Abstract
The halophyte plant species Salicornia europaea and Salicornia ramosissima were investigated for their potential to serve as a substrate for biogas production. Salicornia europaea was cultivated in hydroponic systems under varying salt concentrations (0, 10, 20, and 30 g/L NaCl), while S. ramosissima was grown in greenhouse farming with aquaculture effluent irrigation. The biomethane potential of the two halophyte feedstocks was determined through batch experiments, and correlations to the plant biochemical composition were investigated. Ash and mineral content of S. europaea was correlated to the increasing salt concentration used for plant cultivation in hydroponic systems. No indication of inhibition of the anaerobic digestion process was detected for sodium concentrations of up to 2400 mg/L in the anaerobic batch-test assays. The highest biomethane yield of S. europaea of 250 mL CH4/gVS was obtained when grown under 20 g/L NaCl and up to 300 mL CH4/gVS for S. ramosissima. By concentrating the dry matter content, the biomethane yield per ton of feedstock could be increased from 24 m3 CH4/t of the fresh halophyte plant to 74 m3 CH4/t by fractionation into a pulp fraction and to 149 m3 CH4/t by drying of the plant at room temperature for 1 week.
1. Introduction
Halophytes have the ability to thrive optimally in high salt conditions and possibly irrigate using saline water by sustaining favorable biomass or seed yields and eventually assuring food security in land areas affected by salinity. For this reason, halophytes and their residues from food production are potential candidates also as bioenergy crops for saline environments that, due to their perennial life cycle, permit harvesting for many years without reseeding and maintaining high biomass yields with relatively low lignocellulosic content [1,2,3]. According to the findings of Glen et al. [4], members of the Salicornia genus can be cultivated for food, fodder/forage, medicinal, and as oilseed crops, with the most productive species yielding 13 to 25 tons/ha of dry matter using seawater irrigation and on average 2 tons/ha of seeds over a 200-day developing cycle, comparable to conventional crops. Therefore, based on the extent of their diversity, salt-tolerant plant species can be considered a valuable feedstock for the production of bioenergy through anaerobic digestion (AD).
Anaerobic digestion of complex organic matter encompasses a sequence of conversion processes, from hydrolysis to methanogenesis, that is carried out by a consortium of microorganisms that operate in synergy to produce methane (CH4) and carbon dioxide (CO2). The accumulation of intermediate products such as volatile fatty acids (VFA) depends largely on the balance of the activity of the different microbial groups, while the formation of other compounds such as hydrogen sulfide (H2S), ammonia, depends on, e.g., the sulfur and protein content of the biomass [1,5]. The AD process is firmly established for the conversion of organic waste such as agricultural residues, food waste, and animal slurry and of energy crops such as cereals, maize, and clover and their residues and other organic materials into biogas/biomethane [6,7,8]. However, limited research has been carried out on AD of halophytic biomass for biogas production.
One unambiguous challenge encountered in the AD of halophytic biomass is associated with the high salinity within the cells and tissues of the biomass as well as of the saltwater attached to the plant material. More specifically, the potential and possible inhibitory effect of halophyte biomass as a substrate for the AD process is dependent on the content of carbohydrates, proteins, fats and lignin, and salt, namely the salts of sodium (Na), potassium (K), calcium (Ca), magnesium (Mg) and phosphorous (P) [2,9]. According to literature, the main inhibiting element, Na, of the light earth metals has been reported to cause strong inhibition of the methanogens at mesophilic conditions at concentrations of 8000 mg/L of anaerobic medium [10,11,12]. Likewise, the presence of some secondary metabolites in biomass feedstocks can potentially hinder the activity of biomass-degrading microbes, to differing degrees, during the AD process [13,14]. As reported in previous studies, halophytic plant material contains a variety of phytochemicals [15,16,17], in particular phenolic compounds, which are considered antimicrobial and can probably cause inhibition in the production of biomethane [10]. Nevertheless, the composition of halophytic biomass differs significantly based on the genotype, specific species, life cycle status, mode of cultivation (whether using a hydroponic system, greenhouse seawater irrigation system, or naturally grown), and climate conditions [1].
The aim of this study was to determine the biomethane potential (BMP) of the halophyte plant material and to investigate correlations of the BMP to the plant composition. In order to identify thresholds for salt inhibition and the influence of other plant compounds, the experimental BMP values are compared to the theoretical biomethane potential (TBMP) derived from elemental analysis and are correlated to the ash, sodium, and lignin content of the halophyte plant material. Based on the thresholds for salt inhibition, the maximum admixing ratios of halophyte plant material in co-digestion with other non-saline biomass such as manure or sewage sludge are calculated.
2. Materials and Methods
2.1. Halophyte Plant Material and Residues Used for the Biogas Potential Tests
The halophytic biomass used in this study was the above-ground plant part, collected from different cultivation conditions and under varying saline conditions.
Salicornia europaea was cultivated in hydroponic systems at the Institute of Botany, Leibniz University Hannover (LUH), Germany, with variations in NaCl concentrations (10, 20, 30 g/L NaCl) in the nutrient solution as well as under non-saline conditions. Each experimental unit consisted of eight plants per container, with three replicates per treatment. Polypropylene containers (L400 × W300 × H175 mm) with a capacity of 16 L were used. Each container had 13 L of modified Hoagland solution, containing 606 mg/L KNO3, 944 mg/L Ca(NO3)2·4H2O, 230 mg/L NH4H2PO4, 246 mg/L MgSO4·7H2O, 3.73 mg/L KCl, 1.55 mg/L H3BO3, 0.34 mg/L MnSO4·H2O, 0.58 mg/L ZnSO4·7H2O, 0.12 mg/L CuSO4·5H2O, 0.12 mg/L MoNa2O4·2H2O, and 9.16 mg/L Fe-EDDHA (0.56 mg Fe/L). The water was aerated constantly by small compressors and one air stone in the middle of each tank (Eheim, Deizisau, Germany). The hypocotyl was fixed with soft foam in 35 mm holes. The water level was adjusted constantly in each tank with tap water to compensate the evapotranspiration. The halophytes were cultivated in a greenhouse at temperatures of 35/21 °C during day/night. A total of 14 h of artificial light was provided (sodium vapor lamps, SON-T Agro 400W, Philips, Amsterdam, The Netherland). Light intensity ranged from 65 µmol/m2 s to 740 µmol/m2 s depending on the season, the time of the day, and the weather conditions. The plants were harvested after 5 weeks in the hydroponic cultures. To avoid further modification of the biomass composition and to facilitate the transport and storage of the biomass, the plant material was dried at 60 °C for 48 h prior to composition and BMP analysis. For scaling-up experiments, these drying conditions can be used as the reference standard conditions. However, it is important to note that both dry and fresh biomass can be used for anaerobic digestion.
Salicornia ramosissima was cultivated in greenhouse conditions, under natural photoperiod, at Riasearch (RSR) facilities in Murtosa, Portugal. Prior to germination of locally harvested seeds, freshwater was used to maintain the soil moisture. The seeds were sown in a 2 g/m2 density and irrigated twice daily utilizing a mixture of the recirculating aquaculture system (RAS) effluents, with salinity around 18 g/L NaCl, and freshwater, in order to keep soil salinity around 12 g/L NaCl. The aquaculture effluents were from shrimp (Penaeus vannamei) and European seabass (Dicentrarchus labrax) cultivation. After a growth phase of approximately 5 months, S. ramosissma biomass was harvested after it began to flower. The estimated green biomass production was around 1.85 kg/m2 per year. The fresh green succulent plants were harvested, and the roots discarded. After using the green tips for food production, the plants were divided in two groups. The whole green plants were packed in styrofoam boxes with cold packs and shipped for approximately 48 h to Flensburg University of Applied Sciences (FUAS) for composition and BMP analysis. At FUAS, a fraction of the S. ramosissima whole green plant samples was separated into a pulp and a juice fraction by wet-pressing in a juice extractor (Green Star GS-2000, Tribest, Anaheim, California, USA). In Portugal, the mature plants were dried on mesh trays in the shade, for 1 week, at room temperature (min. 18 °C, max. 28 °C), simulating the large-scale drying in a greenhouse without additional heat supply. The plants were moved on the trays daily in order to prevent the development of fungus and to accelerate the drying process. The dried mature plants were packed in styrofoam boxes and shipped at room temperature to FUAS.
2.2. Biomass Composition Analysis
The total solids (TS), volatile solids (VS), and ash content of the halophyte plant biomass were determined according to standard methods DIN EN 12880 and DIN EN 12879, respectively [18,19]. The TS content of the substrates was determined by drying at 105 °C until constant weight (approximately 24 h). The VS content was measured by subsequent incineration of the TS at 550 °C for 3 h in a muffle furnace (M104, Thermo Fisher Scientific Corporation, Waltham, MA, USA).
2.2.1. Content of Carbohydrates, Lignin, and Proteins
Carbohydrates and Klason lignin (acid-insoluble lignin) of the substrates were analyzed according to the standard protocols developed by the National Renewable Energy Laboratory (NREL) [20]. The dried (>85% TS), milled and sieved (1 mm screen) plant material was subjected to strong acid hydrolysis (SAH) using 72 w/w% sulfuric acid (H2SO4) for 1 h at 30 °C, followed by diluting the acid to 4% and autoclavation at 121 °C for 1 h. A mixture of glucose, xylose, and arabinose was used as spike solution. The hydrolysate from SAH was filtered, and Klason lignin content was measured as the difference between the ash content and weight of insoluble residue. The monomeric sugars glucose, xylose, and arabinose released in the hydrolysate were analyzed using a Hitachi Chromaster High-Performance Liquid Chromatography (HPLC, Hitachi High-Tech Corporation, Tokio, Japan), mounted with a Macherey Nagel (Düren, Germany) Nucleogel Sugar 810 H column and reflective index (RI) detector at 63 °C using 5 mM H2SO4 as the eluent (mobile phase) with a 0.4 mL/min flowrate.
To analyze for easily dissolvable sugars in the liquid fraction after fractionation of S. ramosissima the liquid sample was subjected to weak acid hydrolysis (WAH) by digesting with 8 w/w% H2SO4 at 121 °C with subsequent analysis of the three monomeric sugars using HPLC.
Crude protein content was determined based on the nitrogen content by a nitrogen-to-protein conversion factor of 6.25.
2.2.2. Elemental Analysis
The elemental analysis of carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) contents was performed using a CHNS-O Thermo Scientific FlashSmart elemental analyzer with BBOT (2,5-Bis(5-tert-butyl-benzoxazol-2-yl)thiophene) as standard. The oxygen (O) content was determined by assuming C + H + N + S + O + ash = 99.5% (on TS basis) [21,22,23].
2.2.3. Mineral Content in Substrates and Inoculum
The mineral content of the plant material with respect to Na, K, Ca, Mg, and P was analyzed by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) (iCAP 6000 ICP Spectrometer, Thermo Fisher Scientific Corporation, Waltham, MA, USA), and the procedure was carried out as described by Weese et al. [24]. Briefly, about 38 mg of fine powder from each sample was incinerated for 8 h in a muffle furnace (M104, Thermo Fisher Scientific Corporation, Waltham, MA, USA). After cooling the samples to room temperature, 1.5 mL of nitric acid (diluted 1:3, Rotipuran® Supra 69%) was added. After 10 min, 13.5 mL of ultrapure water was added. The solution was filtered (0.45 μm pore size, Carl Roth) and stored in vials at 4 °C before final analysis. An empty vial was treated in parallel with the samples and later used as a blank. The mineral content of juice fraction of S. ramosissima was calculated based on the TS content as a difference between the whole plant and pulp fraction post fractionation.
2.3. Theoretical BMP and Biodegradability
The TBMP was calculated based on the elemental composition C, H, O, N, and S of the halophyte plant material and on the extended reaction equation Equations (1) and (2) proposed by Boyle [25]. This equation includes nitrogen and sulfur to calculate the production of ammonia and hydrogen sulfide. The general chemical formula CcHhOoNnSs was determined from the elemental analysis. The TBMP was hinged on the subsequent assumptions [26]:
- optimal microbial condition, constant temperature, and ideal mixing, leading to complete anaerobic degradation of the organic matter content (no differentiation of biodegradable and non-biodegradable compounds);
- input substrate organic matter consists only of the elements C, H, O, N and S, and gaseous products resulting from anaerobic digestion are CH4, CO2, NH3, and H2S.
The anaerobic biodegradability (BDCHONS) was determined by the ratio of the cumulative methane yield from experimental BMP batch assay to TBMP, as shown in Equation (3).
2.4. Experimental Biomethane Potential (BMP)
The BMP of the different halophyte samples was determined using the Automated Methane Potential Test System II (AMPTS II, Bioprocess Control Sytems, Lund, Sweden). The AMPTS II is equipped with pressure and temperature sensors that are used to normalize the produced gas volume at standard temperature and pressure (STP). All batch fermentation assays were conducted in 500 mL flasks in triplicates under mesophilic conditions 37 ± 2 °C according to German Standard, VDI 4630 using an inoculum to substrate ratio (ISR) ≥ 2 (on VS basis) and a TS concentration in the batch assay of ≤10% to ensure sufficient mass transfer [27].
Inoculum for the biomethane potential (BMP) tests was sludge taken from an anaerobic digester of the Wastewater Treatment Plant (WWTP) in Flensburg, Germany, operating at mesophilic conditions (38–42 °C). The fresh inoculum was incubated at 37 ± 2 °C for up to 5 days to minimize the production of background gas and influence of the blanks prior to inoculation. The TS and VS concentration of the inoculum was 2.0 wt% (weight percent) TS and 60 wt% VS of TS, respectively.
The batch tests were operated until the daily gas production was less than 1 vol% of the accumulated gas production on three consecutive days. Since the inoculum also contains biodegradable material, the gas yield of blank samples only containing inoculum was deducted from the gas produced from the batches containing substrate and inoculum. Microcrystalline cellulose was used as a reference to evaluate inoculum activity and its ability to degrade cellulosic polymers.
3. Results and Discussion
3.1. Salt Content of Halophyte Plant Material
The TS, VS, and ash content of the different Salicornia samples used in this study are shown in Table 1. The results of the ash content show that the content of inorganics is relatively high for halophytes, ranging from 22 wt% to 52 wt% of TS in the plant materials, similar to the findings for halophytic plants [17,28,29,30,31]. After green fractionation of the halophyte plants, a major part of the minerals is found in the juice fraction, resulting in ash content of 58 wt% of TS in the juice, while the ash content in the separated fibers is only 12 wt% of TS.

Table 1.
TS, VS and ash content of Salicornia samples in wt% of fresh mass (FM) and wt% of TS, standard deviation of triplicates in parentheses. For Salicornia europaea the biomass values after drying of the fresh plant material are given.
For the plant samples of S. europaea cultivated at different NaCl concentrations, the ash content increases for the halophytes cultivated at 10 and 20 g/L NaCl, but not as much for higher NaCl concentrations (Table 1).
The same tendency can be seen for the Na content measured in the plant tissue of S. europaea (Table 2), which increases from 68.0 mg/gTS under non-saline conditions to 190 mg/gTS for 20 g/L NaCl, but no further for 30 g/L NaCl in the culture medium. This indicates that higher NaCl concentration in the culture medium leads to a higher NaCl uptake in the plant tissue, only up to 20 g/L NaCl in the culture medium, which shows that the plant is able to regulate its salinity level to maintain a certain salt concentration in their cells and tissues to sustain its water potential gradient and osmotic pressure required for water intake [1].

Table 2.
Mineral content of Salicornia samples in mg/gTS, standard deviation of triplicates in parentheses.
The concentrations of K, Ca, Mg, and P, on the other hand, decreased with the higher NaCl concentration used for cultivation (Table 2), indicating that the higher osmotic pressure is counterbalanced by a lower uptake of these ions. In this research, the increasing content of Na in the cultivating conditions of S. europaea and plant material could have caused low uptake of K owing to the antagonism and synergism between the elements [1,32]. Likewise, the Ca content was significantly suppressed at salinity higher than 10 g/L NaCl cultivating condition.
The content of Na, Mg, and P content of S. ramosissima cultivated in a greenhouse equipped with seawater irrigation was similar to the respective concentrations of S. europaea cultivated in the hydroponic system at varying salt concentrations. The concentrations of K and Ca were, however, much lower in S. ramosissima. Applying fractionation of the fresh succulent material into juice and pulp fraction showed that the mineral content in the pulp fraction was except for Ca, less than 50% of the content in the whole green plant. Furthermore, the majority of the mineral elements are left in the juice fraction post fractionation pretreatment. The mass balance and the separation of the different minerals, C, N, S, and lignin, are shown in Figure 1. Compared to the fractionation of red clover and clover grass by Santamaria-Fernandez et al. [33], the separation of C and N was very similar to the fractionation of red clover (60% and 53% in pulp fraction) while after fractionation of red clover the percentage of total mass, K and P was much higher in the pulp fraction (40%, 43%, and 53%) and of S much lower (52% in pulp fraction).
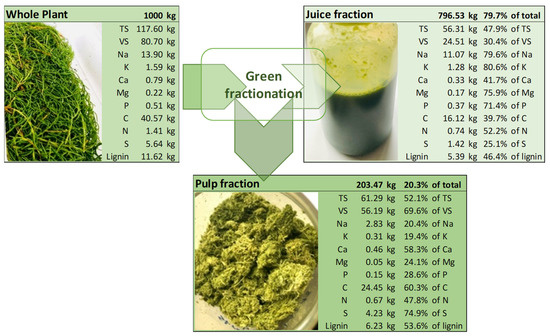
Figure 1.
Mass balance of green fractionation of S. ramosissima whole plant biomass into pulp and juice fraction in mass per 1000 kg of whole plant input and % of mass found in juice and pulp fraction.
In the dry mature S. ramosissima plant residues, the concentration of Na and Ca was 50% lower compared to the whole fresh plant, while the K concentration was about twice, indicating an increased uptake of K at this plant growth stage [32].
3.2. Content of Carbohydrates, Lignin and Proteins
The content of total sugars, acid-insoluble lignin, and proteins in the samples is shown in Table 3. The total sugars are the sum of the monomeric sugars analyzed by strong acid hydrolysis, i.e., glucose, xylose, and arabinose, while the protein content is based on the N content determined by the elemental analysis (Table 4). Of the three analyzed sugars, based on the total sugar content, 57% glucose, 33% xylose, and 10% arabinose were generally present in all Salicornia samples, which is in accordance with the analyses of Salicornia bigelovii [17,31,34]. The carbohydrate and lignin content of S. europaea was lower, while the protein content was higher compared to the S. ramosissima samples. The low sugar content measured in the S. europaea samples is not consistent with the content of organic matter in the dry matter of 49–79 wt% VS of TS (Table 1). This may indicate either the presence of another monomeric sugar that was not analyzed or a general deviation of the strong acid hydrolysis analysis of samples with high ash content.

Table 3.
Total sugars, lignin, and protein content of Salicornia samples in g/100 gTS, standard deviation of triplicates in parentheses.

Table 4.
Elemental analysis, C/N ratio and overall composition of Salicornia samples, standard deviation of triplicates in parentheses.
The difference in protein content between the two Salicornia species may be due to the difference in the growth stage at the time of harvesting. Salicornia ramosissima was harvested at the maturity stage with a higher lignification and, therefore, higher lignin and lower protein content. In contrast, S. europaea was harvested in the vegetative growth phase, not yet lignified with a highly active metabolism and, therefore, higher protein content.
For S. ramosissima, the sugar content was highest in the pulp fraction and similar in the dry mature plant and the whole green plant. The lignin content was higher in the dry, mature plant compared to all other samples, indicating that lignification increases as the plant matures. For S. europaea cultivated at different salinity, the sugar and lignin content generally increased while the protein content decreased with increasing NaCl concentration used for hydroponic cultivation. This inverse relationship between salinity and protein content of halophytic biomass was previously confirmed for Tripolium pannonicum and Chenopodium quinoa [29,30].
3.3. Elemental Composition of Organic Matter
The results of the elemental analysis of C, H, N, O, and S were used to derive the overall composition formula of the organic matter (VS) of the halophyte material and the C/N ratio (Table 4). The TBMP of the different halophyte samples calculated from the composition formula can be seen in Table 5. Furthermore, the sum of the elements C, H, N, O, and S reflects the wt% VS of TS, as shown in Table 1, of the substrates.

Table 5.
Experimental BMP, TBMP and BDCHONS of Salicornia samples in mL CH4/gVS, m3 CH4/tFM and %, standard deviation of triplicates in parentheses.
The C content of S. europaea (20–30 wt% of TS) was much lower than for S. ramosissima (34–40 wt% of TS), which is in accordance with the lower total sugars measured (Table 3), but in contrast with the similar concentration of VS of TS measured for the two Salicornia species (Table 1).
Due to the significant difference in C and N content of the two Salicornia species, the C/N ratio of S. europaea (7–8) was much lower than for S. ramosissima (30–41), probably due to harvesting at different physiological stages, S. europaea at a relatively early stage and S. ramosissima at a mature stage, rather than dependent on characteristics of the different species or growing conditions. According to the findings of Amon et al. [35], the optimum C/N range for a stable AD process is between 10–30, which was found to be below the minimum for S. europaea and above the maximum for S. ramosissima. Therefore, AD of S. europaea can result in a high release of ammonia, which may hinder the activity of methanogenic bacteria leading to the accumulation of VFA. On the contrary, utilizing S. ramosissima will eventually cause N deficiency in the entire microbial community, which may lead to decreasing methane production when digested as mono-substrate, based on reported research [35,36]. Consequently, to achieve an optimal C/N ratio, co-digestion is suggested with a carbon-rich substrate such as grass or straw for S. europaea and for S. ramosissima with a protein-rich substrate such as cow manure or lipid-rich sources. Likewise, Chen et al. [37] showed that co-digesting the halophyte Spartina alterniflora with cow manure lowered the C/N ratio, which ultimately improved degradation with lower VFA content and higher biomethane yields of 7–44%.
The results for S in Table 4 show that the sulfur content of S. ramosissima ranges between 5–7% TS, whereas for S. europaea 4–6% TS. The sulfur concentration of the S. europaea samples was lower with the increasing salinity of the culture medium. Compared to the composition analysis of red clover and clover grass by Santamaria-Fernandez et al. [33], the sulfur concentration per kgTS was 25 to 45 times higher in the halophyte samples. This difference in S content should be considered for the selection of feedstock for biogas production since anaerobic degradation of S-containing compounds leads to the formation of H2S with its toxic and corrosive effects.
3.4. Biomethane Potential of Halophytic Substrates
The course of the BMP batch assays is shown for S. europaea in Figure 2 and for S. ramosissima in Figure 3. Table 5 shows the final methane yields of all samples compared to the calculated TBMP based on the composition analysis.
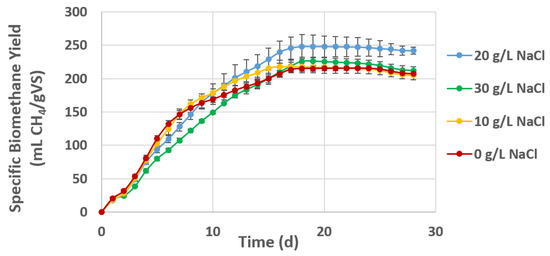
Figure 2.
Cumulative BMP of S. europaea cultivated under hydroponic conditions at different salinity concentrations (0, 10, 20 and 30 g/L NaCl). Values of each curve represent the average biomethane yield and standard deviation of triplicate BMP batch assays.
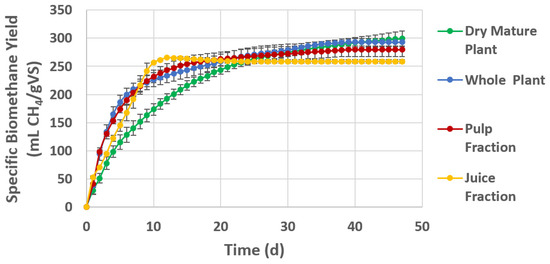
Figure 3.
Cumulative BMP of S. ramosissima cultivated through saline water irrigated greenhouse farming as whole green plant, dry mature plant and pulp and liquid fraction after fractionation. Values of each curve represent the average biomethane yield and standard deviation of triplicate BMP batch assays.
The course of the experimental BMP assay shows a relatively rapid degradation for all S. europaea samples with a digestion duration of less than 20 d (Figure 2). For S. ramosissima, the degradation generally takes longer, especially for the dry mature plant material, indicating a higher content of less degradable organic matter (Figure 3).
The final methane yields of S. ramosissima (280–299 mL CH4/gVS) were, however, higher than the BMP of S. europaea, which were in the range of 200–250 mL CH4/gVS. The biodegradability values are accordingly higher for S. ramosissima compared to S. europaea. The highest final yield for S. europaea of 248 mL CH4/gVS was found for the plant cultivated at 20 g/L NaCl.
The similar methane yield of the pulp fraction compared to the whole green plant of S. ramosissima indicates a similar degradability of the pulp fraction. However, the longer digestion time of more than 30 d indicates a slower hydrolysis process due to the lignocellulosic content (Figure 3).
Compared to methane yields of non-halophyte biomass, the measured values are lower than of green grass biomass and similar to the yields of lignocellulosic biomass. Previous literature reported an average specific methane yield of 250–450 mL CH4/gVS for maize silage, 200–250 mL CH4/gVS for straw, and 300–450 mL CH4/gVS for grass [7,8].
The biomethane yields per ton of FM of the halophyte feedstock were 110–164 m3 CH4/tFM for the dried samples of S. europaea and 23.6, 74.4, 7.8, and 149 m3 CH4/tFM for the whole green plant, pulp fraction, juice fraction and dry mature plant of S. ramosissima, respectively. This indicates that drying and fractionation of a pulp fraction of the halophyte plant material increase the methane yield per ton by a factor of 3–7. For the different dried samples of S. europaea, it can be seen that the higher ash content of the samples cultivated at higher NaCl concentrations leads to a lower methane yield per ton of the feedstock even though the specific methane yield per gVS is higher.
The lower values of BMP can be due to the recalcitrance nature of lignin present in the substrates as this composite structural constituent of biomass physically shields the available carbohydrates from degrading enzymes to be converted to fermentable sugars, whereas the TBMPs are based on complete anaerobic degradation.
The relatively high TBMP values of the dry mature S. ramosissima are presumably due to higher lignin content that is characterized by a lower oxygen/carbon ratio. The anaerobic biodegradability of the organic matter content of the halophyte biomass based on elemental content varied from 47–71% for S. europaea and 55–63% for S. ramosissima substrates.
3.5. Mineral Concentration in Batch Tests
An overview of the mineral concentration in the BMP batch-test set-up of the mixture of halophyte plant material and inoculum is shown in Table 6. The mineral content in the batch assay was calculated based on the sum of the TS added per liter in the test set-up from the inoculum and substrate samples. The Na content in the batches of S. europaea grown at different salinities increased from 814 mg/L for the samples cultivated under non-saline conditions to 2458 mg/L for the samples cultivated at 30 g/L NaCl. The different fractions of S. ramosissima showed the highest Na content of 2349 mg/L in the juice fraction, then 1434, 887, and 675 mg/L in the whole green plant, dry mature plant, and pulp fraction, respectively.

Table 6.
Mineral concentration in the BMP batch test set-up of mixtures of Salicornia samples and inoculum in mg/L.
The presence of these different elements in the anaerobic process is necessary for microbial development. In particular, sodium is vital for methanogenic bacteria and archaea, but in high concentrations can lead to inhibition [29]. The development of mesophilic anaerobes is beneficial with Na content between 100–200 mg/L [7,8]. McCarty [11] investigated the inhibitory effect of Na contents and reported that sodium is considered the main inhibiting element of light earth metals with moderate inhibitory effects ranging between 3500–5500 mg/L and strong inhibition at 8000 mg/L to the methanogens under mesophilic conditions.
In the conducted experiments, S. europaea cultivated in non-saline conditions had the highest Ca content of 433 mg/L and 246 mg/L in the whole green S. ramosissima plants, which were both slightly above the optimal condition for methanation of acetic acid of 200 mg/L but much below the lower limit of the moderate inhibitory concentration of 2500–4000 mg/L reported by Kugelman and McCarty [12]. However, on the contrary, Jackson-Moss et al. [38] reported no inhibitory effect on AD at 7000 mg Ca/L, with the majority of the calcium content found in the effluent. Furthermore, the potassium concentration in the anaerobic BMP test set-up was below the 400 mg K/L threshold, whereas magnesium content was much lower than the optimum of 720 mg Mg/L for anaerobic bacteria [10].
3.6. Correlation of Mineral and Lignin Content and BMP
An overview of the ash, Na, and lignin content and the biomethane yield of the different samples of S. europaea and S. ramosissima is given in Figure 4. The correlations of ash, Na, and lignin content and experimental BMP of the different samples of S. europaea and S. ramosissima are shown in Figure 5 with the equations and statistical R2 factors of the respective linear regression lines.
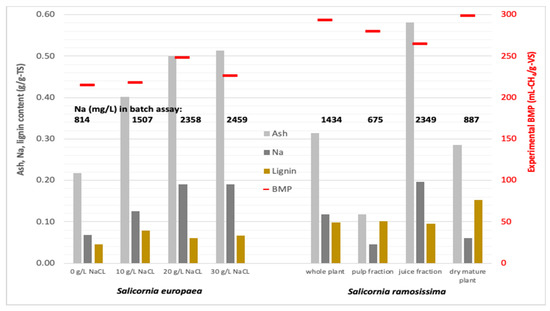
Figure 4.
Experimental BMP and ash, Na and lignin content of the different samples of S. europaea and S. ramosissima.
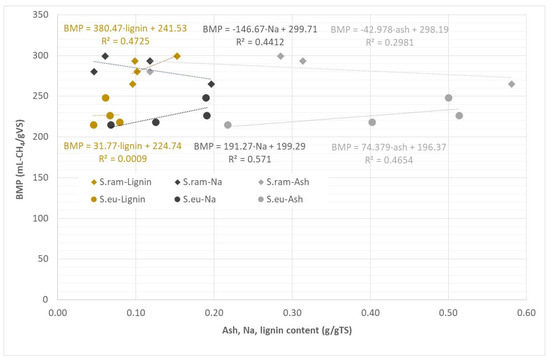
Figure 5.
Correlations of ash, Na and lignin content and experimental BMP of the different samples of S. europaea (S.eu) and S. ramosissima (S.ram), including equations and R2 factors of linear regressions.
For the different samples of S. europaea, the biomethane yield increased with increasing salinity of the plant material resulting in Na concentrations in the batch assays up to 2358 mg Na/L. These findings are in accordance with the results of Turcios et al. [29], who reported the lowest methane yield of T. pannonicum of 301 mL CH4/gVS cultivated under non-saline conditions with a sodium concentration of 929 mg/L and the highest methane yield of 347 mL CH4/gVS grown at 30 g/L NaCl with a sodium content of 1988 mg/L. The lower methane yield of S. europaea cultivated at 30 g/L NaCl in the present study may give an indication that a Na concentration of 2400 mg Na/L is a limit for inhibition of the AD process. Accordingly, there is a moderate linear correlation of Na and ash content to BMP of these samples, which is expressed by R2 values below 0.58 (Figure 5). Furthermore, for the plant and pulp samples of S. ramosissima, the biomethane yield increases with higher Na-concentrations in the batch set-up. Only for the juice fraction of S. ramosissima with a Na concentration in the batch assay of 2349 mg Na/L the biomethane yield is much lower. This non-linear correlation can also be seen in the correlations of ash and Na content and BMP of the S. ramosissima samples with R2 values below 0.45 (Figure 5).
These findings indicate that AD of the investigated Salicornia plants was not inhibited by sodium concentrations below 2400 mg Na/L. This means that halophyte plant material should be added only in limited amounts in co-digestion with other non-saline substrates such as manure or sewage sludge. The amount of halophyte material that can be added to 1 kg of the non-saline substrate until the threshold value of 2400 mg/L is reached is dependent on the TS content and the Na concentration per TS. From these values, it can be calculated that in co-digestion with 1 ton of a substrate with a low sodium concentration of 350 mg Na/L (like the inoculum used), 11–32 kg of S. europaea dry matter plant material or 51.5 kg of dried mature plant or 178 kg of the fresh plant or pulp fraction of S. ramosissima may be added until the sodium concentration of 2400 mg/L is reached (Table 7).

Table 7.
Limit of Salicornia material that can be added in co-digestion with 1 ton (t) of non-saline substrate to reach Na concentration of 2400 mg Na/L, based on TS concentration of the material and Na concentration in g/kg in the BMP batch-test set-up.
With respect to a possible correlation between biomethane yield and lignin content, it can be seen that S. europaea grown at 10 g/L NaCl had a relatively low biomethane yield and contained the highest lignin content of these samples of 7.9 g/100 gTS. For the other samples of S. europaea, however, the biomethane yield was not correlated to the lignin content, which was rather similar. Furthermore, for the S. ramosissima samples, no correlation between the biomethane yield and the lignin content can be detected since the dry mature plant of S. ramosissima with the highest lignin content of 15.3 g/100 gTS had the highest biomethane yield. This indicates that the lignin content of the halophyte plant material is too low to have a significant effect on the degradation of this biomass.
Overall, the influence of salt and lignin content on the biomethane potential cannot be clearly allocated. For the Na content, a threshold for AD process inhibition could be estimated, while the lignin concentration of the halophyte plant material was obviously too low to have a clear effect on the degradation. Furthermore, antagonism and synergism may occur between different salt ions as well as organic compounds of the substrates. According to Chen et al. [10], for example, combinations of cations induce antagonisms advantageous to that of an individual cation. It was reported that a mixture of Na and K or Mg and Na achieved a methane yield 10% greater as compared to the yield of solely Na.
4. Conclusions
In the present study, the biomethane potential of the halophyte plants S. europaea and S. ramosissima was analyzed and correlated to their biomass composition, focusing on the content of carbohydrates, lignin, proteins, and minerals. A higher NaCl concentration used in the culture medium in a hydroponic system for S. europaea showed an increase in Na content of the plant tissue while the concentration of K, Ca, Mg, and P decreased with increasing NaCl concentration used for the cultivation. The highest methane yield of 248 mL CH4/gVS was found for S. europaea cultivated at 20 g/L NaCl. The methane yield of S. ramosissima cultivated in a greenhouse with seawater irrigation was generally higher, with yields of up to 300 mL CH4/gVS for both the whole green plant, pulp, and juice fraction. In general, the anaerobic digestion process showed no signs of inhibition at final sodium concentrations of up to 2400 mg Na/L with methane yields similar to the yields of conventional grass crops. Up to this sodium concentration, a moderate correlation between sodium content and biomethane yield was identified. Furthermore, no clear correlation between the relatively low lignin content and the biomethane yield could be found in the samples. The results show that the halophyte crop Salicornia either cultivated in hydroponic systems or in a greenhouse equipped with seawater irrigation, can be utilized as a feedstock for biogas production in co-digestion with a non-saline feedstock. It can be estimated that up to 10–30 kg of dry matter, 50 kg of dried mature plant material, or up to 178 kg of whole green plant or separated pulp fraction of halophytes can be added to 1 ton of non-saline feedstocks without causing inhibition of the process. Attention must be paid to the 25–45 times higher sulfur content in halophyte plant material at least compared to grass biomass that may cause significant H2S production during the biogas process. Although this higher sulfur content did obviously not hinder the anaerobic digestion process in the present study, H2S has to be removed from the biogas to avoid corrosion in subsequent technical applications.
Author Contributions
Conceptualization, A.C., J.P. and H.U.; methodology, analysis and investigation of biomass composition and biomethane potential, A.C.; methodology, analysis and investigation of halophyte cultivation, A.E.T., J.P. (S. europaea) and R.M.R. (S. ramosissima); writing—original draft preparation, A.C.; writing—review and editing, A.C., A.E.T., M.H.T., R.M.R., J.P. and H.U.; project leadership, M.H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No. 862834.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Turcios, A.; Cayenne, A.; Uellendahl, H.; Papenbrock, J. Halophyte Plants and Their Residues as Feedstock for Biogas Production—Chances and Challenges. Appl. Sci. 2021, 11, 2746. [Google Scholar] [CrossRef]
- Kamel, M.; Hammad, S.; Khalaphallah, R.; Elazeem, M.A. Halophytes and Salt Tolerant Wild Plants as a Feedstock for Biogas Production. J. BioSci. Biotechnol. 2019, 8, 151–159. [Google Scholar]
- Abideen, Z.; Ansari, R.; Khan, M.A. Halophytes: Potential Source of Ligno-Cellulosic Biomass for Ethanol Production. Biomass Bioenergy 2011, 35, 1818–1822. [Google Scholar] [CrossRef]
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt Tolerance and Crop Potential of Halophytes. Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- Deublein, D.; Angelika, S. (Eds.) Biogas from Waste and Renewable Resources—An Introduction; 3. Nachdr.; Wiley-VCH-Verl: Weinheim, Germany, 2010; ISBN 9783527318414. [Google Scholar]
- Al Seadi, T.; Rutz, D.; Prassl, H.; Köttner, M.; Finsterwalder, T.; Volk, S.; Janssen, R. Biogas Handbook; University of Southern Denmark Esbjerg: Esbjerg, Denmark, 2008; ISBN 9788799296200. [Google Scholar]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Machmüller, A.; Hopfner-Sixt, K.; Bodiroza, V.; Hrbek, R.; Friedel, J.; Pötsch, E.; Wagentristl, H.; et al. Methane production through anaerobic digestion of various energy crops grown in sustainable crop rotations. Bioresour. Technol. 2007, 98, 3204–3212. [Google Scholar] [CrossRef]
- Murphy, J.; Braun, R.; Weiland, P.; Wellinger, A. Biogas from crop digestion. IEA Bioenergy Task 2011, 37, 1–23. [Google Scholar]
- Yang, S.; Li, J.; Zheng, Z.; Meng, Z. Characterization of Spartina alterniflora as Feedstock for Anaerobic Digestion. Biomass Bioenergy 2009, 33, 597–602. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- McCarty, P.L. Anaerobic Waste Treatment Fundamentals—Part Four—Process Design. Public Works 1964, 95, 95–99. [Google Scholar]
- Kugelman, I.J.; McCarty, P.L. Cation Toxicity and Stimulation in Anaerobic Waste Treatment. J. Water Pollut. Control Fed. 1965, 37, 97–116. [Google Scholar]
- Oleszek, M.; Kowalska, I.; Oleszek, W. Phytochemicals in bioenergy crops. Phytochem. Rev. 2019, 18, 893–927. [Google Scholar] [CrossRef] [Green Version]
- Li, H.L.; Zhang, X.M.; Deng, F.D.; Han, X.G.; Xiao, C.W.; Han, S.J.; Wang, Z.P. Microbial methane production is affected by secondary metabolites in the heartwood of living trees in upland forests. Trees 2020, 34, 243–254. [Google Scholar] [CrossRef]
- Cybulska, I.; Brudecki, G.; Alassali, A.; Alassali, M.; Brown, J.J. Phytochemical composition of some common coastal halophytes of the United Arab Emirates. Emir. J. Food Agric. 2014, 26, 1046–1056. [Google Scholar] [CrossRef] [Green Version]
- Isca, V.M.S.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, A.M.S. An overview of Salicornia genus: The phytochemical and pharmacological profile. In Natural Products: Research Reviews; Gupta, V.K., Ed.; Daya Publishing House: New Delhi, India, 2014; Volume 2, pp. 145–176. [Google Scholar]
- Cybulska, I.; Chaturvedi, T.; Alassali, A.; Brudecki, G.P.; Brown, J.J.; Sgouridi, S.; Thomsen, M.H. Characterization of the chemical composition of the halophyte Salicornia bigelovii under cultivation. Energy Fuels 2014, 28, 3873–3883. [Google Scholar] [CrossRef]
- DIN EN 12880; Characterization of Sludges e Determination of Dry Residue and Water Content; German Version. Beuth Verlag: Berlin, Germany, 2000.
- DIN EN 12879; Characterization of Sludges e Determination of the Loss on Ignition of Dry Mass; German Version. Beuth Verlag: Berlin, Germany, 2000.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Laboratory (NREL): Golden, CO, USA, 2008; pp. 1–15. [Google Scholar]
- Raposo, F.; Fernández-Cegrí, V.; De la Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.C.; et al. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Rincón, B.; Heaven, S.; Banks, C.J.; Zhang, Y. Anaerobic Digestion of Whole-Crop Winter Wheat Silage for Renewable Energy Production. Energy Fuels 2012, 26, 2357–2364. [Google Scholar] [CrossRef] [Green Version]
- Ugwu, S.; Enweremadu, C. Biodegradability and kinetic studies on biomethane production from okra (Abelmoschus esculentus) waste. S. Afr. J. Sci. 2019, 115, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Weese, A.; Pallmann, P.; Papenbrock, J.; Riemenschneider, A. Brassica napus L. cultivars show a broad variability in their morphology, physiology and metabolite levels in response to sulfur limitations and to pathogen attack. Front. Plant Sci. 2015, 6, 9. [Google Scholar] [CrossRef]
- Boyle, W.C. Energy recovery from sanitary landfills—A review. Micro Energy Conv. 1977, 119–138. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G. Theoretical analysis of biogas potential prediction from agricultural waste. Resour. Effic. Technol. 2016, 2, 143–147. [Google Scholar] [CrossRef] [Green Version]
- VDI 4630; VDI Guideline: Fermentation of Organic Materials. Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. Beuth Verlag: Berlin, Germany, 2016.
- Akinshina, N.; Naka, D.; Toderich, K.; Azizov, A.; Yasui, H. Anaerobic degradation of halophyte biomass for biogas production. J. Arid Land Stud. 2012, 22, 227–230. [Google Scholar]
- Turcios, A.; Weichgrebe, D.; Papenbrock, J. Effect of salt and sodium concentration on the anaerobic methanisation of the halophyte Tripolium pannonicum. Biomass Bioenergy 2016, 87, 69–77. [Google Scholar] [CrossRef]
- Turcios, A.; Weichgrebe, D.; Papenbrock, J. Potential use of the facultative halophyte Chenopodium quinoa Willd. as substrate for biogas production cultivated with different concentrations of sodium chloride under hydroponic conditions. Bioresour. Technol. 2016, 203, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, I.; Chaturvedi, T.; Brudecki, G.P.; Kádár, Z.; Meyer, A.S.; Baldwin, R.M.; Thomsen, M.H. Chemical characterization and hydrothermal pretreatment of Salicornia bigelovii straw for enhanced enzymatic hydrolysis and bioethanol potential. Bioresour. Technol. 2014, 153, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.; Colmer, T. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Santamaria-Fernandez, M.; Ytting, N.K.; Lübeck, M.; Uellendahl, H. Potential Nutrient Recovery in a Green Biorefinery for Production of Feed, Fuel and Fertilizer for Organic Farming. Waste Biomass Valoriz. 2019, 11, 5901–5911. [Google Scholar] [CrossRef]
- Brown, J.J.; Cybulska, I.; Chaturvedi, T.; Thomsen, M.H. Halophytes for the Production of Liquid Biofuels. In Sabkha Ecosystems: Volume IV: Cash Crop Halophyte and Biodiversity Conservation; Springer: Dordrecht, The Netherlands, 2014; pp. 67–72. [Google Scholar]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas production from maize and dairy cattle manure—Influence of biomass composition on the methane yield. Agric. Ecosyst. Environ. 2007, 118, 173–182. [Google Scholar] [CrossRef]
- Ward, A.J.; Lewis, D.M.; Green, F.B. Anaerobic Digestion of Algae Biomass: A Review. Algal Res. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, Z.; Yang, S.; Fang, C.; Zou, X.; Zhang, J. Improving conversion of Spartina alterniflora into biogas by co-digestion with cow feces. Fuel Process. Technol. 2010, 91, 1416–1421. [Google Scholar] [CrossRef]
- Jackson-Moss, C.A.; Duncan, J.R.; Cooper, D.R. The effect of calcium on anaerobic digestion. Biotechnol. Lett. 1989, 11, 219–224. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).