Abstract
The aims of this study are to investigate the structure of four historical Moroccan cedar softwood samples of different aging time duration (16th, 17th, 19th, 21st centuries) and compare among these four samples, using two analytical methods, FTIR and XRD, in order to confirm some structural changes and determine the degree of deterioration. The pronounced hemicellulose deterioration was highlighted by a breakdown of IR acetyl groups at 1738 cm−1 from the 19th century sample until aged ones. The cellulose XRD crystallinity index showed an important decrease from recent to oldest samples (51.8 to 20.2%) justifying the damages mainly in the two oldest samples (17th and 16th centuries), also confirmed by FTIR. The alteration of lignin was manifested in the case of the two ancient samples (16th and 17th centuries), proven by the decrease in IR bands related to aromatic nuclei (1595, 1500, 1230 cm−1) evolving towards a new diconjugate C=O formers at 1647 cm−1 (quinone, Ar-CO-Ar, Ar-CO-C=C). For accurate elucidation, the data of two combined techniques were compared and correlated. The obtained results depended on the part of the wood exposed to weathering effects (internal or external) and were influenced by both extended time of aging and effects of natural deterioration agents. The effects of natural aging were investigated in four historical Moroccan cedar softwood samples (16th, 17th, 19th, 21st centuries) using two analytical tools: FTIR and XRD. The pronounced hemicellulose deterioration was highlighted by a breakdown of IR acetyl groups at 1738 cm−1 and declines in the absorption signal at 1268 cm−1 from the 19th century sample until aged ones. The cellulose XRD crystallinity index (CrI) estimation showed an important decrease from recent to oldest samples (51.8 to 20.2%) justifying the damages mainly in the two oldest samples (17th and 16th centuries). These data were also confirmed by FTIR showing a significant reduction in both area profiles of C-O-C (1150–1000 cm−1) and C-H crystalline cellulosic bands (1375, 1318, and 1268 cm−1), respectively. The lignin alteration in both old samples (16th and 17th centuries) was proven by the decrease in IR aromatic skeleton (1595, 1500, and 1230 cm−1) evolving towards a new diconjugate C=O formers at 1647 cm−1 (quinone, Ar-CO-Ar, Ar-CO-C=C). To determine the structural difference and the degree of deterioration, the IR area of C=O band intensities ranging from 1550 to 1800 cm−1 was exploited. For accurate elucidation, the data of two combined techniques were compared and correlated. The obtained results depended on the part of the wood (internal or external) exposed to weathering effects and were influenced by both extended time of aging and effects of natural deterioration agents.
1. Introduction
Cedar wood is located in the following three continents: Africa (northwest), Europe (southeast), and Asia. According to A.M. Saab et al. [1], the four types of cedar are: Cedrus atlantica in Morocco and Algeria, Cedrus libanotica ssp. atlantica regrouping the region of Lebanon, Syria, and Turkey; Cedrus brevefolia located in Cyprus Island; and finally, Cedrus deodara which is present in Asia and extends to the Himalayan Mountains. Cedrus atlantica is an endemic tree species to northwest Africa of Morocco and Algeria [2].
In Morocco, Atlas cedar softwood presents many advantages, such as its potential energy source (domestic fire) and traditional economic support to the local mountaineer population [3]. Additionally, it presents a natural durability against wood decay fungi attack [4,5]. Today, as before, it is greatly solicited in different sectors as a source of bio-energy during cold seasons, as biomaterial to construct ecological building, cabinetmaking, and transportation such as ships and carriages, carpentry (staircases, cabinets, etc.). Additionally, it is used as an archeomaterial for historical monuments, such as wood art museums, traditional and Koranic schools, ancient mosques, mausoleums, door decorations in museums, and wooden ceilings, as well as portals of ancient archaeological buildings and artists’ palettes in imperial cities of Morocco [6], and also their noble timber is used in cabinetmaking and marquetry [4]. Finally, it represents a source of economic prosperity of many people throughout history.
Cedar softwood, like other wood biomaterials, is exposed to several environmental factors of alteration: physico-chemical such humidity and temperature [7,8,9,10,11], oxidation (atmospheric oxygen), photochemical and hydrolysis reactions [12,13], as well as biological effect as fungi (soft, white and brown rots), bacteria and fauna (termites, weevils) [14,15,16,17]. The Penicillium commune fungal spores are known to be the main mechanisms of cedar ancient wood biodeterioration [5].
The lignocellulosic wood biomaterial is a complex mixture of organic constituent composed of carbohydrates (cellulose and hemicellulose) and lignin. Hemicellulose consists of several polysaccharides with a lower degree of polymerization, a higher oxygen/carbon ratio, containing ester groups (-CO2CH3) and a greater fraction of available hydroxyl groups than the other constituents of the biomass [18].
Cellulose is bio-polymeric chain constituted of several polysaccharides (xylans ring) connected with the β-(1-4)-glycosidic bonds. It presents a higher degree of polymerization, and an important proportion of hydroxyl groups conferring the establishment of intra- and intermolecular hydrogen bonds. The two fractions of cellulose constituents are crystalline and amorphous, the first one is well known by its lower reactivity to deterioration than the second one (more reactive) [19,20].
Lignin is a multi-constituent of stable phenolic units (Ar-C3-OH) variously substituted (guaiacyl, syringyl, etc.) and linked to sugar molecules through the functionality of the side chain (conjugate ester groups) susceptible to hydrolysis reaction as well as the oxidative process.
Environmental effects engender wood weakening or even disappearing of its physical and mechanical properties [10,18]. It results in a deep defibrillation of the material by breaking down lignin and cellulose structures [14], accompanied by change of color and aesthetic aspects [9,11,21,22], as well as a loss of mass during heat treatment of wood [23] and decrease in consolidation strength and thermal insulation properties [24].
The degradation of cellulose bio-polymeric chains is manifested by breaking down of the β-(1-4)-glycosidic bonds, broadening and shifting of OH stretching vibrations, or varying the constituent groups under oxidative processes, and thus promoting multi-structural modifications and loss of degree of polymerization in the fiber strength [25]. It results in an evolution in the polymorphism of crystalline form inducing a change and disruption in the conformation of cellulose.
The structural complexity of wood constituents and the conformational changes under the effect of degradation conditions make the characterization with mono-instrumental analytical tools very difficult. That is why combined techniques, such as XRD and FTIR vibrational spectroscopy were selected for this type of study [12,18,20].
FTIR provides information on the deacetylation of hemicellulose and alteration of lignin aromatic skeleton as well as their degree of deterioration, while XRD represents the accurate method for measuring the crystallinity index of cellulose and its degree of polymorphism [12].
To quantify the content of crystalline cellulose (CrI) and the degree of polymorphism, previous procedures were established, using the following empirical methods: CrI % = It − Ia/It, with It crystalline plane at 2~22.7 and Ia amorphous cellulose at 2~18 [26], as well as CrI % = ∑Acryst/∑Atotal, with an area ratio of crystalline planes to the total area [27], the latest method based on Rietveld analysis. Some partial discordance with L. Segal’s model has been reported by A.D. French et al. [28], concerning the consideration of neighboring profiles of both I200 and I002 giving rise to a greater extent, hence the difficulty in exploring their Miller’s indices related to their reflection contributions (200) and (020). It results in a reduction in the crystal size, which contributes to an increase in the amorphous fraction of the Segal empirical approach, leading to the determination of wrong crystalline cellulose polymorph type [29,30].
Referring to previous works of A.L. Barnette et al. [31] and J. Zhang et al. [32], the crystalline cellulose I (native state) consists of two types of allomorphs, Iα and Iβ, and is considered more recalcitrant than other regenerated forms of cellulose (II, III, IV) [20,33]. Referring to Z. Ling et al. [19], the amorphized part of the cellulose could convert into a crystalline phase, which is influenced by the neighboring effect of cellulosic chains or by mechanochemical processes, and which subsequently becomes an activating agent in the evolution of cellulose Iβ toward cellulose II.
The main purpose of this study is to investigate the effect of natural environmental conditions on the alteration progress of both recent and ancient cedar softwood in order to preserve the endemic archaeological timbers of Moroccan cultural patrimony. The impact of this knowledge constitutes a scientific database for the agency of safeguard of the old city of Fez, during the restoration of this old cedar wood, which was often used as a biomaterial in the construction of ancient buildings. It also helps restorers to establish efficient treatments and appropriate preventive safeguards.
The present work was conducted on four samples of historical Moroccan cedar softwood, one of which is recent (21st century: reference), while the other three are considered aged (16th, 17th, and 19th centuries). For each sample, the analysis was carried out on both internal (non-degraded) and external (degraded) parts, constituting a set of eight samples. In order to assess the structural changes and evaluate the degree of damage produced on lignocellulosic biomaterial, two combined analytical approaches were used: ATR-FTIR spectroscopy and XRD. The results obtained were compared and correlated with each other to ensure the effectiveness of the analysis and provide reliable and accurate data.
2. Materials and Methods
2.1. Sampling
In the old city of Fez (Morocco), cedar wood was often used as a material in the construction of building because of its strength and mechanical properties, as well as its potential for natural durability against fungi.
Eight wood samples were collected from four archaeological cedar woods dating to the 16th, 17th, 19th, and 21st centuries, used as a raw material (wood beam) in the construction of ancient building in the imperial old city of Fez (Morocco) without any prior treatment. They were dated by specialist researchers using the radiocarbon dating method. The degraded wood samples were taken from the outside surface (superficial face) exposed to the effects of weathering conditions and compared with non-degraded ones, which are taken from 5 mm below the surface of the two wood pieces. This comparison aims at the evaluation of the effects of natural degradation process. The dimensions of wood samples are 10 × 5 × 20 mm (tangential × radial × longitudinal directions). The characteristics of the experimental materials are presented in the Table 1.

Table 1.
Non-degraded samples (A, B, C, D) and degraded samples (A’, B’, C’, D’), and their relative age (century).
It should be noted that the ATR-FTIR and XRD analyses were done directly on the surface of the samples.
2.2. ATR-FTIR Spectroscopy
Fourier transform infrared (FTIR) transmission spectra were carried out with a BRUCKER VERTEX 70® spectrometer coupled to a Hyperion® microscope. All samples were scanned using Platinum diamond ATR (attenuated total reflectance) in the wave number region between 4000 and 400 cm−1 with a resolution of 4 cm−1. At each position, 16 scans were averaged. The temperature and humidity room were controlled during analysis. In addition, the spectra were normalized to the band maximum around 1375 cm−1 related to C-H in plane deformation of crystalline cellulosic. According to L. Bejo et al. [22], this band is often used as internal standard, due to its high intensity and central position, as well as strong stability.
The following acronyms were used: : stretching vibration mode; as: asymmetric stretching vibration; s: symmetric stretching vibration; as asymmetric bending vibration; s symmetric bending vibration; : in plane deformation mode; : out of plane bending mode; r: rocking vibration; oop: out of plane bending vibration.
2.3. X-ray Diffraction Measurement
The X-ray diffraction (XRD) analyses were obtained using an X’Pert Pro diffractometer operating with monochromatic Cu K radiation (λ = 1.5406 Å), generated at a current of 30 mA and a voltage of 40 kV. The scattering angle of 2 was detected from 10 to 70 using a step size of 0.016 with 40 s exposure at each step. The crystallinity study was based on the determination of two parameters:
CrI (%) crystallinity index that was described by L. Segal method [26], using the following equation:
where is the total intensity of diffraction of the (200) plane at a 2θ~22.6, (10) plane at a 2θ~16.7, (101) plane at a 2 of 14.5, (040) plane at a 2 of 34.52 for cellulose , and of the (020) lattice peak for cellulose II at a 2θ~21.6, and is the intensity of diffraction of amorphous cellulose content at a 2θ~18 for cellulose , and at a 2θ~16.5 for cellulose II.
The average size of crystallite that was calculated from the Scherrer equation based on the width of the diffraction patterns. In this work, crystalline size was calculated from the 002-lattice plane of microcrystalline cellulose (MCC) samples.
where D(hkl) is crystalline size (nm), K is Scherrer constant (0.89), is X-ray wavelength (1.5406 Å), H is the full width at half maximum (FWHM) in radians, and the corresponding Bragg angle.
3. Results and Discussions
3.1. ATR-FTIR Spectroscopy
The spectra carried out using ATR-FTIR spectroscopy enabled us to study the chemical structure by identifying the functional groups and wood parts of the eight analyzed samples. The FTIR spectra of the four non-degraded samples are plotted in Figure 1, while the four degraded ones are illustrated in Figure 2. The FTIR superposition of eight samples (non-degraded + degraded) are reported in Figure 3 and their assignments are summarized in Table 2.
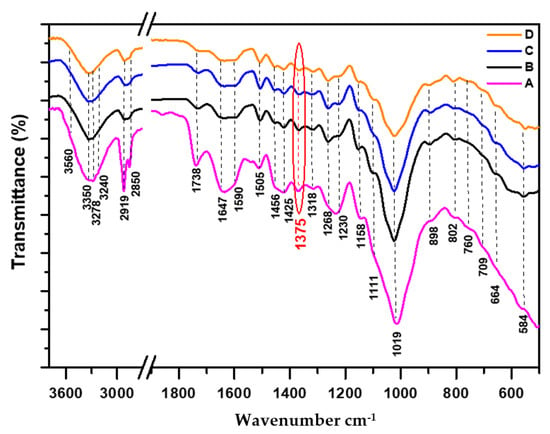
Figure 1.
FTIR spectra of non-degraded cedar wood samples normalized at 1375 cm−1: (A: 21st century, B: 19th century, C: 17th century, D: 16th century).
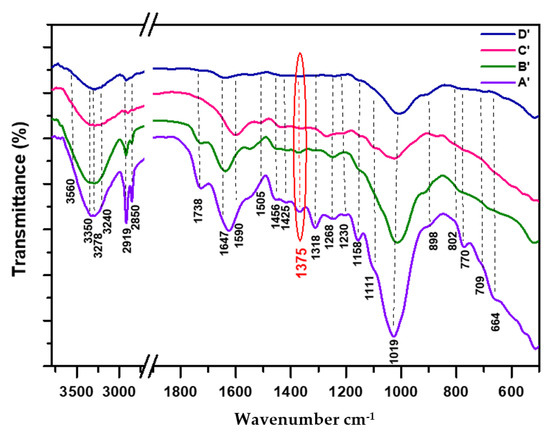
Figure 2.
FTIR spectra of degraded cedar wood samples normalized at 1375 cm−1: (A’: 21st century, B’: 19th century, C’: 17th century, D’: 16th century).
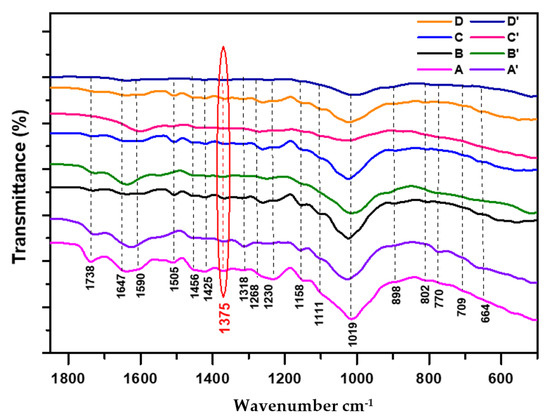
Figure 3.
Superposition of FTIR spectra of both cedar wood samples normalized at 1375 cm−1: non-degraded (A: 21st century, B: 19th century, C: 17th century, D: 16th century) and degraded (A’: 21st century, B’: 19th century, C’: 17th century, D’: 16th century).
The difficulties encountered stem from the ascription of the majority of IR bands that are not directly related to a single family of constituents (cellulose, hemicellulose, lignin), generating contributions from all components, and might induce some confusion leading to erroneous results, particularly in the region of 1800–700 cm−1. Thus, exhaustive literature data were exploited and careful interpretations were considered to better examine and assign the presence of different bands. Regarding the complexity of IR spectra profiles of non-degraded parts (Figure 1) compared to those of degraded ones (Figure 2), many structural modifications were detected in the following three ranges of 3750–2800 cm−1 (O-H + C-H aliphatic), 1800–1550 cm−1 (C=O + C=C + δO-H), and 1550–600 cm−1 (C=C + δC-H, C-O + δO-H), reflecting the essential transformations of molecular structure that occurred in wood components exposed to the natural degradation process.
The spectra area of 3000–2800 cm−1 is due to the C-H aliphatic stretching vibration of the primary, secondary, and tertiary alkyls groups (methyl, methylene, and methylidene, respectively). The intense band at 2919 cm−1 is ascribed to asymmetric stretching vibration asCH2 and CH3, while the weak one at 2850 cm−1 is assigned to both symmetric stretching vibration sCH2 + sCH3 [18,34]. The two latest bands are clearly visible and well resolved in the IR spectra of recent samples (Figure 1 (A)) and (Figure 2 (A’)), as well as that corresponding to the less aged one (Figure 2 (B’)). Their intensities decrease in the case of aged and degraded wood samples dating from the 17th and 16th centuries (Figure 2 (C’) and (D’)), indicating the damage that occurred in the structural constituents of cellulose, hemicellulose and lignin. As for the tertiary C-Hmethylidene (cellulose and hemicellulose), it overlaps with the area signals of the above-mentioned bands, usually expected at 2909 and 2896 cm−1 [19,20].
Concerning the high-frequency region (3750–3000 cm−1), there is a strong and broadening peak related to the O-H stretching vibration of bonded hydroxyl groups in cellulose, hemicellulose, and lignin, which decreases gradually in intensity as the sample’s age increases. This result is in total accordance with our XRD finding (Section 3.2). Moreover, this range contains a mixture of inter- and intra-molecular hydrogen bonds responsible for various properties of native cellulose and lignin, as well as the broadening and reduction in the IR signal of O-H shown in Figure 1 and Figure 2.
3.1.1. Behavior of Hemicellulose in Both Non-Degraded and Degraded Samples
Referring to numerous literature data [12,18,20,34,35,36,37,38], in the IR spectra profiles (Figure 1 and Figure 2), the presence of hemicellulose was highlighted by the following IR absorptions bands at 3350 cm−1 (OH), 2919 and 2850 cm−1 (as+sCH2, as+sCH3, C-H), 1738 cm−1 (C=O unconjugate acetyl and carboxyl groups), 1462 and 1375 cm−1 (as+sCH3 of acetyl), 1230 cm−1: C-O-C of ester groups in glucomannan and C-O of carboxyl groups in glucoronoxylan, 1158 cm−1 (asC-O-C) and 664 cm−1 (oopO-H).
The hemicellulosic acidic character is manifested by the presence of broad deviation in the IR spectra from 2250 to 3600 cm−1 (Figure 1 and Figure 2), informing of the bonded OH-acidic group (dimer), also confirmed by the presence of an associated C=O carboxylic signal at 1738 cm−1 related to glucuronic acid in hemicellulose [38,39]. The accentuated acidic character was shown in the case of recent non-degraded and degraded samples (Figure 1 (A)) and (Figure 2 (A’)), respectively, but less sensitive in the case of the most degraded sample (Figure 2 (D’)). In the two mentioned recent samples, the intense band observed at 1738 cm−1 also characterizes the presence of the unconjugate carbonyl ester group (C=O acetyl), which is mostly present in hemicellulose [18,20,37]. This band could overlap with other carbonyl ester groups [40], such as ferulate [41,42] and esterified phenolic acids related to the side chain of lignin [43]. Additionally, the higher intensity observed in this band, as well as that of 1230 cm−1 which is associated with C-O-C ester of xyloglucan, mirrored the inalterability of hemicellulose content [18,35,41,44].
Comparing IR profiles from recent samples (Figure 3 (A) and (A’)) to less aged ones (Figure 3 (B) and (B’)), the observed decrease in the intensity of this band suggests a breakdown in the unconjugate carbonyl ester group (deacetylation in the hemicellulose structure). However, its persistence in the case of very aged non-degraded samples (Figure 1 (C) and (D)) might be justified by the presence of both remaining carboxylic groups resulting from the hydrolysis reaction of acetyl (ester) groups, and esterified phenolic acid groups belonging to the side chain of lignin [38,43]. According to recent work conducted by A. Boukir et al. [18], as well as previous literature data [36,45], the resulting alcohols and acidic groups from hydrolysis and oxidative reactions could react and recombine together, leading to the formation of a new heterocyclic C=O ester group (δ-lactone), absorbing around 1732–1715 cm−1 [36,45]. In the case of very aged non-degraded samples (Figure 1 (C) and (D)), the new carbonyl former partially contributes to the increase and persistence of the mentioned signal [36].
Regarding the degraded samples (Figure 2 (C’) and (D’)), the disappearance of IR C=O acetyl hemicellulosic band is clearly visible, accompanied by the decrease in the intensity bands of holocellulosic fingerprint regions (1200–950 cm−1), as well as the band at 1230 cm−1, clearly proving the total deterioration of hemicellulosic backbone (glucoronoxylan and/or glucomannan).
It could be concluded that the decay in hemicellulosic fractions in both studied parts occurs during the early period of aging (19th century), as shown in both IR samples (Figure 1 (B)) and (Figure 2 (B’)).
It should be highlighted that some IR peak frequencies are subject to opposite attributions mainly in the range of 1470–950 cm−1. The controversies concern the assignment of O-H in plane deformation mode, which is associated to the value of 1460 cm−1 according to K.J. Nagarajan et al. [39], however C. Trilokesh et al. [42] correlated it to the value of 1384 cm−1. Additionally, E. Garskaite et al. [34] connected the band assignment of functional groups according to their vibrational mode of 1460 cm−1 to C-H and O-H in plane deformation, as well as C=C and CH3 symmetric bending in lignin. However, numerous literature data ascribed the following band assignment of functional groups according to their vibrational mode of 1460 cm−1 to asCH3 in plane bending of the acetyl group (CH3-(C=O)-) in hemicellulose and asCH2 in plane deformation of crystalline cellulose I, as well as asCH3 in the plane bending mode of CH3-O in lignin [18,35,37,46,47,48]. As for us, our attribution corroborates the results reported by the last authors (Table 1).
Regarding the signal at 1230 cm−1, some authors assigned it to the C-O-C asymmetric stretching mode of ester groups in xylopyranose [35,36], while other authors ascribed it to the in plane bending mode of acidic OH [41,49]. As for us, it corresponds to C-O-C of acetyl (ester) and C-O of glucuronoxylan carboxyl groups in the hemicellulosic fraction, as well as to the contribution of both Car-O of phenolic groups (Ar-O-H) and Car-O of aromatic ether groups (Ar-O-CH3) in the lignin matrix [18,40,49].
3.1.2. Behavior of Cellulose in Both Non-Degraded and Degraded Samples
Based on numerous literature data [8,12,13,18,19,20,34,38,50,51,52,53], in addition to a deep examination of IR spectra of studied samples (Figure 1 and Figure 2), the cellulose constituents were characterized by the presence of the following IR wavenumbers: 3350 cm−1 (OHintra), 3278 and 709 cm−1 (OHinter of crystalline ), 3240 and 770 cm−1 (OHinter of crystalline ), 2919 and 2850 cm−1 (as+sCH2, C-H), 1456 cm−1 (O-H), 1425 cm−1 (CH2 scissoring), 1375 cm−1 (C-H), 1318 cm−1 (CH2 wagging), 1163–1156 cm−1 (C-O-C bridge in -anomer), 1111 cm−1 (asC-O: secondary alcohol), 1019 cm−1 (C-O: primary alcohol and/or ether groups), 898 cm−1 (C-O-C: -(1-4)-glycosidic linkage), and 664 cm−1 (oopO-H).
Concerning the C-O cellulosic fingerprint region (1200–950 cm−1) and the conformation related to glucopyranose constituents, recently, I. Noda [54] used the 2D-FTIR method for identifying and distinguishing between the presence of the conformers in D-glucose ( and anomers). The author showed that conformer was characterized by the absorption band values at 1145, 1104, 1074, 1055, 1030, and 995 cm−1, while that of was elucidated by 1158, 1108, 1080, 1035, 1020, and 992 cm−1. In our findings (Figure 1 and Figure 2), the characteristic three IR bands at 1158, 1111, and 1019 cm−1 justified the presence of -anomeric conformation in the glucopyranose ring. However, it should be noted that the assignment of some shoulders was still quite complex, due to their overlapping with C-O peaks related to carbohydrates in both holocellulose and side chain of lignin.
In non-degraded IR spectra of cellulose from the recent sample (Figure 1 (A)) to aged ones (Figure 1 (D)), no exhaustive change was revealed in the case of C-H crystalline cellulose peaks (1318 cm−1, and asC-O-C bridge of -(1-4)-glycosidic linkage: 1158 cm−1). The relative observed differences were manifested in the profiles of 3700–3000 cm−1, 3000–2800 cm−1, and 1200–950 cm−1, suggesting that weak structural modifications and small depletion occurred in the cellulose structure. The IR crystalline cellulose results agree with those obtained by XRD, showing a slight decrease in crystallinity indices oscillating between 51.8% in the recent sample (Figure 1 (A)) to 44.7% in the case of the very aged one (Figure 1 (D)) (see XRD Section 3.2).
Regarding the case of degraded samples from recent (Figure 2 (A’)) to aged ones (Figure 2 (D’)), the observed changes in crystalline cellulose bands (1318, and 1158 cm−1) were highly visible, highlighted by a gradual reduction in their intensity bands from recent to aged samples, more accentuated in the case of very aged ones (Figure 2 (C’) and (D’)). This trend is also supported by a drastic decrease in the following intensity regions related to cellulose: C-O (1200–950 cm−1), aliphatic C-H (3000–2850 cm−1), and O-H of crystallized cellulose corresponding to glucopyranose (3500–3210 cm−1). Our findings are in agreement with a recent study conducted by D. Huang et al. [55], after treatment of holocellulose by a fungal community, showing a reduction in the band intensity at 1318 cm−1, justifying the partial damage that occurred in the cellulose structure. Similar results were shown in the case of biodegraded oak wood, enabling us to support and consolidate our proposals [36].
According to L. Bejo et al. [22], the peak at 1375 cm−1 is known by its high intensity and strong stability and is used for normalization in the IR spectra of cellulose. The stability of this later signal (Figure 1) could be explained by its interference with other absorption peaks related to sCH3 in both hemicellulose and stable lignin fraction [18,19], and probably to the contribution of O-H of the carboxyl group [36,56].
As for the area of 3500–3210 cm−1 (Figure 2), the observed regression and flattening in the intensity band around 3350 cm−1, which is linked to the intramolecular hydrogen bond O5-H5···O3, indicates the decrease in cellulose content [44]. As reported recently, this band is adjacent to the β-glycosidic peak of cellulose I [56], clearly explaining its importance in the loading of the cellulose chain comparable to that of the C-O-C bridge. The shift toward low intensity in the area band of 3280–3210 cm−1 related to intermolecular hydrogen bond O6-H6···O3 indicates the reduction in the cellulose I fraction and informs on the presence of both crystallized cellulose allomorphs: (monoclinic: 3278 and 709 cm−1) and (triclinic: 3240 and 770 cm−1), respectively [18,19,20,51,57]. According to M. Broda et al. [44], the presence of a dual peak at 3273 and 3213 cm−1 is assigned to the monoclinic phase and triclinic phase . Meanwhile, the value of 3565 cm−1 is ascribed to free OH(6) and OH(2) in cellulose, as well as to the O-H phenolic group in lignin, and to weakly adsorbed water. As for the following authors, J. Zhicheng et al. [35] and C.M. Popescu et al. [36], the band area position ranging from 3500 to 3430 cm−1 (band II) was imputed to intramolecular hydrogen bond O2-H2···O6 in crystalline cellulose. Our ascriptions corroborate those obtained by the mentioned authors.
Moreover, the remarkable decrease shown in the area of O-H bonds gives us an insight into weakening in intra- and inter-molecular hydrogen bond content in cellulose conformation (breakdown of their linkage), conferring a depolymerization phenomenon to the amorphous phase (hemicellulose + lignin) and, thus, promoting the alteration of crystalline cellulose structure in cedar wood [12,42,58].
In the less aged sample (Figure 2 (B’): 19th century), the early stage of alteration manifested in the cellulose fraction could be explained by the oxidation of the primary alcohol groups in the side chain of the glucopyranose ring, which are structurally more exposed to natural weathering phenomena than their corresponding secondary alcohols and, therefore, are more sensitive to degradation processes, oxidizing faster than others. According to recent work conducted by L. Amoroso et al. [56], the oxidation of cellulose affects preferentially primary alcohols at C6 rather than secondary ones at C2 and C3.
The investigation of the most aged samples (Figure 2 (C’): 17th century and (D’): 16th century) showed an important decrease in crystalline cellulose bands (pronounced alteration), which could be explained by the oxidation of secondary alcohols groups of the glucopyranose backbone, leading to a partial structural alteration of cellulose. This proposal corroborates a recent study [56], indicating that the oxidation of sugar occurs at the alcohol carbons (C2, C3), inducing a cleavage in the glucopyranose ring, resulting in a lowering of cellulose crystallinity. In our case, the arising phenomenon evidences the influence of a prolonged period of natural aging and potentially points to the partial contribution of fungi to the biodeterioration of wood.
Concerning the -O-4 glycosidic linkages (898 cm−1), previous literature data showed that a portion of this band is sensitive to change, highlighting the slower alteration of cellulose with respect to hemicellulose; thus, the amorphous form underwent crystallization [20,45,52,59]. In addition, the relative decay in -(1-4) cellulosic bonds under hydrolysis reaction affects the polymeric structure of cellulose by forcing the rearrangement in the hydrogen bond network, causing changes in the structural modes of C-C-H, O-C-H, and C-O-H, in-plane bending of H-C-H, and bending vibrations of water molecules. Hence, it results in the formation of a new structural arrangement which is responsible for the disruption of the crystalline cellulosic fraction and, thus, its evolution toward an amorphous form [12,20,45,55,59,60]. In the same tendency, A.L. Sullivan et al. [61] pointed out that thermal degradation constitutes the main decomposition mechanism in the breakage of -O-4 glycosidic bonding. According to M.A. Dahlem et al. [46], the enhancement of this band indicated the removal of crystalline cellulose with strong bonds and the pure cellulose fibers could probably be exposed. In a recent study, L. Amoroso et al. [56] showed that the absence of the peak at 880 cm−1 referred to hemiacetal formation, confirming the presence of a crystalline structure.
It could be concluded that cellulose alteration in aged, degraded samples is more affected than their corresponding non-degraded ones, influenced by a long period of exposure to natural aging conditions (temperature, UV irradiation, moisture, etc.), and probably by a partial contribution of fungi to the biodeterioration process.
The IR findings (degraded samples) align well with the results obtained by XRD measurements, showing a gradual regression in cellulose crystallinity indices from the recent sample (A’) (CrI 36.1%) to the very aged one (D’) (CrI 20.2%) (see XRD Section 3.2).
3.1.3. Behavior of Lignin in Both Non-Degraded and Degraded Samples
Referring to numerous literature reports [8,13,15,18,22,34,36,37,40,44,47,59,62], in our case study (Figure 1 and Figure 2), the cedar wood lignin content was identified by the presence of characteristic signals related to aromatic guaiacyl monomers, absorbing at the following frequencies: 3560–3550 cm−1 (OH), 2919 and 2850 cm−1 (as+s CH3), 1647 cm−1 (C=O: diconjugate), 1590 and 1505 cm−1 (Car=Car), 1462 and 1375 cm−1 (as+s CH3 of ether group), 1456 cm−1 (CH2), 1425 cm−1 (C-H), 1268 cm−1 (Car-O of guaiacyl), 1230 cm−1 (Car-O of syringyl), 1111 cm−1 (C-O-C dialkyl ether), 1019 cm−1 (C-O of CH3-O ether group), 802 cm−1 (oopCar-H), and 664 cm−1 (oopO-H).
The band at 3560 cm−1 is connected to intramolecular hydrogen bonds in phenolic lignin (band I). This finding is supported by a recent study at 3565 cm−1 [44], or around 3565–3550 cm−1 in previous literature data [18,36,63]. The presence of a broad slope beginning from 3000 cm−1 (Figure 1) is in favor of a strong intermolecular hydrogen bond between phenolic nuclei and another biphenol [36,44], engendering a loss in its intensity and slightly shifting up in the case of degraded samples (Figure 2). Furthermore, the gradual regression and remarkable difference in the intensity areas of the O-H phenolic band, and the C=C aromatic skeleton stretching mode (1600–1500 cm−1), as well as the C-H aliphatic (mainly CH3) stretching vibration mode (3000–2850 cm−1), is a good indicator for evaluating the deterioration that occurred in the lignin matrix (Figure 2 (C’) and (D’)).
To determine to which wood family (soft or hard) our cedar belongs to, the following two round and flattened Car-O bands (polar character) were exploited at 1268 cm−1 (predominant) and 1230 cm−1, clearly highlighting the presence of a guaiacyl nucleus as a major constituent of the lignin matrix [18,22,41,63]. It should be noted that sometimes the dual bands emerged into a single broad signal, as shown in recent samples (Figure 1 (A)) and (Figure 2 (A’)). Furthermore, the degree of aromatic substitution of guaiacyl (substituted-phenol compound), called ferulyl and/or coniferyl, can also be confirmed by exploring the fingerprint region of C-Har aromatic at 802 cm−1, highlighting its tri-substitution degree at positions 1, 2, and 4, which corroborates the above-mentioned finding [18,37,47,64,65]. Hence, the obtained results indicated clearly the appurtenance of cedar wood–lignin fraction to the softwood family [41,66].
As for the effect of the oxidation mechanism of Car-O lignin bonds (1268 and 1230 cm−1), they convert toward a new quinonic or diaryl carbonyl forms in the range of 1700–1550 cm−1. The decrease in their intensity bands (Figure 2), accompanied by an enhancement in the intensity bands of C=O (formation of new C=O groups), is a sign of the structural alteration of the lignin matrix [13,18,36,44,47,48,49,64].
Regarding the IR profiles of lignin (1750–800 cm−1) in non-degraded samples, from the recent sample (Figure 1 (A)) to the aged one (Figure 1 (D)), a few structural modifications affected mainly the large area signal of 1700–1550 cm−1, which is quite difficult and complex to analyze, due especially to the overlapping between the O-H in-plane bending vibration of adsorbed water (1620 cm−1) and neighboring peaks of conjugate carbonyl groups, as well as C=C aromatic bands [12,13,15,22].
The observed decrease in the intensity profile of this region from the recent non-degraded sample (Figure 1 (A)) to the most aged ones (Figure 1 (C) and (D)) indicates the loss of the contribution of water molecules, justified by the disappearance of the large overlapping signal of adsorbed water which is influenced by the prolonged time of natural degradation conditions. In all IR spectra of aged samples (Figure 1 (B), (C), (D)), the reduced area band splits into two regrouped sub-bands (large and intense shoulders) at 1647 cm−1 and 1590 cm−1, assigned to diconjugate C=O (quinonic form) and C=C aromatic, respectively 12,13,18,20,36,44]—the first of which, at 1647 cm−1, provides information about the starting step of lignin-alteration, preferentially affecting the lignin side chain, while the persistence of the second one (relatively intense) clearly indicates the partial preservation and stability of the aromatic skeletal group constituting the monomer of the lignin matrix.
In non-degraded cedar wood, the relative lignocellulosic degradation observed could be explained by a partial contribution of moisture and fungi (Penicillium commune), as revealed by M. Sadiki et al. [5] in Moroccan aged cedar wood used in artwork.
Comparing the IR features of lignin (1700–1550 cm−1) in both non-degraded and degraded samples (Figure 3 (C) and (C’), as well as (D) and (D’)), the observed decrease in the intensity bands at 1647 cm−1 and 1590 cm−1 clearly highlighted the serious continuity in lignin alteration, affecting the more stable aromatic skeleton of lignin (monomeric nucleus), particularly in degraded and aged samples (C’) and (D’).
Concerning the IR profiles of lignin (1700–800 cm−1) in degraded samples (Figure 2), all IR bands underwent a decrease in their intensities, promoting an increase in their corresponding oxidative region between 1700–1550 cm−1 and, thus, proving the structural deterioration in the aromatic lignin skeleton.
From the recent degraded sample (A’) to the less aged one (B’: 19th century), the few changes concern the decrease in the intensity band at 1268 cm−1 related to Car-O of ether group in guaiacyl monomer (methoxyl group branched to aromatic), highlighting the starting stage of structural modification (oxidation) in Car-O (guaiacyl-lignin), which converts toward a conjugate carbonyl former (1700–1550 cm−1), showing an increase in this area. In sample (Figure 2 (B’)), one should note the persistence of the C=O band at 1738 cm−1, which is probably due to the presence of ester and/or carboxylic group in the side chain of lignin. This carbonyl band disappears after a prolonged time of aging in both samples (C’) and (D’) (Figure 2), indicating its removal and thus, justifying the complete alteration of the side chain in lignin, which is more reactive than the stable aromatic part of the lignin matrix.
From the less aged sample (Figure 2 (B’)) to the most aged ones (Figure 2 (C’) and (D’)), the decrease observed in both broadening intensity bands at 1590 cm−1 (Car=Car) and 1647 cm−1 (C=O) indicates the reduction in the content of the lignin (aromatic character and oxidized form: diconjugate carbonyls fraction), clearly highlighting the serious and accentuated deterioration that occurred in the structural lignin matrix. Similar results have been reported recently by M. Broda et al. [44], using a fungi community in the biodegradation of oak wood, supporting our findings well. Indeed, the Car-O peaks were significantly weakened in spectra of degraded samples (Figure 2 (C’), (D’)), indicating their evolution toward new conjugate or diconjugate carbonyl formers (Ar-CO-Ar, o- and p-quinone, and Ar-CO-C=C) and, thus, providing information about the higher level of alteration in cedar wood lignin [18,36,44,64,65]. In a recent study conducted by J.J. Lucejko et al. [62], the authors showed that oak wood burial exposed to natural aging conditions (10 years) underwent a relative increase in the proportion of conjugate carbonyl groups. According to L. Bejo et al. [22], the conjugate C=O could be responsible for the color change in wood.
It could be concluded that cedar wood lignin belongs to the softwood family. The higher depletion is more accentuated in the case of very age-degraded samples (Figure 2 (C’) and (D’)) than their corresponding non-degraded ones (Figure 1 (C) and (D)). The observed phenomenon could be attributed to the effects of the prolonged time of natural aging conditions and environmental effects (moisture, temperature, UV irradiation, etc.), as well as to the probable contribution of microorganisms, such as fungi [5].
For easy understanding and exploration of all IR figures given previously, the IR ascription results of different constituents in cedar wood (hemicellulose, cellulose, lignin) are summarized in the following Table 2.

Table 2.
Assignments of IR bands for the cedar softwood samples.
Table 2.
Assignments of IR bands for the cedar softwood samples.
| Wavenumbers (cm−1) | Band Attributions | References |
|---|---|---|
| 3750–3000 | OH: cellulose + hemicellulose + lignin (intra- + inter-molecular hydrogen bond) | [18,35,36] |
| 3278 | OH: allomorph cellulose (O6-H6···O3: intermolecular hydrogen-bonds in carbohydrates) | [18,19,20,44] |
| 3240 | OH: allomorph cellulose (O6-H6···O3: intermolecular hydrogen-bond in carbohydrate) | [18,19,20,44,67] |
| 3000–2850 | C-H aliphatic: CH2 + CH3 + CH in holocellulose + lignin | [36,52] |
| 2919; 2850 | as+sCH2 + as+sCH3: holocellulose + lignin | [18,19,44] |
| 2909, 2896 | C-Hmethylidene of holocellulose: overlapped bands | [20,44,64] |
| 1738 | C=O of hemicellulose: unconjugate carbonyl of acetyl and carboxyl and/or glucoronate; C=O esterified phenolic acid in lignin and/or ferulate | [38,39,40,42,43,62] |
| 1647 | C=O of diconjugate carbonyl ketone in lignin: quinone, o- or p-quinone, Ar-CO-Ar, Ar-CO-C=C | [18,44,49,65] |
| 1635–1621 | O-H of adsorbed water; hydrogen bond between hemicellulose-lignin | [12,20,35,45,49] |
| 1590, 1505 | Car = Car of lignin | [13,18,47] |
| 1462 | asCH3 of acetyl pyranose in hemicellulose; asCH3 of methoxyl in lignin; asCH2 of cellulose; asCH2 in lignin and carbohydrates | [13,18,20,35,44,46,63] |
| 1456 | CH2 in lignin + O-H of cellulose | [34,39,46,52] |
| 1425 | CH2: scissoring in crystalline cellulose; lignin; hemicellulose + lignin | [13,18,20,25,35,56] |
| 1375 | C-H: crystalline cellulose; sCH3: acetyl group in hemicellulose; oopO-H; O-H of carboxyl group | [18,19,20,22,35,36,52,56] |
| 1318 | CH2 of crystalline cellulose: wagging; CH2: rocking | [18,34,40,51,52,56] |
| 1268 | Car-O: guaiacyl skeleton in lignin + C-O of cellulose | [18,35,37,63] |
| 1223–1235 | Car-O syringyl skeleton in lignin + C-O-C ester groups in hemicellulose (xyloglucan)/lignin + C-O carboxyl group; O-H of carbohydrate and COOH | [18,35,36,37,41,49] |
| 1158–1163 | asC-O-C: bridge of -(1-4)-glycosidic in crystalline cellulose; C-O of glucopyranose; C-O-C of carbohydrate; C-H of carbohydrate | [18,35,36,37,47,51,52,54] |
| 1111 | C-O: alcohol in carbohydrate, C-O side chain lignin | [12,36,53,54,57] |
| 1019 | C-O: primary alcohol of carbohydrate; C-O- ether of cellulose or CH3-O in lignin; CH3-O- of ester and -O-4 linkages in lignin | [35,36,37,39,49,53,54] |
| 960 | oopC-Har: deformation mode in lignin | [36,37] |
| 898 | C-O-C of -(1-4)-glycosidic linkage: amorphous or crystalline; C-H: rocking of -glycosidic bonds; C-H holocellulose | [18,19,20,35,42,45,46,53,59,62] |
| 802 | C-Har: fingerprint of 1,2,4-trisubstituted aromatic of guaiacyl-lignin | [36,37,46,64,65] |
| 770 | oopO-H of allomorph crystalline cellulose | [18,19,20,51,67] |
| 709 | oopO-H of crystalline cellulose ; r(CH2)n rocking | [18,19,20,51,52,67] |
| 664 | oopO-H of cellulose; or cellulose/hemicellulose/lignin | [20,51,52,68] |
: stretching vibration mode; as: asymmetric stretching vibration; s: symmetric stretching vibration; as asymmetric bending vibration; s symmetric bending vibration; : in plane deformation mode; : out of plane bending mode; r: rocking vibration; oop: out of plane bending vibration.
In summary of this IR section, cedar wood is known by its softwood character. The hemicellulosic fraction presents a faster vulnerability to deterioration as compared to cellulose and lignin fractions, during a short period of natural aging (19th century). In addition, from recent to aged samples, the cellulosic fraction in non-degraded samples is slightly affected as compared to the corresponding one in degraded samples, which is seriously altered. However, in the case of lignin, two degraded samples (Figure 2 (C’) and (D’)) underwent drastic damage. The observed alteration in all fractions (holocellulose, lignin) could be correlated to the extended period of natural aging conditions (photolytic reactions, atmospheric oxygen, temperature, moisture, etc.), and also suspecting the contribution of microorganisms to the biodeterioration process, such as fungi. The IR results obtained are in total accordance with the XRD results mentioned in Section 3.2.
3.2. X-ray Diffraction Analysis
Cedar softwood samples have been analyzed by X-ray diffraction to investigate the change that has been produced, to study the evolution of polymorph during natural degradation process, and to measure their crystallinity content using L. Segal CrI (%) [26] (Table 3). The interval of XRD samples studies ranged from 2θ~5 to 2θ~80, covering significant peak intensities of both crystalline and amorphous parts of cellulose. The XRD diffractograms of four non-degraded samples have been illustrated in Figure 4, showing some modifications in their peak intensities, while those of degraded samples plotted in Figure 5 presented difference in intensity of areas and in some shifted peaks.

Table 3.
Crystallinity indices values (CrI %) and crystallites seize: degraded and non-degraded wood samples.
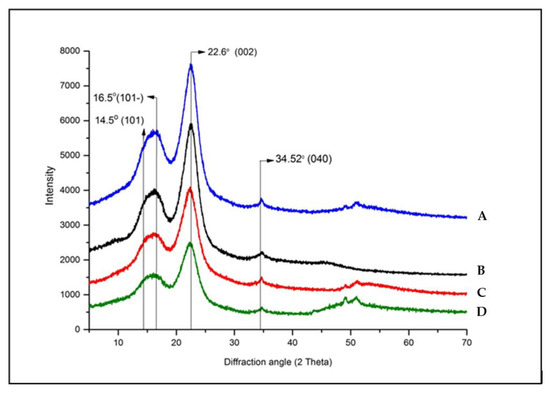
Figure 4.
XRD diffractogram of non-degraded cedar wood samples (A: 21st century, B: 19th century, C: 17th century, D: 16th century).
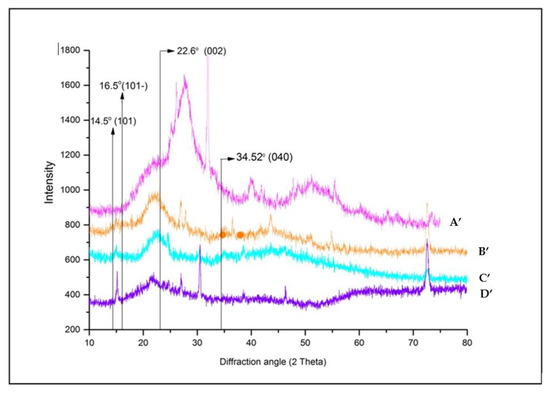
Figure 5.
XRD diffractogram of degraded cedar wood samples (A’: 21st century, B’: 19th century, C’: 17th century, D’: 16th century).
The qualitative evaluation of X-ray profile of all softwood samples (Figure 4) clearly showed the same diffraction features of crystalline cellulose as the dominant phase [60].
The analyzed samples displayed similar diffractions which can be resolved into three fundamental peaks at 2~14.5, 2~16.5, and 2~22.6 characteristic of typical profile of the cellulose (Figure 4). These diffractions were ascribed to the (101), (10), and (002) crystallographic planes, respectively [69,70]. Additionally, a weak and sharp peak was highlighted between 2~34.16 to 2~34.69 of 2 in all samples, assigned to the (040) crystallographic plane (composite of several reflections) of crystalline cellulose mixture (I, II, ). The peak resolve method enabled us to confirm the presence of cellulose allomorph . Figure 4 showed that in all wood material, the diffraction peaks of the (101) and (10) crystallographic planes merged together. Referring to several previous studies, the main crystalline peaks of cellulose I () appear at 2~14.7 to 2~14.88, 2~16.4 to 2~16.68, 2~22.3 to 2~22.9 and 2~34.5 to 2~35.2; while cellulose II is manifested by the presence of three signals at 2~12.1 to 2~12.2, 2~19.9 to 2~22.5 [12,18,20,28,29,30,45,59,71,72]. Our findings are in line with those cited references and also supported by numerous and recent literature data [18,20,68,73]. Some studies conducted by Y. Song et al. [59] have shown that the main peak of cellulose I appeared at 2~22.5 and cellulose II at 2~21.7. The same results were afterward supported by S. Nam et al. [30], who showed that cellulose is revealed by 2~22.7 and cellulose II by 2~21.7. However, some inadequacy literature data were reported by L. Manzato et al. [74]., characterizing the cellulose II at other positions of 2~10, 2~20.6 and 2~25. In the same trend, some controversies were noted in the attribution of crystallographic reflection planes related to the above-mentioned 2 angle [26,45,68], diverging in opinion with those reported by A.D. French and M. Santiago Cintrón [29], S. Nam et al. [30], I. Carrillo-Varela et al. [72,73], concerning the position and choice of crystallographic plane.
For amorphous sections, the diffracted profile was described at 2~18.5. However, xylan and lignin do not display any diffraction peaks but diffuse scattering halos in the range of 2~12 to 2~27, which overlap with the crystalline diffraction peak positions. According to Y. Song et al. [59], the amorphous portions of cellulose I is manifested at 2~19 and cellulose II at 2~16, while S. Nam et al. [30] attributed the amorphous intensities at 2~18 for cellulose I () and 2~16 for cellulose II. The same findings have been provided in recent studies by E.J. Foster et al. [68] for cellulose I.
The most noticeable changes occur in the intensity of the (200) reflection especially in the aged samples. Interestingly, the (004) reflection experienced no change in the diffractograms of non-degraded samples plotted in Figure 5 suggesting that the cellulose molecular chains remained largely unaffected by the chemical and physical effects of degradation [75].
Comparing the degraded samples (Figure 5) to non-degraded ones (Figure 4), the peak at 2~22.6 related to the (200) crystallographic planes of cellulose I in the oldest samples dating from the 16th century (Figure 5 (D’)) shifted towards 2~27.2. This means even the cellulose I lattice lost its ordered structure or transformed on other crystalline form as of cellulose II lattice. According to Z. Ling et al. [19], in XRD diffractograms, the evolution of broad signals toward sharp ones could be associated with the contribution of small crystallite size that enhances the breadth peak or induces the defects in the crystal network, and could derive from the lacks of organization material responsible for the more diffusion in crystalline peaks.
Additionally, the appearance of additional diffraction peaks at 2~43 and 2~73 refers to extraneous components on wood composition which could be due to the residual fraction resulting from the decay of wood components and/or the secretion of substances due to the living organisms.
The obtained results indicate that all experimental samples possessed low values of crystallinity index (below 50%) especially for the oldest ones (20% for sample dating from the 16th century). It stands to reason that the kind of studied wood has a fragile structure to degradation and/or the samples did suffer deterioration to a large extent. Therefore, we can suggest that the cedar wood belongs to the softwood species. This proposition was already reported and confirmed by FTIR analyses (Section 3.1).
For degraded samples (Table 3 (A), (B), (C) and (D)), no significant difference was observed on the size of crystallites values, meaning that the microcrystalline structure was not seriously broken by natural degradation process. Similar observations were applied for the non-degraded samples (Table 3 (A’), (B’), (C’), and (D’)), except for the sample dating back to the 19th century when the significant size value of 0.129 nm could be justified by re-crystallization of degraded cellulose after the removal of the amorphous fraction, consequently, formation of larger crystals.
On the other hand, the measured crystallinity index decreased almost with the decrease of the sample age in the following order A > B > C > D (Table 3) for non-degraded samples, and in the order A’ > B’ > C’ > D’ (Table 3) for the degraded ones. Consistent with this result, the CrI parameter lowers its value passing from the non-degraded part to the degraded one for all wood samples. This was further supported by the indication that the native state of wood cellulose was originally crystalline and became more amorphous when wood was subjected to the natural degradation process in absence of continuous control of environmental preservation conditions.
As manifested in Figure 4 and Figure 5, the peak intensity decreases in as the degradation level increases and is accompanied by slight line broadening. This suggests that several chemical type of degradation mechanism as hydrolysis and oxidation occur with almost equal facility in both amorphous and crystalline regions of the cellulose. H. Li et al. [76] investigated the influence of oxidation in the crystalline domains, and proposed that the oxidation proceeds highly heterogeneously, forming isolated oxidized domains which are more sensitive to degradation; hence, the decrease in crystallinity level. This preposition can be confirmed by the appearance of new vibration band ascribable to carbonyl groups (1738 cm−1) previously discerned in FTIR spectra (ATR-FTIR spectroscopy Section 3.1).
U.J. Kim and Kuga [77], as well as H. Li et al. [76] found out that the loss of crystallinity is considered to result from opening of glucopyranose rings and destruction of their ordered packing. Therefore, when the oxidation level is in an advanced stage, it confers a very low degree of crystallinity. Generally, the crystalline cellulose structure is a result of intermolecular hydrogen bonding between hydroxyl groups and includes water in wood matrix [78]. The decrease of wood crystallinity during weathering process suggests that when the temperature or oxidation level increases, hydrogen bonds in the crystalline cellulose become lower. As a result, the cellulose micro-fibrils become harder and more brittle, causing serious breakdown of hydrogen bonds and so, conferring an easily destroyed cellulose structure. These results are in accordance with our FTIR findings (Section 3.1) which suggest that the evaporation of water molecules during degradation process limits the chain mobility of cellulose. Thus, its alignment decreases the amount of cellulose crystallinity [79]. Similarly, the stability and the rigidity of wood depend more particularly on the crystalline fraction which inhibits degradation effects because the reduction of the amount of hydroxyl groups accessible for water absorption (during thermal degradation) introduces significant changes on cellulose crystallinity, and thus, influences the physical properties of studied materials.
As discussed by Y. Nishiyam et al. [80], the crystallographic structures and hydrogen bonding arrangements for cellulose I and cellulose provide important insights into cellulose stability and transformation and contribute to a scientific basis for understanding cellulose bio-generation and reactivity.
XRD investigation showed that the outer parts of degraded samples, particularly, the ancient ones (16th and 17th centuries) were the most affected, compared to those of the recent samples, and the internal parts (non-degraded samples). In degraded samples, the deep alteration of crystalline cellulose (important depolymerization) has been shown in very aged samples, justified by the reduction in their crystallinity indices, from 36.1% (recent sample) to 20.19% (very aged sample: 16th century). The XRD results corroborate well with the data obtained by IR analyses (Section 3.1).
It should be noted that the CrI calculation method developed by Segal et al. (1959) [26] is considered as a more traditional method (very outdated) and does not give real values of crystallinity but remains debated [81]. It compares the intensity of the highest peak (I200) and the local minimum Imin in the diffraction pattern 2θ range of 17−19°. According to recent literature data reported by Foster et al. (2018) [68], Carrillo-Varela et al. (2018 and 2019) [72,73], Del Cerro et al. (2020) [82], Mudedla et al. (2021) [83], and Redlinger-Pohn (2022) [81], the objective values of crystallinity index (CrI) from XRD can be measured using modern calculation methods such as a deconvolution approach (fitted peaks). The latter takes into account the amorphous model and compares the areas of the peaks [81]. It is defined as the ratio of the crystalline area divided by the total area (crystalline + amorphous) multiplied by a factor of 100.
4. Conclusions
Based on the obtained results, the texture of all cedar wood samples became rougher during the exposure to the natural degradation process and showed significant changes in physico-chemical properties related to wood dimensional stability and durability. The atmospheric and biological damage is the main cause of an important reduction in the amount of wooden materials. Consequently, wood resistance to degradation is substantially reduced.
The degraded sample results revealed a strong reduction in the peak intensities related to cellulose, hemicellulose, and lignin components accompanied by the regeneration of new diconjugate carbonyl groups at 1647 cm−1 (quinone type and/or Ar-CO-Ar) resulted from lignin oxidation and new carbonyl acidic groups at 1738 cm−1 due to the cellulose degradation that might recombine and evolve towards a new carbonyl ester groups (δ-lactone). These IR results agree with those obtained by XRD analysis which ascertained the state of degradation of cellulose fibers by an important decline in CrI values, as well as in peak intensities assigned to the crystalline cellulose fraction.
The obtained data showed that the non-destructive and non-invasive analytical approaches developed in this research present a powerful tool and respond effectively to the wood material study by offering valuable structural information without damaging the material and helping the scientific community to evaluate the appropriate conditions for preservation and restoration of the cultural patrimony material very solicited by the future generation and with important scientific implications.
Author Contributions
Conceptualization, S.F. and Y.B.; methodology, A.B.; software, F.E.M., S.F. and Y.B.; validation, A.B.; formal analysis, S.F. and Y.B.; investigation, Y.B. and S.F.; resources, A.B.; writing—original draft preparation, Y.B., S.F. and A.B.; writing—review and editing, A.B. and F.E.M.; visualization, A.B.; supervision, A.B.; project administration, A.B.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to Shimadzu and Merck Life Science Corporations for the continuous support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saab, A.M.; Lampronti, I.; Borgatti, M.; Finotti, A.; Harb, F.; Safi, S.; Gambari, R. In vitro Evaluation of the anti-proliferative activities of the wood essential oils of three Cedrus species against K562 human chronic myelogenous leukaemia cells. Nat. Prod. Res. 2012, 26, 2227–2231. [Google Scholar] [CrossRef]
- Quezel, P. Réflexions sur L’évolution de la Flore et de la Végétation au Maghreb Méditerranéen [Reflections on the Evolution of Flora and Vegetation in the Mediterranean Maghreb]; Ibis: Paris, France, 2000. [Google Scholar]
- Ruas, M.-P.; Ros, J.; Terral, J.-F.; Ivorra, S.; Andrianarinosy, H.; Ettahiri, A.S.; Fili, A.; Van Staëvel, J.-P. History and archaeology of the emblematic argan tree in the medieval Anti-Atlas Mountains (Morocco). Quat. Int. 2016, 404, 114–136. [Google Scholar] [CrossRef]
- Fidah, A.; Salhi, N.; Janah, T.; Rahouti, M.; Kabouchi, B.; El Alami, A.; Ziani, M.; Famiri, A. Comparative natural durability of four Mediterranean softwoods against wood decay fungi. J. Indian Acad. Wood Sci. 2016, 13, 132–137. [Google Scholar] [CrossRef]
- Sadiki, M.; Barkay, H.; Maryem, A.; Mohammed, E.; Saad, I.K.; Soumya, E. The Anti-Adherent Activity of Plants Extracts on Penicillium commune spores Causing Cedar Wood Decay. J. Adhes. 2016, 92, 295–305. [Google Scholar]
- Lluveras-Tenorio, A.; Andreotti, A.; Boujamid, A.; Castelvetro, V.; Ibnoussina, M.; Lorenzetti, G.; Raihane, M.; Salvadori, B.; Colombini, M.P. Characterization of the artist’s palette from the polychrome decorations of the El Bahia Palace doors (Marrakesh, Morocco). J. Cult. Herit. 2018, 33, 213–221. [Google Scholar] [CrossRef]
- El Hajjam, M.; Idrissi Kandri, N.; Harrach, A.; El Khomssi, A.; Zerouale, A. Physicochemical characterization of soft waste “Cedar” and hard waste “Mahogany”: Comparative study stud. Materials Today: Proceedings. 2019, 13, 803–811. [Google Scholar]
- Pandey, K.K. Study of the effect of photo-irradiation on the surface chemistry of wood. Polym. Degrad. Stab. 2005, 90, 9–20. [Google Scholar] [CrossRef]
- Rosu, D.; Teaca, C.-A.; Bodirlau, R.; Rosu, L. FTIR and color change of the modified wood as a result of artificial light irradiation. J. Photochem. Photobiol. B: Biol. 2010, 99, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Herrera, R.; Erdocia, X.; Llano-Ponte, R.; Labidi, J. Characterization of hydrothermally treated wood in relation to changes on its chemical composition and physical properties. J. Anal. Appl. Pyrolysis 2014, 107, 256–266. [Google Scholar] [CrossRef]
- Mattonai, M.; Watanabe, A.; Shiono, A.; Ribechini, E. Degradation of wood by UV light: A study by EGA-MS and Py-GC/MS with on line irradiation system. J. Anal. Appl. Pyrolysis 2019, 139, 224–232. [Google Scholar] [CrossRef]
- Hajji, L.; Boukir, A.; Assouik, J.; Kerbal, A.; Kajjout, M.; Doumenq, P.; Carvalho, M.L. A Multi-Analytical Approach for the Evaluation of the Efficiency of the Conservation–Restoration Treatment of Moroccan Historical Manuscripts Dating to the 16th, 17th, and 18th Centuries. Appl. Spectrosc. 2015, 69, 920–938. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, D.; Lucejko, J.J.; Zoborowska, M.; Modugno, F.; Cantisani, E.; Mamoňová, M.; Colombini, M.P. The short term degradation of cellulosic pulp in lake water and peat soil: A multi-analytical study from the micro to the molecular level. Int. Biodeter. Biodegr. 2017, 116, 243–259. [Google Scholar] [CrossRef]
- A Blanchette, R. A review of microbial deterioration found in archaeological wood from different environments. Int. Biodeterior. Biodegrad. 2000, 46, 189–204. [Google Scholar] [CrossRef]
- Pandey, K.; Pitman, A. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Howell, C.; Hastrup, A.C.S.; Jara, R.; Larsen, F.H.; Goodell, B.; Jellison, J. Effects of hot water extraction and fungal decay on wood crystalline cellulose structure. Cellulose 2011, 18, 1179–1190. [Google Scholar] [CrossRef]
- Walsh-Korbs, Z.; Avérous, L. Recent developments in the conservation of materials properties of historical wood. Prog. Mater. Sci. 2019, 102, 167–221. [Google Scholar] [CrossRef]
- Boukir, A.; Fellak, S.; Doumenq, P. Structural characterization of Argania spinosa Moroccan wooden artifacts during natural degradation progress using infrared spectroscopy (ATR-FTIR) and X-Ray diffraction (XRD). Heliyon 2019, 5, e02477. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, T.; Makarem, M.; Cintrón, M.S.; Cheng, H.N.; Kang, X.; Bacher, M.; Potthast, A.; Rosenau, T.; King, H.; et al. Effects of ball milling on the structure of cotton cellulose. Cellulose 2019, 26, 305–328. [Google Scholar] [CrossRef]
- Boukir, A.; Mehyaoui, I.; Fellak, S.; Asia, L.; Doumenq, P. The effect of the natural degradation process on the cellulose structure of Moroccan hardwood fiber: A survey on spectroscopy and structural properties. Mediterr. J. Chem. 2019, 8, 179. [Google Scholar] [CrossRef]
- Müller, U.; Rätzsch, M.; Schwanninger, M.; Steiner, M.; Zöbl, H. Yellowing and IR-changes of spruce wood as result of UV-irradiation. J. Photochem. Photobiol. B: Biol. 2003, 69, 97–105. [Google Scholar] [CrossRef]
- Bejo, L.; Tolvaj, L.; Kannar, A.; Preklet, E. Effect of water leaching on photodegraded spruce wood monitored by IR spectroscopy. J. Photochem. Photobiol. A: Chem. 2019, 382, 111948. [Google Scholar] [CrossRef]
- Hakkou, M.; Pétrissans, M.; El Bakali, I.; Gérardin, P.; Zoulalian, A. Wettability changes and mass loss during heat treatment of wood. Holzforschung 2005, 59, 35–37. [Google Scholar] [CrossRef]
- Bennouna, F.; Sadiki, M.; Elabed, S.; Koraichi, S.I.; Lachkar, M. The Effect of Different Vegetable Oils on Cedar Wood Surface Energy: Theoretical and Experimental Fungal Adhesion. Int. J. Biomater. 2022, 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Caro, L.; Giannini, C.; Lassandro, R.; Scattarella, F.; Sibillano, T.; Matricciani, E.; Fanti, G. X-ray Dating of Ancient Linen Fabrics. Heritage 2019, 2, 171. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Ruland, W. X-ray determination of crystallinity and diffuse disorder scattering. Acta Crystallogr. 1961, 14, 1180–1185. [Google Scholar] [CrossRef]
- French, A.D.; Cintron, M.S. Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2013, 21, 885–896. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Barnette, A.L.; Lee, C.; Bradley, L.C.; Schreiner, E.P.; Park, Y.B.; Shin, H.; Cosgrove, D.J.; Park, S.; Kim, S.H. Quantification of crystalline cellulose in lignocellulosic biomass using sum frequency generation (SFG) vibration spectroscopy and comparison with other analytical methods. Carbohydr. Polym. 2012, 89, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Zhang, L.; Zhang, R.; Liu, G.; Cheng, G. Understanding changes in cellulose crystalline structure of lignocellulosic biomass during ionic liquid pretreatment by XRD. Bioresour. Technol. 2014, 151, 402–405. [Google Scholar] [CrossRef]
- Atalla, R.H.; Vanderhart, D.L. Native Cellulose-A Composite of Two Distinct Crystalline Forms. Science 1984, 223, 283–285. [Google Scholar] [CrossRef]
- Garskaite, E.; Karlsson, O.; Stankeviciute, Z.; Kareiva, A.; Jones, D.; Sandberg, D. Surface hardness and flammability of Na2SiO3and nano-TiO2reinforced wood composites. RSC Adv. 2019, 9, 27973–27986. [Google Scholar] [CrossRef] [PubMed]
- Zhicheng, J.; Jian, Y.; Jianmei, L.; Ting, H.; Changwei, H. Promoting Effect of Sodium Chloride on the Solubilization and Depolymerization of Cellulose from Raw Biomass Materials in Water. ChemSusChem 2015, 8, 1901–1907. [Google Scholar]
- Popescu, C.-M.; Gradinariu, P.; Popescu, M.-C. Structural analysis of lime wood biodegraded by white rot fungi through infrared and two dimensional correlation spectroscopy techniques. J. Mol. Struct. 2016, 1124, 78–84. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Differentiation between pine woods according to species and growing location using FTIR-ATR. Wood Sci. Technol. 2017, 52, 487–504. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, C.; Shi, H.; Zhou, W.; Zhang, Q.; Chen, X. Combination of steam explosion pretreatment and anaerobic alkalization treatment to improve enzymatic hydrolysis of Hippophae rhamnoides. Bioresour. Technol. 2019, 289, 121693. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, K.; Balaji, A.; Ramanujam, N. Extraction of cellulose nanofibers from cocos nucifera var aurantiaca peduncle by ball milling combined with chemical treatment. Carbohydr. Polym. 2019, 212, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Yadav, J.; Kumar, R.; Tesarova, D.; Ekielski, A.; Mishra, P.K. On the rapid and non-destructive approach for wood identification using ATR-FTIR spectroscopy and chemometric methods. Vib. Spectrosc. 2020, 110, 103097. [Google Scholar] [CrossRef]
- Tintner, J.; Smidt, E.; Aumüller, C.; Martin, P.; Ottner, F.; Wriessnig, K.; Reschreiter, H. Taphonomy of prehistoric bark in a salt environment at the archaeological site in Hallstatt, Upper Austria–An analytical approach based on FTIR spectroscopy. Vib. Spectrosc. 2018, 97, 39–43. [Google Scholar] [CrossRef]
- Trilokesh, C.; Uppuliri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Gan, T.; Su, C.; Han, Y.; Liu, Z.; Cao, Y. Structural characterization and antioxidant activity of water-soluble lignin-carbohydrate complexes (LCCs) isolated from wheat straw. Int. J. Biol. Macromol. 2020, 161, 315–324. [Google Scholar] [CrossRef]
- Broda, M.; Popescu, C.-M. Natural decay of archaeological oak wood versus artificial degradation processes—An FT-IR spectroscopy and X-ray diffraction study. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2018, 209, 280–287. [Google Scholar] [CrossRef]
- Hajji, L.; Boukir, A.; Assouik, J.; Pessanha, S.; Figueirinhas, J.; Carvalho, M.L. Artificial aging paper to assess long-term effects of conservative treatment. Monitoring by infrared spectroscopy (ATR-FTIR), X-ray diffraction (XRD), and energy dispersive X-ray fluorescence (EDXRF). Microchem. J. 2016, 124, 646–656. [Google Scholar] [CrossRef]
- Dahlem, M.A.; Borsoi, C.; Hansen, B.; Catto, A.L. Evaluation of different methods for extraction of nanocellulose from yerba mate residues. Carbohydr. Polym. 2019, 218, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, S.; Özgenç, Ö.; Boyacı, I.H.; Yıldız, Ü.C.; Erişir, E. Examination of the chemical changes in spruce wood degraded by brown-rot fungi using FT-IR and FT-Raman spectroscopy. Vib. Spectrosc. 2016, 85, 202–207. [Google Scholar] [CrossRef]
- Santoni, I.; Callone, E.; Sandak, A.; Sandak, J.; Dirè, S. Solid state NMR and IR characterization of wood polymer structure in relation to tree provenance. Carbohydr. Polym. 2015, 117, 710–721. [Google Scholar] [CrossRef]
- Lehto, J.; Louhelainen, J.; Huttunen, M.; Alén, R. Spectroscopic Analysis of hot-water- and dilute-acid-extracted hardwood and softchips, Spectrochim. Acta. Part A 2017, 184, 184–190. [Google Scholar] [CrossRef]
- Kesari, K.; Leppänen, M.; Ceccherini, S.; Seitsonen, J.; Väisänen, S.; Altgen, M.; Johansson, L.-S.; Maloney, T.; Ruokolainen, J.; Vuorinen, T. Chemical characterization and ultrastructure study of pulp fibers. Mater. Today Chem. 2020, 17, 100324. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef]
- Acharya, S.; Hu, Y.; Moussa, H.; Abidi, N. Preparation and characterization of transparent cellulose films using an improved cellulose dissolution process. J. Appl. Polym. Sci. 2017, 134, 44871. [Google Scholar] [CrossRef]
- Noda, I. Two-dimensional correlation and codistribution spectroscopy (2DCOS and 2DCDS) analyses of time-dependent ATR IR spectra of d-glucose anomers undergoing mutarotation process in water. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2018, 197, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Li, T.; Xu, P.; Zeng, G.; Chen, M.; Lai, C.; Cheng, M.; Guo, X.; Chen, S.; Li, Z. Deciphering the Fenton-reaction-aid lignocellulose degradation pattern by Phanerochaete chrysosporium with ferroferric oxide nanomaterials: Enzyme secretion, straw humification and structural alteration. Bioresour. Technol. 2019, 276, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, L.; Muratore, G.; Ortenzi, M.A.; Gazzotti, S.; Limbo, S.; Piergiovanni, L. Fast Production of Cellulose Nanocrystals by Hydrolytic-Oxidative Microwave-Assisted Treatment. Polymers 2020, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Liu, Y.; French, A.D.; Lee, C.M.; Kim, S.H. Comparison and validation of Fourier transform infrared spectroscopic methods for monitoring secondary cell wall cellulose from cotton fibers. Cellulose 2017, 25, 49–64. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Popescu, M.-C.; Vasile, C. Structural analysis of photodegraded lime wood by means of FT-IR and 2D IR correlation spectroscopy. Int. J. Biol. Macromol. 2011, 48, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, J.; Zhang, X.; Tan, T. The correlation between cellulose allomorphs (I and II) and conversion after removal of hemicellulose and lignin of lignocellulose. Bioresour. Technol. 2015, 193, 164–170. [Google Scholar] [CrossRef]
- Hajji, L.; Boukir, A.; Assouik, J.; Lakhiari, H.; Kerbal, A.; Doumenq, P.; Mille, G.; De Carvalho, M.L. Conservation of Moroccan manuscript papers aged 150, 200 and 800 years. Analysis by infrared spectroscopy (ATR-FTIR), X-ray diffraction (XRD), and scanning electron microscopy energy dispersive spectrometry (SEM–EDS). Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2015, 136, 1038–1046. [Google Scholar] [CrossRef]
- Sullivan, A.; Ball, R. Thermal decomposition and combustion chemistry of cellulosic biomass. Atmos. Environ. 2012, 47, 133–141. [Google Scholar] [CrossRef]
- Lucejko, J.J.; Tamburini, D.; Zborowska, M.; Babiński, L.; Modugno, F.; Colombini, M.P. Oak wood degradation processes induced by the burial environment in the archaeological site of Biskupin (Poland). Herit. Sci. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Macchioni, N.; Pizzo, B.; Bernabei, M.; Visintin, G. Dating trials of wooden historic artefacts through FT-IR spectroscopy. J. Cult. Herit. 2020, 43, 303–310. [Google Scholar] [CrossRef]
- Boukir, A.; Guiliano, M.; Asia, L.; El Hallaoui, A.; Mille, G. A fraction to fraction study of photo-oxidation of BAL 150 crude oil asphaltenes. Analusis 1998, 26, 358–364. [Google Scholar] [CrossRef]
- Boukir, A.; Aries, E.; Guiliano, M.; Asia, L.; Doumenq, P.; Mille, G. Subfractionation, characterization and photooxidation of crude oil resins. Chemosphere 2001, 43, 279–286. [Google Scholar] [CrossRef]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and harwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stabil. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Santos, S.M.; Carbajo, J.M.; Quintana, E.; Ibarra, D.; Gomez, N.; Ladero, M.; Eugenio, M.E.; Villar, J.C. Characterization of purified bacterial cellulose focused on its use on paper restoration. Carbohydr. Polym. 2015, 116, 173–181. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef]
- Tomé, L.C.; Brandão, L.; Mendes, A.; Silvestre, A.; Neto, C.; Gandini, A.; Freire, C.S.R.; Marrucho, I. Preparation and characterization of bacterial cellulose membranes with tailored surface and barrier properties. Cellulose 2010, 17, 1203–1211. [Google Scholar] [CrossRef]
- Cairul, M.; Mohd, I.; Abadi, V.; Katas, H. Purification, Characterization and comparative studies of spray-dried bacterial cellulose microparticles. Carbohydr. Polym. 2014, 99, 180–189. [Google Scholar]
- Ju, X.; Bowden, M.; Brown, E.E.; Zhang, X. An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohydr. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef]
- Carrillo-Varela, I.; Pereira, M.; Mendonça, R.T. Determination of polymorphic changes in cellulose from Eucalyptus spp. fibres after alkalization. Cellulose 2018, 25, 6831–6845. [Google Scholar] [CrossRef]
- Carrillo-Varela, I.; Retamal, R.; Pereira, M.; Mendonça, R.T. Structure and reactivity of cellulose from bleached kraft pulps of different Eucalyptus species upgraded to dissolving pulp. Cellulose 2019, 26, 5731–5744. [Google Scholar] [CrossRef]
- Manzato, L.; Rabelo, L.C.A.; de Souza, S.M.; da Silva, C.G.; Sanches, E.A.; Rabelo, D.; Mariuba, L.A.M.; Simonsen, J. New approach extraction of cellulose tucuma’s endocarp and its structural characterization. J. Mol. Struct. 2017, 1143, 229–234. [Google Scholar] [CrossRef]
- Yin, J.; Yuan, T.; Lu, Y.; Song, K.; Li, H.; Zhao, G.; Yin, Y. Effect of compression combined with steam treatment on the porosity, chemical composition and cellulose crystalline structure of wood cell walls. Carbohyd. Polym. 2017, 155, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, B.; Mu, C.; Lin, W. Concomitant degradation in periodate oxidation of carboxymethyl cellulose. Carbohydr. Polym. 2011, 84, 881–886. [Google Scholar] [CrossRef]
- Kim, U.-J.; Kuga, S. Ion-exchange chromatography by dicarboxyl cellulose gel. J. Chromatogr. A 2001, 919, 29–37. [Google Scholar] [CrossRef]
- Shimur, R.; Nishiok, A.; Kano, I.; Kod, T.; Nishio, T. Novel method for producing amorphous cellulose only by milling. Carbohyd. Polym. 2014, 102, 645–648. [Google Scholar] [CrossRef]
- Rojas, J.; López, A.; Gamboa, Y.; González, C.; Montoya, F. Assessment of processing and polymorphic form effect on the powder and tableting properties of microcrystalline celluloses I and II. Chem. Pharm. Bull. 2011, 59, 603–607. [Google Scholar] [CrossRef]
- Nishiyam, Y.; Sugiyam, J.; Chanzy, H.; Langan, P. Crystal Structure and Hydrogen Bonding System in Cellulose I_α from Synchrotron X-ray and Neutron Fiber Diffraction. J. Amer. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef]
- Redlinger-Pohn, J.D.; Petkovšek, M.; Gordeyeva, K.; Zupanc, M.; Gordeeva, A.; Zhang, Q.; Dular, M.; Söderberg, L.D. Cavitation Fibrillation of Cellulose Fiber. Biomacromolecules 2022, 23, 847–862. [Google Scholar] [CrossRef] [PubMed]
- del Cerro, D.R.; Koso, T.V.; Kakko, T.; King, A.W.T.; Kilpeläinen, I. Crystallinity reduction and enhancement in the chemical reactivity of cellulose by non-dissolving pre-treatment with tetrabutylphosphonium acetate. Cellulose 2020, 27, 5545–5562. [Google Scholar] [CrossRef]
- Mudedla, S.K.; Vuorte, M.; Veijola, E.; Marjamaa, K.; Koivula, A.; Linder, M.B.; Arola, S.; Sammalkorpi, M. Effect of oxidation on cellulose and water structure: A molecular dynamics simulation study. Cellulose 2021, 28, 3917–3933. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).