1. Introduction
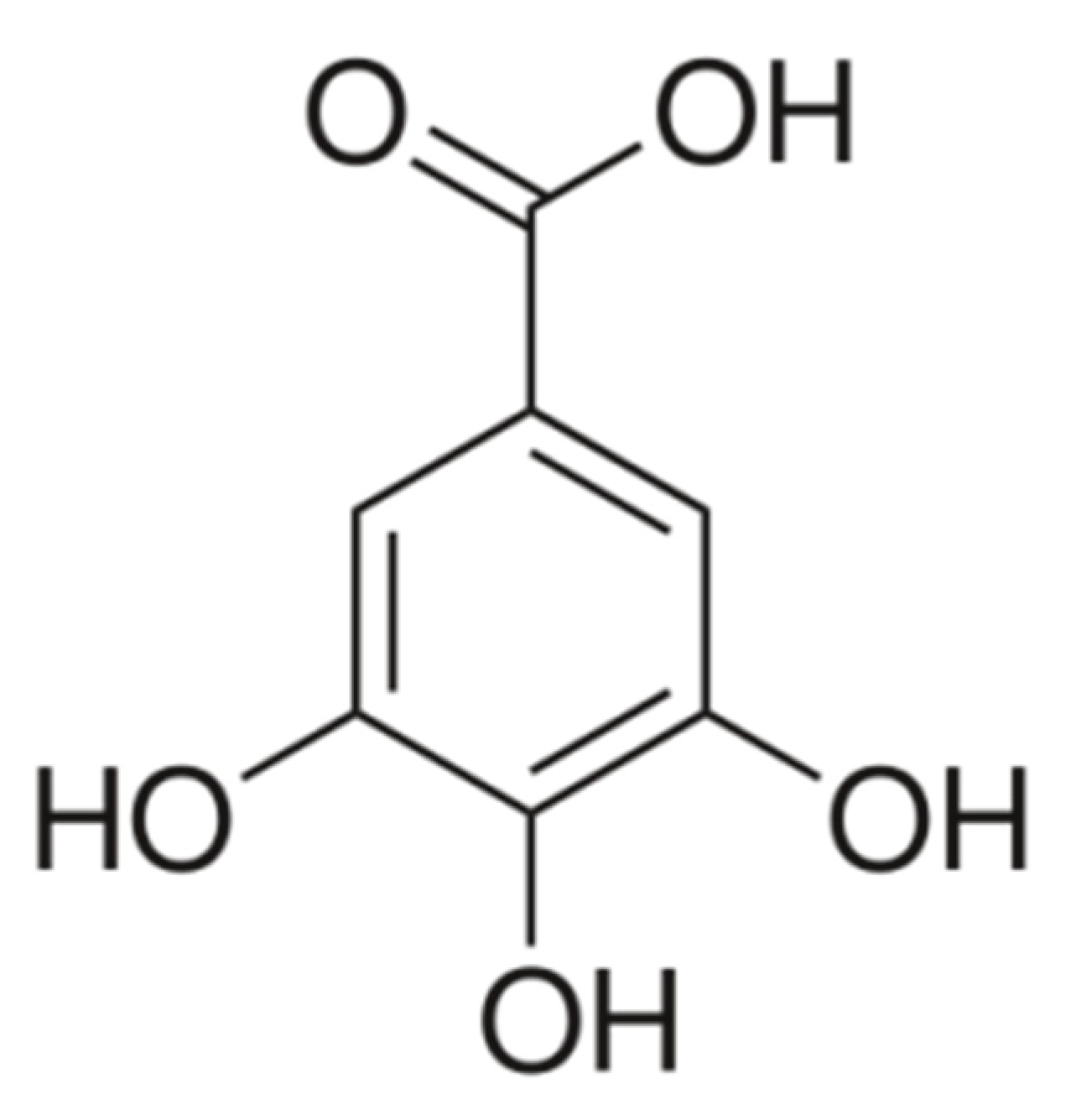
Gallic acid (3,4,5-trihydroxybenzoic acid,
Figure 1) (GA) is a phenolic acid of natural origin, found in the leaves, roots, flowers, or stem of a large number of plant species (
Bergia suffruticosa,
Ceratonia siliqua,
Tectonagrandis, and
Casuarina equisetifolia) with numerous applications in the food and pharmaceutical industries [
1].
GA is a white or pale-yellow crystalline compound, with the structural formula presented in
Figure 1 and the main physicochemical properties in
Table 1.
From the pharmacological point of view, GA has antimicrobial, anti-inflammatory and antioxidant action [
1,
3]. In addition, GA inhibits the adhesion and development of microorganisms from the species
Pseudomonas aeruginosa,
Staphylococcus aureus,
Streptococcus mutans,
Chromobacterium violaceum,
Listeria monocytogenes, and
Shigella flexneri [
4,
5,
6,
7,
8,
9]. Moreover, it is able to exhibit degradative action on the cell wall of some Gram-negative microorganisms and an inhibitory effect on the activity of some enzymes (HIV-1 integrase, HIV-1 transcriptase, HCV serine protease, etc.) [
10,
11,
12]. Additionally, a few studies present models for the use of GA and its derivatives in various diseases, including cancer [
13]. The cytotoxic and antitumoral effect of GA results from modulating pro- and anti-oxidant activities [
3].
Conventionally, GA can be obtained by acid hydrolysis of tannic acid. However, it implies high cost, low yield, low purity, and generation of toxic effluents. A more efficient process involves microbial hydrolysis of tannic acid (
Table 2) using tannase (tannin-acyl-hydrolase, EC 3.1. 1.20, an enzyme that catalyzes the breakdown of ester bonds in gallotannins) produced by fungal and bacterial strains.
Several studies have analyzed reactive extraction for the GA separation [
19,
20,
21,
22], showing its potential. Rewatkar et al. [
19] obtained-under optimum conditions of 2.34 g/L GA, 65.65%
v/
v tri-n-butyl phosphate in hexanol, 19 °C, and pH 1.8-an extraction efficiency of 99.43% for gallic acid from an aqueous stream. Joshi et al. [
20] obtained the following distributions coefficient of GA 1.94–27.57 for 2-octanone: 1.12–8.83 for lauryl alcohol, and 0.20–22.07 for n-heptane, increased values being obtained by adding 0.3652 mol/L TBP (tri-butyl phosphate) as an extractant. Pandey et al. [
21,
22] obtained the maximum GA extraction of 90.1%, at an initial acid concentration of 0.0588 mol/L, initial TOA concentration of 0.2762 mol/L, pH 2, and temperature of 25.0 °C. However, none of these studies have analyzed GA back extraction from organic solvents.
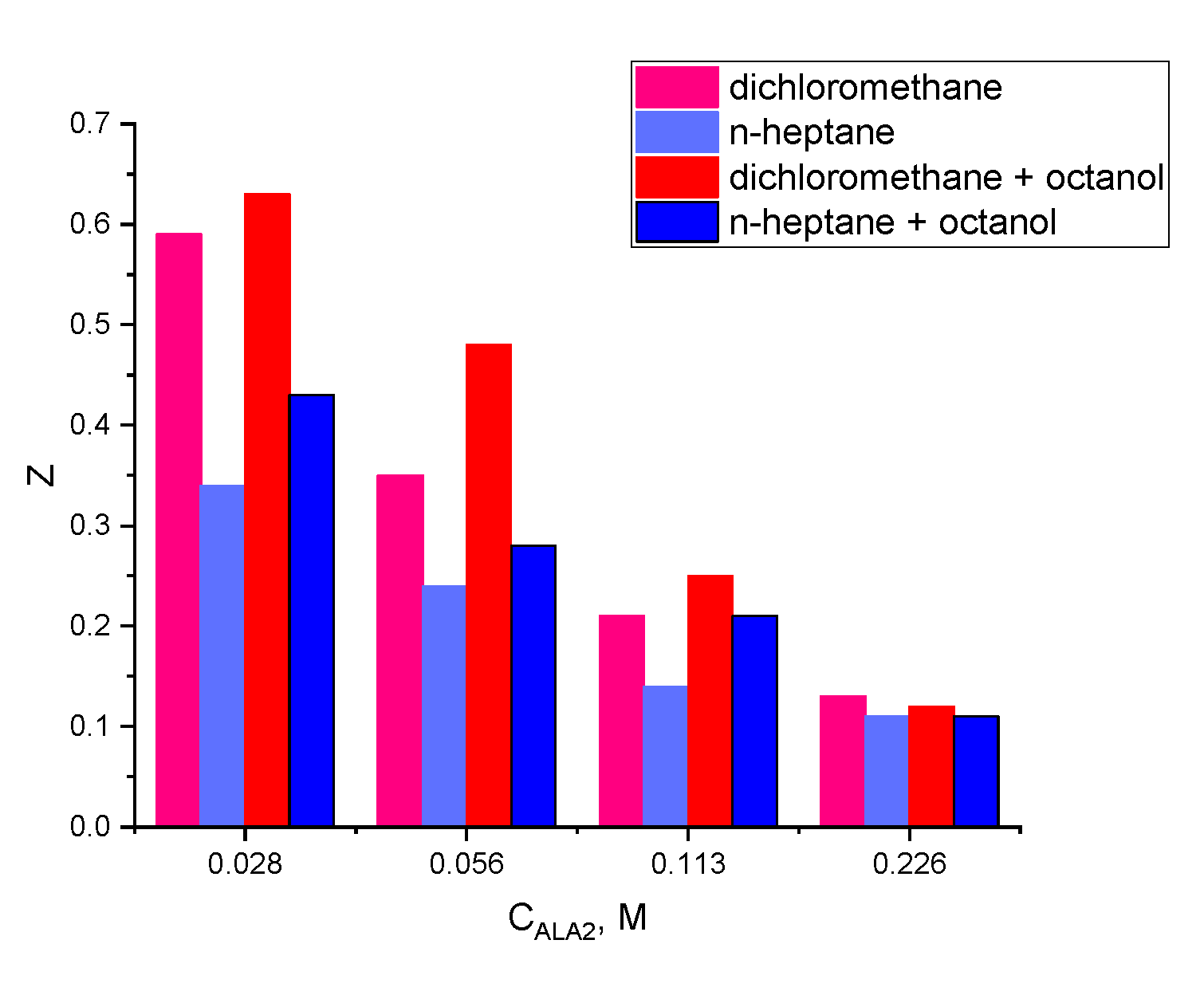
This study aims to provide a complete analysis (extraction, stripping, and optimization) of the GA reactive extraction process using Amberlite LA2 dissolved into two solvents with different dielectric constants: dichloromethane and n-heptane and a phase modifier (1-octanol). For reactive extraction of carboxylic acids during the intense mixture necessary for a good mass transfer, a third phase is formed—a stable emulsion very difficult to separate. The addition of a phase modifier, 1-octanol, prevents its formation, facilitating the organic and aqueous phase separation, and, it also increases the solvent polarity increasing the reactive extraction efficiency [
23,
24,
25]. The results were discussed from the viewpoint of the extraction mechanism, separation yield and distribution coefficient, for different extraction conditions. Back extraction was successfully performed using NaOH solutions. In addition, the process was modeled and optimized using statistical regression and Artificial Neural Networks (ANNs) determined with the Differential Evolution (DE) algorithm. The objective is to rapidly identify the extraction efficiency without additional experiments and determine the optimal conditions that lead to maximum efficiency.
2. Materials and Methods
2.1. Chemicals
All chemicals, including gallic acid (98.0%), dichloromethane (99%), 1-octanol (99%), heptane (99%), sulfuric acid (95.0–98.0%), sodium hydroxide (>97%), lauryl tri-alkylmethylamine—Amberlite LA2 (99%), and acetonitrile (99.99%), were purchased by Sigma Aldrich (Burlington, VT, USA) and used as received without further processing.
2.2. Reactive Extraction Experiments
Reactive extraction and stripping experiments for GA separation were carried out using equal volumes of aqueous and organic phases (20 mL) in an extraction column with vibratory mixing (50 s
−1 frequency and 5 mm amplitude, the stirrer being maintained at the initial interface between the two phases), consisting in a glass column with a diameter of 36 mm and 250 mm height, provided with a thermostatic jacket that allowed temperature control: 25 ± 0.02 °C for all the reactive extraction experiments and 50 ± 0.02 °C for the back extraction. The reactive extraction experiments were performed using two solvents with different polarities: dichloromethane (dielectric constant 9.08 [
26]) and n-heptane (dielectric constant 1.9 [
26]). The first solvent, suitable for polar compounds, was chosen based on efficient reactive extraction obtained for other carboxylic acids (2-ketogluconic acid, formic acid, pseudomonic acid, acetic acid, etc. [
23,
24,
25,
27]), while the second one was chosen due to its green classification [
28]. As a phase modifier, 1-octanol (dielectric constant 10.3 at 25 °C [
26]) was added to the organic solution to increase the polarity of the solvent and to facilitate the phases separations (1-octanol prevents the formation of a third phase—a stable emulsion between the two phases). Solvent selection is an essential parameter since it influences the extraction efficiency and the design of a continuous extraction process and solvent regeneration cycle.
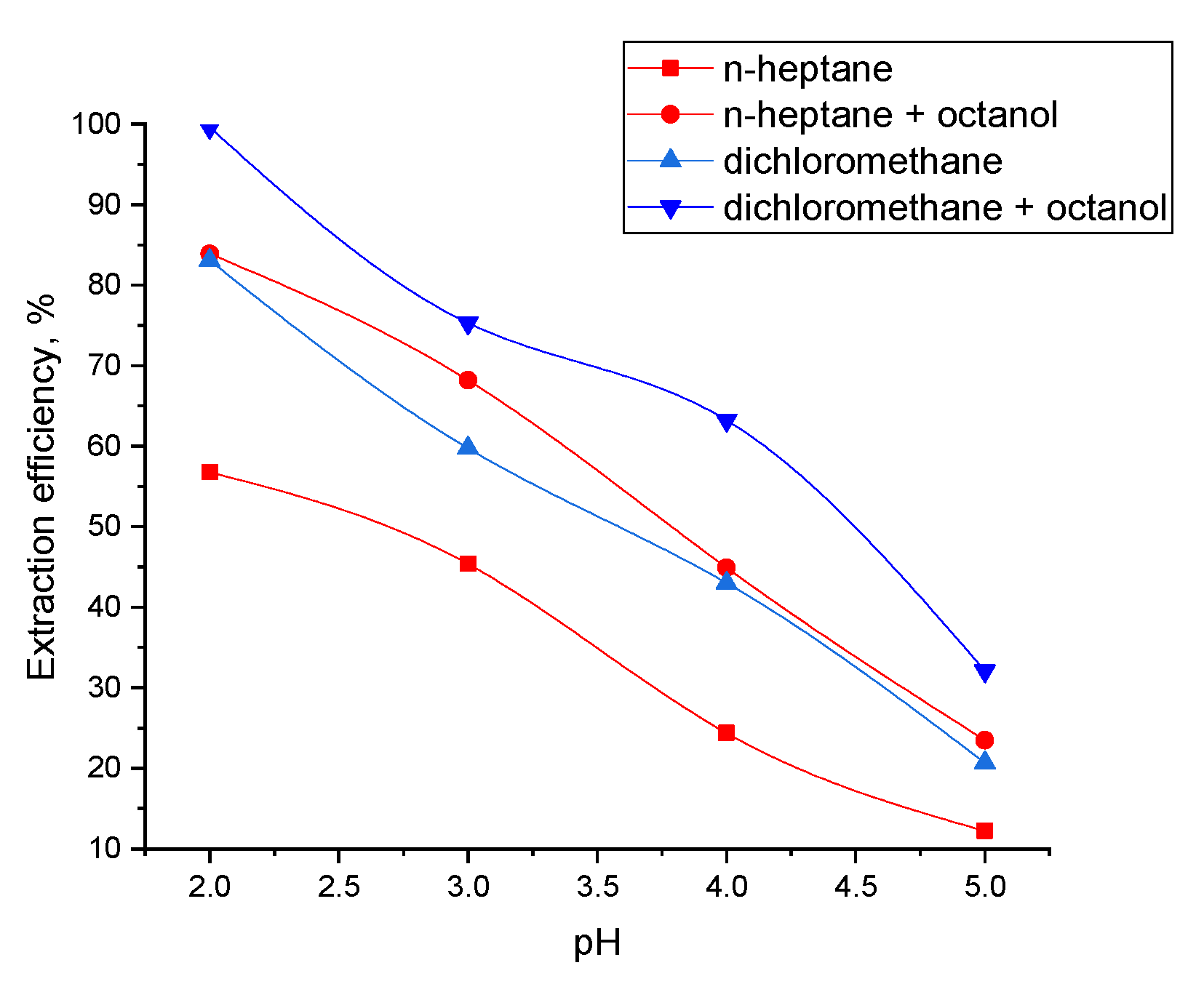
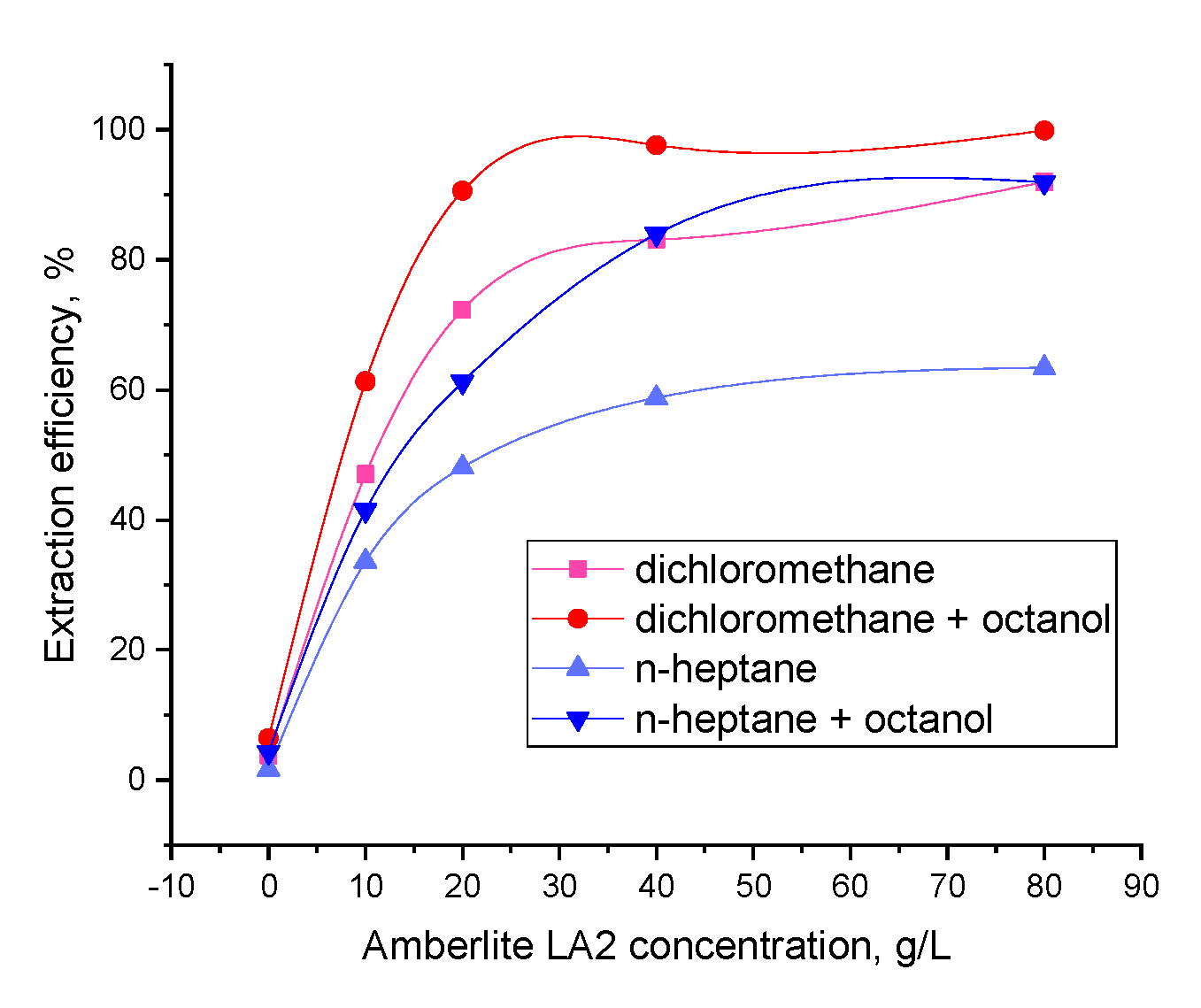
After the extraction and the stripping (each process having a duration of 1 min), phases were separated in a centrifugal separator at 4000 rpm. GA initial concentration in the aqueous phase was 5 g/L (2.29 × 10−2 M), and the concentrations of the amine extractant, Amberlite LA-2, in the organic phase varied between 0 and 80 g/L (0.215 M). The pH value of the aqueous phase was between 2 and 5 for the extraction and 8 for the stripping experiments, modified using solutions of 3% sulfuric acid or 3% sodium hydroxide, by means of the indications of the digital pH meter (CONSORT C 836) (Turnhout, Belgium).
2.3. Analytical Procedures
The processes were analyzed on the basis of distribution coefficients and extraction efficiency, calculated using the mass balance based on the GA concentration in the aqueous phases measured by the high-performance liquid chromatography technique (HPLC) as described in the literature [
19]. For this purpose, an HPLC system, UltiMate 3000 Dionex (Sunnyvale, CA, USA), provided with an Acclaim C18 (150 mm × 4.1 mm, 5 μm) column (Sunnyvale, CA, USA), 90% water, and 10% acetonitrile solution were used as mobile phase, with detection by UV absorbance at 264 nm wavelength, and the flow rate of 0.8 mL/min.
2.4. Modelling and Optimization
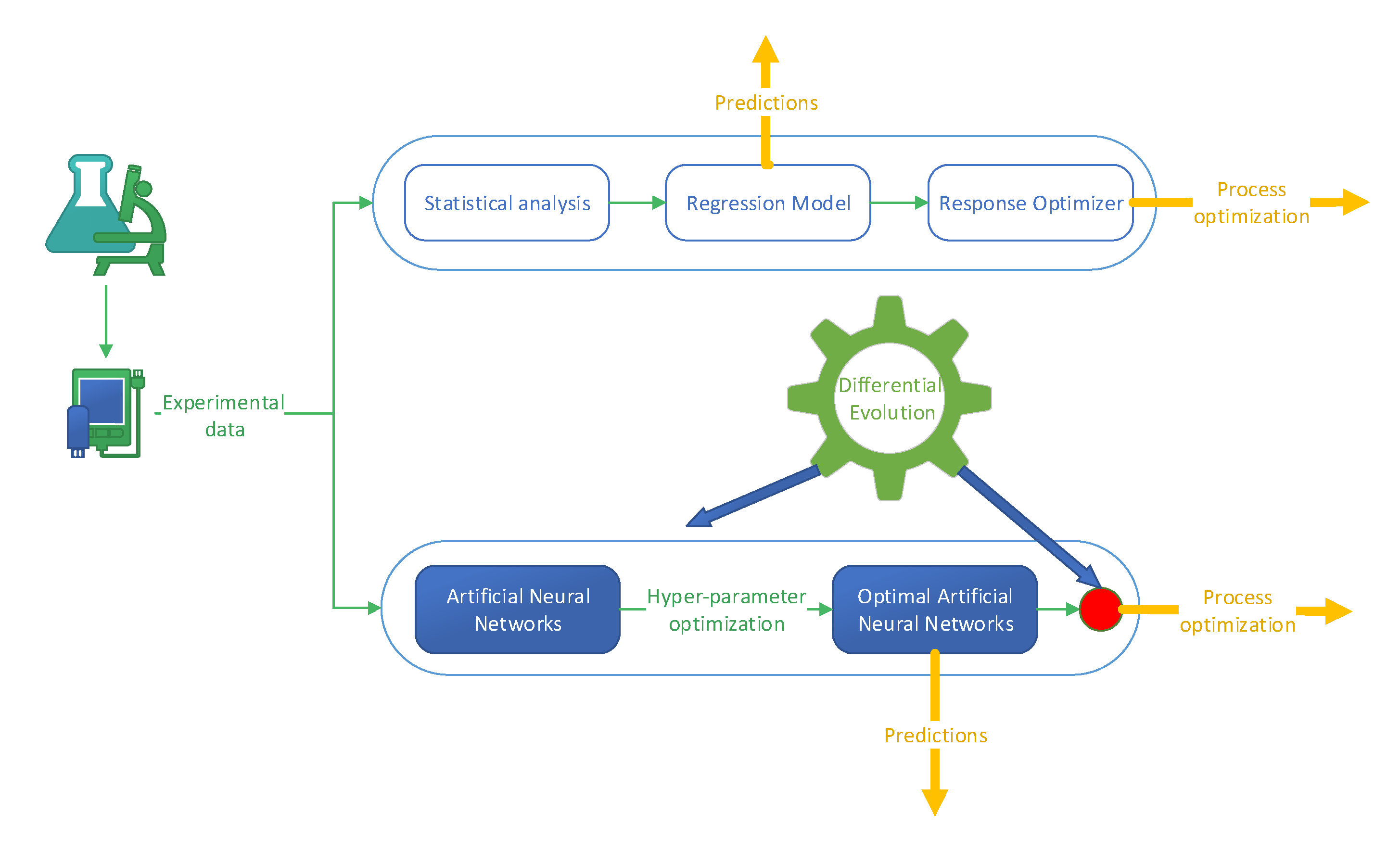
In order to model and optimize the considered process, two strategies were considered. One is based on the classical approaches that use statistical methods, and the other uses artificial intelligence techniques: ANNs and bio-inspired metaheuristics.
2.4.1. Statistic Approaches
The statistical analysis of the process was performed using Minitab (Coventry, UK), with a regression model with interactions through order 2 and terms through order 2, with a 95% confidence level for all intervals, and a forward selection strategy for parameter optimization. For process optimization, the Response Optimizer option was used.
2.4.2. Artificial Neural Networks and Differential Evolution
In this case, the process modeling was performed using ANNs. Although ANNs are relatively easy to use, their optimal configuration and hyper-parameters are a problem in themselves. Thus, a Keras sequential model was considered in this work, with an Adam optimizer and ReLU activation function for the hidden layers and a linear activation function for the output layer. Consequently, the training of the ANN is performed using the Keras framework. However, before training, an optimal architecture must be selected. This step is performed by DE, a population-based bio-inspired metaheuristic that showed a good capability to solve various problems from different areas. For example, in simple or different combinations, it was applied for: the prediction of polycyclic aromatic hydrocarbons formation in grilled meats [
29] prediction of reactive extraction of pseudomonic acids [
23], modeling the biogas production from anaerobic wastewater treatment plant [
30], and optimization of biogas power plant feedstock [
31].
Except for the determination of the ANN model architecture, in this work, DE is also applied to optimize the process. Since this step cannot be performed without a model, in this work, the ANN model was used in combination with DE.
Since DE is a population-based algorithm that uses vectors of real numbers, each of the two optimization problems is individually solved. In each case, a direct encoding transforms the problem into a structure that DE can work with. In the case of ANN optimization, the parameters considered are strictly related to the architecture: the number of hidden layers and neurons in each hidden layer. In the case of process optimization, the parameters considered are related to the process parameters: solvent, octanol addition, pH, and ALA-2 concentration.
Compared with the standard DE algorithm [
32], the variant used in this work uses a self-adaptive procedure for the control parameters. This strategy is applied to eliminate the need for manually tuning the F and CR parameters that influence the algorithm’s performance. The F parameter controls the mutation rate of the individuals in the population. CR is the crossover rate that influences how the trial individuals are generated from the current and mutated population. Overall, the DE algorithm has fewer steps: initialization, mutation, crossover, and selection. In the initialization phase, the population is randomly generated using the limits of the considered problem. The limits are previously set for the ANN problem depending on how large a model is allowed. For process optimization, the limits are the ones used in the experimental phase. The mutation and the crossover phases are the ones responsible for generating new individuals. The mutation introduces changes in the individuals, and the crossover combines the characteristics of two parents to create children. Finally, in the selection phase, the best individuals are selected to participate in the next generation. The steps mutation, crossover, and selection are repeated until a stop criterion is reached (maximum number of generations).
The general schema of the workflow of the steps performed in this work is presented in
Figure 2.
4. Conclusions
This article reports a comprehensive study on gallic acid separation including: reactive extraction, stripping, and modeling. The optimum conditions for the reactive extraction onto a biphasic organic-aqueous system were: pH of aqueous phase 2, dichloromethane used as solvent, 80 g/L Amberlite LA2 used as extractant, and 10% phase modifier (1-octanol). The extraction mechanism analysis confirmed the formation of complexes involving hydrogen bonds between 1 molecule of GA and 1 of extractant, for dichloromethane (with or without 1-octanol) and n-heptane with 10% 1-octanol, and 1:2 (acid:extractant) complexes for n-heptane. The organic phase is regenerated at a high temperature (323 K) with sodium hydroxide, allowing its simultaneous regeneration. Moreover, the process was modeled and optimized with statistical approaches and artificial intelligence tools (ANNs and DE). The results indicated that an effective model process could be further used to generate predictions and eliminate the need for additional experimental work.