Enhancement of Melanogenic Inhibitory Effects of the Leaf Skin Extracts of Aloe barbadensis Miller by the Fermentation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Regents
2.2. Preparation of the Samples
2.3. Determination of Aloenin Contents in the Extracts
2.4. Measurement of the Cell Cytotoxicity of the Extracts
2.5. Measurement of the Antioxidant Activity of the Extracts
2.6. Assessment of the Inhibition of Tyrosinase Activities
2.7. Assessment of the Inhibition of Cellular Tyrosinase Activities and Melanin Synthesis
2.8. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.9. Statistical Analysis
3. Results and Discussion
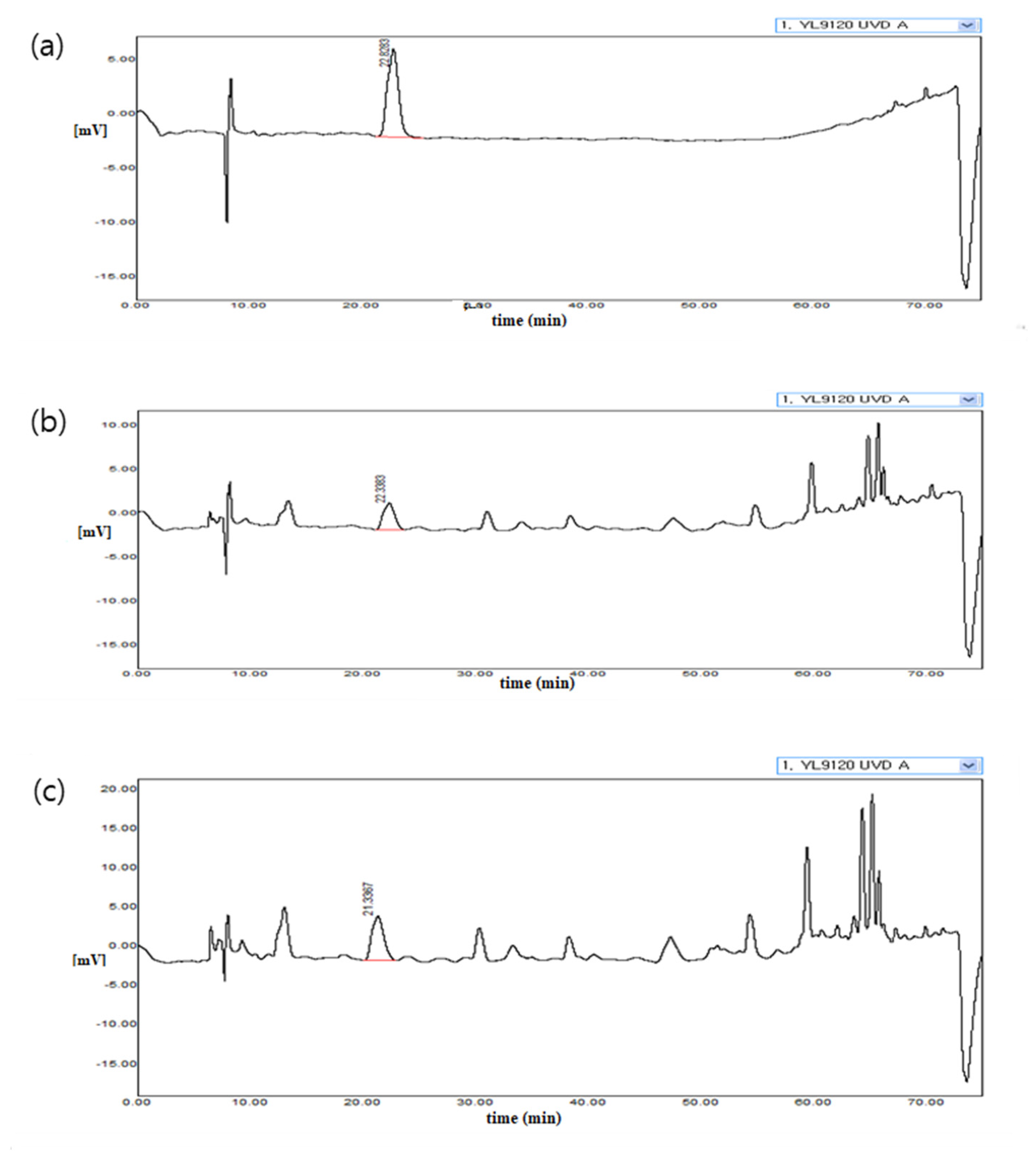
3.1. Amounts of Aloesin in the Extracts
3.2. Cell Cytotoxicity and Antioxidant Effects of the Fermented Extract
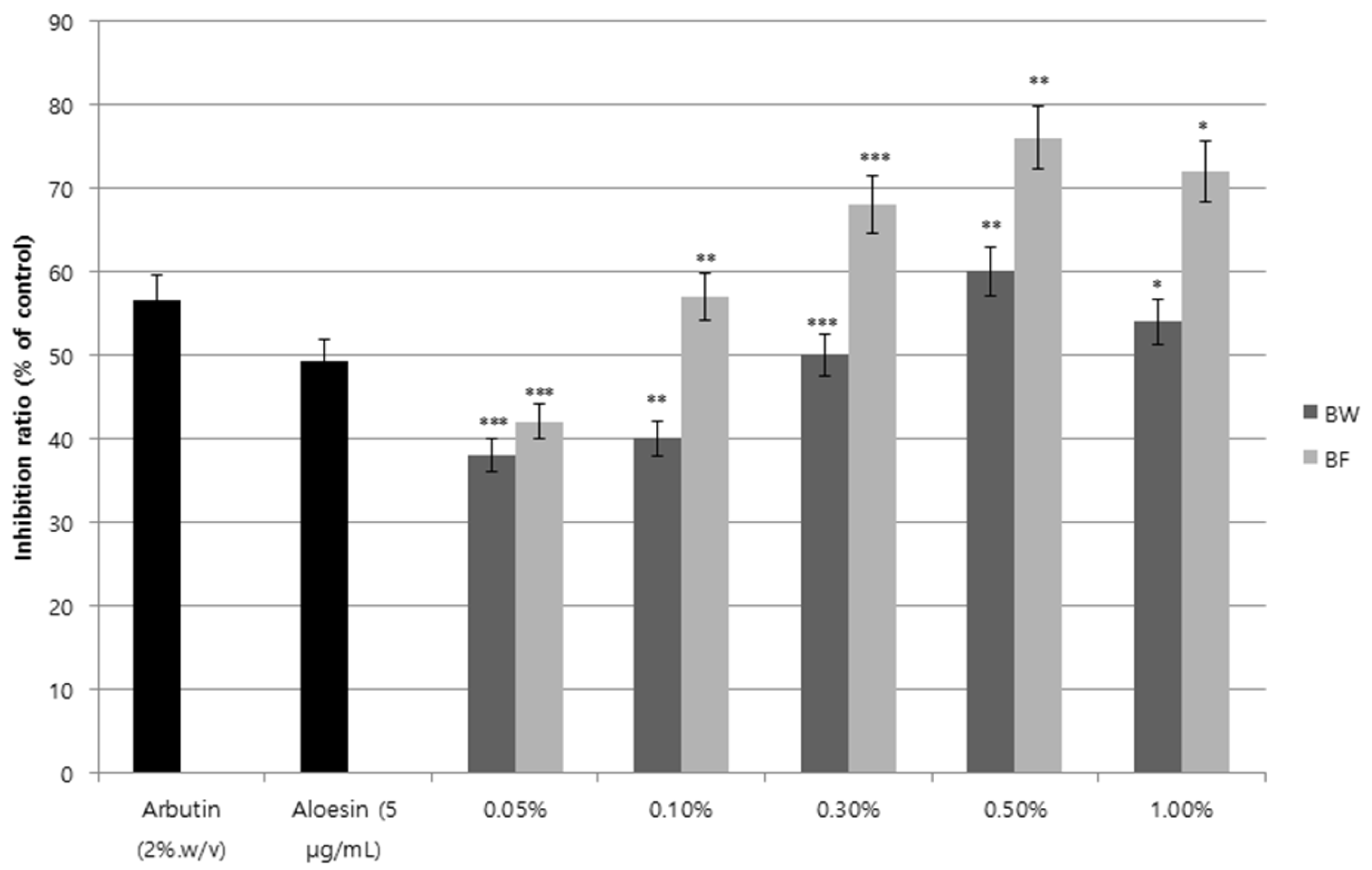
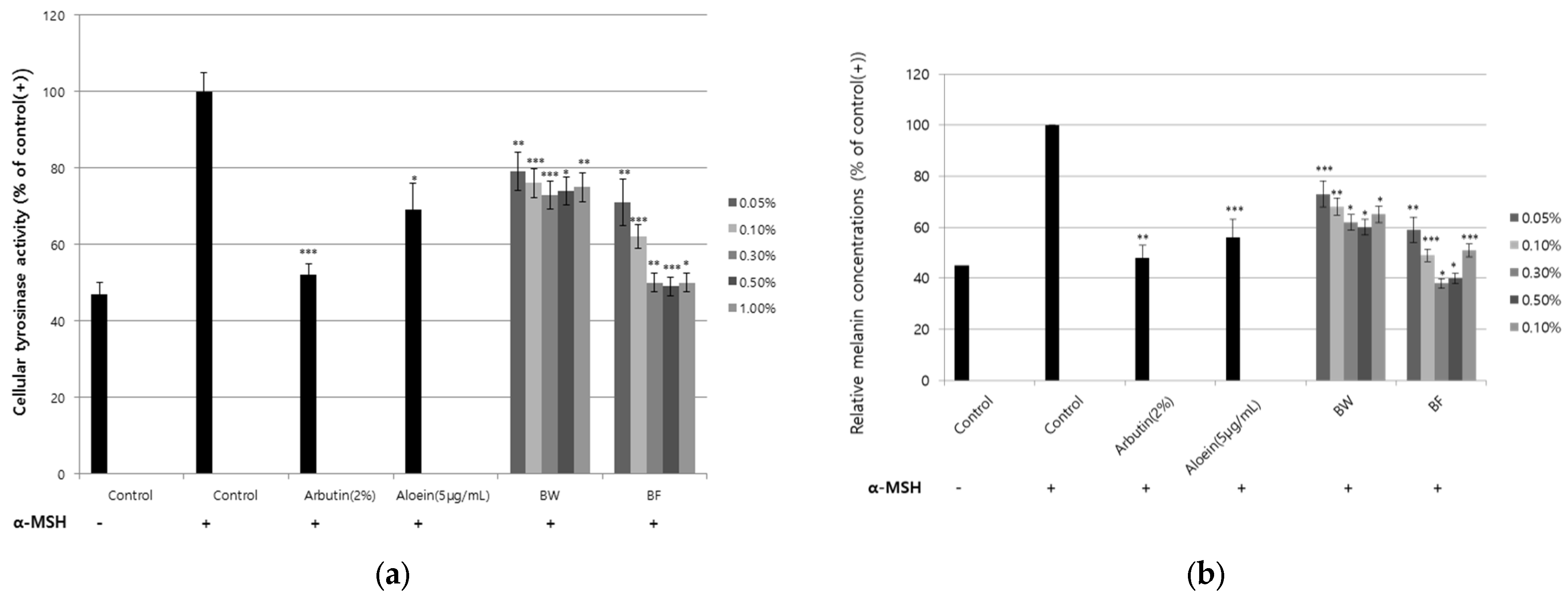
3.3. Inhibition of Tyrosinase Activity and Melanin Synthesis by the Fermented Extract
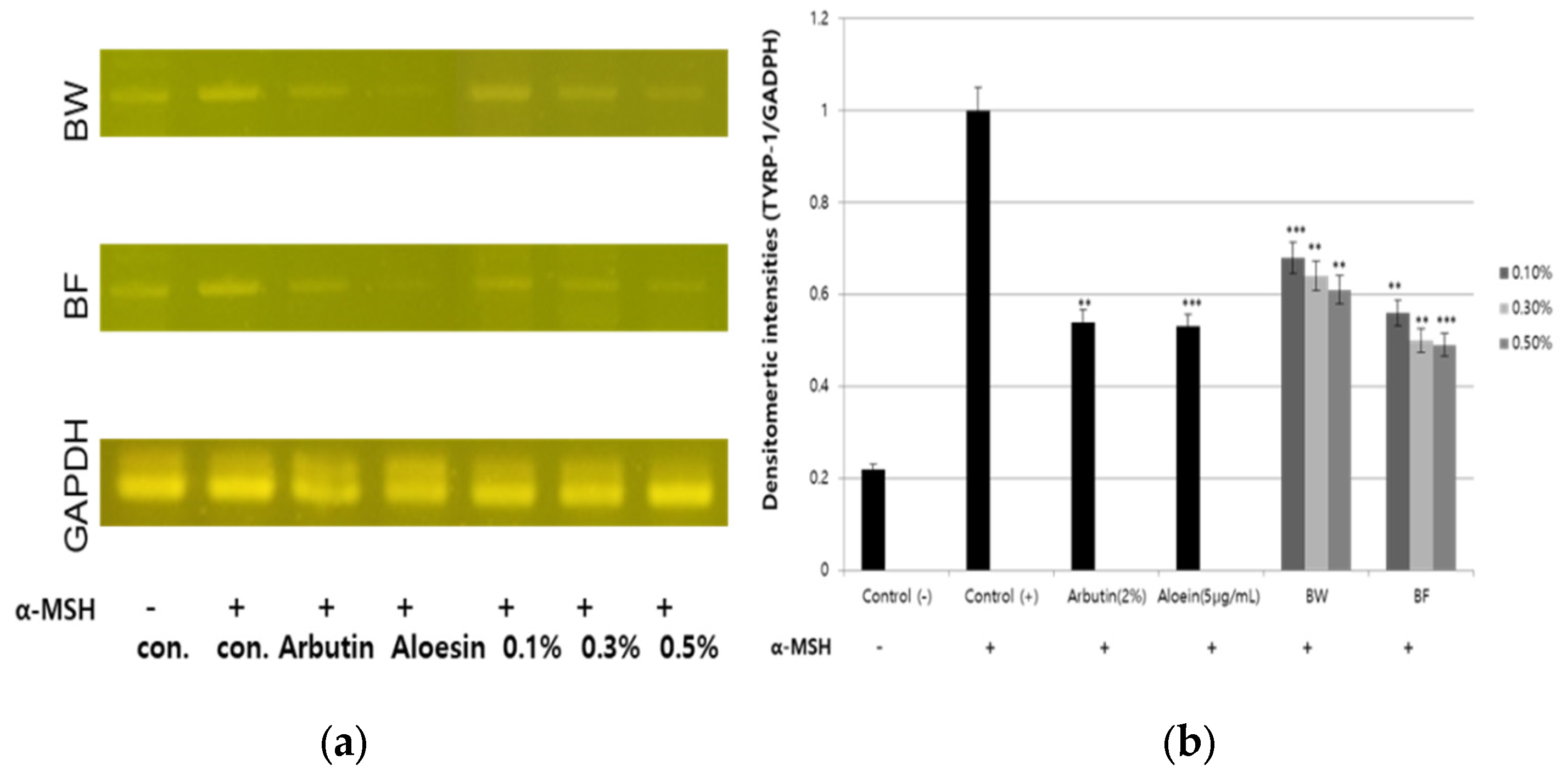
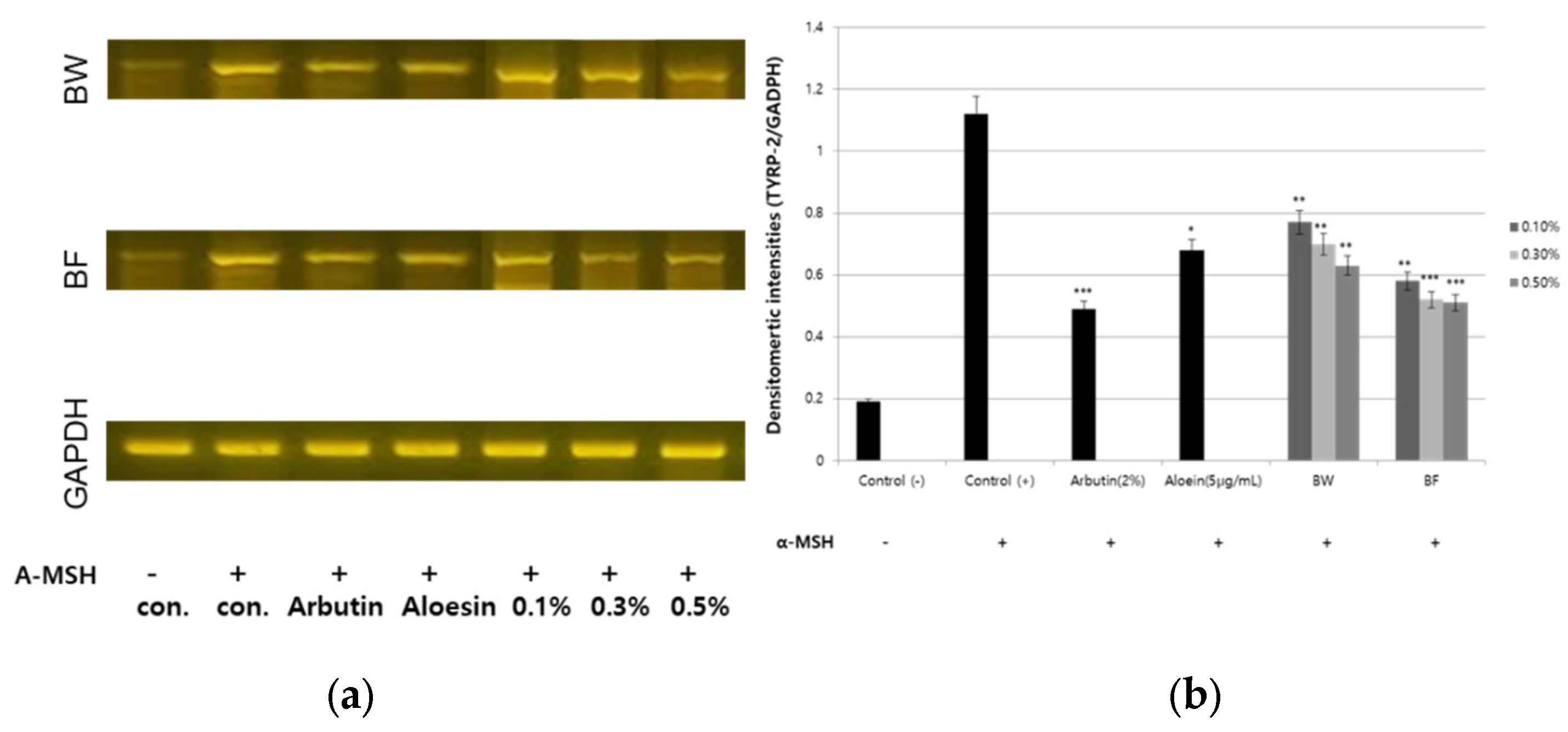
3.4. Downregulation of TYR, TYPR-1, TYRP-2 and MITF Gene Expression by the Fermented Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hart, P.H.; Norval, M. Ultraviolet radiation-induced immunosuppression and its relevance for skin carcinogenesis. Photochem. Photobiol. Sci. 2018, 17, 1872–1884. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.R.; Kim, D.H.; Kim, S.R.; An, H.J.; Lee, E.K.; Tanaka, T.; Kim, N.D.; Yokozawa, T.; Park, J.N.; Chung, H.Y. Anti-wrinkle effect of magnesium lithospermate B from Salvia miltiorrhiza BUNGE: Inhibition of MMPs via NF-kB signaling. PLoS ONE 2014, 9, e102689. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Herlyn, M. The transformed phenotype of melanocytes. In The Pigmentary System: Physiology and Pathophysiology, 2nd ed.; Norlund, J.J., Biossy, R.E., Hearing, V.J., King, R.A., Ortonne, J.P., Eds.; Blackwell Publishing: Oxford, UK, 2006; p. 489. [Google Scholar]
- Park, J.A. Melanin inhibitory activity and skin effect of Rumex crispus L. leaf extracts in B16F10 melanoma cells. Asian J. Beauty Cosmetol. 2015, 13, 509–515. [Google Scholar]
- Clark, A.K.; Sivamani, R.K. Phytochemicals in the treatment of hyperpigmentation. Bot. Targets Ther. 2016, 6, 89–96. [Google Scholar] [CrossRef]
- Snchez-Ferre, A.; Rodriguez-Lopez, N.J.; Garcia-Cánovas, F.; Garcia-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta (BBA)—Protein Struct. Mol. Enzymol. 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Oh, T.I.; Yun, J.M.; Park, E.J.; Kim, Y.S.; Lee, Y.M.; Lim, J.H. Plumbagin suppresses α-MSH-induced melanogenesis in B16F10 mouse melanoma cells by inhibiting tyrosinase activity. Int. J. Mol. Sci. 2017, 18, 320. [Google Scholar] [CrossRef]
- Nakagawa, M.; Kawa, K. Contact allergy to kojic acid in skin care products. Contact Dermat. 1995, 32, 9–13. [Google Scholar] [CrossRef]
- Seo, E.S.; Hong, M.H.; Choi, K.; Kim, K.S.; Lee, S.J. Antioxidant and skin whitening effects of Rhamnus yoshinoi extracts. Korean J. Food Sci. Technol. 2010, 42, 750–754. [Google Scholar]
- Yagi, A.; Kanbara, M.A.; Morinobu, T.N. Inhibition of mushroom-tyrosinase by aloe extract. Planta Medica. 1987, 53, 515–517. [Google Scholar] [CrossRef]
- Wahedi, H.M.; Jeong, M.S.; Chae, J.K.; Do, S.G.; Yoon, H.K.; Kim, S.Y. Aloesin from Aloe vera accelerates skin wound healing by modulating MAPK/Rho and Smad signaling pathways in vitro and in vivo. Phytomedicine 2017, 28, 19–26. [Google Scholar] [CrossRef]
- Jin, Y.H.; Lee, S.J.; Chung, M.H.; Park, J.H.; Park, Y.I.; Cho, T.H.; Lee, S.K. Aloesin and arbutin inhibit tyrosinase activity in a synergistic manner via a different action mechanism. Arch. Pharmacal. Res. 1999, 22, 232–236. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and applications of Aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, H.J.; Park, J.H.; Shin, Y.G. Determination of aloesin in aloe preparations by HPLC. Yakhak Hoeji 1996, 40, 177–182. [Google Scholar]
- Hidehiko, B.; Kan, S.; Takeshi, C.; Takaaki, K.; Ikuko, T.; Sachiyo, Y.; Sayaka, O.; Hiroshi, K.; Shigeru, S. Antidiabetic effects of dietary administration of Aloe arborescens Miller components on multiple low-dose streptozotocin-induced diabetes in mice: Investigation on hypoglycemic action and systemic absorption dynamics of aloe components. J. Ethnopharmacol. 2006, 103, 468–477. [Google Scholar] [CrossRef]
- Ha, J.H.; Jeong, M.H.; Seo, Y.C.; Choi, W.Y.; Kim, J.S.; Kim, H.H.; Ahn, J.H.; Lee, H.Y. Enhancement of antioxidant activities of bark of Berberis koreana Palibin by lactic acid fermentation. Korean J. Med. Crop Sci. 2010, 18, 421–428. [Google Scholar]
- Perdigón, G.; Fuller, R.; Raya, R. Lactic acid bacteria and their effect on the immune system. Curr. Issues Intest. Microbiol. 2001, 2, 27–42. [Google Scholar]
- Yang, M.C.; Jeong, S.W.; Ma, J.Y. Analysis of constituents in Sipjundaebo-tangs fermented by lactic acid bacteria. Microbiol. Biotech. Lett. 2011, 39, 350–356. [Google Scholar]
- Lee, H.Y. Improvement of skin barrier dysfunction by Scutellaria baicalensis GEOGI extracts through lactic acid fermentation. J. Cosmet. Dermatol. 2019, 18, 183–191. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, H.M.; Lee, E.D.; Park, S.D.; Kim, Y.T.; Kim, Y.J.; Jeong, J.W.; Jang, S.S.; Ahn, Y.T.; Sim, J.H.; et al. Oral administration of Lactobacillus plantarum HY7714 protects hairless mouse against ultraviolet B-induced photoaging. J. Microbiol. Biotechnol. 2014, 24, 1583–1591. [Google Scholar] [CrossRef]
- Park, Y.E.; Kim, B.H.; Yoon, Y.C.; Kim, J.K.; Lee, J.H.; Kwon, S.S.; Soo, H.H.; Lee, J.B. Total polyphenol contents, flavonoid contents, and antioxidant activity of roasted-flaxseed extracts based on lactic-acid bacteria fermentation. J. Life Sci. 2018, 28, 547–554. [Google Scholar] [CrossRef]
- Park, H.J.; Cho, J.H.; Hong, S.H.; Kim, D.H.; Jung, H.Y.; Kang, I.K.; Cho, Y.J. Whitening and anti-wrinkle activities of ferulic acid isolated from Tetragonia tetragonioides in B16F10 melanoma and CCD-986sk fibroblast cells. J. Nat. Med. 2018, 72, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Frezzini, M.A.; Castellani, F.; Francesco, N.; Ristorini, M.; Canepari, S. Application of DPPH assay for assessment of particulate matter reducing properties. Atmosphere 2019, 10, 816. [Google Scholar] [CrossRef]
- Kobashigawa, L.C.; Xu, Y.C.; Padbury, J.F.; Tseng, Y.T.; Yano, N. Metformin protects cardiomyocyte from doxorubicin induced cytotoxicity through an AMP-activated protein kinase dependent signaling pathway: An in vitro study. PLoS ONE 2014, 15, e104888. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, S.; Mapunya, B.M.; Houghton, P.J.; Edgerly, C.; Hussein, A.; Naidoo, S.; Lall, N. Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. J. Ethnopharmacol. 2008, 119, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Pintus, F.; Spanò, D.; Corona, A.; Medda, R. Antityrosinase activity of Euphorbia characias extracts. PeerJ 2015, 3, e1305. [Google Scholar] [CrossRef][Green Version]
- Matsuda, H.; Kawaguchi, Y.; Yamazaki, M. Melanogenesis stimulation in murine B16 melanoma cells by Piper nigrum leaf extract and its lignan constituents. Biol. Pharm. Bull. 2004, 27, 1611–1616. [Google Scholar] [CrossRef]
- Kang, H.C.; Cha, M.Y.; Kim, J.Y. A study of the antioxidant activities and whitening activities of Areca semen extracts as cosmetic ingredient. J. Korean Soc. Cosmet. Sci. 2015, 41, 269–277. [Google Scholar] [CrossRef]
- Seol, N.G.; Jang, E.Y.; Sung, J.H.; Moon, G.W.; Lee, J. Antioxidant capacities of Aloe vera (Aloe vera Linne) from Jeju Island, Korea. Korean J. Food Sci. Technol. 2012, 44, 643–647. [Google Scholar] [CrossRef][Green Version]
- Kang, D.H.; Kim, J.W.; Youn, K.S. Antioxidant activities of extracts from fermented mulberry (Cudrania tricuspidata) fruit and inhibitory actions on elastase and tyrosinase. Korean J. Food Preserv. 2011, 18, 236–243. [Google Scholar] [CrossRef]
- Kim, C.H.; Kwon, M.C.; Kim, H.S.; Ahn, J.H.; Choi, G.P.; Choi, Y.B.; Ko, J.R.; Lee, H.Y. Enhancement of immune activities of Kadsura japonica Dunal through conventional fermentation process. Korean J. Med. Crop Sci. 2007, 15, 162–169. [Google Scholar]
- Mikayoulou, M.; Fabian, M.; Veronika, T.; Ajuran, P.; IIze, V.; Chen, W.; Kormane, B.; Stuppner, H.; Viljoen, A. Anti-tyrosinase activity of South African Aloe species and isolated compounds plicataloside and aloesin. Fitoterapia 2021, 150, e104828. [Google Scholar] [CrossRef]
- Shao, A.; Broadmeadow, A.; Goddard, G.; Bejar, F.; Frankos, V. Safety of purified decolorized (low anthraquinone) whole leaf Aloe vera (L.) Burm. f. juice in a 3-month drinking water toxicity study in F344 rats. Food Chem. Toxicol. 2013, 57, 21–31. [Google Scholar] [CrossRef]
- Nalimu, F.; Oloro, J.; Kahwa, I.; Ogwang, P.E. Review on the phytochemistry and toxicological profiles of Aloe vera and Aloe ferox. Future J. Pharma. Sci. 2021, 7, 1–21. [Google Scholar] [CrossRef]
- Choi, W.S.; Kwon, H.S.; No, R.H.; Choi, G.P.; Lee, H.Y. Enhancement of anti-inflammatory activities of fermented Scutellaria baicalensis extracts using Lactobacillus rhamnosus. J. Soc. Cosmetic Sci. 2013, 39, 303–311. [Google Scholar] [CrossRef]
- Yoon, T.J.; Yang, W.S.; Park, S.M.; Jung, H.Y.; Lee, A.N.; Jung, J.H.; Kang, T.B.; Yoo, Y.C.; Kim, J.B. In vivo toxicity and immune adjuvant activity of Korean mistletoe (Viscum album coloratum) extract fermented with Lactobacillus. Korean J. Food Sci. Technol. 2009, 41, 560–565. [Google Scholar]
- Hanif, N.; Al-Shami, A.M.; Khalid, K.A.; Hadi, H. Plant-based skin lightening agents: A review. J. Phytopharm. 2020, 9, 54–60. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeon, Y.D.; Cha, J.H.; Hwang, S.W.; Lee, H.Y.; Park, M.; Lee, B.R.; Shin, M.K.; Kim, S.I.; Shin, S.M.; et al. Antioxidant and skin-whitening effects of aerial part of Euphorbia supina Raf. Extract. BMC Complement. Altern. Med. 2018, 18, 256. [Google Scholar] [CrossRef]
- Coleman, M.D.; Fermandes, S.; Khanderia, L.A. A preliminary evaluation of a novel method to monitor a triple antioxidant combination (vitamin E, C and a-lipoic acid) in diabetic volunteers using in vitro methaemoglobin formation. Environ. Toxicol Pharmacol. 2003, 14, 69–75. [Google Scholar] [CrossRef]
- Saeedi, M.; Khezri, K.; Seyed, Z.A.; Mohammadamini, H.A. Comprehensive review of the therapeutic potential of α-arbutin. Phytother. Res. 2021, 35, 4136–4154. [Google Scholar] [CrossRef]
- Sarkar, R.; Arora, P.; Garg, K.V. Cosmeceuticals for hyperpigmentation: What is available? J. Cutan Aesthet. Surg. 2013, 6, 4–11. [Google Scholar] [CrossRef]
- Schiaffino, M.V. Signaling pathways in melanosome biogenesis and pathology. Int. J. Biochem. Cell Biol. 2010, 42, 1094–1104. [Google Scholar] [CrossRef]
- Lim, D.; Lee, K.J.; Kim, Y.; Kim, M.S.; Ju, H.M.; Kim, M.J.; Choi, D.W.; Choi, J.; Kim, S.; Kang, D.M.; et al. A basic domain-derived tripeptide inhibits MITF activity by reducing its binding to the promoter of target genes. J. Investig. Dermatol. 2021, 141, 2459–2469. [Google Scholar] [CrossRef]
- Morag, M.; Nawrot, J.; Siatkowski, I.; Admski, Z.; Fedorowicz, F.; Dawid, R.; Nowak, G. Double-blind, placebo-controlled randomized trial of Serratulae quinquefoliae folium, a new source of β-arbutin, in selected skin hyperpigmentations. J. Cosmet. Dermatol. 2015, 14, 185–190. [Google Scholar] [CrossRef]
- Sarkar, R.; Chugh, S.; Garg, V.K. Newer and upcoming therapies for melasma. Ind. J. Dermatol. Venereol. Leprol. 2012, 78, 417–423. [Google Scholar] [CrossRef]
- Vachtenheim, J.; Ondrušová, L. MITF: A critical transcription factor in melanoma transcriptional regulatory network. In Recent Advances in the Biology, Therapy and Management of Melanoma; Davids, L.M., Ed.; Intech Pub.: Rijeka, Croatia, 2013; p. 392. [Google Scholar] [CrossRef][Green Version]
- Chang, T. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Alcala, A.M.; Flaherty, K.T. BRAF inhibitors for the treatment of metastatic melanoma: Clinical trials and mechanisms of resistance. Clin. Cancer Res. 2012, 18, 33–39. [Google Scholar] [CrossRef]
- Huh, S.Y.; Shin, J.W.; Na, J.I.; Huh, C.H.; Youn, S.W.; Park, K.C. The efficacy and safety of 4-n-butylresorcinol 0.1% cream for the treatment of melasma: A randomized controlled split-face trial. Ann. Dermatol. 2010, 22, 21–25. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, G.; Ro, H.-S.; Kim, G.-R.; Lee, H.-Y. Enhancement of Melanogenic Inhibitory Effects of the Leaf Skin Extracts of Aloe barbadensis Miller by the Fermentation Process. Fermentation 2022, 8, 580. https://doi.org/10.3390/fermentation8110580
Jeon G, Ro H-S, Kim G-R, Lee H-Y. Enhancement of Melanogenic Inhibitory Effects of the Leaf Skin Extracts of Aloe barbadensis Miller by the Fermentation Process. Fermentation. 2022; 8(11):580. https://doi.org/10.3390/fermentation8110580
Chicago/Turabian StyleJeon, Gibeom, Hyang-Sun Ro, Gyu-Rae Kim, and Hyeon-Yong Lee. 2022. "Enhancement of Melanogenic Inhibitory Effects of the Leaf Skin Extracts of Aloe barbadensis Miller by the Fermentation Process" Fermentation 8, no. 11: 580. https://doi.org/10.3390/fermentation8110580
APA StyleJeon, G., Ro, H.-S., Kim, G.-R., & Lee, H.-Y. (2022). Enhancement of Melanogenic Inhibitory Effects of the Leaf Skin Extracts of Aloe barbadensis Miller by the Fermentation Process. Fermentation, 8(11), 580. https://doi.org/10.3390/fermentation8110580





