Effect of Lactobacillus plantarum Fermentation on Metabolites in Lotus Leaf Based on Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Fermentation of a Water Extract from Lotus Leaves
2.3. Metabolite Extraction
2.4. LC–MS/MS Conditions
2.5. Statistical Analysis
3. Results and Discussion
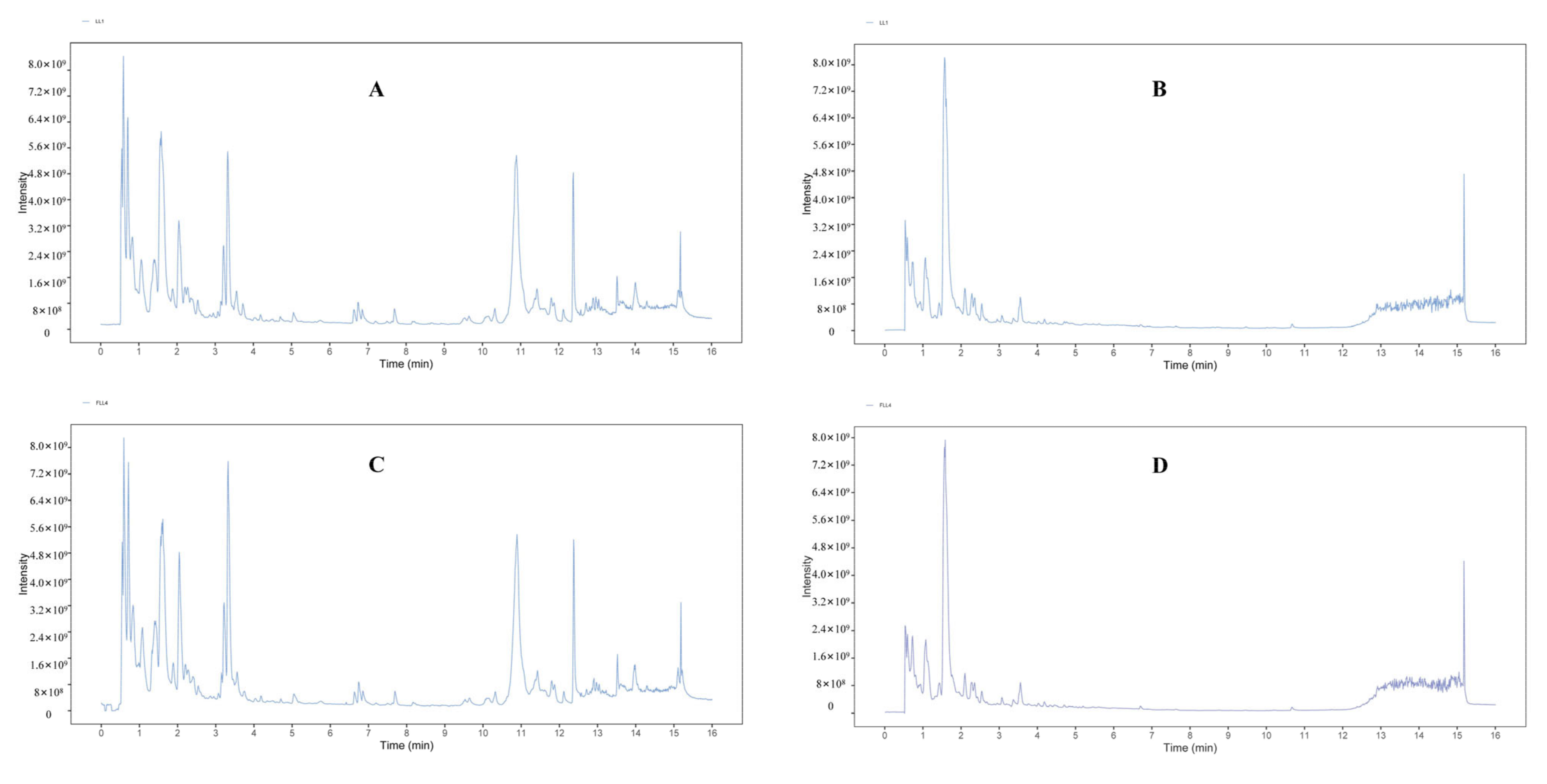
3.1. UHPLC–HR-MS Metabolic Profile Analysis
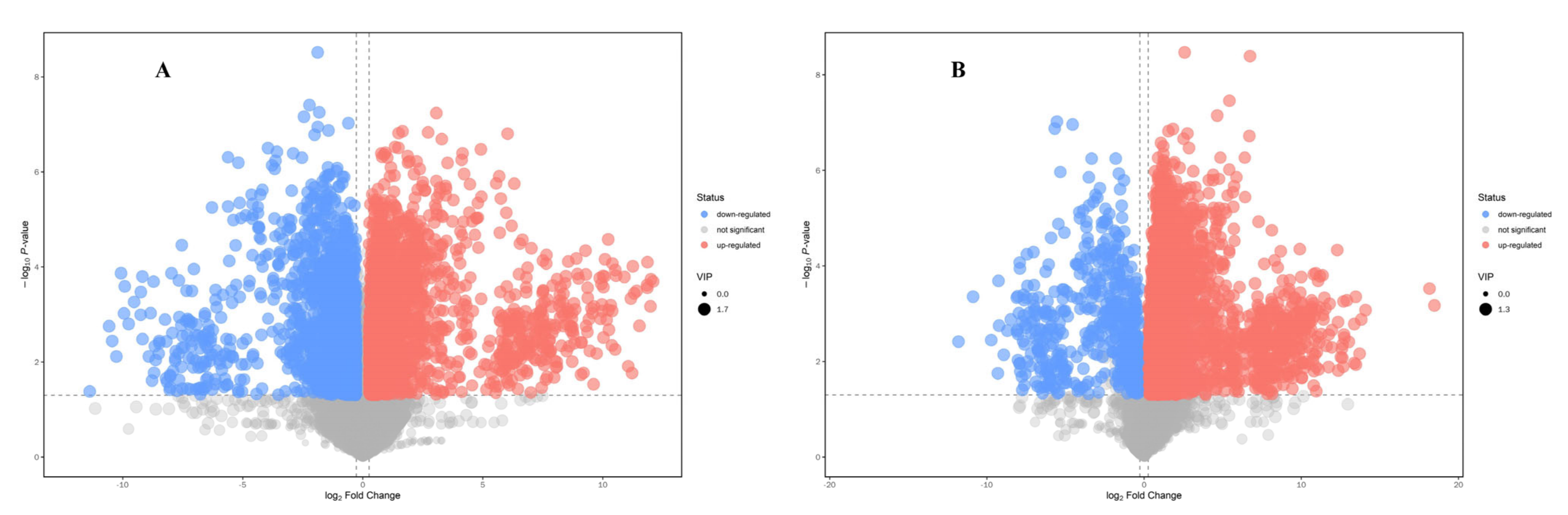
3.2. Multivariate Statistical Analysis
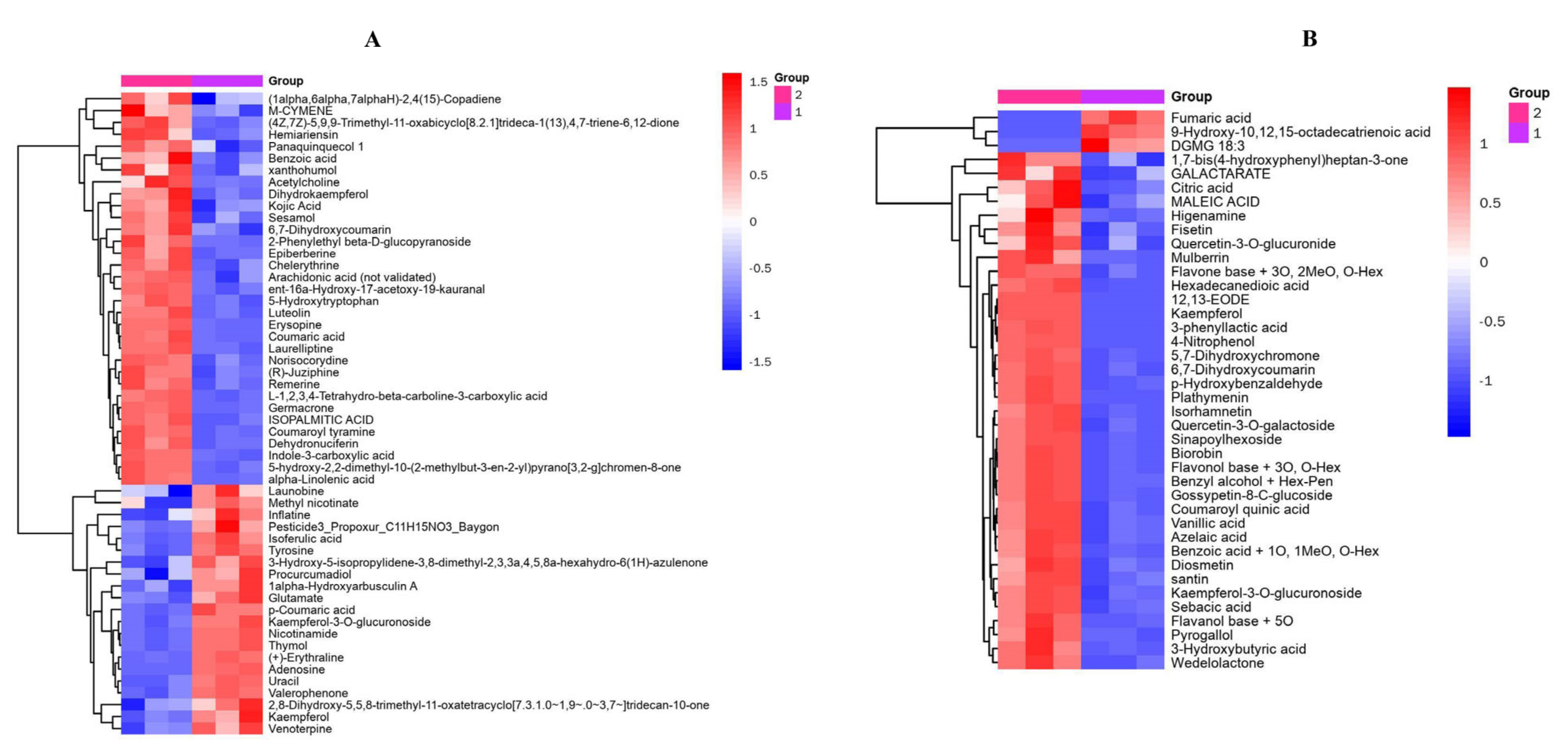
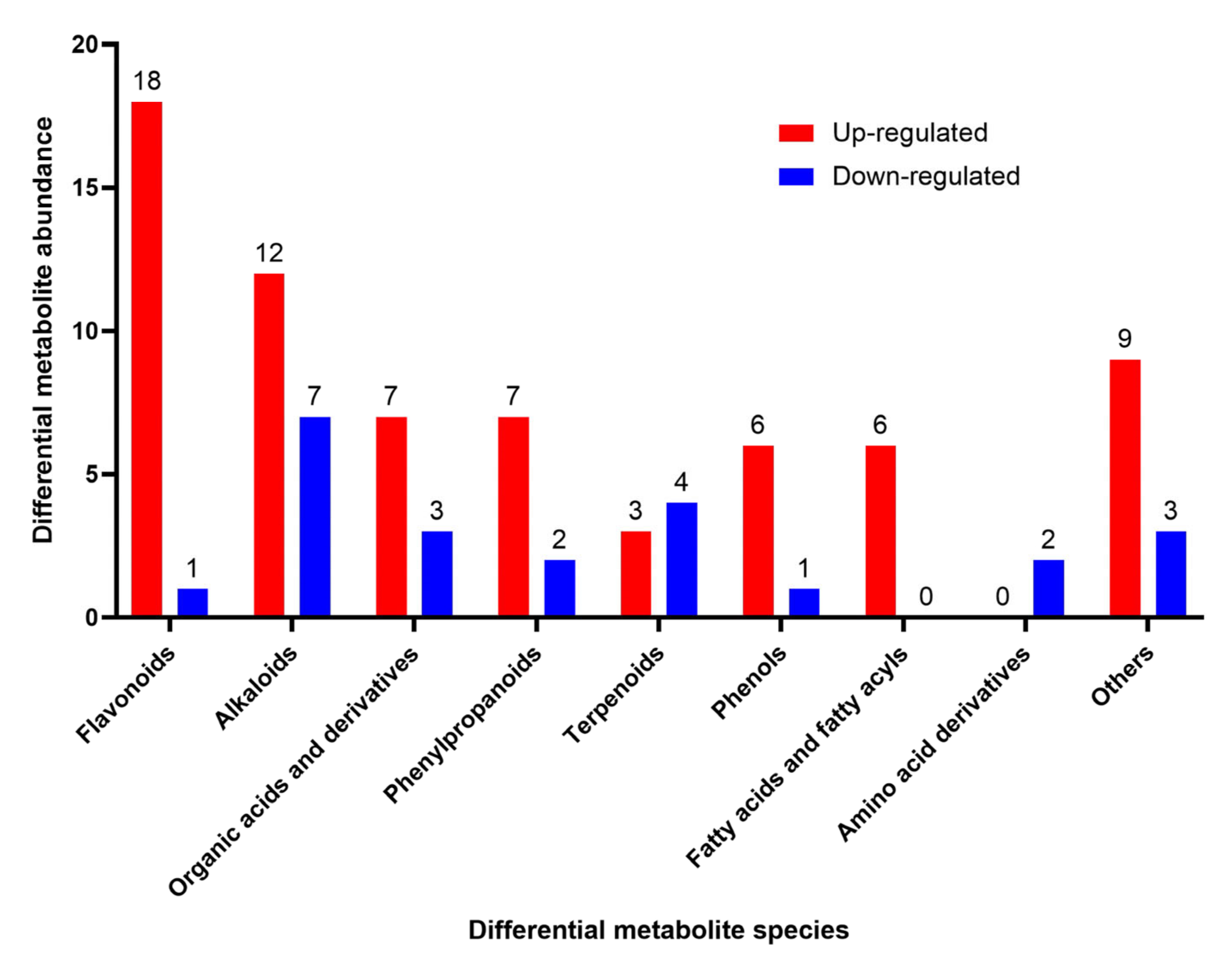
3.3. Analysis of Differential Metabolites
3.3.1. Flavonoid Compounds
3.3.2. Alkaloid Compounds
3.3.3. Organic Acids and Their Derivatives
3.3.4. Phenylpropanoid Compounds
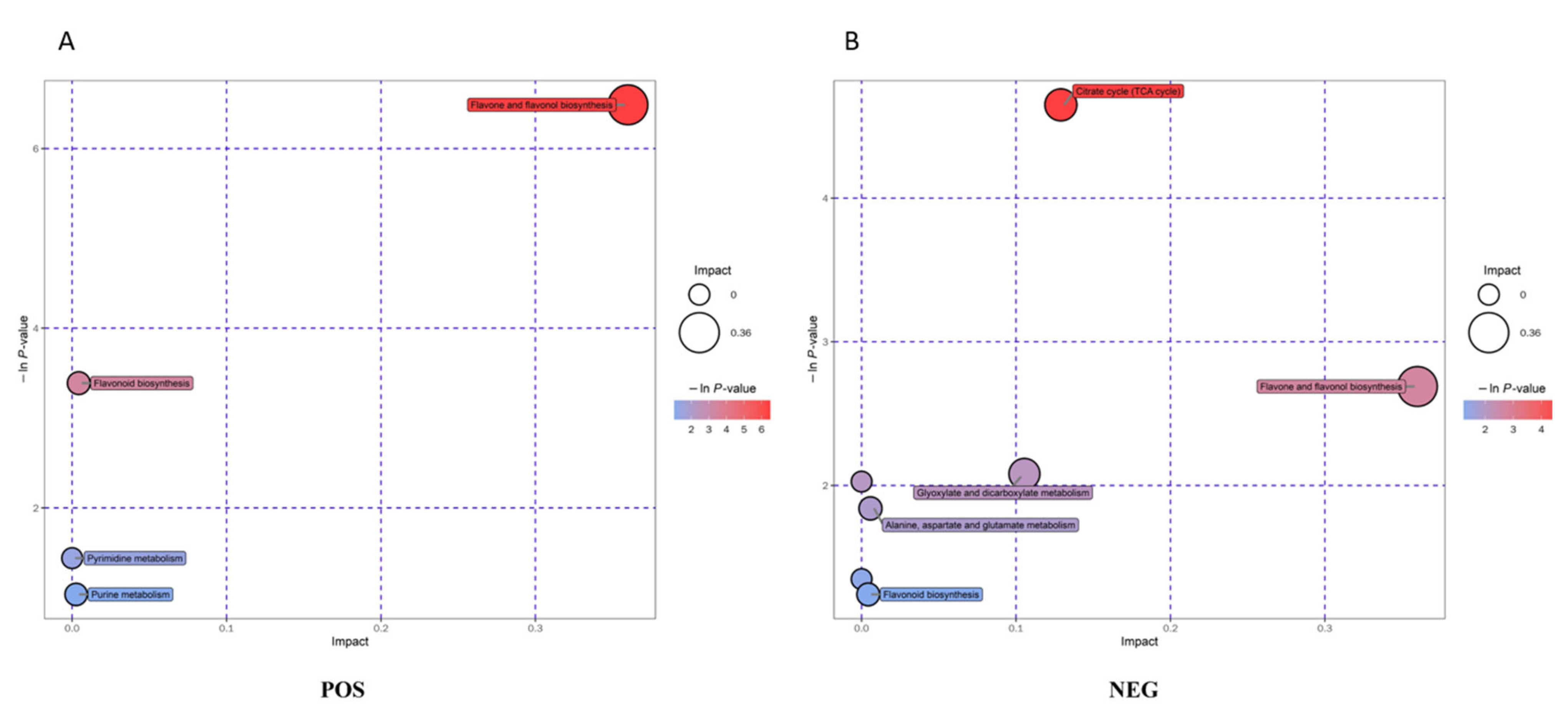
3.4. Metabolic Pathway Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, J.H.; Park, E.J.; Kim, S.H.; Lee, H.J. Gastroprotective Effects of Fermented Lotus Root against Ethanol/HCl-Induced Gastric Mucosal Acute Toxicity in Rats. Nutrients 2020, 12, 808. [Google Scholar] [CrossRef]
- Zheng, H.; Han, L.; Shi, W.; Fang, X.; Hong, Y.; Cao, Y. Research Advances in Lotus Leaf as Chinese Dietary Herbal Medicine. Am. J. Chin. Med. 2022, 50, 1423–1445. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Panth, N. Phytochemical Profile and Biological Activity of Nelumbo nucifera. Evid. -Based Complement. Altern. Med. 2015, 2015, 789124. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Patel, P.A.; Sharma, P.; Thoutireddy, S.; Das, N. Lotus (Nelumbo nucifera Gaertn.) and Its Bioactive Phytocompounds: A Tribute to Cancer Prevention and Intervention. Cancers 2022, 14, 529. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhu, M.; Guo, M. Research advances in traditional and modern use of Nelumbo nucifera: Phytochemicals, health promoting activities and beyond. Crit. Rev. Food Sci. Nutr. 2019, 59, S189–S209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, W.; Li, B.; Qian, S.; Wei, B.; Gong, S.; Wang, J.; Liu, M.; Wei, M. Nuciferine modulates the gut microbiota and prevents obesity in high-fat diet-fed rats. Exp. Mol. Med. 2020, 52, 1959–1975. [Google Scholar] [CrossRef]
- Li, C.; He, Y.; Yang, Y.; Gou, Y.; Li, S.; Wang, R.; Zeng, S.; Zhao, X. Antioxidant and Inflammatory Effects of Nelumbo nucifera Gaertn. Leaves. Oxidative Med. Cell Longev. 2021, 2021, 8375961. [Google Scholar] [CrossRef]
- Hammi, K.M.; Essid, R.; Khadraoui, N.; Ksouri, R.; Majdoub, H.; Tabbene, O. Antimicrobial, antioxidant and antileishmanial activities of Ziziphus lotus leaves. Arch. Microbiol. 2022, 204, 119. [Google Scholar] [CrossRef]
- Hwang, D.; Charchoghlyan, H.; Lee, J.S.; Kim, M. Bioactive compounds and antioxidant activities of the Korean lotus leaf (Nelumbo nucifera) condiment: Volatile and nonvolatile metabolite profiling during fermentation. Int. J. Food Sci. Technol. 2015, 50, 1988–1995. [Google Scholar] [CrossRef]
- Shukla, S.; Park, J.; Park, J.H.; Lee, J.S.; Kim, M. Development of novel Meju starter culture using plant extracts with reduced Bacillus cereus counts and enhanced functional properties. Sci. Rep. 2017, 7, 11409. [Google Scholar] [CrossRef]
- Gao, P.; Xu, G. Mass-spectrometry-based microbial metabolomics: Recent developments and applications. Anal. Bioanal. Chem. 2015, 407, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.M.; Zha, Q.L.; Chen, T.B.; Xiao, S.Y.; Xie, Y.; Luo, P.; Wang, Y.P.; Liu, L.; Zhou, H. Discovery of markers for discriminating the age of cultivated ginseng by using UHPLC-QTOF/MS coupled with OPLS-DA. Phytomedicine 2018, 45, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Wu, Q.; Battino, M.; Bai, W.; Tian, L. Using untargeted metabolomics to profile the changes in roselle (Hibiscus sabdariffa L.) anthocyanins during wine fermentation. Food Chem. 2021, 364, 130425. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, T.; Melzig, M.F. Polyphenolic Compounds as Pancreatic Lipase Inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Drouet, S.; Hano, C. Phytochemical Diversity and Antioxidant Potential of Natural Populations of Nelumbo nucifera Gaertn. throughout the Floristic Regions in Thailand. Molecules 2022, 27, 681. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Lei, J.; Zhong, J.; Wang, B.; Wan, Y.; Li, J.; Liao, C.; He, Y.; Liu, Z.; Ito, K.; et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J. Nutr. Biochem. 2022, 99, 108840. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, Z.; Li, X.; Zhang, X. Kaempferol’s Protective Effect on Ethanol-Induced Mouse Primary Hepatocytes Injury Involved in the Synchronous Inhibition of SP1, Hsp70 and CYP2E1. Am. J. Chin. Med. 2018, 46, 1093–1110. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Sheng, H.; Liang, C.; Liu, H.; Moran Guerrero, J.A.; Lu, Z.; Mao, W.; Dai, Z.; Liu, X.; et al. Hyperoside Suppresses Renal Inflammation by Regulating Macrophage Polarization in Mice with Type 2 Diabetes Mellitus. Front. Immunol. 2021, 12, 733808. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef] [PubMed]
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from Hop: Hope for cancer prevention and treatment. IUBMB Life 2021, 73, 1016–1044. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.F.; Frank, J.; Venturelli, S.; Egert, S. Bioavailability and Cardiometabolic Effects of Xanthohumol: Evidence from Animal and Human Studies. Mol. Nutr. Food Res. 2022, 66, e2100831. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.B.; Zhang, Q.; Xu, L.; Yao, W.J.; Wei, L. Lotus leaf flavonoids induce apoptosis of human lung cancer A549 cells through the ROS/p38 MAPK pathway. Biol. Res. 2021, 54, 7. [Google Scholar] [CrossRef]
- Zhou, G.; Feng, X.; Tao, A. Explore the Lipid-Lowering and Weight-Reducing Mechanism of Lotus Leaf Based on Network Pharmacology and Molecular Docking. Evid. -Based Complement. Altern. Med. 2021, 2021, 1464027. [Google Scholar] [CrossRef]
- Zhang, N.; Lian, Z.; Peng, X.; Li, Z.; Zhu, H. Applications of Higenamine in pharmacology and medicine. J. Ethnopharmacol. 2017, 196, 242–252. [Google Scholar] [CrossRef]
- Rangelov Kozhuharov, V.; Ivanov, K.; Ivanova, S. Higenamine in Plants as a Source of Unintentional Doping. Plants 2022, 11, 354. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; He, Y. Multifunctional epiberberine mediates multi-therapeutic effects. Fitoterapia 2020, 147, 104771. [Google Scholar] [CrossRef]
- Zou, Z.Y.; Hu, Y.R.; Ma, H.; Feng, M.; Li, X.G.; Ye, X.L. Epiberberine reduces serum cholesterol in diet-induced dyslipidemia Syrian golden hamsters via network pathways involving cholesterol metabolism. Eur. J. Pharmacol. 2016, 774, 1–9. [Google Scholar] [CrossRef]
- Zarev, Y.; Foubert, K.; Cos, P.; Maes, L.; Elgorashi, E.; Apers, S.; Ionkova, I.; Pieters, L. HPLC-DAD-SPE-NMR isolation of tetracyclic spiro-alkaloids with antiplasmodial activity from the seeds of Erythrina latissima. Nat. Prod. Res. 2020, 34, 1037–1040. [Google Scholar] [CrossRef]
- Mu, W.; Yu, S.; Zhu, L.; Zhang, T.; Jiang, B. Recent research on 3-phenyllactic acid, a broad-spectrum antimicrobial compound. Appl. Microbiol. Biotechnol. 2012, 95, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Gerez, C.L.; Carbajo, M.S.; Rollan, G.; Torres Leal, G.; Font de Valdez, G. Inhibition of citrus fungal pathogens by using lactic acid bacteria. J. Food Sci. 2010, 75, M354–M359. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, N.; Salgado, J.M.; Cortés, S.; Domínguez, J.M. Antimicrobial activity of d-3-phenyllactic acid produced by fed-batch process against Salmonella enterica. Food Control 2012, 25, 274–284. [Google Scholar] [CrossRef]
- Marques, E.S.; Salles, D.B.; Maistro, E.L. Assessment of the genotoxic/clastogenic potential of coumarin derivative 6,7-dihydroxycoumarin (aesculetin) in multiple mouse organs. Toxicol. Rep. 2015, 2, 268–274. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Lin, B.; Li, Z. Effect of Lactobacillus plantarum Fermentation on Metabolites in Lotus Leaf Based on Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry. Fermentation 2022, 8, 599. https://doi.org/10.3390/fermentation8110599
Wang Y, Lin B, Li Z. Effect of Lactobacillus plantarum Fermentation on Metabolites in Lotus Leaf Based on Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry. Fermentation. 2022; 8(11):599. https://doi.org/10.3390/fermentation8110599
Chicago/Turabian StyleWang, Yubao, Bingjun Lin, and Zhengxu Li. 2022. "Effect of Lactobacillus plantarum Fermentation on Metabolites in Lotus Leaf Based on Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry" Fermentation 8, no. 11: 599. https://doi.org/10.3390/fermentation8110599
APA StyleWang, Y., Lin, B., & Li, Z. (2022). Effect of Lactobacillus plantarum Fermentation on Metabolites in Lotus Leaf Based on Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry. Fermentation, 8(11), 599. https://doi.org/10.3390/fermentation8110599




