Abstract
Cytidine is an antiviral and anticancer drug intermediate, its primary method of manufacture being fermentation. Uridine-cytidine kinase (UCK) catalyzes the reverse process of phosphorylation of cytidine to produce cytidylic acid, which influences cytidine accumulation in the Escherichia coli cytidine biosynthesis pathway. The cytidine-producing strain E. coli NXBG-11 was used as the starting strain in this work; the udk gene coding UCK was knocked out of the chromosomal genome using clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 technology. The mutant strain E. coli NXBG-12 was obtained; its transcriptomics were studied to see how udk gene deletion affected cytidine synthesis and cell-wide transcription. The mutant strain E. coli NXBG-12 generated 1.28 times more cytidine than the original strain E. coli NXBG-11 after 40 h of shake-flask fermentation at 37 °C. The udk gene was knocked out, and transcriptome analysis showed that there were 1168 differentially expressed genes between the mutant and original strains, 559 upregulated genes and 609 downregulated genes. It was primarily shown that udk gene knockout has a positive impact on the cytidine synthesis network because genes involved in cytidine synthesis were significantly upregulated (p < 0.05) and genes related to the cytidine precursor PRPP and cofactor NADPH were upregulated in the PPP and TCA pathways. These results principally demonstrate that udk gene deletion has a favorable impact on the cytidine synthesis network. The continual improvement of cytidine synthesis and metasynthesis is made possible by this information, which is also useful for further converting microorganisms that produce cytidine.
1. Introduction
As a pyrimidine nucleoside, cytidine is a structural component of RNA in organisms that are involved in several cellular processes [1]. Cytidine has recently been a research hotspot, primarily as an intermediate in various antiviral, anticancer, and anti-AIDS medicines [2,3,4]. As the market demand for these pharmaceuticals grows, so does the supply of cytidine, emphasizing the importance of developing a cost-effective and environmentally friendly cytidine production process [5]. Cytidine is presently generated using microbial fermentation, which has the advantages of low cost, simple conditions, high production, short cycle time, and easy control, among other things [6]. Bacillus subtilis is frequently chosen as the foundation strain in traditional breeding techniques to create a nucleoside-producing strain [7,8]. In contrast to B. subtilis, each gene in Escherichia coli is controlled by a separate mechanism. Furthermore, because of its distinct genetic background, E. coli has emerged as a new industrial production strain, progressively displacing B. subtilis and being widely employed for large-scale synthesis of amino acids, nucleotides, vitamins, and fatty acids [9,10,11,12,13].
The development of high-yield strains is essential for effective cytidine production; the methods used mostly include random mutation, directed evolution, and genetic engineering [14,15,16]. Some studies choose cytidine-producing bacteria by mutagenesis screening of structural analogue-resistant strains; nevertheless, mutagenic strains frequently acquire negative mutations, and strain improvement is restricted [17,18]. In recent years, it has become increasingly promising to build optimum cytidine-generating strains through rational metabolic engineering [19]. Haitian Fang et al. [20], in shaker-flask culture, overexpressed prs, zwf, and gnd in E. coli CYT15 to boost intracellular metabolites in the pentose phosphate pathway (PPP) and pyrimidine biosynthesis pathway, boosting cytidine production by 128% to around 735 mg/L, compared to the original strain (CYT15). Yang et al. [21] conducted batch-fed fermentation in a 5 L reactor by deleting genes related to cytidine catabolism and overexpressing genes related to the cytidine synthesis pathway in E. coli MG1655, boosting the titre of cytidine by 11.13-fold to 7.84 g/L in 48 h. Wang et al. [22] created a strain of B. subtilis with a high uridine yield by metabolically engineering the UMP biosynthesis pathway, which resulted in a uridine yield of 11.03 g/L.
E. coli NXBG-12 was obtained in this investigation by knocking out udk in E. coli NXBG-11 (Figure 1A). The fermentation data revealed that after udk knockout, cytidine production was marginally increased. RNA-Seq has been proven to be an effective strategy to investigate gene activity [23,24,25]. Therefore, we conducted transcriptome analysis on the starting strain E. coli NXBG-11 and the mutant strain E. coli NXBG-12, delving into the pyrimidine metabolism, central carbon metabolism, and cofactor metabolism involved in cytidine production. The study of cytidine biosynthesis is expected to elucidate the transcriptional mechanism of cytidine biosynthesis and provide possible intervention strategies for the production of cytidine.
Figure 1.
Schematic diagram of relevant experimental processes of this study. (A) Strategy of strain transformation used in this study. (B) Shake-flask fermentation experiment. (C) Determination of cytidine concentration by HPLC. (D) Transcriptome analysis process.
2. Materials and Methods
2.1. Strains, Primers, and Plasmids
All strains and plasmids used in this study are listed in Table S1. Details regarding primer pairs are listed in Table S2. Primers were synthesized, and sequencing reactions were completed by Sangon Biotech (Shanghai, China). Specifically, E. coli DH5α was used for all recombinant genetic manipulations as the host, whereas E. coli NXBG-11 was used as the host strain. Strains were cultured in Luria–Bertani (LB) medium at 37 °C with vigorous shaking (220 rpm). When necessary, the medium was supplemented with 30 μg/mL kanamycin and 55 μg/mL chloramphenicol to maintain the plasmid. Seed medium (10 g/L peptone, 5 g/L glucose, 2 g/L (NH4)2SO4, 10 g/L NaCl) and fermentation medium (50 g/L glucose, 1.8 g/L (NH4)2SO4, 20 mL/L corn syrup, 10 mg/L FeSO4·7H2O, 10 g/L K2HPO4, 0.5 g/L KH2PO4, 0.4 g/L MgSO4·7H2O, 1.5 μg/L MnSO4·H2O, 0.1% Phenol red, 20 mL/L, pH 7.2) were used.
2.2. Genetic Manipulation
The CRISPR/Cas9 gene-editing system was applied to knock out the udk gene in E. coli NXBG-11 [26,27]. Chromosomal DNA was isolated from E. coli K-12 MG1655 (NC_000913.3) and used as a PCR template to amplify udk. Sequencing and verification results after udk knockout are shown in Table S3 and Figure S1. First, to amplify the udk gene, the plasmid pTargetF was utilized as a template, and the primers gRNA-udk-S and gRNA-udk-A were used. Following the generation of competent E. coli DH5α cells, the target gene DNA for transformation was introduced, the transformants were developed after culture, and the presence of the knockout-targeting recombinant plasmid pTargetF-udk confirmed. Then, using the primers udk-up-S/udk-up-A and udk-down-S/udk-down-A, the upstream homologous arm segment M1 and downstream homologous arm segment M2 were amplified from E. coli NXBG-11 genomic DNA. The donor DNA was acquired by using overlapping extension (SOE) PCR to join M1 and M2. The pCas plasmid was then introduced into competent E. coli NXBG-11 cells, L-arabinose was given to stimulate the pCas plasmid to produce red recombinant protein, and proper positive recombinants were screened by colony PCR. The plasmid pTargetF-udk and knockout DNA pieces were electrotransformed into E. coli NXBG-11 competent cells; the cultivated bacterial suspension was uniformly coated on a plate with spectinomycin and kanamycin resistance, and grown overnight at 30 °C. When a single colony formed on the plate, the primer udk-S/udk-A was utilized for colony PCR verification to get a positive clone single colony. Finally, it was inoculated into an LB test tube, a specific dosage of IPTG and kanamycin was added, and it was grown overnight at 42 °C to destroy the mutant’s temperature-sensitive pCas plasmid; the strain with the right band was E. coli NXBG-12 mutant.
2.3. Shake-Flask Fermentation
First, a seed culture was prepared by inoculating 5 mL of medium with a single colony from a fresh LB agar plate. The cells were then inoculated into a 220 mL flask containing 30 mL of seed medium and incubated for 8–10 h at 37 °C at 220 rpm. Three millilitres of this culture were transferred to a 500 mL baffled flask containing 50 mL of production medium and incubated at 37 °C with vigorous shaking (220 rpm) for 40 h for flask cultures. The cultures were taken and examined at regular intervals. Cell density was estimated by measuring optical density at 600 nm with a spectrophotometer. A biosensor analyzer was used to measure the glucose concentration. Cytidine content was measured by HPLC, using water/methyl cyanide (96:4 v/v) as the mobile phase at 1 mL/min and detection at 270 nm (Figure 1C).
2.4. RNA-Seq Analysis
Total RNA was extracted from recombinant E. coli NXBG-11 and wild E. coli NXBG-12 using an appropriate amount of bacterial solution, according to the TIANGEN kit’s instructions. Please refer to the text for precise operation instructions [28]. Transcriptome sequencing was completed on the Illumina sequencing platform of Majorbio (Shanghai, China) (Figure 1D). The obtained sequences were mapped to the reference genome of E. coli K-12 MG1655 (NCBI Reference Sequence: NC_000913.3). The sequenced image signal is converted into text signal by CASAVA base calling, and stored in fasta format as the original data. The software used is based on Burrows Wheeler method to compare the high-quality sequence obtained after quality control with the designated reference genome. The quantitative analysis of gene and transcript expression levels was performed using the expression quantitative software RSEM, and the quantitative indicator was TPM. The criteria for identifying differences in gene expression between various samples were |log2Ratio| ≥ 1 and p-value ≤ 0.05. The KEGG and GO databases were used to perform functional annotation on the genes with significant differential expression.
3. Results and Discussion
3.1. Effect of udk Deletion on Cytidine Production
To achieve the accumulation of cytidine, the uridine-cytidine kinase encoded by the gene udk was successively knocked out to block the generation of cytidylic acid after the reverse reaction of cytidine phosphorylation. To investigate the effect of the produced E. coli NXBG-12 gene deletion mutant strain on cytidine synthesis by fermentation, the strain and the starting strain E. coli NXBG-11 were treated to 40 h of shake-flask fermentation (Figure 1B). During the fermentation process, samples were obtained every 4 h to identify the bacterial cytidine content and cell density. As may be seen in Figure 2A, E. coli NXBG-12 grew somewhat faster than the initial strain E. coli NXBG-11, demonstrating that the deletion of the udk gene had no effect on bacterial growth. This is consistent with previous reports [21]. The rate of glucose intake increased in 8~20 h (Figure 2B), possibly due to the fast growth and cytidine production of bacteria utilizing glucose in the fermentation broth. The mutant strain’s sugar consumption was 1.08 times that of the original strain and its cytidine content was 1.28 times that of the original strain (Figure 2C). After 40 h of fermentation, the cytidine content reached 2.1 g/L and its glycoside conversion rate was 1.19 times that of the original strain (Table 1), indicating that it was significant for the accumulation of cytidine by cutting off the reverse reaction of cytidine to produce CMP. Although the cytidine titre of E. coli NXBG-12 in this study was lower than that of some improved strains, the growth of E. coli NXBG-12 was not greatly affected, indicating its potential for further reconstruction. and other unknown cytidine catabolic pathways may exist.
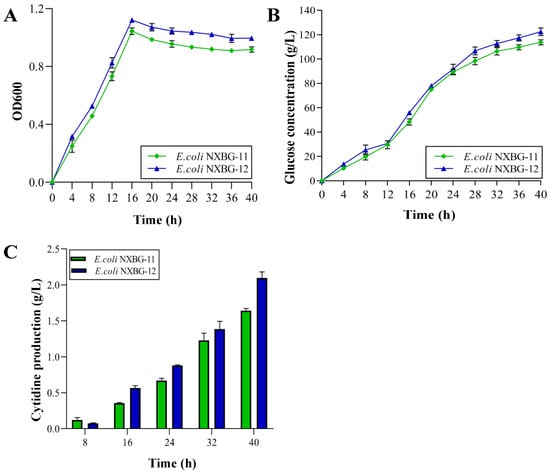
Figure 2.
Effect of udk deletion on cytidine production. (A) Cell growth. (B) Glucose consumption. (C) Yield of cytidine.

Table 1.
Cytidine production in recombinant E. coli strains.
3.2. Transcriptome Analysis of the Influence of Gene Deletion on the Cytidine-Producing Strain
The total RNA was isolated with high purity from the aforesaid wild E. coli and recombinant strains and the transcriptome sequenced using an Illumina HiSeq system. To achieve clean reads, the original data were filtered by quality control. The sequence quality evaluation values Q20 were all >98%, Q30 were all >95%, and the error rates were all <0.235, suggesting that the sequencing data were of high quality and dependable, and could be used for further information analysis. Specific statistics can be found in Table S4. Clean reads of six samples after quality control and comparison with the reference genome E. coli K12 MG1655 revealed that, on average, 99.08% of the three E. coli NXBG-11 samples matched the reference genome and 98.1% matched the unique site of the reference genome. On average, 77.75% of the three mutant strain E. coli NXBG-12 samples matched the reference genome, and 76.53% matched the unique site of the reference genome, indicating that the sequencing data are reliable (Table S5).
In order to understand the global effect of udk deletion on cytidine synthesis after gene knockout, the logarithmic RNA of the strain was isolated for RNA-Seq analysis. The starting strain E. coli NXBG-11 and mutant E. coli NXBG-12 are separated into two different groups with strong intra-group correlation, which is compatible with the experimental predictions, according to the study of the correlation between samples based on the sequencing data (Figure 3A). The sequencing findings can then be utilized for transcriptome analysis. The differentially expressed genes were filtered from the sequencing library of the initial bacterium and mutant bacterium using p < 0.05. As a consequence, a volcanic map (Figure 3B) was created, with red representing strongly upregulated genes and green representing considerably downregulated genes. In comparison to the original strain E. coli NXBG-11, mutant E. coli NXBG-12 has 1168 significantly differentially expressed genes, 559 upregulated and 609 downregulated, indicating that udk gene knockout in E. coli has a significant impact on the overall gene transcription level.
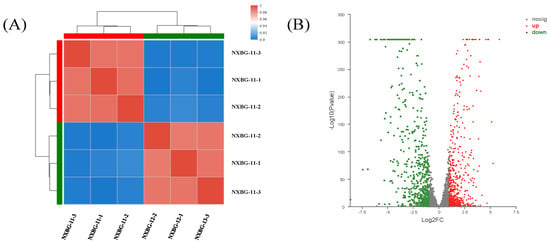
Figure 3.
(A) Sample correlation analysis. Squares with different colors represent correlation between the two samples. (B) DEG volcano map. The abscissa is the multiple change value of gene expression difference between two groups of samples, namely FC value. The ordinate is the statistical test value of the difference in gene expression changes, namely the p-value.
The differentially expressed genes of the mutant strains were subjected to GO enrichment analysis, and a histogram was created (Figure 4). Significant differences in up- and downregulated genes in the GO database are mostly categorized into biological process, cellular processes, and metabolic processes. In biological processes, differentially expressed genes are mainly involved in cell processes, metabolic processes, and response to stimulus; in cell process, differentially expressed genes are mainly annotated to cell parts, membranes, and membrane part and in molecular function, differentially expressed genes are annotated to catalytic activity, binding and transporter activity. According to the data, genes involved in cell functions and metabolic processes are expressed in E. coli NXBG-11 and E. coli NXBG-12. The changes in E. coli NXBG-12 are large, demonstrating that udk deletion impacts several metabolic pathways in cells.
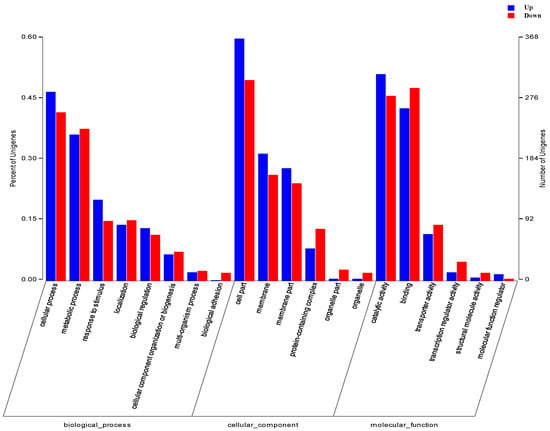
Figure 4.
GO enrichment analysis histogram of DEGs. The abscissa represents the three branches of GO, namely biological processes, cell components, and molecular functions, and further level 2 classification; the ordinate represents the relative proportion of genes.
We may extract the metabolic processes or signal pathways, in which differentially expressed genes mostly participate and investigate the metabolic pathways that may be involved in cytidine anabolism using KEGG annotation and enrichment (Figure 5). KEGG annotated 193 upregulated and 212 downregulated genes based on metabolic pathway analysis of 559 genes significantly upregulated and 609 genes downregulated. They were categorized into six groups, according to the results: metabolism, genetic information processing, environmental information processing, cellular processes, organic systems, and human diseases. The differentially expressed genes are primarily found in metabolism and environmental information processing; the upregulated genes are primarily found in carbohydrate metabolism, membrane transport, energy metabolism, and other pathways, while the downregulated genes are primarily found in carbohydrate metabolism, amino acid metabolism, and membrane transport.
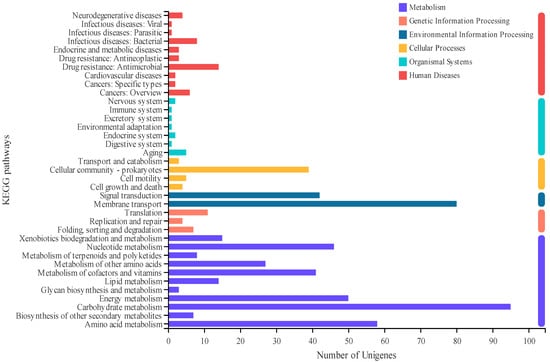
Figure 5.
KEGG histogram of DEG. The ordinate is the name of the KEGG metabolic pathway, and the abscissa is the number of genes annotated to the pathway.
These genes were further screened and investigated for pathways and genes relevant to the cytidine synthesis pathway using KEGG enrichment analysis (Table 2). Enzymes encoded by upregulated genes are primarily involved in pyrimidine metabolism, oxidative phosphorylation, the citrate cycle, and other metabolic processes; whereas enzymes encoded by downregulated genes are primarily involved in glycolysis/gluconeogenesis, pyrimidine metabolism, fructose and mannose metabolism, and other metabolic processes. All of these are linked to the cytidine production pathway. Interestingly, the genes involved in energy metabolism and nucleotide metabolism differ dramatically between the mutant and control strains. When the udk gene is knocked out, gene expression is thought to fluctuate to variable degrees. The upregulation of genes involved in energy metabolism suggests that udk deletion disrupts energy metabolism in cells. Furthermore, genes involved in nucleotide metabolism changed dramatically, which may be related to the udk-encoded uridine-cytidine kinase, which is involved in nucleotide metabolism.

Table 2.
KEGG pathway functional annotation of differentially expressed genes.
3.3. Effect of Deleting udk on Pyrimidine Metabolism
Transcription analysis revealed that, as compared to the initial strain, the genes involved in the pyrimidine biosynthesis pathway of mutant strains were considerably upregulated (Figure 6), demonstrating that udk deletion has a favorable influence on pyrimidine synthesis. The transcription level of numerous critical operons was upregulated during de novo pyrimidine biosynthesis, and reducing UMP and CMP production would lessen the feedback inhibition of these operons to some extent. The enzyme encoded by pyrBI catalyzes the formation of carbamoyl phosphate from aspartic acid, a critical step in the de novo synthesis of pyrimidines [29]. Its expression was increased, which could be due to increased TCA cycle activity, which strengthens aspartic acid synthesis, or it could be due to the deletion of the udk gene, which blocks the reverse reaction of uridine to produce UMP [30], or the downregulation of the cytidine kinase-encoding gene cmk. Furthermore, the expression of ndk, which encodes nucleoside diphosphate kinase, and pyrH, which encodes uridine kinase, was increased upstream of cytidine production. As a nucleoside transporter, the product of nupC is primarily responsible for the transport of pyrimidine nucleosides, which is critical for maintaining pyrimidine nucleoside levels both within and outside cells. The nucleotide hydrolase produced by the rihB gene will degrade cytidine into cytosine, which is not favorable for cytidine accumulation and will also result in waste of precursor materials, which may be one of the reasons why cytidine synthesis does not rise considerably after udk deletion.
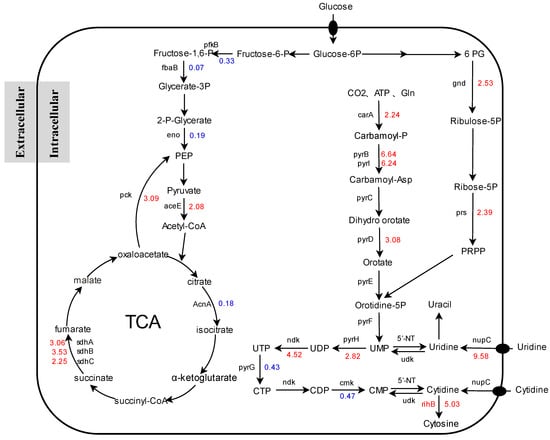
Figure 6.
Schematic diagram of gene expression involved in cytidine synthesis-related pathways. Numbers represent the difference multiple of a gene between E. coli NXBG-11 and E. coli NXBG-12, blue representing downward adjustment and red representing upward adjustment.
3.4. Effect of Deleting udk on the Central Carbon Metabolism Pathway
The primary source of energy for bacteria is central carbon metabolism (CCM). The glycolysis route (EMP), tricarboxylic acid cycle (TCA), and PPP have always been included. As a branch of glucose metabolism, the PPP can provide bacteria with NADPH and the pyrimidine nucleoside precursor PRPP [31]. The transcriptional levels of gnd and prs, two key alteration genes in this pathway, were dramatically increased. gnd encodes the most important enzyme in the non-oxidative PPP; prs encodes a ribose phosphate diphosphate kinase that can catalyse R5P to create the cytidine precursor PRPP, which is a key regulatory step in cytidine production [32]. Overexpression has been reported in several studies. As a result, the upregulation of gnd and prs potentially increases the supply of PRPP and stimulates cytidine production. The TCA cycle is a critical mechanism in microbial carbon catabolism [33]. sdhABCD encodes four subunits of succinate dehydrogenase, which catalyzes the conversion of succinic acid to fumaric acid and is upregulated in mutant strains, leading to an increased TCA cycle at succinate nodes [34]. The EMP pathway is an essential link in E. coli carbohydrate metabolism that produces adenosine triphosphate (ATP) for cell growth and metabolism. However, transcription of genes involved in the EMP pathway, such as pfkB, fbaB, and eno, was considerably reduced. The downregulation of genes in the EMP pathway may result in an imbalance of carbon metabolism or cofactors, resulting in delayed cell growth or the build-up of by-products, therefore affecting cytidine production. The imbalance of CCM of mutant strains may be one of the reasons for their slow growth rate.
3.5. Effect of Deleting udk on Reduction of Cofactor Synthesis
The balance of cofactors in microbial cells is critical for sustaining normal cell metabolism. According to research, PPP produces 35–45% of NADPH in E. coli [35]. As a result, the increased expression of gnd and prs in the PPP pathway may be more favorable for the provision of cofactors and PRPP. Furthermore, TCA cycle improvement can swiftly convert NADH to NADPH, providing more NADPH for cytidine production. However, ATP demand and supply are also important in the process of cell development and metabolism. According to this study, the downregulation of EMP pathway genes may influence ATP generation, reducing cell growth and metabolism. Although numerous studies have demonstrated that increasing NADPH supply may boost target chemical production in E. coli, an imbalance of cofactor concentration leading to an imbalance of intracellular redox reaction is also detrimental to host strain development and fermentation efficiency. Typically, one or more genes are not knocked out sufficiently to accomplish the desired results because carbon/nitrogen metabolism or cofactor metabolism may be out of balance, resulting in delayed cell growth or the build-up of by-products [36]. As a result, further research into ways to decrease by-product accumulation during cytidine production while still balancing intracellular redox metabolism is required.
4. Conclusions
CRISPR/Cas9 gene-editing technology was employed in this study to knock out the uridine-cytidine kinase-expressing gene udk in the original strain E. coli NXBG-11, resulting in mutant E. coli NXBG-12. The findings of shake-flask fermentation revealed that the mutant strain produced 1.28 times more cytidine than the parent strain. The yield of cytidine can reach 2.1 g/L. Transcription study showed that the pyrimidine production pathway genes in mutant strains were highly upregulated, which is theoretically conducive to the build-up of cytidine. Furthermore, certain genes associated with CCM in the PPP and TCA cycles were upregulated, thereby raising the cytidine synthesis precursor PRPP and cofactor NADPH. The balance of intracellular redox reactions can be reached in the future by modulating redox-related enzymes and transcription factors. In conclusion, this study’s fermentation analysis of single-gene knockout strains, global transcription analysis of the intracellular carbon metabolic network, and intracellular cofactor level provide new methods and ideas for re-regulating the metabolic network of E. coli and improving E. coli’s ability to produce cytidine.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation8110586/s1, Figure S1: Verification results after the removal of udk; Table S1: Plasmids and strains used in this experiment; Table S2: Primers used in this experiment; Table S3: Sequencing results after udk knockout; Table S4: Sequencing data quality control comparison results; Table S5: Sequencing data quality control comparison results.
Author Contributions
Conceptualization, F.L., H.L. and H.F.; methodology, F.L., T.Y. and X.Z.; formal analysis, F.L. and X.Z.; investigation, F.L., T.Y. and C.M.; writing—original draft preparation, F.L., H.L. and H.F.; writing—review and editing, F.L., H.L. and H.F.; project administration, H.L.; funding acquisition, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China grant number 31560445, 31860020; Ningxia Hui Autonomous Region Youth Top Talent Training Project grant number 022004000010; and Ningxia Key Laboratory of Food Microbial Application Technology and Safety Control Platform Construction Project grant number 2019YDDF0062, 2021JCTJ0030, 2021DPC05003.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dong, H.; Liu, Y.; Zu, X.; Li, N.; Li, F.; Zhang, D. An enzymatic assay for high-throughput screening of cytidine-producing microbial strains. PLoS ONE 2015, 10, e0121612. [Google Scholar] [CrossRef] [PubMed]
- Galmarini, C.M.; Jordheim, L.; Dumontet, C. Pyrimidine nucleoside analogs in cancer treatment. Expert Rev. Anticancer. Ther. 2003, 3, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Jordheim, L.P.; Durantel, D.; Zoulim, F.; Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013, 12, 447–464. [Google Scholar] [CrossRef]
- Iglesias, L.E.; Lewkowicz, E.S.; Medici, R.; Bianchi, P.; Iribarren, A.M. Biocatalytic approaches applied to the synthesis of nucleoside prodrugs. Biotechnol. Adv. 2015, 33, 412–434. [Google Scholar] [CrossRef] [PubMed]
- Garavaglia, M.; Rossi, E.; Landini, P. The pyrimidine nucleotide biosynthetic pathway modulates production of biofilm determinants in Escherichia coli. PLoS One 2012, 7, e31252. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, H.; Fang, H.; He, J.; He, X.; Yu, L. The New Strategy of Breeding Cytidine Excessive Biosynthesis Mutants by pyr Operon Rearrangement of Bacillus amyloliquefaciens. In Advances in Applied Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 649–656. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Liu, L.; Ban, R. Improve uridine production by modifying related metabolic pathways in Bacillus subtilis. Biotechnol. Lett. 2020, 42, 551–555. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, S.-M.; Yuan, Z.-M.; Ban, R. Metabolic and genetic factors affecting the productivity of pyrimidine nucleoside in Bacillus subtilis. Microb. Cell Factories 2015, 14, 1–12. [Google Scholar] [CrossRef]
- Chen, L.; Chen, M.; Ma, C.; Zeng, A.-P. Discovery of feed-forward regulation in L-tryptophan biosynthesis and its use in metabolic engineering of E. coli for efficient tryptophan bioproduction. Metab. Eng. 2018, 47, 434–444. [Google Scholar] [CrossRef]
- You, J.; Yang, C.; Pan, X.; Hu, M.; Du, Y.; Osire, T.; Yang, T.; Rao, Z. Metabolic engineering of Bacillus subtilis for enhancing riboflavin production by alleviating dissolved oxygen limitation. Bioresour. Technol. 2021, 333, 125228. [Google Scholar] [CrossRef]
- Shimaoka, M.; Kawasaki, H.; Takenaka, Y.; Kurahashi, O.; Matsui, H. Effects of edd and pgi disruptions on inosine accumulation in Escherichia coli. Biosci. Biotechnol. Biochem. 2005, 69, 1248–1255. [Google Scholar] [CrossRef][Green Version]
- Liu, M.; Fu, Y.; Gao, W.; Xian, M.; Zhao, G. Highly efficient biosynthesis of hypoxanthine in Escherichia coli and transcriptome-based analysisof the purine metabolism. ACS Synth. Biol. 2020, 9, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Orive-Milla, N.; Delmulle, T.; de Mey, M.; Faijes, M.; Planas, A. Metabolic engineering for glycoglycerolipids production in E. coli: Tuning phosphatidic acid and UDP-glucose pathways. Metab. Eng. 2020, 61, 106–119. [Google Scholar] [CrossRef]
- Fang, H.; Liu, H.; Chen, N.; Zhang, C.; Xie, X.; Xu, Q. Site-directed mutagenesis studies on the uridine monophosphate binding sites of feedback inhibition in carbamoyl phosphate synthetase and effects on cytidine production by Bacillus amyloliquefaciens. Can. J. Microbiol. 2013, 59, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Asahi, S.; Tsunemi, Y.; Izawa, M.; Doi, M. Cytidine Production by Mutants of Bacillus subtilis. Biosci. Biotechnol. Biochem. 1994, 58, 1399–1402. [Google Scholar] [CrossRef]
- Asahi, S.; Tsunemi, Y.; Izawa, M.; Doi, M. A 3-deazauracil-resistant mutant of Bacillus subtilis with increased production of cytidine. Biosci. Biotechnol. Biochem. 1995, 59, 915–916. [Google Scholar] [CrossRef][Green Version]
- Doi, M.; Tsunemi, Y.; Asahi, S. Opimization of Conditions for Production of Uridine by a Mutant of Bacillus subtilis. J. Agric. Chem. Soc. Jpn. 2014, 58, 1608–1612. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, C.; Xie, X.; Xu, Q.; Zhou, Y.; Chen, N. Enhanced cytidine production by a recombinant Escherichia coli strain using genetic manipulation strategies. Ann. Microbiol. 2014, 64, 1203–1210. [Google Scholar] [CrossRef]
- Fan, X.; Wu, H.; Li, G.; Yuan, H.; Zhang, H.; Li, Y.; Xie, X.; Chen, N. Improvement of uridine production of Bacillus subtilis by atmospheric and room temperature plasma mutagenesis and high-throughput screening. PLoS ONE 2017, 12, e0176545. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Xie, X.; Xu, Q.; Zhang, C.; Chen, N. Enhancement of cytidine production by coexpression of gnd, zwf, and prs genes in recombinant Escherichia coli CYT15. Biotechnol. Lett. 2013, 35, 245–251. [Google Scholar] [CrossRef]
- Yang, K.; Li, Z. Multistep construction of metabolically engineered Escherichia coli for enhanced cytidine biosynthesis. Biochem. Eng. J. 2019, 154, 107433. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, R.; Liu, L.; He, L.; Ban, R. Improvement of uridine production in Bacillus subtilis by metabolic engineering. Biotechnol. Lett. 2018, 40, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, J.; Huang, Y.; Wang, J. Recent developments in single-cell RNA-seq of microorganisms. Biophys. J. 2018, 115, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Park, J.; Lee, E.; Lee, J.-H.; Jeong, D.-W. Transcriptomic Analysis of Staphylococcus equorum KM1031, Isolated from the High-Salt Fermented Seafood Jeotgal, under Salt Stress. Fermentation 2022, 8, 403. [Google Scholar] [CrossRef]
- Pei, D.; Liu, Z.; Wu, W.; Hu, B. Transcriptome analyses reveal the utilization of nitrogen sources and related metabolic mechanisms of Sporosarcina pasteurii. PloS ONE 2021, 16. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Huang, C.; Zhang, Y.; Wang, Z.; Tang, Y.-J.; Chen, T.; Zhao, X. Metabolic engineering of Escherichia coli using CRISPR–Cas9 meditated genome editing. Metab. Eng. 2015, 31, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef]
- Oshima, T.; Aiba, H.; Masuda, Y.; Kanaya, S.; Sugiura, M.; Wanner, B.L.; Mori, H.; Mizuno, T. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 2002, 46, 281–291. [Google Scholar] [CrossRef]
- Liu, C.G.; Turnbough, C.L. Multiple control mechanisms for pyrimidine-mediated regulation of pyrBI operon expression in Escherichia coli K-12. J. Bacteriol. 1989, 171, 3337. [Google Scholar] [CrossRef]
- Shi, S.; Chen, T.; Zhang, Z.; Chen, X.; Zhao, X. Transcriptome analysis guided metabolic engineering of Bacillus subtilis for riboflavin production. Metab. Eng. 2009, 11, 243–252. [Google Scholar] [CrossRef]
- Zakataeva, N.P.; Romanenkov, D.V.; Skripnikova, V.S.; Vitushkina, M.V.; Livshits, V.A.; Kivero, A.D.; Novikova, A.E. Wild-type and feedback-resistant phosphoribosyl pyrophosphate synthetases from Bacillus amyloliquefaciens: Purification, characterization, and application to increase purine nucleoside production. Appl. Microbiol. Biotechnol. 2012, 93, 2023–2033. [Google Scholar] [CrossRef]
- Jishage, M.; Dasgupta, D.; Ishihama, A. Mapping of the Rsd contact site on the sigma 70 subunit of Escherichia coli RNA polymerase. J. Bacteriol. 2001, 183, 2952–2956. [Google Scholar] [CrossRef] [PubMed]
- A Van Hoek, M.J.; Merks, R.M.H. Redox balance is key to explaining full vs. partial switching to low-yield metabolism. BMC Syst. Biol. 2012, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Dauner, M.; Bailey, J.E.; Sauer, U. Metabolic flux analysis with a comprehensive isotopomer model in Bacillus subtilis. Biotechnol. Bioeng. 2001, 76, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Rossmo, K.; Harries, K. The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J. Biol. Chem. 2004, 279, 6613–6619. [Google Scholar] [CrossRef]
- Deng, C.; Lv, X.; Li, J.; Zhang, H.; Liu, Y.; Du, G.; Amaro, R.L.; Liu, L. Synergistic improvement of N-acetylglucosamine production by engineering transcription factors and balancing redox cofactors. Metab. Eng. 2021, 67, 330–346. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).