Development of a Culture Medium for Microalgae Production Based on Minimal Processing of Oil Palm Biomass Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Oil Palm Ash
2.2. Nutrient Extraction from Ash
2.3. Kinetics of Phosphate Extraction
2.4. Microorganisms and Culture Media
2.5. Analytical Methods
2.6. Statistical Analysis
3. Results and Discussion
3.1. Phosphate Extraction
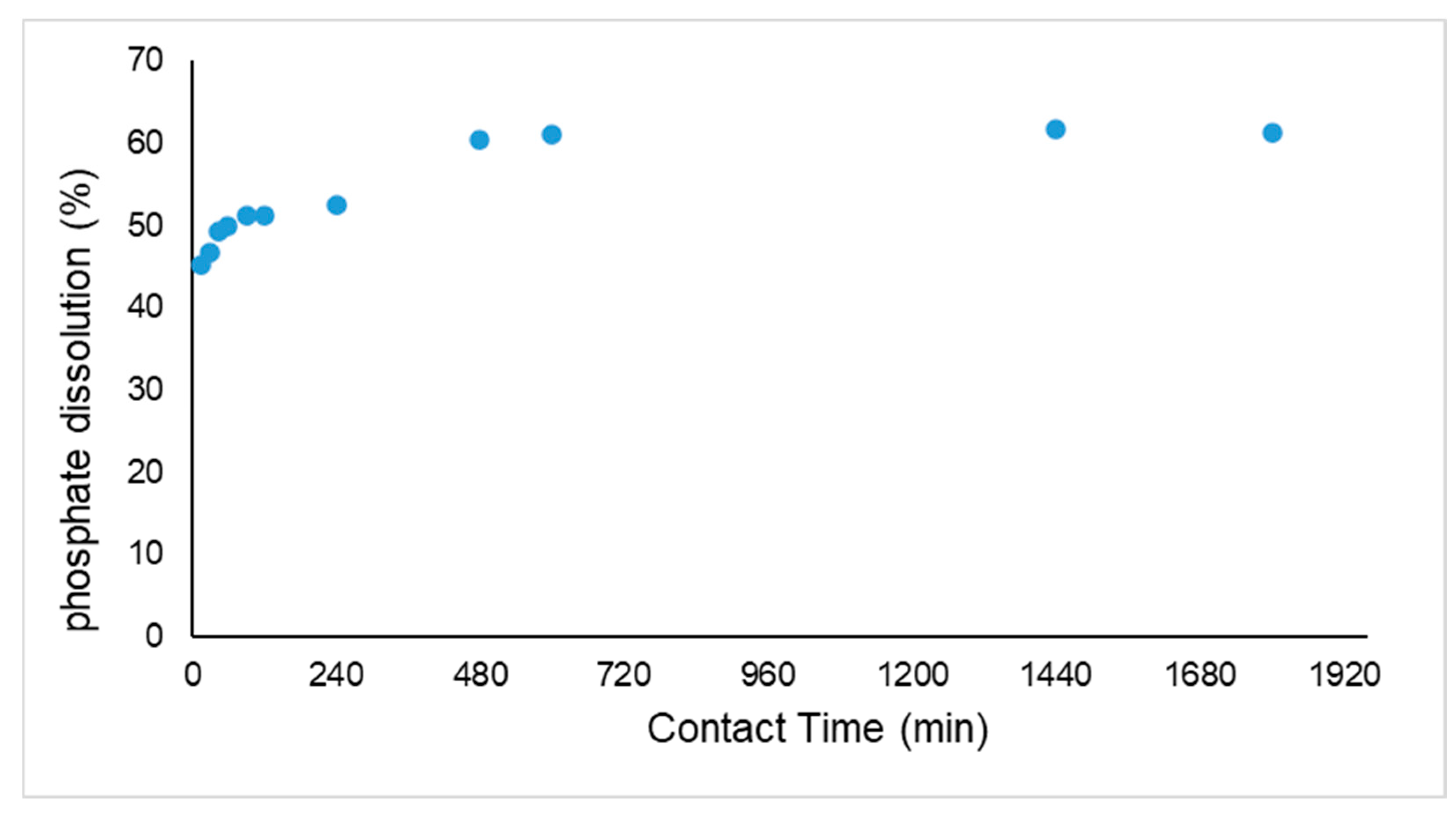
3.2. Kinetics of Extraction
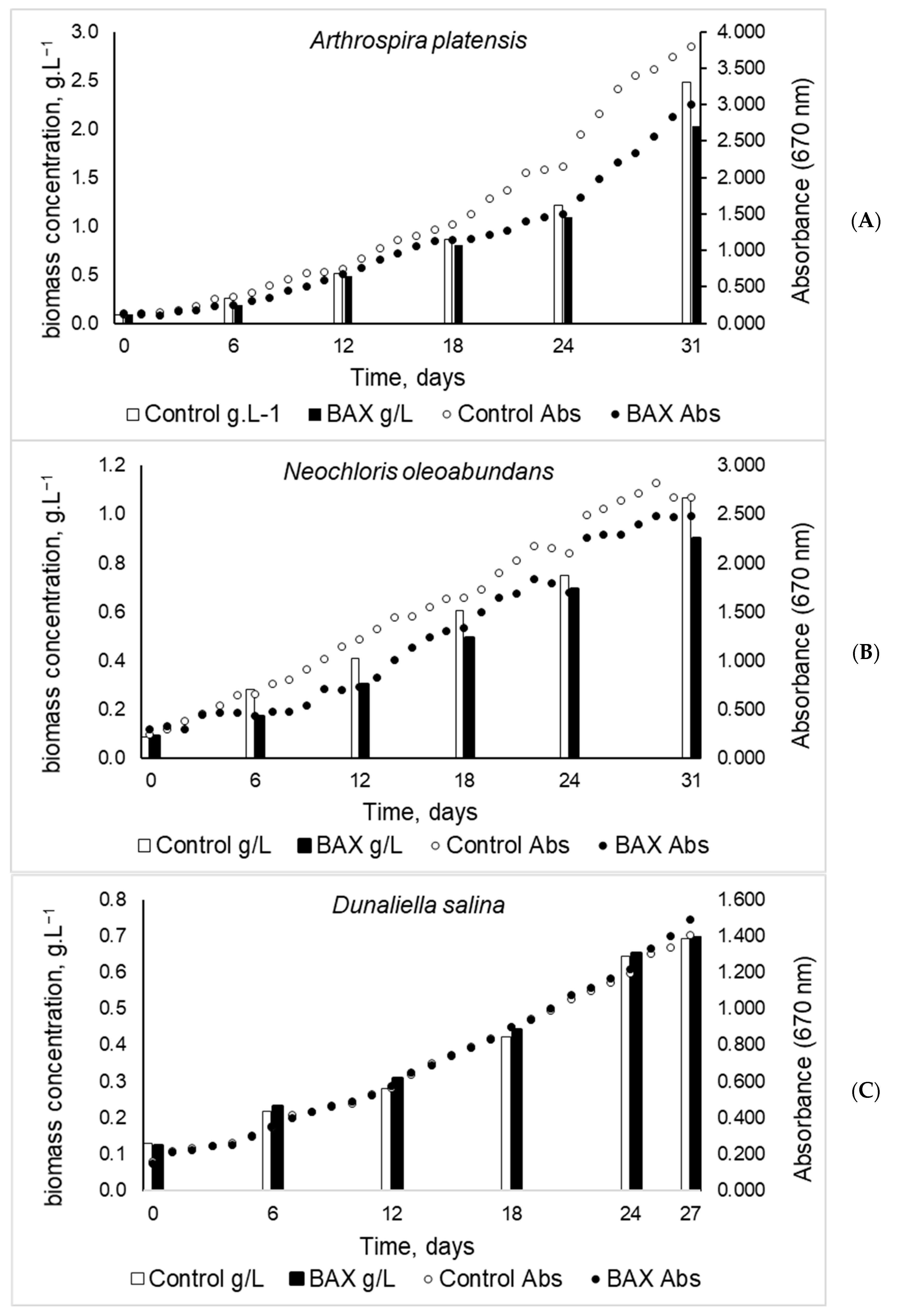
3.3. Cultivation of A. platensis, N. oleoabundans, and D. salina on BAX Medium
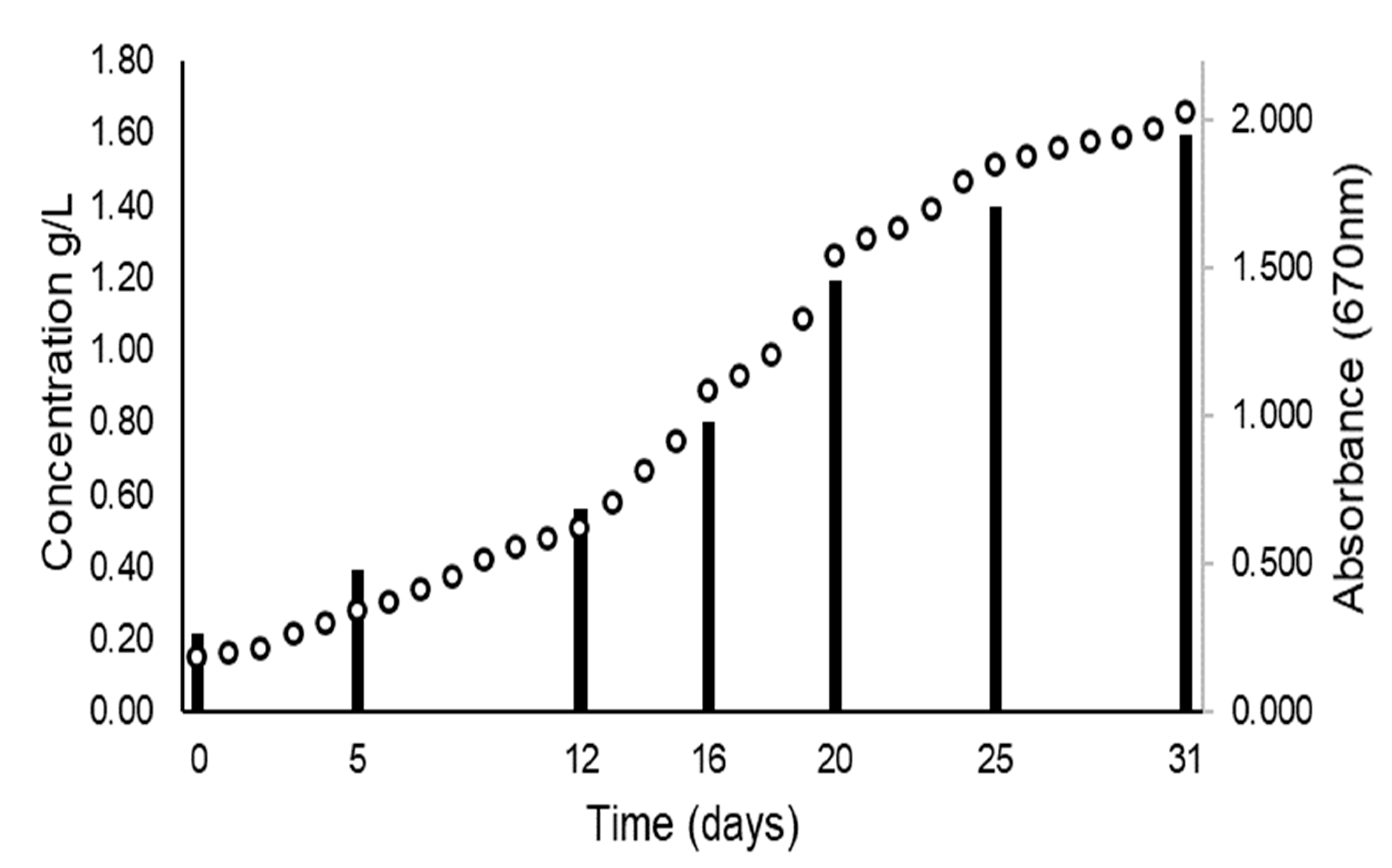
3.4. Scaling Up
3.5. Practical Applications and Future Research
- (i)
- Scale-up was similar to laboratory cultures, but with modest productivity. It is possible that warmer temperatures lead to better growth. Repeated batches in a long running experiment will also show if some nutrient lacking, e.g., sodium for Spirulina, or in excess such as iron (usually present in low concentrations in synthetic media) limit the growth or affect the composition.
- (ii)
- Oil palm ash has a high concentration of potassium, a most needed element in plant fertilizers, but not important enough for microalgae growth. Fractionation of palm ash, e.g., through selective or sequential extraction could make even better use of this residue and maybe integrate it to palm production, reducing the need for fertilizer.
- (iii)
- Large-scale processing of palm oil ash would require reactors, pumps, and filters. Even if the equipment is simple compared to the large-scale equipment used in palm processing, it involves a capital cost that would have to be compensated by the income from biomass products. This requires a process simulation and sensitivity analysis to indicate if microalgae production, although technically feasible, can be profitable in its biorefinery integration with palm processing.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Birol, F.; Cozzi, L.; Gould, T.; Bromhead, A.; Priddle, R. World Energy Outlook 2015; Organisation for Economic Cooperation and Development: Paris, France, 2015. [Google Scholar]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase-mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Schill, S.R.; IEA Task 40: Biomass Provides 10 Percent of Global Energy Use. Biomass Magazine. Grand Forks 2013. Available online: http://biomassmagazine.com/articles/9444/iea-task40-biomass-provides-10-percent-of-global-energy-use (accessed on 20 January 2022).
- Chuah, T.G.; Wan Azlina, A.G.K.; Robiah, Y.; Omar, R. Biomass as the renewable energy sources in Malaysia: An overview. Int. J. Green Energy 2006, 3, 323–346. [Google Scholar] [CrossRef]
- Siddique, R. Utilization of wood ash in concrete manufacturing. Resour. Conserv. Recycl. 2012, 67, 27–33. [Google Scholar] [CrossRef]
- Wiselogel, A.E.; Agblevor, F.A.; Johnson, D.K.; Deutch, S.; Fennell, J.A.; Sanderson, M.A. Compositional changes during storage of large round switchgrass bales. Bioresour. Technol. 1996, 56, 103–109. [Google Scholar] [CrossRef]
- Thy, P.; Jenkins, B.M.; Grundvig, S.; Shiraki, R.; Lesher, C.E. High temperature elemental losses and mineralogical changes in common biomass ashes. Fuel 2006, 85, 783–795. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Algal nutrition‒mineral nutrition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Blackwell Publishing: Oxford, UK, 2004; pp. 95–115. [Google Scholar]
- De Carvalho, J.C.; Sydney, E.B.; Assú Tessari, L.F.; Soccol, C.R. Culture media for mass production of microalgae. In Biomass, Biofuels, Biochemicals, 2nd ed.; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.-J., Chisti, Y.B.T.-B., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. ISBN 978-0-444-64192-2. [Google Scholar]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Rittmann, B.E.; Mayer, B.; Westerhoff, P.; Edwards, M. Capturing the lost phosphorus. Chemosphere 2011, 84, 846–853. [Google Scholar] [CrossRef]
- Bennett, E.M.; Carpenter, S.R.; Caraco, N.F. Human impact on erodable phosphorus and eutrophication: A global perspective. Bioscience 2001, 51, 227–234. [Google Scholar] [CrossRef]
- Liu, H.; Hu, G.; Basar, I.A.; Li, J.; Lyczko, N.; Nzihou, A.; Eskicioglu, C. Phosphorus recovery from municipal sludge-derived ash and hydrochar through wet-chemical technology: A review towards sustainable waste management. Chem. Eng. J. 2021, 417, 129300. [Google Scholar] [CrossRef]
- Leng, L.; Bogush, A.A.; Roy, A.; Stegemann, J.A. Characterisation of ashes from waste biomass power plants and phosphorus recovery. Sci. Total. Environ. 2019, 690, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, D.-J. Identification of phosphorus forms in sewage sludge ash during acid pre-treatment for phosphorus recovery by chemical fractionation and spectroscopy. J. Ind. Eng. Chem. 2017, 51, 64–70. [Google Scholar] [CrossRef]
- Gorazda, K.; Tarko, B.; Wzorek, Z.; Nowak, A.K.; Kulczycka, J.; Henclik, A. Characteristic of wet method of phosphorus recovery from polish sewage sludge ash with nitric acid. Open Chem. 2016, 14, 37–45. [Google Scholar] [CrossRef]
- Liang, S.; Chen, H.; Zeng, X.; Li, Z.; Yu, W.; Xiao, K.; Hu, J.; Hou, H.; Liu, B.; Tao, S. A comparison between sulfuric acid and oxalic acid leaching with subsequent purification and precipitation for phosphorus recovery from sewage sludge incineration ash. Water Res. 2019, 159, 242–251. [Google Scholar] [CrossRef]
- Melia, P.M.; Cundy, A.B.; Sohi, S.P.; Hooda, P.S.; Busquets, R. Trends in the recovery of phosphorus in bioavailable forms from wastewater. Chemosphere 2017, 186, 381–395. [Google Scholar] [CrossRef]
- Carey, D.E.; Yang, Y.; McNamara, P.J.; Mayer, B.K. Recovery of agricultural nutrients from biorefineries. Bioresour. Technol. 2016, 215, 186–198. [Google Scholar] [CrossRef]
- Marchi, A.; Geerts, S.; Saerens, B.; Weemaes, M.; De Clercq, L.; Meers, E. Struvite recovery from domestic wastewater. In Biorefinery of Inorganics: Recovering Mineral Nutrients from Biomass and Organic Waste; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 107–119. [Google Scholar]
- Aziz, M.; Kurniawan, T.; Oda, T.; Kashiwagi, T. Advanced power generation using biomass wastes from palm oil mills. Appl. Therm. Eng. 2016, 114, 1378–1386. [Google Scholar] [CrossRef]
- Letti, L.A.J.; Woiciechowski, A.L.; Medeiros, A.B.P.; Rodrigues, C.; de Carvalho, J.C.; de Souza Vandenberghe, L.P.; Karp, S.G.; Torres, L.A.Z.; Guarnizo, A.F.C.; Colonia, B.S.O. Valorization of solid and liquid wastes from palm oil industry. In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 235–265. [Google Scholar]
- Madhiyanon, T.; Sathitruangsak, P.; Sungworagarn, S.; Pipatmanomai, S.; Tia, S. A pilot-scale investigation of ash and deposition formation during oil-palm empty-fruit-bunch (EFB) combustion. Fuel Process. Technol. 2012, 96, 250–264. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohamed, A.R.; Mohammadi, M. Ash of palm empty fruit bunch as a natural catalyst for promoting the CO2 gasification reactivity of biomass char. Bioresour. Technol. 2013, 132, 351–355. [Google Scholar] [CrossRef]
- Acquah, C.; Sie Yon, L.; Tuah, Z.; Ling Ngee, N.; Danquah, M.K. Synthesis and performance analysis of oil palm ash (OPA) based adsorbent as a palm oil bleaching material. J. Clean. Prod. 2016, 139, 1098–1104. [Google Scholar] [CrossRef]
- Mohamed, A.R.; Lee, K.T.; Noor, N.M.; Zainudin, N.F. Oil palm ash/Ca(OH)2/CaSO4 absorbent for flue gas desulfurization. Chem. Eng. Technol. 2005, 28, 939–945. [Google Scholar] [CrossRef]
- Lee, K.T.; Bhatia, S.; Mohamed, A.R. Preparation and characterization of sorbents prepared from ash (waste material) for sulfur dioxide (SO2) removal. J. Mater. Cycles Waste Manag. 2005, 7, 16–23. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.A.; Aziz, N. Isotherms, kinetics and thermodynamics of acid dye adsorption on activated palm ash. Chem. Eng. J. 2007, 133, 195–203. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Alengaram, U.J.; Metselaar, H.S.C.; Jumaat, M.Z. Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar under elevated temperatures. Constr. Build. Mater. 2014, 65, 114–121. [Google Scholar] [CrossRef]
- Bashar, I.I.; Alengaram, U.J.; Jumaat, M.Z.; Islam, A.; Santhi, H.; Sharmin, A. Engineering properties and fracture behaviour of high volume palm oil fuel ash based fibre reinforced geopolymer concrete. Constr. Build. Mater. 2016, 111, 286–297. [Google Scholar] [CrossRef]
- Zainudin, N.F.; Lee, K.T.; Kamaruddin, A.H.; Bhatia, S.; Mohamed, A.R. Study of adsorbent prepared from oil palm ash (OPA) for flue gas desulfurization. Sep. Purif. Technol. 2005, 45, 50–60. [Google Scholar] [CrossRef]
- Tyrrell, T. Redfield ratio. In Encyclopedia of Ocean Sciences; Steele, J.H., Thorpe, S.A., Turekian, K.K., Eds.; Academic Press: San Diego, CA, USA, 2001; Volume 83, pp. 2377–2387. [Google Scholar]
- Richmond, A.; Hu, Q. Handbook of Microalgal Culture. Richmond, A., Hu, Q., Eds.; Wiley: New York, NY, USA, 2013; ISBN 0632059532. [Google Scholar]
- Biswas, B.K.; Inoue, K.; Harada, H.; Ohto, K.; Kawakita, H. Leaching of phosphorus from incinerated sewage sludge ash by means of acid extraction followed by adsorption on orange waste gel. J. Environ. Sci. 2009, 21, 1753–1760. [Google Scholar] [CrossRef]
- Matjie, R.H.; Bunt, J.R.; Van Heerden, J.H.P. Extraction of alumina from coal fly ash generated from a selected low rank bituminous South African coal. Miner. Eng. 2005, 18, 299–310. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Kirkelund, G.M.; Jensen, P.E. Extracting phosphorous from incinerated sewage sludge ash rich in iron or aluminum. Chemosphere 2013, 91, 963–969. [Google Scholar] [CrossRef]
- Parés Viader, R.; Jensen, P.E.; Ottosen, L.M.; Ahrenfeldt, J.; Hauggaard-Nielsen, H. Electrodialytic extraction of phosphorus from ash of low-temperature gasification of sewage sludge. Electrochimica Acta 2015, 181, 100–108. [Google Scholar] [CrossRef][Green Version]
- Wzorek, Z.; Jodko, M.; Gorazda, K.; Rzepecki, T. Extraction of phosphorus compounds from ashes from thermal processing of sewage sludge. J. Loss Prev. Process Ind. 2006, 19, 39–50. [Google Scholar] [CrossRef]
- Xu, H.; He, P.; Gu, W.; Wang, G.; Shao, L. Recovery of phosphorus as struvite from sewage sludge ash. J. Environ. Sci. 2012, 24, 1533–1538. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, H.; Liu, J.; Luo, J.; Qian, G.; Wang, A. Ph dependent phosphorus release from waste activated sludge: Contributions of phosphorus speciation. Chem. Eng. J. 2015, 267, 260–265. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution à l’étude d’une Cyanophycée. Influence de Divers Facteurs Physiques et Chimiques Sur la Croissance et la Photosynthèse de Spirulina maxima. Ph.D. Thesis, Université de Paris, Paris, France, 1996. [Google Scholar]
- Stanier, R.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. BG11 (blue green medium). Cult. Collect. Algae Protozoa 1971, 11, 559001. [Google Scholar]
- Borowitzka, M.A.; Borowitzka, L.J. Micro-Algal Biotechnology; Cambridge University Press: Cambridge, UK, 1988; ISBN 0521323495. [Google Scholar]
- D’Angelo, E.; Crutchfield, J.; Vandiviere, M. Rapid, sensitive, microscale determination of phosphate in water and soil. J. Environ. Qual. 2001, 30, 2206–2209. [Google Scholar] [CrossRef]
- De Freitas, B.C.B.; Brächer, E.H.; de Morais, E.G.; Atala, D.I.P.; de Morais, M.G.; Costa, J.A.V. Cultivation of different microalgae with pentose as carbon source and the effects on the carbohydrate content. Environ. Technol. 2019, 40, 1062–1070. [Google Scholar] [CrossRef]
- Silva, H.R.; Prete, C.E.C.; Zambrano, F.; de Mello, V.H.; Tischer, C.A.; Andrade, D.S. Combining glucose and sodium acetate improves the growth of Neochloris oleoabundans under mixotrophic conditions. AMB Express 2016, 6, 10. [Google Scholar] [CrossRef]
- Walter, A.; De Carvalho, J.C.; Soccol, V.T.; De Faria, A.B.B.; Ghiggi, V.; Soccol, C.R. Study of phycocyanin production from Spirulina platensis under different light spectra. Braz. Arch. Biol. Technol. 2011, 54, 675–682. [Google Scholar] [CrossRef]
- Zhu, C.J.; Lee, Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997, 9, 189–194. [Google Scholar] [CrossRef]
- Tan, Z.; Lagerkvist, A. Phosphorus recovery from the biomass ash: A review. Renew. Sustain. Energy Rev. 2011, 15, 3588–3602. [Google Scholar] [CrossRef]
- Cohen, Y. Phosphorus dissolution from ash of incinerated sewage sludge and animal carcasses using sulphuric acid. Environ. Technol. 2009, 30, 1215–1226. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Zessner, M. Overview and description of technologies for recovering phosphorus from municipal wastewater. Resour. Conserv. Recycl. 2015, 105, 325–346. [Google Scholar] [CrossRef]
- Redfield, A.C. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 205–221. [Google Scholar]
- Geider, R.J.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- Hong, K.-J.; Tarutani, N.; Shinya, Y.; Kajiuchi, T. Study on the recovery of phosphorus from waste-activated sludge incinerator ash. J. Environ. Sci. Health Part A 2005, 40, 617–631. [Google Scholar] [CrossRef]
- Hedges, J.I.; Baldock, J.A.; Gélinas, Y.; Lee, C.; Peterson, M.L.; Wakeham, S.G. The biochemical and elemental compositions of marine plankton: A NMR perspective. Mar. Chem. 2002, 78, 47–63. [Google Scholar] [CrossRef]
- Azimatun Nur, M.M. Evaluation of carbon, nitrogen and phosphorus ratio of palm oil mill effluent digested (POMED) wastewater as replacement synthetic medium for Spirulina sp. growth. Am. J. Agric. Environ. Sci. 2014, 14, 536–540. [Google Scholar] [CrossRef]
- Jeanfils, J.; Canisius, M.F.; Burlion, N. Effect of high nitrate concentrations on growth and nitrate uptake by free-living and immobilized Chlorella vulgaris cells. J. Appl. Phycol. 1993, 5, 369–374. [Google Scholar] [CrossRef]
- Markou, G.; Iconomou, D.; Sotiroudis, T.; Israilides, C.; Muylaert, K. Exploration of using stripped ammonia and ash from poultry litter for the cultivation of the cyanobacterium Arthrospira platensis and the green microalga Chlorella vulgaris. Bioresour. Technol. 2015, 196, 459–468. [Google Scholar] [CrossRef]
- Colla, L.M.; Furlong, E.B.; Alberto, J.; Costa, V. Antioxidant properties of Spirulina (Arthospira) platensis cultivated under different temperatures and nitrogen regimes. Braz. Arch. Biol. Technol. 2007, 50, 161–167. [Google Scholar] [CrossRef]



| [25] | [26] | [27] | [28] | [29] | [30] | [31] | [32] | [33] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Components | EFB | EFB | PS | OPA | OPA | OPA | APA | POFA | POFA | POFA |
| SiO2 | 12.12 | 27.00 | 49.70 | 86.44 | 35.60 | 37.00 | 40.00 | 64.17 | 67.72 | 40.00 |
| CaO | 9.65 | 8.00 | 10.20 | - | 12.00 | 9.20 | 10.00 | 5.80 | 5.57 | 10.00 |
| K2O | 55.48 | 44.00 | 12.20 | - | 11.00 | 11.00 | 12.10 | 8.25 | 7.67 | 12.10 |
| Al2O3 | 0.26 | - | 7.70 | 6.49 | 4.80 | 14.30 | 6.10 | 3.73 | 3.71 | 6.10 |
| MgO | 1.90 | 4.80 | 6.90 | 1.51 | 7.20 | 6.10 | 6.40 | 4.87 | 4.04 | 6.40 |
| P2O5 | 3.58 | 3.60 | 8.40 | - | 6.80 | 6.20 | 8.32 | 5.18 | - | 8.20 |
| Fe2O3 | - | 3.00 | 2.70 | 4.08 | 2.00 | 2.50 | - | 6.33 | 4.71 | 2.50 |
| SO3 | 1.66 | 2.70 | - | 0.20 | - | - | - | 0.72 | 1.07 | - |
| Na2O | 0.09 | - | - | 0.10 | - | 0.10 | - | 0.18 | 0.16 | - |
| Cl | 6.84 | 5.30 | - | - | - | 2.90 | - | - | - | - |
| C | - | - | - | - | - | - | - | - | - | 5.40 |
| TiO2 | - | - | - | - | - | - | - | 0.19 | - | - |
| MnO | - | - | - | - | - | - | - | 0.18 | - | - |
| MnO2 | - | - | - | 0.10 | - | - | - | - | - | - |
| ZnO | - | - | - | - | - | - | - | 0.03 | - | - |
| Rb2O | - | - | - | - | - | - | - | 0.06 | - | - |
| Cr2O3 | - | - | - | 0.69 | - | - | - | 0.03 | - | - |
| Rh2O3 | - | - | - | 0.41 | - | - | - | - | - | - |
| CuO | - | - | - | - | - | - | - | 0.08 | - | - |
| RuO2 | - | - | - | 0.29 | - | - | - | - | - | - |
| SrO | - | - | - | - | - | - | - | 0.02 | - | - |
| ZrO2 | - | - | - | - | - | - | - | < 0.01 | - | - |
| NiO | - | - | - | - | - | - | - | 0.02 | - | - |
| Y2O3 | - | - | - | - | - | - | - | < 0.01 | - | - |
| Components | wt% of Dry Solids |
|---|---|
| SiO2 | 49.40 |
| K2O | 11.60 |
| CaO | 8.70 |
| P2O5 | 8.60 |
| MgO | 5.80 |
| Fe2O3 | 5.60 |
| Al2O3 | 1.60 |
| SO3 | 1.60 |
| Cl | 0.70 |
| TiO2 | 0.20 |
| MnO | 0.10 |
| SrO | 0.10 |
| Na2O | 0.10 |
| ZrO2 | <0.10 |
| CuO | <0.10 |
| ZnO | <0.10 |
| LOI. | 5.99 |
| Temperature (°C) | Moles of Acid | L/S (mL g−1) |
|---|---|---|
| 20 | 0.01 | 50 |
| 45 | 0.02 | 100 |
| 70 | 0.03 | 150 |
| Sample | Experiment Conditions | Recovered P2O5 (g) | Dissolution of P2O5 (%) | ||
|---|---|---|---|---|---|
| Temperature (°C) | Acid Added, Mols | Liquid/Solid Ratio (mL g−1) | |||
| 1 | 70 | 0.01 | 50 | 0.067 | 78.39 |
| 2 | 70 | 0.01 | 100 | 0.065 | 75.31 |
| 3 | 70 | 0.01 | 150 | 0.058 | 67.31 |
| 4 | 70 | 0.02 | 150 | 0.084 | 97.52 |
| 5 | 70 | 0.03 | 150 | 0.073 | 85.21 |
| 6 | 70 | 0.03 | 100 | 0.069 | 79.94 |
| 7 | 70 | 0.03 | 50 | 0.072 | 83.91 |
| 8 | 70 | 0.02 | 50 | 0.073 | 85.18 |
| 9 | 70 | 0.02 | 100 | 0.076 | 88.59 |
| 10 | 45 | 0.01 | 50 | 0.059 | 68.76 |
| 11 | 45 | 0.01 | 100 | 0.064 | 74.86 |
| 12 | 45 | 0.01 | 150 | 0.057 | 66.64 |
| 13 | 45 | 0.02 | 150 | 0.060 | 69.33 |
| 14 | 45 | 0.03 | 150 | 0.065 | 76.04 |
| 15 | 45 | 0.03 | 100 | 0.070 | 81.43 |
| 16 | 45 | 0.03 | 50 | 0.069 | 79.88 |
| 17 | 45 | 0.02 | 50 | 0.060 | 69.96 |
| 18 | 45 | 0.02 | 100 | 0.065 | 75.46 |
| 19 | 45 | 0.02 | 100 | 0.066 | 76.65 |
| 20 | 45 | 0.02 | 100 | 0.065 | 75.31 |
| 21 | 20 | 0.01 | 50 | 0.062 | 72.42 |
| 22 | 20 | 0.01 | 100 | 0.059 | 68.90 |
| 23 | 20 | 0.01 | 150 | 0.049 | 57.02 |
| 24 | 20 | 0.02 | 150 | 0.063 | 73.35 |
| 25 | 20 | 0.03 | 150 | 0.064 | 74.25 |
| 26 | 20 | 0.03 | 100 | 0.074 | 86.50 |
| 27 | 20 | 0.03 | 50 | 0.062 | 72.12 |
| 28 | 20 | 0.02 | 50 | 0.062 | 72.27 |
| 29 | 20 | 0.02 | 100 | 0.063 | 72.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessari, L.F.A.; Soccol, C.R.; Rodrigues, C.; González, E.G.; Tanobe, V.O.d.A.; Kirnev, P.C.d.S.; de Carvalho, J.C. Development of a Culture Medium for Microalgae Production Based on Minimal Processing of Oil Palm Biomass Ash. Fermentation 2022, 8, 55. https://doi.org/10.3390/fermentation8020055
Tessari LFA, Soccol CR, Rodrigues C, González EG, Tanobe VOdA, Kirnev PCdS, de Carvalho JC. Development of a Culture Medium for Microalgae Production Based on Minimal Processing of Oil Palm Biomass Ash. Fermentation. 2022; 8(2):55. https://doi.org/10.3390/fermentation8020055
Chicago/Turabian StyleTessari, Lorenzo Ferrari Assú, Carlos Ricardo Soccol, Cristine Rodrigues, Estefania García González, Valcineide Oliveira de Andrade Tanobe, Paulo Cesar de Souza Kirnev, and Júlio Cesar de Carvalho. 2022. "Development of a Culture Medium for Microalgae Production Based on Minimal Processing of Oil Palm Biomass Ash" Fermentation 8, no. 2: 55. https://doi.org/10.3390/fermentation8020055
APA StyleTessari, L. F. A., Soccol, C. R., Rodrigues, C., González, E. G., Tanobe, V. O. d. A., Kirnev, P. C. d. S., & de Carvalho, J. C. (2022). Development of a Culture Medium for Microalgae Production Based on Minimal Processing of Oil Palm Biomass Ash. Fermentation, 8(2), 55. https://doi.org/10.3390/fermentation8020055






