Abstract
This study focused on applying a patented medium-chain fatty acids (MCFAs) mixture at the end of alcoholic fermentation and monitoring its residues. MCFAs are a promising agent that has the potential to increase the efficiency of sulfur dioxide and ultimately minimize its doses, which is one of the important goals of wine research today. Detailed octanoic, decanoic, and dodecanoic acid contents were observed during the experiment. The MCFA mixture was applied at doses of 0, 10, 20, and 60 mg/L. GC–MS determined the content of individual fatty acids. The results showed that the use of the investigated mixture of fatty acids at doses of 10 and 20 mg/L did not cause an increase in the content of individual fatty acids residues. The octanoic acid content after application of the 20 mg/L MCFA mixture was 8.24 mg/L after 744 h, while the untreated control variant showed a value of 7.71 mg/L. The performed sensory analysis also did not show a negative effect of MCFA application on the sensory properties of wine. Therefore, applying an MCFA mixture at 10 and 20 mg/L can be recommended as a safe alternative following alcoholic fermentation. However, the results obtained can also serve as a valuable basis for permitting the use of MCFA in the proceeding OIV approval process. The research thus opens the possibility of expanding a new oenological agent capable of reducing SO2 doses.
1. Introduction
Sulphur dioxide is one of the most common additives in wine technology. It is used to terminate alcoholic fermentation and as an antioxidant effect during the ageing and storage of wines. However, its presence harms human health and, at higher concentrations, causes a burning headache. Minimizing SO2 doses is thus one of the main topics of today’s wine society [,].
One alternative to the use of SO2 may be applying medium-chain fatty acids (MCFAs). These are compounds naturally formed in wine during alcoholic fermentation []. The path of their formation has been known for a relatively long time: it is an intermediate product of lipid metabolism [,]. Examples of MCFAs in wines are hexanoic, octanoic. and decanoic acid. Their negative effect on the fermentation of clear musts has also been known for a long time. MCFAs reduce the resistance of microorganisms to alcohol [,] and SO2 by changing the properties of cell membranes [,,]. The presence of MCFA causes changes in plasma membrane organization [] and affects H+ transport and ATPase activity []. Ultimately, the presence of octanoic acid and especially decanoic acid lowers the intracellular pH in yeast cells and increases the permeability of the plasma membrane [] to an effective form of molecular SO2 []. However, this effect can be used to reduce SO2 doses at the end of alcoholic fermentation by a targeted application at the appropriate time []. MCFAs could also serve to prevent the re-fermentation of sweet wines []. In addition to inhibitory effects on the activity of the yeast Saccharomyces cerevisiae, the inhibition of bacteria, specifically Oenococcus oeni, has also been demonstrated [].
The experimental process has shown the effectiveness of applying a mixture of C8, C10, and C12 fatty acids following alcoholic fermentation []. Based on these results, a mixture of C8:C10:C12 was patented in the ratio 2:7:1 []. This roughly corresponds to the natural content of these MCFAs in wines [], and decanoic acid also has the highest proportion in the mixture, due to its highest inhibitory effects [,]. The undeniable advantage of this method is the fact that MCFAs are naturally occurring compounds in wine [], unlike other alternatives to SO2 used today (DMDC, sorbic acid, etc.) []. In addition, up to a 50% reduction in acetaldehyde and other carbonyl compounds content has been demonstrated compared to other methods of terminating alcoholic fermentation []. Thus, the content of carbonyl compounds is closely related to the management of SO2 in wines []. The patented mixture of MCFA [] is now in the OIV approval process for use as an additive in winemaking.
However, the application of MCFA may carry certain risks in the form of a negative effect on the sensory properties of the wine. MCFAs are active sensory compounds; they can cause rancid butter, soap, coconut oil, and metallic tones at higher concentrations [,]. However, MCFAs are partially esterified in wine [,] and these fatty acid esters, in turn, can promote fruity aromas []. Above all, MCFAs are bound to the dead yeast bodies and removed together during racking [,]. The ability to reduce MCFA by binding to yeast bodies was described as early as 1984 []. This fact has also been reported in studies dealing with the mixture we studied []. At the same time, MCFA residues were not measured after application in this research. However, detailed data of residue observation after application of the observed MCFA mixture have not yet been published. It is this development that is the subject of this experiment.
2. Materials and Methods
2.1. Design of Experiment
Grapes used in this experiment were of the Hibernal variety harvested in 2020. The pressed must was allowed to settle independently for 24 h without additives. After clarification to a turbidity value of 100 NTU, the must was inoculated with active dry yeasts. The alcoholic fermentation took place in a 600 L stainless-steel vessel at 14–16 °C. In phase 20–25 g/L of residual sugar, the fermenting must was divided into 8 (2 × 4) glass vessels with a volume of 50 L. To the first 4 vessels, 20 mg/L SO2 (40% ammonium bisulfite) + doses of 0, 10, 20, and 60 mg/L MCFA were applied. To the other 4 vessels, 40 mg/L SO2 (40% ammonium bisulfite) + doses of 0, 10, 20, and 60 mg/L MCFA were applied. Sampling was performed at 0, 12, 24, 48, 168, and 744 h. Based on our experiences with the results of previous unpublished experiments, we omitted the control variant, which never provided relevant results for comparison. At the same time, we did not create duplicates of variants but focused on precise analysis with triplicates just in time.
The samples were analyzed in the laboratories of Mendel University in Lednice. Sensory analysis was performed at 24 and 744 h.
2.2. Sensory Analysis
A panel of 7 wine judges evaluated the intensity of fatty acid expression (rancid butter, coconut, and cheese) in the submitted samples on a scale of 1–10 (least–most). The judging was blind. The temperature of the evaluated wine was 12 °C. All judges held tasting tests according to ČSN EN ISO 8586. The panel of judges was trained by a triangular test, where a sample with the addition of 60 mg/L MCFA was known.
2.3. Determination of the Fatty Acids by GC-MS
The concentration of individual volatile compounds in wine was determined according to the unpublished extraction method with methyl-t-butyl ether (MTBE). An amount of 20 mL of wine was pipetted into a 25 mL volumetric flask together with 50 µL of 2-nonanol ethanol solution and 5 mL of a saturated (NH4)2SO4 solution. 2-nonanol was used as an internal standard (in a concentration of 400 mg/L). The flask content was thoroughly stirred, and then a volume of 0.75 mL of the extraction solvent (MTBE with 1% of cyclohexane added) was added. After another thorough stirring and separation of individual phases, the upper organic layer (supernatant) was placed into a micro-test-tube with the produced emulsion and centrifuged; the clear organic phase was dried with anhydrous magnesium sulphate. Extract samples (adjusted in this way) were used for the GC–MS analysis.
Validation of this unpublished method was performed by the spiking of standard: 2-octanol and 2-pentylbyturate. The measurement accuracy was 97%.
Instruments: Shimadzu GC-17A, Autosampler: AOC-5000, Detector: QP-5050A, Software: GC solution. (Shimadzu; Kyoto, Japan) Separation conditions: column: DB-WAX 30 m × 0.25 mm; 0.25 µm stationary phase (polyethylene glycol). (Merck KGaA; Darmstadt, Germany) The voltage of the detector was 1.5 kV.
Separation conditions: Injection chamber temperature: 200 °C. The initial column space temperature of 45 °C was maintained for 3.5 min, followed by the following temperature gradient: up to 90 °C at 15 °C/min, up to 135 °C at 6 °C/min, up to 207 °C at 9 °C/min, and up to 252 °C at 15 °C/min. The final temperature was maintained for 5 min. The total length of the analysis was 30 min. Individual substances were identified on the basis of MS spectrum and retention time. Quantification was performed by comparing the peak area of the sample and the external standard with the correction to the internal standard.
All chemicals used were purchased from Merck KGaA; Darmstadt, Germany.
2.4. Determination of Glucose and Fructose Content
The glucose and fructose content was determined using the Alpha Bruker FTIR Analyzer (Bruker; Billerica, MA, USA), which determines a substance’s content by spectrometry. This analysis used 1 mL of the clear sample. The application was performed using a syringe. The content of each substance was automatically evaluated by the software, depending on the calibration equations. All samples were measured in triplicate.
2.5. Preparation of the MCFA Mixture Solution
The mixture of MCFA was prepared according to European Patent No. 2681301. First, a mixture of fatty acids at the desired weight ratio (C8:C10:C12—2:7:1) was prepared. An amount of 100 g of a mixture of MCFA and 38 g of potassium hydroxide (KOH) were used per 1 L of the solution. The appropriate procedure was to weigh the MCFA mixture in a volumetric flask and gradually add the KOH solution, dissolved in two-thirds of the required water. After the dissolution and temperature stabilization, the pH was optionally adjusted to a value above 10, and the diluted KOH solution was regulated to the desired volume. The resulting solution should have had an average density of 1.0128 kg/L.
2.6. Statistical Analysis
Statistical evaluation of the data was performed using software Statistica 12 (TIBCO Software; Palo Alto, CA, USA). The data were subjected to ANOVA analysis followed by Fisher’s LSD test. Graphs were also generated in this software.
3. Results and Discussion
The following section graphically shows the results of the samples taken. Detailed results, including analysis of variance (ANOVA) and Fisher’s LSD test, are available in the Appendix A (Table A1).
3.1. Octanoic Acid
Figure 1 shows the development of the octanoic acid content (C8). The lowest values during the whole measurement can be observed in the control variant. However, the 10 MCFA and 20 MCFA variants did not show a statistically significant increase compared to the control. At 744 h, the values for these three variants were almost identical (7.62–8.24 mg/L). In contrast, the content of octanoic acid in variant 60 MCFA was significantly higher during the whole measurement, showing values about 50% higher (11.29 and 11.84 mg/L) than other variants in the last term. The effect of the application dose of SO2 was practically not observed. The results of the C8 content thus confirmed the hypothesis that fatty acids are bound to yeast bodies [,], which is the explanation for the observed decrease in all observed doses of MCFA. Similar results were obtained by Baron et al. [], who determined 4.62 mg/L in the variant without MCFA application and 4.3 mg/L in the variant with MCFA application. In our case, the overall higher values can be explained by the naturally higher MCFA content [].
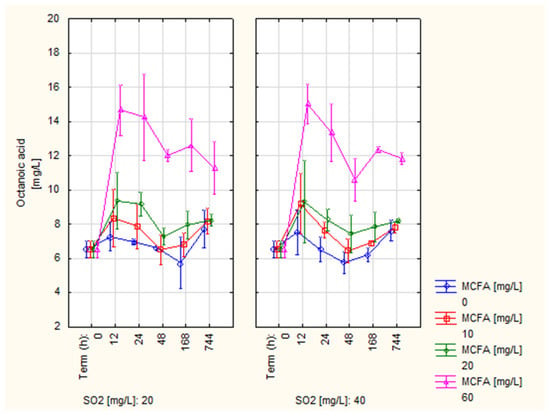
Figure 1.
Development of octanoic acid content (C8).
Compared to the values published by some authors, the values of C8 content measured in all variants were higher. These authors [,] reported values in the range of 0.18–6.61 mg/L. In contrast, much higher concentrations were reported by Li et al. [] of 26 mg/L and Lee and Noble [] of 10.15–50.56 mg/L. Similar values to this experiment were measured in the experiment by Lukic et al. [] of 6.07–10.93 mg/L. Based on a confrontation with data from other authors, it can be stated that the measured values of all variants were in the normal range.
Most authors [,,,] have reported the threshold for the perception of octanoic acid as 0.5 mg/L. However, this is a value obtained from a model solution of alcohol and ethanol with pH adjustment. Compared to this value, the C8 content was higher in all variants. In contrast, Shinohara [] reported a perception threshold for octanoic acid of 10 mg/L, only exceeded by variant 60 MCFA. However, the overall sensory properties of the wine are affected by the content of all active sensory compounds present, not by the concentration of only one compound [,]. Therefore, the measured values in this experiment cannot automatically be considered harmful from the perspective of organoleptic properties. A sensory test also ruled out the negative effect of MCFA application in variants 10 and 20 MCFA on sensory properties (Section 3.5).
3.2. Decanoic Acid
Figure 2 shows the decanoic acid (C10) content development, which was a crucial monitored parameter, as it made up 70% of fatty acids in the examined preparation. Here, we can observe a change similar to the development of the octanoic acid content. Control variant 0 MCFA showed the lowest values throughout the experiment. For variants 10 and 20 MCFA, a similar content was observed, slightly higher than that of the control variant. Approximately four-fold higher concentrations were measured in variant 60 MCFA within 24 h than in the control variant, but the C10 content in this variant had a decreasing trend during the experiment. In the final term (744 h), a significantly lower C10 content was observed in variant 0 MCFA (2.28 and 2.3 mg/L), variants 10 and 20 MCFA showed statistically identical values (2.93–3.28 mg/L), and the highest value was measured for variant 60 MCFA (5.02 and 5.41 mg/L). The effect of the SO2 dose was again not observed.
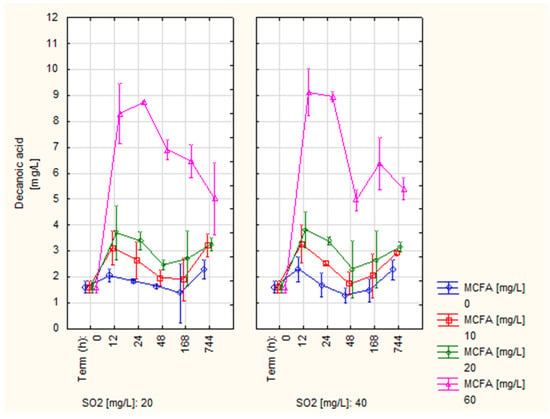
Figure 2.
Development of decanoic acid content (C10).
The C10 content of variants 0, 10, and 20 of MCFA showed values similar to those published by some authors [,] of 1.75–3.59 mg/L. Lower values were recorded by other authors [,,] of 0.13–1.27 mg/L. Lee and Noble [] published a wide range of measured values as 0.55–14.15 mg/L. Thus, the C10 content values obtained in this experiment can be considered normal, including variant 60 MCFA (5.02–5.41 mg/L).
Due to the sensory activity of C10, the measured content in all variants can be considered problematic. According to some authors [,,,], the threshold of perception is 1 mg/L. Again, this is the value obtained by the model solution test. On the contrary, other authors [,] have stated values of the perception threshold of 6 mg/L and 15 mg/L, respectively. Compared to these values, the C10 content was lower in all variants.
3.3. Dodecanoic Acid
The development of the dodecanoic acid (C12) content can be observed in Figure 3. Based on the results, it can be stated that the development of the C12 content was very similar for all variants up to 168 h and showed a decreasing trend. However, a sharp increase in C12 content was recently observed in control variant 0 MCFA (189.99 and 208.30 µg/L). For variants 10 and 20 MCFA, the content increased slightly (114.82–157.32 g/L). For variant 60 MCFA, the content remained stable and decreased slightly (68.79 and 79.20 g/L), respectively. This result may be due to the esterification of C12 induced by an overall higher MCFA content [], combined with the fact that C12 makes up only 10% of the MCFA in the preparation. Again, changes in C12 content due to different doses of SO2 were not observed.
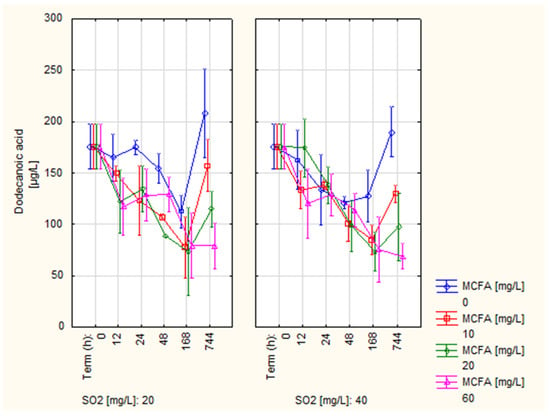
Figure 3.
Development of dodecanoic acid content (C12).
The content of dodecanoic acid was not part of the research of many authors, probably due to relatively lower concentrations compared to octanoic and decanoic acid. Li et al. [] indicated the measured value of 90 µg/L as the threshold of perception of 1000 µg/L. In comparison, the concentrations measured for all variants appeared to be comparable and without affecting the sensory properties of the wine.
3.4. Glucose + Fructose
The content of glucose and fructose (reducing carbohydrates) was monitored as an indicator of yeast activity. Figure 4 shows the development of its content. The initial value of the content of reducing sugars before MCFA application was 24.82 g/L. Stable development can be observed for all variants with the addition of MCFA; at the end of the experiment, they showed values in the range of 23.05–26.79 g/L. Inevitable fluctuations in the measured values can be attributed to error. However, it can be stated that after the application of MCFA in all doses, there was no fermentation of sugars by yeast [].
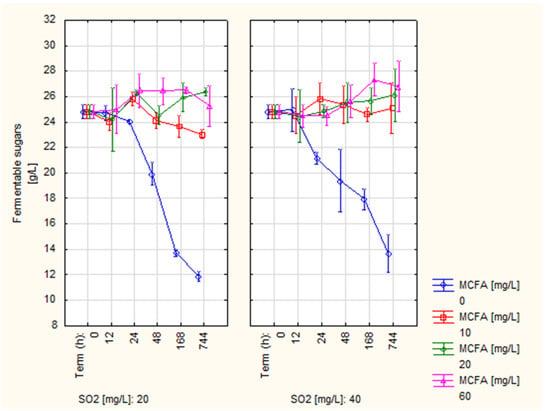
Figure 4.
Development of fermentable sugars content.
Conversely, in control samples without the addition of MCFA, we can see a sharp decrease in the content of reducing sugars throughout the experiment. The final value of 11.86 g/L was measured for the variant with the addition of 20 mg/L SO2. The value was 13.65 g/L for the 40 mg/L SO2 variant. Thus, in the control variants, the alcoholic fermentation was not terminated at all. The results confirm the fact that MCFA is a significant inhibitor of alcoholic fermentation [,,]. At the same time, the hypothesis was confirmed that with the help of MCFA, it is possible to terminate alcoholic fermentation even at lower doses of SO2 [,].
3.5. Sensory Analysis
The sensory analysis results (Table 1) showed that the highest measurements in evaluating fatty acid perception were achieved in variant 60 MCFA (4.50–6.83 g/L). The evaluation of variants 10 MCFA and 20 mg/L MCFA ranged from 2.17 g/L to 5.00 g/L. However, the control variant with 0 mg/L MCFA showed similar values (2.50–3.67 g/L). Statistically, the control variant was placed at the level of the variants with 10 and 20 mg/L MCFA. In general, the panel of experienced judges, who focused on the MCFA expression, could not safely separate samples with the addition of 10 and 20 mg/L MCFA from samples without the addition of MCFA. However, the addition of 60 mg/L MCFA already appeared to be risky concerning the negative effect on the organoleptic properties of wines.

Table 1.
Results of sensory analysis. The average values (n = 3) are supplemented by the contribution into homogeneous groups (a, b) according to Fisher’s LSD (least significant difference) test (α = 0.05).
4. Conclusions
Based on the results, it can be stated that the application of the investigated mixture of MCFA at doses of 10 and 20 mg/L did not cause an increase in the content of individual fatty acids in comparison with the control variant. The content of octanoic acid at 744 h after application was statistically identical to the control variant in these variants. Similar results were obtained when monitoring the concentration of decanoic acid. In contrast, administration of a dose of 60 mg/L MCFA resulted in significantly higher octanoic and decanoic acid content values than the control. In addition, the sensory analysis excluded a negative effect on the organoleptic properties of wine by applying a dose of 10 and 20 mg/L MCFA. At the same time, the positive effect of MCFA application on the termination of alcoholic fermentation was confirmed. All observed application doses of MCFA, in conjunction with doses of 20 and 40 mg/L SO2, led to the termination of alcoholic fermentation, in contrast to the control variant without the addition of MCFA. In conclusion, it is recommended to apply the MCFA mixture at a dose of 10–20 mg/L without the risk of adversely affecting the sensory properties of wine.
Author Contributions
Conceptualization, J.L., M.B. and J.S.; methodology, J.L., M.K. and M.B.; software, J.L.; validation, M.B., J.M., M.K. and J.S.; formal analysis, J.L. and M.K.; investigation, J.L. and M.B.; resources, J.L.; data curation, M.B.; writing—original draft preparation, J.L.; writing—review and editing, M.B., J.M. and J.S.; visualization, J.L.; supervision, M.B. and J.M.; project administration, M.B.; funding acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the project “Study of the application of medium-chain fatty acids to the terminating of alcohol fermentation” IGA-ZF/2021-SI1011 and by the project CZ.02.1.01/0.0/0.0/16_017/0002334 Research Infrastructure for Young Scientists; this is co-financed by Operational Program Research, Development and Education.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Detailed results of octanoic acid, decanoic acid, dodecanoic acid, and fermentable sugars content. Data normality was verified by the Shapiro–Wilk test, followed by ANOVA analysis (p-value). The average values (n = 3) are supplemented by the contribution into homogeneous groups (a, b, c, d) according to Fisher’s LSD (least significant difference) test (α = 0.05).
Table A1.
Detailed results of octanoic acid, decanoic acid, dodecanoic acid, and fermentable sugars content. Data normality was verified by the Shapiro–Wilk test, followed by ANOVA analysis (p-value). The average values (n = 3) are supplemented by the contribution into homogeneous groups (a, b, c, d) according to Fisher’s LSD (least significant difference) test (α = 0.05).
| Octanoic Acid (mg/L) | ||||
| Dose | Term | |||
| SO2 (mg/L) | MCFA (mg/L) | 0 h | 24 h | 744 h |
| 20 | 0 | 6.53 ± 0.12 a | 6.99 ± 0.04 a | 7.71 ± 0.25 a |
| 10 | 6.53 ± 0.12 a | 7.89 ± 0.30 b | 8.18 ± 0.17 a | |
| 20 | 6.53 ± 0.12 a | 9.16 ± 0.16 c | 8.24 ± 0.08 a | |
| 60 | 6.53 ± 0.12 a | 14.27 ± 0.59 d | 11.29 ± 0.36 b | |
| p-value | 1.00 | p < 0.01 | p < 0.01 | |
| 40 | 0 | 6.53 ± 0.12 a | 6.53 ± 0.37 a | 7.62 ± 0.14 a |
| 10 | 6.53 ± 0.12 a | 7.67 ± 0.11 b | 7.81 ± 0.08 a,b | |
| 20 | 6.53 ± 0.12 a | 8.26 ± 0.14 b | 8.21 ± 0.03 b | |
| 60 | 6.53 ± 0.12 a | 13.35 ± 0.39 c | 11.84 ± 0.08 c | |
| p-value | 1.00 | p < 0.01 | p < 0.01 | |
| Decanoic Acid (mg/L) | ||||
| Dose | Term | |||
| SO2 (mg/L) | MCFA (mg/L) | 0 h | 24 h | 744 h |
| 20 | 0 | 1.61 ± 0.06 a | 1.84 ± 0.02 a | 2.30 ± 0.09 a |
| 10 | 1.61 ± 0.06 a | 2.64 ± 0.17 b | 3.21 ± 0.10 b | |
| 20 | 1.61 ± 0.06 a | 3.41 ± 0.08 c | 3.28 ± 0.06 b | |
| 60 | 1.61 ± 0.06 a | 8.73 ± 0.01 d | 5.02 ± 0.32 c | |
| p-value | 1.00 | p < 0.01 | p < 0.01 | |
| 40 | 0 | 1.61 ± 0.06 a | 1.70 ± 0.11 a | 2.28 ± 0.09 a |
| 10 | 1.61 ± 0.06 a | 2.53 ± 0.02 b | 2.93 ± 0.03 b | |
| 20 | 1.61 ± 0.06 a | 3.40 ± 0.04 c | 3.17 ± 0.05 b | |
| 60 | 1.61 ± 0.06 a | 8.95 ± 0.05 d | 5.41 ± 0.10 c | |
| p-value | 1.00 | p < 0.01 | p < 0.01 | |
| Dodecanoic Acid (µg/L) | ||||
| Dose | Term | |||
| SO2 (mg/L) | MCFA (mg/L) | 0 h | 24 h | 744 h |
| 20 | 0 | 176.08 ± 5.12 a | 175.15 ± 1.58 b | 208.30 ± 9.96 d |
| 10 | 176.08 ± 5.12 a | 123.14 ± 7.82 a | 157.32 ± 5.94 c | |
| 20 | 176.08 ± 5.12 a | 134.47 ± 5.24 a | 114.82 ± 4.09 b | |
| 60 | 176.08 ± 5.12 a | 128.73 ± 5.81 a | 79.20 ± 5.17 a | |
| p-value | 1.00 | p < 0.01 | p < 0.01 | |
| 40 | 0 | 176.08 ± 5.12 a | 133.75 ± 7.86 a | 189.99 ± 5.63 d |
| 10 | 176.08 ± 5.12 a | 138.27 ± 1.05 a | 129.89 ± 1.91 c | |
| 20 | 176.08 ± 5.12 a | 138.04 ± 4.23 a | 97.77 ± 7.59 b | |
| 60 | 176.08 ± 5.12 a | 128.92 ± 4.76 a | 68.79 ± 2.93 a | |
| p-value | 1.00 | 0.55 | p < 0.01 | |
| Fermentable Sugars (g/L) | ||||
| Dose | Term | |||
| SO2 (mg/L) | MCFA (mg/L) | 0 h | 24 h | 744 h |
| 20 | 0 | 24.82 ± 0.13 a | 24.02 ± 0.04 a | 11.86 ± 0.09 a |
| 10 | 24.82 ± 0.13 a | 25.82 ± 0.13 b | 23.05 ± 0.09 b | |
| 20 | 24.82 ± 0.13 a | 26.29 ± 0.05 b | 26.35 ± 0.07 d | |
| 60 | 24.82 ± 0.13 a | 26.46 ± 0.31 c | 25.26 ± 0.37 c | |
| p-value | 1.00 | p < 0.01 | p < 0.01 | |
| 40 | 0 | 24.82 ± 0.13 a | 21.17 ± 0.37 a | 13.65 ± 0.35 a |
| 10 | 24.82 ± 0.13 a | 25.83 ± 0.29 c | 25.09 ± 0.46 b | |
| 20 | 24.82 ± 0.13 a | 24.85 ± 0.12 b | 26.12 ± 0.48 c | |
| 60 | 24.82 ± 0.13 a | 24.49 ± 0.17 b | 26.79 ± 0.47 c | |
| p-value | 1.00 | p < 0.01 | p < 0.01 | |
References
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Guerrero, R.; Cantos-Villar, E. Demonstrating the efficiency, of sulphur dioxide replacements in wine: A parameter review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- Viegas, C.A.; Rosa, M.F.; Sacorreia, I.; Novais, J.M. Inhibition of yeast growth by octanoic and decanoic acids produced during ethanolic fermentation. Appl. Environ. Microbiol. 1989, 55, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Taylor, G.T.; Kirsop, B.H. Origin of medium chain-length fatty-acids present in beer. J. Inst. Brew. 1977, 83, 241–243. [Google Scholar] [CrossRef]
- Restrepo, S.; Espinoza, L.; Ceballos, A.; Urtubia, A. Production of Fatty Acids during Alcoholic Wine Fermentation under Selected Temperature and Aeration Conditions. Am. J. Enol. Vitic. 2019, 70, 169–176. [Google Scholar] [CrossRef]
- Sacorreia, I. Synergistic effects of ethanol, octanoic, and decanoic acids on the kinetics and the activation parameters of thermal death in Saccharomyces bayanus. Biotechnol. Bioeng. 1986, 28, 761–763. [Google Scholar] [CrossRef]
- Pina, C.; Santos, C.; Couto, J.; Hogg, T. Ethanol tolerance of five non-Saccharomyces wine yeasts in comparison with a strain of Saccharomyces cerevisiae-influence of different culture conditions. Food Microbiol. 2004, 21, 439–447. [Google Scholar] [CrossRef]
- Sajbidor, J.; Malik, F.; Kissantalova, H. The relationship between the viability of selected dried wine yeasts and their lipid-composition and fatty-acid profile. Food Biotechnol. 1992, 6, 187–196. [Google Scholar] [CrossRef]
- Lafonlafourcade, S.; Geneix, C.; Ribereaugayon, P. Inhibition of alcoholic fermentation of grape must by fatty-acids produced by yeasts and their elimination by yeast ghosts. Appl. Environ. Microbiol. 1984, 47, 1246–1249. [Google Scholar] [CrossRef] [Green Version]
- Stevens, S.; Hofmeyr, J. Effects of ethanol, octanoic and decanoic acids on fermentation and the passive influx of protons through the plasma-membrane of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1993, 38, 656–663. [Google Scholar] [CrossRef]
- Viegas, C.; Sacorreia, I. Activation of plasma-membrane atpase of Saccharomyces cerevisiae by octanoic-acid. J. Gen. Microbiol. 1991, 137, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Viegas, C.A.; Almeida, P.F.; Cavaco, M.; Sa-Correia, I. The H+-ATPase in the plasma membrane of Saccharomyces cerevisiae is activated during growth latency in octanoic acid-supplemented medium accompanying the decrease in intracellular pH and cell viability. Appl. Environ. Microbiol. 1998, 64, 779–783. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, T.W.; Howe, P.A.; Sacks, G.L.; Waterhouse, A.L. Determination of Molecular and “Truly” Free Sulfur Dioxide in Wine: A Comparison of Headspace and Conventional Methods. Am. J. Enol. Vitic. 2020, 71, 222–230. [Google Scholar] [CrossRef]
- Bábíková, P.; Baroň, M.; Kumšta, M.; Sotolář, R. Increasing the efficiency of sulfur dioxide in wine by using of saturated higher fatty acids. Acta Univ. Agric. Silvic. Mendel. Brun. 2012, 60, 2. [Google Scholar] [CrossRef] [Green Version]
- Horvath, B.O.; Fazekas, E.; Kellner, N.; Magyar, I. Influence of medium chain fatty acids on some botrytised wine-related yeast species and on spontaneous refermentation of Tokaj essence. Acta Aliment. 2020, 49, 339–347. [Google Scholar] [CrossRef]
- Guilloux-Benatier, M.; Le Fur, Y.; Feuillat, M. Influence of fatty acids on the growth of wine microorganisms Saccharomyces cerevisiae and Oenococcus oeni. J. Ind. Microbiol. Biotechnol. 1998, 20, 144–149. [Google Scholar] [CrossRef]
- Mendel University in Brno; Baron, M.; Kumsta, M.; Babikova, P. Composition of Saturated Fatty Acids and Its Use for Inhibition of Alcoholic or Malolactic Fermentation and Dose Reduction of Sulphur Dioxide in Wine Making Technology. European Patent EP2681301A2, 8 January 2014. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20140108&CC=EP&NR=2681301A2&KC=A2# (accessed on 29 January 2022).
- Ancin, C.; Ayestaran, B.; Garcia, A.; Garrido, J. Evolution of fatty acid contents in Garnacha and Viura musts during fermentation and the aging of wine. Z. Fur Lebensm.-Unters. und-Forsch. A-Food Res. Technol. 1998, 206, 143–147. [Google Scholar] [CrossRef]
- Banita, C.D.; Antoce, A.O. Preliminary study on the inhibition of alcoholic fermentation using octanoic and decanoic acids to obtain aromatic wines with residual sugar. Sci. Pap.-Ser. B-Hortic. 2021, 65, 299–306. [Google Scholar]
- Baron, M.; Babikova, P. Saturated higher fatty acids as a means of inhibiting alcoholic fermentation and sulphur dioxide reduction in wine. Mitt. Klosterneubg. 2011, 61, 158–165. [Google Scholar]
- Lisanti, M.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative Methods to SO2 for Microbiological Stabilization of Wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef] [Green Version]
- Licek, J.; Baron, M.; Sochor, J. Comparison of MCFA and Other Methods of Terminating Alcohol Fermentation and Their Influence on the Content of Carbonyl Compounds in Wine. Molecules 2020, 25, 5737. [Google Scholar] [CrossRef]
- Jackowetz, J.N.; de Orduna, R.M. Survey of SO2 binding carbonyls in 237 red and white table wines. Food Control 2013, 32, 687–692. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.; Wang, H.; Zhang, L. Impact odorants of Chardonnay dry white wine from Changli County (China). Eur. Food Res. Technol. 2008, 227, 287–292. [Google Scholar] [CrossRef]
- Cullere, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef]
- Bardi, L.; Cocito, C.; Marzona, M. Saccharomyces cerevisiae cell fatty acid composition and release during fermentation without aeration and in absence of exogenous lipids. Int. J. Food Microbiol. 1999, 47, 133–140. [Google Scholar] [CrossRef]
- Diaz-Maroto, M.; Schneider, R.; Baumes, R. Formation pathways of ethyl esters of branched short-chain fatty acids during wine aging. J. Agric. Food Chem. 2005, 53, 3503–3509. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.J.; Mei, W.C.; Li, T.; Tao, Y.S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef]
- Larue, F.; Geneix, C.; Lafon-Lafourcade, S.; Bertrand, A.; Ribéreau-Gayon, P. Premières observations sur le mode d’action des écorces de levure. OENO One 1984, 18, 155–163. [Google Scholar] [CrossRef]
- Baron, M.; Kumsta, M.; Prokes, K.; Tomaskova, L.; Tomkova, M. The inhibition of Saccharomyces cerevisiae population during alcoholic fermentation of grape must by octanoic, decanoic and dodecanoic acid mixture. In Proceedings of the 40th World Congress of Vine and Wine, Sofia, Bulgaria, 29 May–2 June 2017. [Google Scholar]
- San Juan, F.; Cacho, J.; Ferreira, V.; Escudero, A. Aroma Chemical Composition of Red Wines from Different Price Categories and Its Relationship to Quality. J. Agric. Food Chem. 2012, 60, 5045–5056. [Google Scholar] [CrossRef]
- Lee, S.; Noble, A. Characterization of odor-active compounds in Californian Chardonnay wines using GC-olfactometry and GC-mass spectrometry. J. Agric. Food Chem. 2003, 51, 8036–8044. [Google Scholar] [CrossRef]
- Lukic, I.; Plavsa, T.; Sladonja, B.; Radeka, S.; Persuric, D. Aroma compounds as markers of wine quality in the case of Malvazija Istarska young wine. J. Food Qual. 2008, 31, 717–735. [Google Scholar] [CrossRef]
- Shinohara, T. Gas-chromatographic analysis of volatile fatty-acids in wines. Agric. Biol. Chem. 1985, 49, 2211–2212. [Google Scholar] [CrossRef]
- Baron, M.; Fiala, J. Chasing after Minerality, Relationship to Yeast Nutritional Stress and Succinic Acid Production. Czech J. Food Sci. 2012, 30, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Legras, J.; Erny, C.; Le Jeune, C.; Lollier, M.; Adolphe, Y.; Demuyter, C.; Delobel, P.; Blondin, B.; Karst, F. Activation of Two Different Resistance Mechanisms in Saccharomyces cerevisiae upon Exposure to Octanoic and Decanoic Acids. Appl. Environ. Microbiol. 2010, 76, 7526–7535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donéche, B.; Lonvaud, A. Handbook of Enology, Volume 1. The Microbiology of Wine and Vinifications, 2nd ed.; Wiley: Chichester, UK, 2006. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).