Abstract
Sunn hemp (SH, Crotalaria juncea, L.) is a tropical multiple-purpose legume. The green manure SH (GMSH) crop might display protein ecology in sustaining ruminants; however, its silage features remain unclear. To efficiently prepare GMSH crop silage, additive treatments consisting of control (no additive, CON), molasses (MO), Acremonium cellulase (AC), and Lactobacillus casei TH14 strain inoculant (TH14) were implemented using a completely randomized design. Repeated measurements were done after silage (AE conditions) in a small-scale silo system for 120 days and after aerobic instability (AE + AIS conditions). Briefly, ensiling loss and aerobic stability ranged from 150 to 175 g/kg and 8.3 to 104 days, respectively. In AE conditions, the pH ranged from 4.33 to 5.74, and MO or AC was desirable (p < 0.01) for lactic acid fermentation. AC reduced the fiber contents. MO increased soluble non-protein nitrogen by decreasing insoluble nitrogen. TH14 increased the ammonia nitrogen level and in vitro methane production. In AE + AIS conditions, AC led to more air damage to the chemical compositions and reduced digestibility in vitro. The results show that an optimization of additives could effectively modify GMSH crop silage to make it a good protein roughage source; however, more studies are required for effectively feeding ruminants.
1. Introduction
Sunn hemp (SH, Crotalaria juncea, L.) is an annual legume originating from India [1]. This plant species grows fast, has high seed yield, is tolerant of low fertilizer conditions, and is easily harvested or plowed into soil. As a result, SH is widely used for green manure, land cover crops, oil seed, forage, and tourism (for its full yellow flower bloom). The root nodules of SH increase atmospheric nitrogen (N) fixation with increasing age, and the parts above ground contain nutrients and high crude protein (CP) content as a leguminous material [2]. Therefore, green manure SH (GMSH) crop materials are potent and cheap for use in meat and milk production by ruminants. For late cuts, however, the CP content decreases with increasing lignification, and indigestible fiber contents increase. The use of SH as forage crop is increasing in Thailand; however, more information is needed for efficient utilization in animals [3].
Silage is a suitable technique for wet preservation purposes. The technique ferments much plant material by employing epiphytic lactic acid bacteria (LAB) and available water-soluble carbohydrates in lactic acid fermentation [4]. These days, improving ensiling characteristics by adding silage additives is widely known, and it is important to increase the speed of lactic acid fermentation, which ensures or increases silage quality. The quality is critical to avoid pathogens and undesirable end-products [5], preserve available nutrients [6], and increase utilization efficiency (biotransformation) [7], especially the digestibility of the silage by ruminants [8].
Studies of silage additives normally include both chemical and biological compounds such as alkalis, acids, fermentable sugar substrates, LAB inoculant, and cellulase [9,10,11]. The addition of some LAB inoculants was demonstrated to reduce enteric methane production [12,13,14,15]. In our previous energy balance studies, the enteric methane emissions of tropical cattle fed agricultural residues and by-products were a little high and needed mitigation strategies for use on farms [16,17]. Enteric methane emissions are a major source of energy loss and a contributor to ruminants’ greenhouse gas emissions. To date, the fermentation characteristics and response to additive treatments for ensiling and feeding of GMSH silage have not been well studied. Therefore, the objective in this study was to characterize GMSH crop silage prepared with additives and its aerobic instability, N fractions, and in vitro rumen methane production.
2. Materials and Methods
2.1. GMSH Preparation
For ideal green manure crop, SH was planted without fertilizer or soil amendments on rotational perennial grassland run by the Faculty of Natural Resources, Rajamangala University of Technology Isan (17°34′ N, 103°73′ W), Sakon Nakhon, Thailand. At 101 days after planting (late full-bloom stage), 16 sub-plots of GMSH crop were harvested within quadrate samplers (2 × 2 m) at 15 cm above the soil surface. The samples of GMSH crop were chopped individually into 1 cm pieces and divided into two portions. One portion (16 sub-plots × 0.5 kg fresh matter, FM) was pooled and obtained as a representative sample (1 kg FM) for analyzing microbial populations, pH, lactate buffering capacity (LBC), chemical compositions, N fractions, in vitro digestibility, gas production, and methane production. The other portion (16 sub-plots × 0.5 kg FM) was used for making silage.
2.2. Design, Silage Making, and Measurement of Ensiling Loss, and Aerobic Stability
This study was conducted as a completely randomized design with repeated measurements. The GMSH crop was prepared with no additive (control), molasses at 50 g/kg FM (MO), cellulase enzyme at 0.1 g/kg FM (AC), and LAB inoculant at 1 × 108 colony forming units (cfu)/kg FM (TH14) in four silo replications (4 additives × 4 sub-plots, each 500 g FM). After ensiling indoors for 120 days, the parameters of the experimental silages were measured twice to study different conditions after ensiling (AE) and after the addition of aerobic instability (AE + AIS).
The concentrations of silage additives were estimated to be a modest dosage to improve silage quality when prepared from tropical forage material [8,18]. The MO used for increasing sugar substrates was a commercial type for feedstuff and was used undiluted. The AC used for enzymatic saccharification of fiber substrates was a mixture of glucanase and pectinase (7350 U/g), which were obtained from Acremonium cellulolyticus [18]. This enzyme was dissolved with distilled water (0.05 g + 5 mL) for spraying onto 500 g FM of a randomized GMSH crop material. The TH14 strain was used for ensuring lactic acid production and was produced overnight in Lactobacilli de Man, Rogosa, Sharpe (MRS) broth (Difco Laboratories, Detroit, MI, USA) [19]. The TH14 strain was suspended in distilled water at 5.0 × 107 cfu/5 mL [8].
The GMSH crop material was precisely weighed in a plastic bag container. The additives were added and mixed homogeneously, and the mixture was transferred to pre-weighed laboratory-scale silos laminated with nylon and polyethylene (200 mm × 300 mm, Hiryu KN type, Asahi Kasei Pax Co., Tokyo, Japan). The silos were sealed using a vacuum sealer (SQ-303, Asahi Kasei Pax Co., Tokyo, Japan) and weighed to find the total initial weight. The total final weight of silage samples was measured to calculate the ensiling loss, which was reported in g/kg of initial ensiling material.
Aerobic stability was measured as the day when silage temperature increased 2 °C above ambient room temperature (26.1 ± 1.51 °C, mean ± standard deviation) under aerobic conditions [10,20,21]. Briefly, a half portion of silage samples was used to conduct this aerobic stability experiment. The sample was precisely weighed to obtain 200 g FM in a 500 mL glass beaker. The samples were covered with two layers of cheesecloth, left at room temperature, and measured daily for temperature at the center of the silage materials using a thermometer (CyberScan Ion 510, Eutech Instruments Pte Ltd., Singapore). When a sample entered aerobic instability conditions, the day was recorded, and the sample was immediately measured for microbial populations and silage quality.
2.3. Analytical Procedures
The microbial populations in GMSH crop material, AE silage, and AE + AIS silage were evaluated in triplicate using plate-counting methods [8,22]. The sample (10 g FM) was mixed with 90 mL of sterile NaCl solution containing 8.5 g NaCl/L, and serial dilutions were made at 10−1 to 10−5. Each dilution (20 µL) was spread on agar plates. LAB were counted on MRS agar (Difco) after 48 h of incubation at 30 °C in an anaerobic box (Sugiyamagen Ltd., Tokyo, Japan). Coliform bacteria and aerobic bacteria were counted on blue-light agar (Nissui-seiyaku Ltd., Tokyo, Japan) and nutrient agar (Difco), respectively, after 3 days of incubation at 30 °C under aerobic conditions. Yeasts and molds were observed on potato dextrose agar (Nissui-seiyaku Ltd., Tokyo, Japan) and were distinguished by observing the cell morphology. The microorganism counts are reported in cfu/g FM.
The LBC of fresh GMSH crop material was analyzed in triplicate using a volumetric method [4]. The sample was weighed to obtain 10 g, added to 90 mL of deionized water, adjusted to pH 3 using 0.1 M HCl solution, and left for 5 min. The sample was then slowly titrated to increase the pH using 0.1 M NaOH solution. The titration volume of NaOH solution that changed the pH from 4 to 6 was recorded and used to calculate the LBC concentration, which is reported in mEq/kg DM.
The DM content was measured using an air-drying oven for 24 h at 100 °C. The samples for analyses were dried for 48 h at 60 °C and then ground to pass through a 1 mm screen. The organic matter (OM) and ether extract (EE) contents were analyzed using standard methods 942.05 and 920.39, respectively [23]. The CP content was measured us-ing an N analyzer (828 Series, LECO, St. Joseph, MI, USA) with 6.25 as factor for CP conversion. The neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents were evaluated using a fiber analyzer (ANKOM 200, ANKOM Technology, Macedon, NY, USA). The acid detergent lignin (ADL) was measured by solubilization with a sulfuric acid solution [24]. The non-fibrous carbohydrates (NFC) content of GMSH crop material was calculated as DM minus ash, CP, EE, and NDF components [25].
The N fractions were examined in triplicate based on solubility in a carbonate–phosphate buffer (BCP) following the methods described by Krishnamoorthy et al. [26]. The sample (1 g) was weighed in a 100 mL volumetric flask with a tight cap and added to 65 mL of BCP (we dissolved 1.43 g of Na2HPO4, 1.55 g of KH2PO4, and 9.81 g of NaHCO3 in 1 L and adjusted it to pH 6.8 by adding 1 M HCl solution). The sample was incubated for 1 h at 39 °C with shaking at 120 rpm using an incubator (Innova 40, Eppendorf AG, Hamburg, Germany). The sample was filtered to separate the residue and extract using a pre-weighed filter paper (Whatman No. 54). The residue was washed with 300 mL of cold distilled water, dried for 48 h at 60 °C, weighed for residue, and examined for the insoluble N content using an N analyzer (828 Series). The extract (10 mL) was pipetted into a 15 mL screwcap tube, added to 2 mL of 600 g C2HCl3O2/L solution, and left for 10 min. The precipitated protein was centrifuged for 20 min at 4200× g, and we discarded the supernatant. We washed the protein for 3 cycles using 10 mL of cold ethanol resuspensions and centrifugations and dried it for 6 h at 60 °C. The sample was dissolved with the addition of 1 mL of 1.0 M NaOH solution and left overnight at 4 °C. The sample (0.1 mL) was then diluted with 0.9 mL of 0.09 M NaOH solution and spectrophotometrically assayed for true protein using Lowry analysis [27]. We used 0.1 M NaOH solution as a reagent blank and bovine serum albumin as a standard. The soluble true protein N (TPN) content was calculated by dividing true protein with 6.25. The soluble N content was the difference of total N and insoluble N. Non-protein N (NPN) was the total N minus the sum of insoluble N and soluble TPN.
Silage-quality indicator tests were performed in triplicate using cold extract [28]. A 10 g FM sample was mixed with 90 mL of sterilized distilled water and left at 4 °C. After 12 h, the extract was warmed to 25 °C, and then the pH value was measured (pH meter FiveGo, Mettler-Toledo GmbH, Greifensee, Switzerland). The concentrations of organic acids (lactic acid, acetic acid, propionic acid, and butyric acid) were analyzed with a periodic acid reagent [29] using gas chromatography (Nexis GC-2030, Shimadzu Co., Kyoto, Japan). The chromatograph was equipped with a split-mode injector, capillary column (DB-WAX 30 m, 0.25 mm, 0.25 µm, Agilent Technologies, Inc., Santa Clara, CA, USA), and flame ionization detector. The content of ammonia nitrogen (NH3N) was examined using spectrophotometry (UV/VIS Spectrometer, PG Instruments Ltd., London, UK) [30].
The samples were investigated for gas production and in vitro digestibility of DM (IVDMD), NDF (IVNDFD), and ADF (IVADFD) at 48 h after incubation in triplicate using a gas production technique described by Makkar et al. [31]. The in vitro gas produced was stored for measuring the methane production using the method described by Kaewpila et al. [13]. Briefly, rumen fluid was obtained before the morning feeding from two lactating Holstein dairy cows by a stomach-tube sucker. The cattle were fed with a fermented total mixed ration containing rice straw and indigenous concentrated feedstuffs at a ratio of approximately 1:1 on a DM basis. To avoid saliva contamination, the first 500 mL of collected rumen fluid was discarded according to Muizelaar et al. [32]. Rumen fluid was filtered through four layers of cloth sheet and mixed with a mineral buffer solution [31] at a 1:4 ratio under a stream of CO2. The samples were precisely pre-weighed to obtain 0.50 g in 50 mL serum bottles, and three bottles without sample were used as blanks. The bottles were sealed with rubber stoppers and aluminum caps. They were incubated at 39 °C with shaking at 120 rpm (Innova 40, Hamburg, Germany). The gas produced was measured and stored in a gas bag at 3, 6, 12, 24, and 48 h using a 25 mL calibrated glass syringe equipped with an air connector.
At 48 h, gas in the bottle’s headspace was purged into a bag by injecting 100 mL of N2. The methane concentration was analyzed using gas chromatography (Nexis GC-2030) with a capillary column (SH-Rt-Q-BOND 30 m, 0.53 mm, 20 µm, Shimadzu Co., Tokyo, Japan) and high-purity methane as a standard. After 48 h, the residual samples were filtered with fiber bags (F57, Ankom Technology, Macedon, NY, USA), washed with pepsin solution and distilled water, dried at 100 °C for 24 h, and weighed for IVDMD measurement [10]. The bags were sealed and analyzed for NDF and ADF contents, respectively (ANKOM 200, Macedon, NY, USA). The in vitro digestibility is reported in g/kg of substrate.
2.4. Statistical Analysis
The data were analyzed using an ANOVA procedure of SAS (V. 6.12, SAS Institute Inc., Cary, NC, USA) with the following models:
where Yijk = observation, μ = overall mean, αi = additive effect (i = 1 to 4), and εij = error, and
where Yijk = observation, μ = overall mean, αi = additive effect (i = 1 to 4), βj(i) = silo effect (j = 1 to 4) within additive i, γk = condition effect (k = AE to AE + AIS), αγik = additive × condition effect, and εijk = error. Differences among treatment means were done using Duncan’s test and the significance level was accepted when p ≤ 0.05 [33].
Yij = μ + αi + εij,
Yijk = μ + αi + βj(i) + γk + αγik + εijk,
3. Results
3.1. GMSH Crop Material
Table 1 shows the microbial counts, pH, LBC, chemical compositions, N fractions, and in vitro rumen parameters at 48 h incubation of the fresh GMSH crop material. The counts of microorganisms ranged from 103 to 109 cfu/FM with LAB and aerobic bacteria having the lowest and highest values, respectively. The pH value was 6.4. The LBC content was high at a 2343 mEq/kg DM. The DM content was 256 g/kg and contained OM, CP, EE, NDF, ADF, ADL, and NFC at 915, 160, 20, 624, 434, 95, and 112 g/kg DM, respectively.

Table 1.
Microbial counts, pH, lactate buffering capacity (LBC), chemical compositions, N fractions, and in vitro rumen parameters at 48 h incubation of fresh GMSH crop material used to prepare silage in this study.
The insoluble N content was 565 g/kg total N. The soluble TPN content was 179 g/kg total N, and soluble NPN had a greater value. The values of in vitro digestibility ranged from 293 to 558 g/kg. The gas production was 136 L/kg DM, which included methane at 17.2 L/kg DM, and 30.9 L/kg IVDMD.
3.2. Ensiling Loss and Aerobic Stability
Figure 1 shows the effects of additives on the ensiling loss of GMSH crop silage after 120 days’ storage and days of aerobic stability. The ensiling loss ranged from 150 to 175 g/kg, and it was lower (p < 0.05) with MO and TH14 than with CON. The aerobic stability in GMSH crop silage prepared with CON, AC, and TH14 was extremely long at 100 days; however, it decreased (p < 0.01) to 8.3 days with MO.
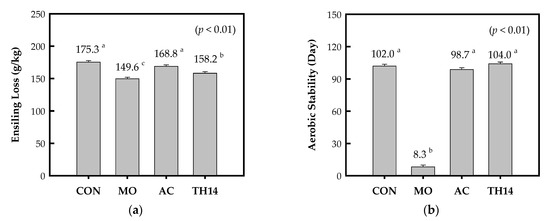
Figure 1.
(a) The ensiling loss of GMSH crop silage prepared with additives and ensiled for 120 days; (b) the aerobic stability test. Bars represented the standard errors of the means. a−c Means within figures with difference superscript letters differ at p ≤ 0.05. CON, no additive; MO, adding molasses; AC, adding Acremonium cellulase; TH14, adding L. casei strain TH14 inoculant.
3.3. Silage Quality of GMSH Crop
The silage quality indicators are revealed in Table 2. In the repeated analysis, the pH value, organic acid concentrations (except propionic acid), and NH3N level of GMSH crop silage were affected (p < 0.01) by additive × condition effects. The propionic acid concentration in AE conditions was higher (p = 0.01) than in AE + AIS conditions. In addition, the propionic acid level with CON or TH14 was consistently greater (p < 0.01) than with MO or AC.

Table 2.
Silage quality of GMSH crop prepared with additives measured after ensiling (AE) and after the addition of aerobic instability (AE + AIS).
For AE conditions, the pH value of GMSH crop silage ranged from 4.33 to 5.74. In comparison with CON, the additive treatments decreased (p < 0.01) pH, although the lactic acid concentration with TH14 was similar. MO or AC decreased the butyric acid concentration; however, TH14 increased it (p < 0.01). The NH3N level with MO was the lowest (p < 0.01), followed by CON or AC, and TH14.
For AE + AIS conditions, the pH value with MO was the lowest, and then it increased with CON, TH14, and AC (p < 0.01). The concentrations of organic acids and NH3N were different among treatments (p < 0.01). The lactic acid concentration with MO was greater than with other three treatments. Acetic acid and butyric acid concentrations with MO or AC were lower, and those with TH14 were greater than with CON. The NH3N concentration was higher with AC, followed by CON or MO, and TH14.
3.4. Microbial Populations of GMSH Crop Silage
The microbial populations in GMSH crop silage are shown in Table 3. The additive × condition effect for microbial populations (except coliform bacteria) was significant (p < 0.01). The coliform bacteria numbers seem to be higher (p = 0.07) for AE + AIS compared with AE conditions. In both conditions, the counts of coliform bacteria were consistently not affected (p = 0.31) by treatments.

Table 3.
Microbial populations of GMSH crop silage prepared with additives measured after ensiling (AE) and after the addition of aerobic instability (AE + AIS).
For AE conditions, the LAB counts of CON and TH14 were greater (p < 0.01) than those of MO and AC conditions. The counts of coliform bacteria and mold were under a detectable level for most treatments. The aerobic bacteria counts were greatest (p < 0.01) for CON, followed by MO and then AC or TH14. Yeasts in CON were highest (p < 0.01), followed by AC or TH14, and MO.
For AE + AIS conditions, the LAB count with MO was 8.09 log10 cfu/g FM, which was greater (p < 0.01) than in CON, TH14, and CON conditions. The aerobic bacteria counts were greater (p < 0.01) for MO and AC compared with CON and TH14. Yeasts in MO conditions (p < 0.01) and molds in AC conditions (p < 0.01) were greater than in other treatments.
3.5. Chemical Compositions of GMSH Crop Silage
The chemical compositions had significant differences (p < 0.05) with an additive × conditions effect (Table 4). In AE conditions, the DM content with MO was greater (p < 0.01) than with the other three treatments. The OM content was similar among treatments (p = 0.43). The CP content was improved by MO and decreased by TH14 compared with CON (p < 0.01). Moreover, TH14 decreased (p < 0.01) the EE content. The NDF and ADF contents with CON and TH14 were greater (p < 0.01) than those with MO and AC. Compared with CON, the additive treatments decreased (p < 0.01) the ADL content.

Table 4.
Chemical compositions of GMSH crop silage prepared with additives measured after ensiling (AE) and after the addition of aerobic instability (AE + AIS).
In AE + AIS conditions, the DM and OM contents had significant differences (p ≤ 0.05) among treatments, with AC having the lowest values. The EE content with CON and MO was greater (p < 0.01) than with TH14, and that with AC was in the middle. Among treatments, MO had the lowest (p < 0.01) NDF and ADF contents. The ADL content was highest (p < 0.01) for AC, followed by CON or TH14, and MO.
3.6. N Fractions of GMSH Crop Silage
The N fractions (except soluble TPN) were not affected (p > 0.05) by additive × condition effects (Table 5). However, the N fractions of GMSH crop silage were not affected (p > 0.05) by different AE and AE + AIS conditions. In both conditions, the insoluble N fraction with AC was consistently greater (p = 0.01) than with CON and MO. The soluble NPN fraction with AC was consistently lower (p = 0.02) than with MO, CON, and TH14. For AE conditions, the soluble TPN fraction with AC was less (p < 0.01) than those of the other three treatments. For AE + AIS conditions, this fraction with AC turned out to be greater (p = 0.01) than those of other treatments.

Table 5.
N fractions of GMSH crop silage prepared with additives measured after ensiling (AE) and after the addition of aerobic instability (AE + AIS).
3.7. In Vitro Digestibility, and Productions of Gas and Methane of GMSH Crop Silage
The in vitro parameters at 48 h of incubation of GMSH crop silage were altered (p ≤ 0.01) by additive × condition effects (Table 6). For AE conditions, the IVDMD ranged from 459 to 614 g/kg, which was higher (p < 0.01) with MO and TH14 than with CON. The IVNDFD of MO and TH14 was greater than that of CON, while the CON result was similar to that of AC (p < 0.01). The IVADFD with AC was lower (p < 0.01) than with CON, while TH14 and MO had higher values. The gas production ranged from 106 to 148 L/kg DM and increased (p < 0.01) with the additive treatments, with MO having the greatest value. The methane production (L/kg DM) was lower with CON because it increased with AC, MO, and TH14 (p < 0.01). The methane production (L/kg IVDMD) with CON and MO was lower than with AC and TH14 (p < 0.01).

Table 6.
In vitro digestibility, gas production, and methane production of GMSH crop silage prepared with additives measured after ensiling (AE) and after the addition of aerobic instability (AE + AIS).
For AE + AIS conditions, the IVDMD of CON was similar to that of TH14, and both of these had lower values than MO and greater than AC (p < 0.01). Compared with CON, MO and TH14 had greater (p < 0.01) IVNDFD, with AC having the lowest value. The IVADFD of CON and MO was greater (p < 0.01) than that of TH14 and AC. The gas production of CON and MO was also greater (p < 0.01) than that of AC. The methane production (L/kg DM) was different (p < 0.01) and was higher with MO, CON, or TH14, and AC in that order. The methane production (L/kg IVDMD) of CON and MO was higher (p < 0.01) than that of TH14 and AC.
4. Discussion
4.1. Characteristics of GMSH Crop as Ensiling Material and Potent Forage
Silage practices require suitable components from plant materials to promote lactic acid fermentation. For green forage, a high epiphytic LAB number and low LBC are highly regarded to achieve a rapid production of lactic acid and rapid acidity for inhibiting undesirable fermentation by anaerobic microorganisms, such as enterobacteria and clostridia [4]. The results in this study showed that these two critical characteristics in the GMSH crop prior to ensiling might not meet requirements as the LAB number was too low (103 cfu/g FM) and the LBC value was too high (2343 mEq/kg DM, Table 1).
A previous study demonstrated that the LAB counts in tropical forages that were less than 105 cfu/g FM could not maximize lactic acid fermentation [8]. High LBC chemically resists a change in pH and requires more acid to be supplied to decrease the pH value. In our previous findings, a Napier grass cultivar had an LBC value of less than 800 mEq/kg DM. Considerably greater LBC occurs in most forage legumes, including GMSH crop, and this is highly related with soluble NPN. Thus, ensiling SH plants in practice with undesirable LAB counts and LBC levels could make it difficult and could require external factors such as silage additives for effective preservation of forage mass and nutrients.
Studies of the chemical compositions, N fractions, and in vitro digestibility have been recommended to elucidate the nutritive values of forage for ruminant feeding regimes [34,35]. The analyzed CP, NDF, and ADF levels for the GMSH crop were consistent with those for SH plant materials reported by Lepcha et al. [36]. The present study was the first report of N fractions determined in GMSH crop. The data suggested that it has a moderate level of soluble TPN fraction (179 g/kg total N), which was approximately equal to that of a haft of oats (430 g/kg total N) [26]. The nutritive value of an SH crop can vary among genotypes and as a result of other factors such as soil, fertilizer, and weather [1].
When applying fertilizers, SH can be cut after 35 days of planning for receiving CP crop [36]. For late cuts, the results show a very high ADL (95 g/kg DM), which probably suppresses fiber utilization in livestock production. Thus, future investigation on pre-treatments for reducing ADL content would be important for effectively using GMSH crop as a good protein roughage source.
4.2. Characteristics of Ensiling Loss, Aerobic Stability, and Silage Quality in GMSH Crop
The ensiling loss in a closed silo system is a biological cost of fermentation, so minimizing this value significantly improves silage production. Adding silage additives could reduce this loss because rapid lactic acid fermentation occurs [8,18]. For GMSH crop silage, the results showed that MO and TH14 resulted in the lowest ensiling loss (Figure 1). In comparison with TH14, however, the advantage with MO remarkably affected the aerobic stability of GMSH crop silage, suggesting an intensive face management in the feed out phase.
In this present finding, the aerobic stability of GMSH crop silage prepared with CON, AC, and TH14 was remarkably high value (99 to 104 days) [37]. The aerobic stability of typical low-pH silages was ranged from 1 to 21 days. In a previous study, air-dried rice straw silage did not spoil over 33 days [38]. Residual water soluble carbohydrates and lactic acid with a lack of volatile fatty acids have a negative effect on the aerobic stable of silage [28]. However, no studies have clearly verified whether acetic acid or propionic acid fermentation has a greater antibiotic effect for prolonging the aerobic stability phase of silage [10]. In this study, the antibiotic feature of acetic acid can presumably be lower than that of propionic acid. The acetic acid in AC and AE + AIS conditions was probably used by aerobic microorganisms, resulting in unstable results (additive × condition effect, p < 0.01, Table 2).
A high pH value (>4.2) of leguminous silage types has consistently occurred in different studies [3,39,40], including this study (pH of CON = 5.74, Table 2). The results showed that the additives can reduce the pH value, so they might improve the silage quality of GMSH crop. The results implied that a low concentration of fermentable sugars was the major barrier for this plant because MO and AC resulted in better ensiling characteristics. The results showed that TH14 increased (p < 0.01) butyric acid and NH3N concentrations. This undesirable amino group degradation in GMSH crop silage when adding the TH14 strain contradicts our previous studies using tropical grasses and agricultural by-products [8,10,13,18,41]. This finding probably resulted from an increase in the LBC level, suggesting the addition of TH14 be done with a fermentable sugar source or a new LAB strain to specifically improve leguminous silage production.
In addition, after aerobic instability enrichment for GMSH crop silage, lactic acid, acetic acid, butyric acid, and NH3N contents were probably reduced by microbial utilization. The results demonstrated that the desirable pH value with AC and TH14 was not stable. This finding revealed that a sufficient period of providing aerobic condition could negate the benefits of the feeding of silage with improved ensiling characteristics.
4.3. Characteristics of Microbial Populations in GMSH Crop Silage
The results showed that GMSH crop silage ensiled for a 120-day period still had abundant LAB, aerobic bacteria, yeasts, and molds (Table 3). These findings are related with the high pH of silage (4.3 to 5.7, Table 2), an important factor that supports microbial survival and living activities [37]. At this high pH, Clostridium species that are detrimental to the nutrients (producing butyric acid) and pathogenic species can occur (such as C. botulinum) [5], so more studies are required to confirm and clarify the microbial safety of GMSH crops prepared silage.
In this study, we suggested that MO (pH = 4.3) should be adequate to achieve critical pH to prevent clostridial growth in GMSH crop silage. AC might also achieve a critical pH if the dose is increased [10], while TH14 was always undesirable for reducing pH without sufficient fermentable sugars provided. The coliform bacteria disappeared because of anaerobic fermentation in silage. In AE + AIS conditions, coliform bacteria and molds were observed, suggesting that they could be the main microorganisms related with the air spoilage of GMSH crop silage.
4.4. Characteristics of Chemical Compositions in GMSH Crop Silage
The additives had a specific effect on chemical compositions of GMSH crop silage (Table 4), as in other silage reports [18,42,43]. The effect of MO can be clearly interpreted for nutrient preservation of CP and NDF contents. The reduction of NDF and ADF contents with AC is associated with enzymatic saccharification, releasing fermentable sugars for lactic acid fermentation. The slight delignification of TH14 in GMSH crop silage was consistent with a previous study on cassava pulp silage [41]. Probably, the TH14 strain stimulated the growth of other fiber-decomposing microbes that result in a reduction in fibrous contents and delignification [41].
In addition, nutrients of silage are able to aerobically deteriorate with obligate microbes. In this study, the results in AE + AIS conditions showed that CON, AC, and TH14 received more air deterioration damage to the chemical compositions (CP, NDF, and ADF) and more ADL condensation. These findings are associated with the shortened days in the aerobic stability phase with MO versus other treatments (Figure 1). The findings demonstrated that prolonging aerobic stability could have a negative effect on the desirable chemical compositions of GMSH crop silage. Thus, benefits from prolonging the aerobic stable period of GMSH crop silage could be omitted to improve nutritive value.
4.5. Characteristics of N Fractions in GMSH Crop Silage
The data of N fractions showed that the differences among additive treatments for the insoluble N and soluble NPN fractions in GMSH crop silage were consistent across AE and AE + AIS conditions (p-values of additive × condition > 0.05, Table 5). Thus, this indicated that an additive modification of N fractions in GMSH crop silage could be stable for a lower feed out rate. In general, insoluble N represents rumen-undegradable protein [26]. Increasing soluble N fractions in forage by degrading insoluble N should improve N digestibility and N balance for ruminants. MO could decrease insoluble N with increases in soluble NPN, probably by acidification of lactic acid fermentation. In contrast, AC preserved insoluble N and reduced soluble NPN. Thus, the critical pH (4.2) of silage is important to improve the N utilization of GMSH crop silage.
4.6. Characteristics of In Vitro Rumen Test in GMSH Crop Silage
In spite of major progress in understanding the utilization of forage by ruminants, in vitro tests are done to determine digestibility with potential treatments [44]. In this study, the data suggested that the IVDMD in CON (459 g/kg, Table 6) was lower than that of the GMSH crop sample (558 g/kg, Table 1) and apparently decreased to about 100 g/kg with silage. However, the greater IVDMD of MO was probably confounded by residual molasses effects. The results suggested that lactic acid fermentation could improve the IVNDFD and IVADFD values obtained with MO and TH14. A lower IVADFD value with AC might result from the condensation of lignocellulose owing to enzymatic saccharification at ensiling [41].
In AE + AIS conditions, the results suggested that AC with air can do greater damage to the in vitro digestibility of GMSH crop silage. Possibly, enzymatic saccharification by AC transformed the fibrous substrates available for aerobic microbial growth. However, the reasons for aerobic degradation of the silage’s feed utilization by cellulase are fairly complex. In addition, an influence of chemical composition on the in vitro fermentation characteristics agreed with Musco et al. [45].
Reduction methanogenesis is a standing issue in ruminant production worldwide to control energy loss in the rumen and to delay atmospheric warming [16,17]. The results indicated that the additives increased in vitro gas and methane productions of GMSH crop silage. The addition of TH14 strain to ensiled GMSH crop can increase methane production (4.11 L/kg DM or 29.7%) and methanogenesis efficiency (5.12 L/kg IVDMD or 17.0%). In a previous study, the TH14 strain inoculant improved the ensiling characteristics, in vitro digestibility, and in vitro methane mitigation in forage–sorghum mixture silage [13]. Other studies showed various effects on methane production of silage in responding to the addition of LAB inoculants [14,15]. The present finding supported those of Ellis et al. [15] in that the probiotic effects of LAB inoculations could depend on the ensiling substrates. Many factors such as a changing microbial community, LBC level, ensiling characteristics, and ensiling period can have a unique methanogenesis effect on TH14. Interestingly, another attempt with rumen manipulation might potently reduce the methanogenesis in GMSH crop silage.
5. Conclusions
This study characterizes GMSH crop silage prepared with some additives. The results demonstrate that an optimization of molasses, Acremonium cellulase, and TH14 strain could effectively improve the ensiling characteristics of GMSH crop silage, resulting in a greater protein roughage source. The insoluble N fraction of GMSH crop silage is released as a soluble NPN fraction when lactic acid fermentation results in the critical pH of the silage. Despite high aerobic stability occurring, a longer feed out phase reduces the nutritive value of GMSH crop silage. Unfortunately, with increasing in vitro digestibility in GMSH crop silage, the in vitro enteric methane mitigation is not achieved by the TH14 strain. More studies on GMSH crop silage using in vitro and in vivo experiments are required to obtain more data.
Author Contributions
Conceptualization, C.K., W.K. and A.C.; formal analysis, C.K., W.K., P.G. and T.K.; investigation, C.K. and W.K.; resources, C.K., W.K., C.S. and A.C.; writing—original draft preparation, C.K. and W.K.; writing—review and editing, C.K., P.K., C.S., W.K. and A.C.; supervision, C.K., W.K. and A.C.; funding acquisition, A.C., C.K. and W.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work has received scholarship under the Post-Doctoral Training Program from Khon Kaen University, Thailand, grant number PD2564-14, the Research Program on the Research and Development of Winged Bean Root Utilization as Ruminant Feed (RP64-6/002), and the Increase Production Efficiency and Meat Quality of Native Beef and Buffalo Research Group, Department of Animal Science, Faculty of Agriculture, Khon Kaen University. The APC was funded by Khon Kaen University.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Animal Ethics Committee of Faculty of Natural Resources, Rajamangala University of Technology Isan (approval number 49/2564 on 4th October 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Rajamangala University of Technology Isan, Khon Kaen University, Animal Nutrition Laboratory (Khon Kaen University), and Japan International Research Center for Agricultural Sciences (JIRCAS) for the infrastructure and laboratory facilities. C.K. and W.K. would like to thank Professor Yimin Cai (JIRCAS) for expert technical support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Garzon, J.; Vendramini, J.M.B.; Silveira, M.L.; Moriel, P.; da Silva, H.M.S.; Dubeux, J.C.B.; Kaneko, M.; Carnelos, C.C.; Mamede, P.A. Harvest management and genotype effects on Sunn hemp forage characteristics. Agron. J. 2021, 113, 298–307. [Google Scholar] [CrossRef]
- Ashworth, A.J.; West, C.P.; Allen, F.L.; Keyser, P.D.; Weiss, S.A.; Tyler, D.D.; Taylor, A.M.; Warwick, K.L.; Beamer, K.P. Biologically fixed nitrogen in legume intercropped systems: Comparison of nitrogen-difference and nitrogen-15 enrichment techniques. Agron. J. 2015, 107, 2419–2430. [Google Scholar] [CrossRef] [Green Version]
- Wanapat, M.; Totakul, P.; Viennasay, B.; Matra, M. Sunnhemp (Crotalaria juncea, L.) silage can enrich rumen fermentation process, microbial protein synthesis, and nitrogen utilization efficiency in beef cattle crossbreds. Trop. Anim. Health Prod. 2021, 53, 187. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.; Henderson, A.; Heron, S. The Biochemistry of Silage; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Driehuis, F.; Wilkinson, J.M.; Jiang, Y.; Ogunade, I.; Adesogan, A.T. Silage review: Animal and human health risks from silage. J. Dairy Sci. 2018, 101, 4093–4110. [Google Scholar] [CrossRef]
- Sun, J.; Wang, T.; Huang, F.; Liu, Y.; Shi, W.; Ma, C.; Zhong, J. Silage fermentation: A potential microbial approach for the forage utilization of Cyperus esculentus L. by-product. Fermentation 2021, 7, 273. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Pateiro, M.; Nawaz, A.; Hano, C.; Walayat, N.; Lorenzo, J.M. Strategies to increase the value of pomaces with fermentation. Fermentation 2021, 7, 299. [Google Scholar] [CrossRef]
- Khota, W.; Pholsen, S.; Higgs, D.; Cai, Y. Natural lactic acid bacteria population of tropical grasses and their fermentation factor analysis of silage prepared with cellulase and inoculant. J. Dairy Sci. 2016, 99, 9768–9781. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.-Y.; Ungerfeld, E.; Ouyang, Z.; Zhou, X.-L.; Han, X.-F.; Zeng, Y.-Q.; Tan, Z.-L. Effect of Lactobacillus plantarum inoculation on chemical composition, fermentation, and bacterial community composition of ensiled sweet corn whole plant or stover. Fermentation 2022, 8, 24. [Google Scholar] [CrossRef]
- Kaewpila, C.; Khota, W.; Gunun, P.; Kesorn, P.; Cherdthong, A. Strategic addition of different additives to improve silage fermentation, aerobic stability and in vitro digestibility of Napier grasses at late maturity stage. Agriculture 2020, 10, 262. [Google Scholar] [CrossRef]
- Wilson, R.F.; Wilkins, R.J. Formic acid as a silage additive for wet crops of cocksfoot and lucerne. J. Agric. Sci. 1973, 80, 225–231. [Google Scholar] [CrossRef]
- Panyawoot, N.; So, S.; Cherdthong, A.; Chanjula, P. Effect of feeding discarded durian peel ensiled with Lactobacillus casei TH14 and additives in total mixed rations on digestibility, ruminal fermentation, methane mitigation, and nitrogen balance of Thai Native-Anglo-Nubian goats. Fermentation 2022, 8, 43. [Google Scholar] [CrossRef]
- Kaewpila, C.; Gunun, P.; Kesorn, P.; Subepang, S.; Thip-Uten, S.; Cai, Y.; Pholsen, S.; Cherdthong, A.; Khota, W. Improving ensiling characteristics by adding lactic acid bacteria modifies in vitro digestibility and methane production of forage-sorghum mixture silage. Sci. Rep. 2021, 11, 1968. [Google Scholar] [CrossRef]
- Cao, Y.; Cai, Y.; Takahashi, T.; Yoshida, N.; Tohno, M.; Uegaki, R.; Nonaka, K.; Terada, F. Effect of lactic acid bacteria inoculant and beet pulp addition on fermentation characteristics and in vitro ruminal digestion of vegetable residue silage. J. Dairy Sci. 2011, 94, 3902–3912. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.L.; Bannink, A.; Hindrichsen, I.K.; Kinley, R.D.; Pellikaan, W.F.; Milora, N.; Dijkstra, J. The effect of lactic acid bacteria included as a probiotic or silage inoculant on in vitro rumen digestibility, total gas and methane production. Anim. Feed Sci. Technol. 2016, 211, 61–74. [Google Scholar] [CrossRef]
- Kaewpila, C.; Sommart, K. Development of methane conversion factor models for Zebu beef cattle fed low-quality crop residues and by-products in tropical regions. Ecol. Evol. 2016, 6, 7422–7432. [Google Scholar] [CrossRef]
- Kaewpila, C.; Sommart, K.; Mitsumori, M. Dietary fat sources affect feed intake, digestibility, rumen microbial populations, energy partition and methane emissions in different beef cattle genotypes. Animal 2018, 12, 2529–2538. [Google Scholar] [CrossRef] [PubMed]
- Khota, W.; Pholsen, S.; Higgs, D.; Cai, Y. Comparative analysis of silage fermentation and in vitro digestibility of tropical grass prepared with Acremonium and Tricoderma species producing cellulases. Asian-Australas. J. Anim. Sci. 2018, 31, 1913–1922. [Google Scholar] [CrossRef] [Green Version]
- Pholsen, S.; Khota, W.; Pang, H.; Higgs, D.; Cai, Y. Characterization and application of lactic acid bacteria for tropical silage preparation. Anim. Sci. J. 2016, 87, 1202–1211. [Google Scholar] [CrossRef]
- Weiß, K.; Kroschewski, B.; Auerbach, H.U. The influence of delayed sealing and repeated air ingress during the storage of maize silage on fermentation patterns, yeast development and aerobic stability. Fermentation 2022, 8, 48. [Google Scholar] [CrossRef]
- Moselhy, M.A.; Borba, J.P.; Borba, A.E.S. Improving the nutritive value, in vitro digestibility and aerobic stability of Hedychium gardnerianum silage through application of additives at ensiling time. Anim. Feed Sci. Technol. 2015, 206, 8–18. [Google Scholar] [CrossRef]
- Kozaki, M.; Uchimura, T.; Okada, S. Experimental Manual for Lactic Acid Bacteria; Asakurasyoten: Tokyo, Japan, 1992. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Faichney, G.; White, G. Methods for the Analysis of Feeds Eaten by Ruminants; Division of Animal Production, Ian Clunies Ross Animal Research Laboratory, Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia, 1983. [Google Scholar]
- Mertens, D.R. Creating a system for meeting the fiber requirements of dairy cows. J. Dairy Sci. 1997, 80, 1463–1481. [Google Scholar] [CrossRef]
- Krishnamoorthy, U.; Muscato, T.V.; Sniffen, C.J.; Van Soest, P.J. Nitrogen fractions in selected feedstuffs. J. Dairy Sci. 1982, 65, 217–225. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Kumai, S. Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J. Dairy Sci. 1999, 82, 520–526. [Google Scholar] [CrossRef]
- Darwin; Charles, W.; Cord-Ruwisch, R. Concurrent lactic and volatile fatty acid analysis of microbial fermentation samples by gas chromatography with heat pre-treatment. J. Chromatogr. Sci. 2018, 56, 1–5. [Google Scholar] [CrossRef]
- Fawcett, J.K.; Scott, J.E. A rapid and precise method for the determination of urea. J. Clin. Pathol. 1960, 13, 156–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makkar, H.P.; Blümmel, M.; Becker, K. Formation of complexes between polyvinyl pyrrolidones or polyethylene glycols and tannins, and their implication in gas production and true digestibility in in vitro techniques. Br. J. Nutr. 1995, 73, 897–913. [Google Scholar] [CrossRef] [Green Version]
- Muizelaar, W.; Bani, P.; Kuhla, B.; Larsen, M.; Tapio, I.; Yáñez-Ruiz, D. Rumen fluid sampling via oral stomach tubing method. In Methods in Cattle Physiology and Behaviour Research—Recommendations from the SmartCow Consortium; Mesgaran, S.D., Baumont, R., Munksgaard, L., Humphries, D., Kennedy, E., Dijkstra, J., Dewhurst, R., Ferguson, H., Terré, M., Kuhla, B., Eds.; PUBLISSO: Cologne, Germany, 2020; p. 6. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach, 2nd ed.; McGraw–Hill Book Co. Inc.: New York, NY, USA, 1980. [Google Scholar]
- Allen, M.S. Effects of diet on short-term regulation of feed intake by lactating dairy cattle. J. Dairy Sci. 2000, 83, 1598–1624. [Google Scholar] [CrossRef]
- Kearl, L.C. Nutrient Requirements of Ruminants in Developing Countries; International Feedstuffs Institute: Logan, UT, USA, 1982. [Google Scholar]
- Lepcha, I.; Naumann, H.D. Partitioning of forage mass and nutritive value in Sunn hemp leaf and stem components. Int. J. Agron. 2021, 2021, 5547120. [Google Scholar] [CrossRef]
- Muck, R.E. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, W.; Yang, J.; Zhao, H.; Pan, C.; Ding, X. Effects of applying lactic acid bacteria to the fermentation on a mixture of corn steep liquor and air-dried rice straw. Anim. Nutr. 2016, 2, 229–233. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, N.; Wang, Y.; Yang, H.; Wei, Y.; Moriel, P.; Palmer, E.; Zhang, Y. Combining orchardgrass and alfalfa: Effects of forage ratios on in vitro rumen degradation and fermentation characteristics of silage compared with hay. Animals 2020, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Heinritz, S.N.; Martens, S.D.; Avila, P.; Hoedtke, S. The effect of inoculant and sucrose addition on the silage quality of tropical forage legumes with varying ensilability. Anim. Feed Sci. Technol. 2012, 174, 201–210. [Google Scholar] [CrossRef]
- Kaewpila, C.; Thip-Uten, S.; Cherdthong, A.; Khota, W. Impact of cellulase and lactic acid bacteria inoculant to modify ensiling characteristics and in vitro digestibility of sweet corn stover and cassava pulp silage. Agriculture 2021, 11, 66. [Google Scholar] [CrossRef]
- Gao, J.L.; Wang, P.; Zhou, C.H.; Li, P.; Tang, H.Y.; Zhang, J.B.; Cai, Y. Chemical composition and in vitro digestibility of corn stover during field exposure and the fermentation characteristics of silage prepared with microbial additives. Asian-Australas. J. Anim. Sci. 2019, 32, 1854–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinne, M.; Winquist, E.; Pihlajaniemi, V.; Niemi, P.; Seppälä, A.; Siika-Aho, M. Fibrolytic enzyme treatment prior to ensiling increased press-juice and crude protein yield from grass silage. Bioresour. Technol. 2020, 299, 122572. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Musco, N.; Koura, I.B.; Tudisco, R.; Awadjihè, G.; Adjolohoun, S.; Cutrignelli, M.I.; Mollica, M.P.; Houinato, M.; Infascelli, F.; Calabrò, S. Nutritional characteristics of forage grown in south of Benin. Asian-Australas. J. Anim. Sci. 2016, 29, 51–61. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).