidative Stress in a Renal Cell Line and Renal Tissue. Evidence-Based Complement. Altern. Med. 2021, 2021, 6667902. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Elmastaş, M.; Gülçin, I.; Işildak, Ö.; Küfrevioğlu, I.; Ibaoğlu, K.; Aboul-Enein, H.Y. Radical scavenging activity and antioxidant capacity of bay leaf extracts. J. Iran. Chem. Soc. 2006, 3, 258–266. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Tembhre, M.; Shammi, Q.J.; Ahirwar, P.; Akram, M.A. In vitro antioxidant activity, total phenolic and total flavonoids contents of Taraxacum officinale leaves. Int. J. Innov. Pharm. Sci. Res. 2015, 3, 697–707. [Google Scholar]
- Gülçın, I.; Oktay, M.; Kıreçcı, E.; Küfrevıoǧlu, Ö. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003, 83, 371–382. [Google Scholar] [CrossRef]
- Gülçin, I.; Elmastaş, M.; Aboul-Enein, H.Y. Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies. Phytother. Res. 2007, 21, 354–361. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Eisen, J.S. Zebrafish Make a Big Splash. Cell 1996, 87, 969–977. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Zhang, H.; Wang, Z.; Wu, Z.; Lu, J.; Di, C.; Zhou, X.; Wang, X. Effects of 12C6+ ion radiation and ferulic acid on the zebrafish (Danio rerio) embryonic oxidative stress response and gene expression. Mutat. Res. Mol. Mech. Mutagen. 2013, 745, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, H.; Gao, Y.; Meng, Y.; Zhao, X.-X.; Pan, S.-N. Effects of Dandelion Extract on the Proliferation of Rat Skeletal Muscle Cells and the Inhibition of a Lipopolysaccharide-Induced Inflammatory Reaction. Chin. Med. J. 2018, 131, 1724–1731. [Google Scholar] [CrossRef]
- Luo, J.; Song, J.; Liu, L.; Xue, B.; Tian, G.; Yang, Y. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult. Sci. 2018, 97, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magied, N.; Abdel Fattah, S.M.; Elkady, A.A. Differential effect of Taraxacum officinale L.(dandelion) root extract on hepatic and testicular tissues of rats exposed to ionizing radiation. Mol. Biol. Rep. 2019, 46, 4893–4907. [Google Scholar] [CrossRef]
- Giribabu, N.; Rao, P.V.; Kumar, K.P.; Muniandy, S.; Rekha, S.S.; Salleh, N. Aqueous Extract of Phyllanthus niruri Leaves Displays In Vitro Antioxidant Activity and Prevents the Elevation of Oxidative Stress in the Kidney of Streptozotocin-Induced Diabetic Male Rats. Evidence-Based Complement. Altern. Med. 2014, 2014, 834815. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Han, X.; Song, X.; Zhang, Y.; Jiang, J.; Han, Q.; Liu, M.; Qiao, G.; Zhuo, R. Overexpressing the Sedum alfredii Cu/Zn Superoxide Dismutase Increased Resistance to Oxidative Stress in Transgenic Arabidopsis. Front. Plant Sci. 2017, 8, 1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lis, B.; Rolnik, A.; Jedrejek, D.; Soluch, A.; Stochmal, A.; Olas, B. Dandelion (Taraxacum officinale L.) root components exhibit anti-oxidative and antiplatelet action in an in vitro study. J. Funct. Foods 2019, 59, 16–24. [Google Scholar] [CrossRef]
- Park, C.M.; Park, J.Y.; Noh, K.H.; Shin, J.H.; Song, Y.S. Taraxacum officinale Weber extracts inhibit LPS-induced oxidative stress and nitric oxide production via the NF-κB modulation in RAW 264.7 cells. J. Ethnopharmacol. 2011, 133, 834–842. [Google Scholar] [CrossRef]
- Chen, D.; Sun, H.; Shen, Y.; Luo, M.; Xin, X.; Xu, Z. Selenium bio-absorption and antioxidant capacity in mice treated by selenium modified rice germ polysaccharide. J. Funct. Foods 2019, 61, 103492. [Google Scholar] [CrossRef]
- Cai, L.; Wan, D.; Yi, F.; Luan, L. Purification, Preliminary Characterization and Hepatoprotective Effects of Polysaccharides from Dandelion Root. Molecules 2017, 22, 1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Y.; Siow, Y.; Isaak, C.K. Downregulation of Glutathione Biosynthesis Contributes to Oxidative Stress and Liver Dysfunction in Acute Kidney Injury. Oxidative Med. Cell. Longev. 2016, 2016, 9707292. [Google Scholar] [CrossRef] [PubMed] [Green Version]


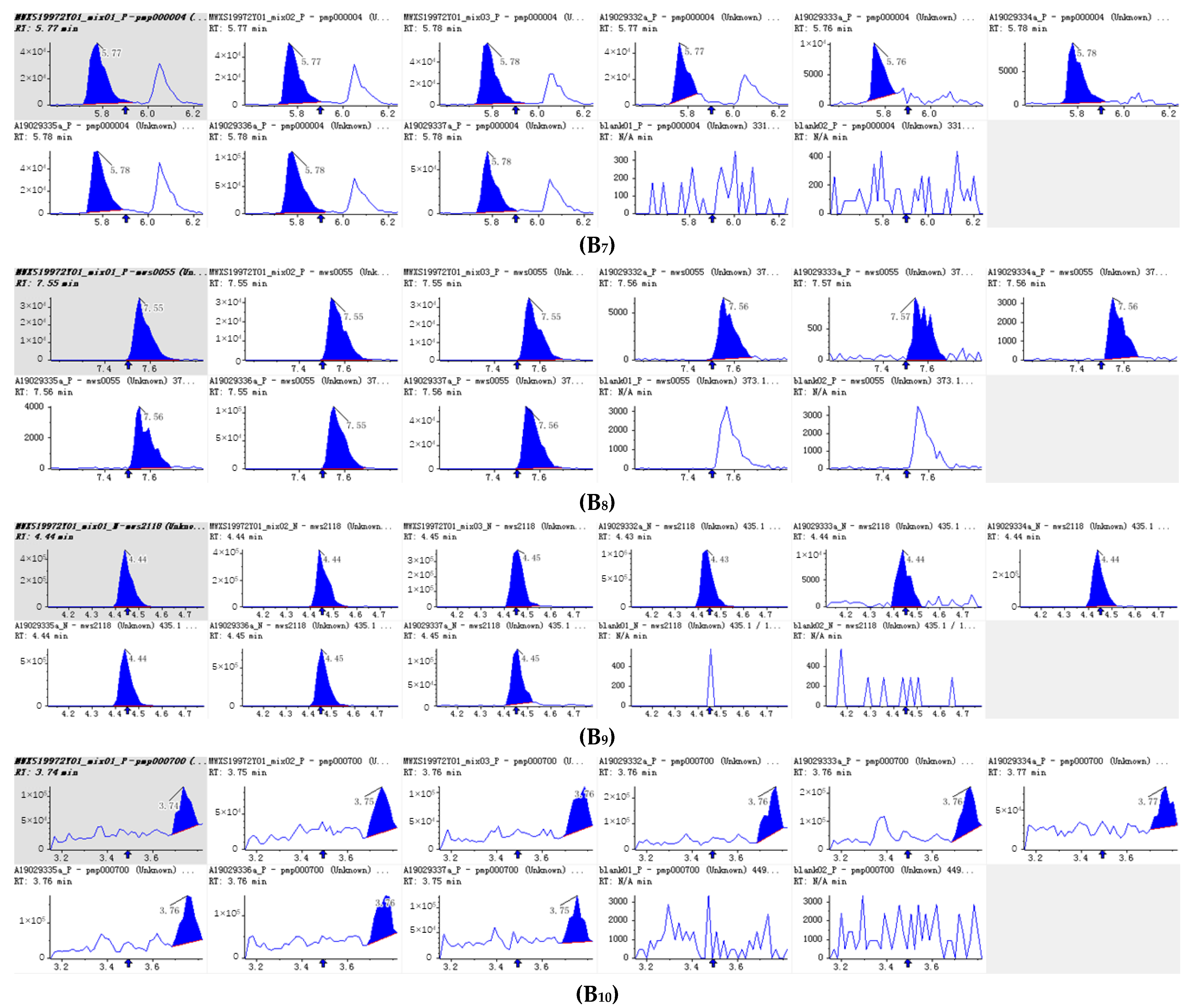

| Molecular Weight (Da) | Formula | Compounds | Class II | Relative Content | |
|---|---|---|---|---|---|
| A1 | 449.1 | C21H20O11 | Luteolin 7-O-glucoside (Cynaroside) | Flavonoid | 23,420,000 |
| A2 | 593.16 | C27H30O15 | Kaempferol 3-O-rutinoside (Nicotiflorin) | Flavonols | 21,362,000 |
| A3 | 449.11 | C21H20O11 | Quercetin-3-O-α-L-rhamnopyranoside | Flavonols | 20,955,000 |
| A4 | 595.16 | C27H30O15 | Kaempferol-3-O-neohesperidoside | Flavonols | 17,111,000 |
| A5 | 595.16 | C27H30O15 | Luteolin-7-O-rutinoside | Flavonoid | 16,898,000 |
| A6 | 593.16 | C27H30O15 | Kaempferol 3-O-robinobioside (Biorobin) | Flavonols | 16,529,000 |
| A7 | 595.16 | C27H30O15 | Kaempferol-3-O-glucoside-7-O-rhamnoside | Flavonols | 13,746,000 |
| A8 | 595.17 | C27H30O15 | Tetrahydroxyflavone-C-rhamnosyl-glucoside | Flavonoid carbonoside | 12,264,000 |
| A9 | 595.16 | C27H30O15 | Lonicerin | Flavonoid | 12,034,000 |
| A10 | 435.08 | C20H18O11 | Avicularin | Flavonols | 11,444,000 |
| Molecular Weight (Da) | Formula | Compounds | Class II | Relative Content | |
|---|---|---|---|---|---|
| B1 | 286.04 | C15H10O6 | Luteolin | Flavonoid | 4,983,700 |
| B2 | 300.05 | C16H12O6 | Hispidulin | Flavonoid | 3,514,700 |
| B3 | 300.05 | C16H12O6 | Diosmetin | Flavonoid | 3,472,700 |
| B4 | 304.05 | C15H12O7 | Taxifolin | Dihydroflavonol | 3,057,300 |
| B5 | 330.07 | C17H14O7 | Tricin | Flavonoid | 1,137,200 |
| B6 | 286.05 | C15H10O6 | Isoscutellarein | Flavonoid | 1,030,300 |
| B7 | 330.07 | C17H14O7 | Jaceosidin | Flavonoid | 522,290 |
| B8 | 372.11 | C20H20O7 | Tangeretin | Flavonols | 500,730 |
| B9 | 274.07 | C15H14O5 | Phloretin | Chalcones | 498,600 |
| B10 | 448.08 | C21H20O11 | Isoorientin | Flavonoid carbonoside | 436,490 |
| PFDF (μg/mL) | Proliferative Activity of IPEC-J2 Cells (%) | Malondialdehyde (nmol/mg Prot) |
|---|---|---|
| control | 100 bc | 0.53 a |
| 0.625 | 97.31 c | 0.41 ab |
| 1.25 | 102.10 abc | 0.37 ab |
| 2.5 | 122.11 a | 0.23 b |
| 5 | 120.49 ab | 0.28 b |
| 10 | 96.10 c | 0.42 ab |
| SEM | 2.40 | 0.02 |
| p-value | 0.0296 | 0.0248 |
| PFDF (μg/mL) | Superoxide Dismutase (U/mg Prot) | Catalase (U/mg Prot) | Glutathione Peroxidase (U/mg Prot) |
|---|---|---|---|
| control | 16.88 | 7.13 | 1.43 a |
| 0.3125 | 16.88 | 4.27 | 5.98 a |
| 0.625 | 15.05 | 6.11 | 4.29 a |
| 1.25 | 16.64 | 7.61 | 21.23 b |
| 2.5 | 15.77 | 7.41 | 18.81 b |
| SEM | 0.36 | 0.62 | 4.03 |
| p-value | 0.4186 | 0.1808 | 0.0011 |
| PFDF (μg/mL) | Superoxide Dismutase (U/mg Prot) | Catalase (U/mg Prot) | Glutathione Peroxidase (U/mg Prot) | Glutathione (μmol/g Prot) |
|---|---|---|---|---|
| control | 3.18 b | 21.42 b | 226.27 | 3.63 c |
| 0.625 | 4.57 b | 22.64 b | 205.80 | 4.84 ab |
| 1.25 | 5.68 ab | 25.43 b | 250.25 | 4.97 ab |
| 2.5 | 6.15 ab | 25.97 b | 235.13 | 5.10 ab |
| 5 | 8.60 a | 34.14 a | 387.27 | 5.46 a |
| 10 | 8.32 a | 33.57 a | 371.65 | 4.11 bc |
| SEM | 0.38 | 0.77 | 12.96 | 0.11 |
| p-value | 0.0073 | 0.0014 | 0.1234 | 0.0288 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, N.; Wang, Y.; Ren, X.; Zhao, Y.; Liu, N.; An, X.; Qi, J. Isolation and Characterization of Flavonoids from Fermented Dandelion (Taraxacum mongolicum Hand.-Mazz.), and Assessment of Its Antioxidant Actions In Vitro and In Vivo. Fermentation 2022, 8, 306. https://doi.org/10.3390/fermentation8070306
Yin N, Wang Y, Ren X, Zhao Y, Liu N, An X, Qi J. Isolation and Characterization of Flavonoids from Fermented Dandelion (Taraxacum mongolicum Hand.-Mazz.), and Assessment of Its Antioxidant Actions In Vitro and In Vivo. Fermentation. 2022; 8(7):306. https://doi.org/10.3390/fermentation8070306
Chicago/Turabian StyleYin, Na, Yuan Wang, Xuerong Ren, Yang Zhao, Na Liu, Xiaoping An, and Jingwei Qi. 2022. "Isolation and Characterization of Flavonoids from Fermented Dandelion (Taraxacum mongolicum Hand.-Mazz.), and Assessment of Its Antioxidant Actions In Vitro and In Vivo" Fermentation 8, no. 7: 306. https://doi.org/10.3390/fermentation8070306
APA StyleYin, N., Wang, Y., Ren, X., Zhao, Y., Liu, N., An, X., & Qi, J. (2022). Isolation and Characterization of Flavonoids from Fermented Dandelion (Taraxacum mongolicum Hand.-Mazz.), and Assessment of Its Antioxidant Actions In Vitro and In Vivo. Fermentation, 8(7), 306. https://doi.org/10.3390/fermentation8070306







