β-Farnesene Production from Low-Cost Glucose in Lignocellulosic Hydrolysate by Engineered Yarrowia lipolytica
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Plasmid Construction
2.2. Fermentation Conditions
2.3. Pretreatment and Enzymatic Hydrolysis
2.4. Fed-Batch Fermentation
2.5. Analytical Methods
2.6. Statistics
3. Results and Discussion
3.1. Construction of β-Farnesene Biosynthesis Pathway in Yarrowia lipolytica
3.2. Further Increasing the Carbon Flux of the MVA Pathway
3.3. Establishing Lignocellulosic Hydrolysate Utilization Strategy in Fermentation Media
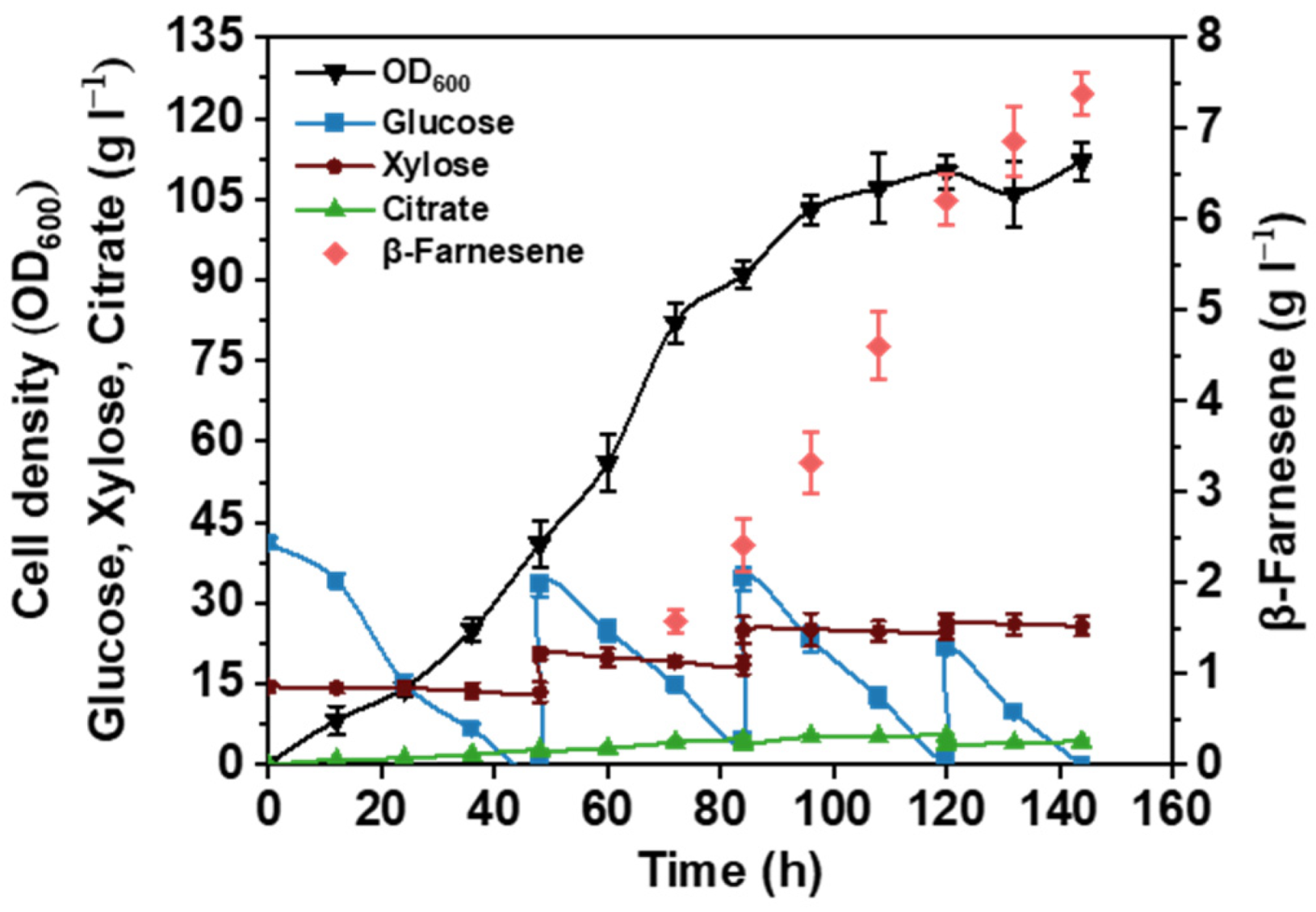
3.4. Upscaling Fermentation in 2 L Fermenter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huelin, F.E.; Murray, K.E. α-Farnesene in the natural coating of apples. Nature 1966, 210, 1260–1261. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Liu, X.; Pan, G.; Hou, X.; Zhang, H.; Yuan, Y. In vitro characterization of a (E)-beta-farnesene synthase from Matricaria recutita L. and its up-regulation by methyl jasmonate. Gene 2015, 571, 58–64. [Google Scholar] [CrossRef]
- Yu, X.; Jones, H.D.; Ma, Y.; Wang, G.; Xu, Z.; Zhang, B.; Zhang, Y.; Ren, G.; Pickett, J.A.; Xia, L. (E)-beta-Farnesene synthase genes affect aphid (Myzus persicae) infestation in tobacco (Nicotiana tabacum). Funct. Integr. Genom. 2012, 12, 207–213. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, X.; Zhan, Y.; Wang, K.; Francis, F.; Liu, Y. New slow release mixture of (E)-β-farnesene with methyl salicylate to enhance aphid biocontrol efficacy in wheat ecosystem. Pest Manag. Sci. 2021, 77, 3341–3348. [Google Scholar] [CrossRef]
- You, S.; Yin, Q.; Zhang, J.; Zhang, C.; Qi, W.; Gao, L.; Tao, Z.; Su, R.; He, Z. Utilization of biodiesel by-product as substrate for high-production of β-farnesene via relatively balanced mevalonate pathway in Escherichia coli. Bioresour. Technol. 2017, 243, 228–236. [Google Scholar] [CrossRef]
- George, K.W.; Alonso-Gutierrez, J.; Keasling, J.D.; Lee, T.S. Isoprenoid Drugs, Biofuels, and Chemicals—Artemisinin, Farnesene, and Beyond. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 355–389. [Google Scholar]
- Akutagawa, S.; Taketomi, T.; Kumobayashi, H.; Takayama, K.; Someya, T.; Otsuka, S. Metal-Assisted Terpenoid Synthesis. V. The Catalytic Trimerization of Isoprene to trans-β-Farnesene and Its Synthetic Applications for Terpenoids. Bull. Chem. Soc. Jpn. 1978, 51, 1158–1162. [Google Scholar] [CrossRef]
- Arkoudis, E.; Stratakis, M. Synthesis of Cordiaquinones B, C, J, and K on the Basis of a Bioinspired Approach and the Revision of the Relative Stereochemistry of Cordiaquinone C. J. Org. Chem. 2008, 73, 4484–4490. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Silver, P.A. Synthetic biology expands chemical control of microorganisms. Curr. Opin. Chem. Biol. 2015, 28, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Woolston, B.M.; Edgar, S.; Stephanopoulos, G. Metabolic Engineering, Past and Future. Annu. Rev. Chem. Biomol. Eng. 2013, 4, 259–288. [Google Scholar] [CrossRef]
- Yao, P.; You, S.; Qi, W.; Su, R.; He, Z. Investigation of fermentation conditions of biodiesel by-products for high production of β-farnesene by an engineered Escherichia coli. Environ. Sci. Pollut. R 2020, 27, 22758–22769. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Chang, H.; Zhang, C.; Gao, L.; Qi, W.; Tao, Z.; Su, R.; He, Z. Recycling strategy and repression elimination for lignocellulosic-based farnesene production with an engineered Escherichia coli. J. Agric. Food Chem. 2019, 67, 9858–9867. [Google Scholar] [CrossRef] [PubMed]
- Tippmann, S.; Anfelt, J.; David, F.; Rand, J.M.; Siewers, V.; Uhlén, M.; Nielsen, J.; Hudson, E.P. Affibody scaffolds improve sesquiterpene production in Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Tippmann, S.; Ferreira, R.; Siewers, V.; Nielsen, J.; Chen, Y. Effects of acetoacetyl-CoA synthase expression on production of farnesene in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2017, 44, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Tippmann, S.; Scalcinati, G.; Siewers, V.; Nielsen, J. Production of farnesene and santalene by Saccharomyces cerevisiae using fed-batch cultivations with RQ-controlled feed. Biotechnol. Bioeng. 2016, 113, 72–81. [Google Scholar] [CrossRef]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef]
- Shi, T.; Li, Y.; Zhu, L.; Tong, Y.; Yang, J.; Fang, Y.; Wang, M.; Zhang, J.; Jiang, Y.; Yang, S. Engineering the oleaginous yeast Yarrowia lipolytica for β-farnesene overproduction. Biotechnol. J. 2021, 16, 2100097. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Markham, K.A.; Palmer, C.M.; Liu, N.; Stephanopoulos, G.; Alper, H.S. Metabolic engineering in the host Yarrowia lipolytica. Metab. Eng. 2018, 50, 192–208. [Google Scholar] [CrossRef]
- Liu, H.-H.; Ji, X.-J.; Huang, H. Biotechnological applications of Yarrowia lipolytica, Past, present and future. Biotechnol. Adv. 2015, 33, 1522–1546. [Google Scholar] [CrossRef]
- Gao, S.; Tong, Y.; Zhu, L.; Ge, M.; Zhang, Y.; Chen, D.; Jiang, Y.; Yang, S. Iterative integration of multiple-copy pathway genes in Yarrowia lipolytica for heterologous β-carotene production. Metab. Eng. 2017, 41, 192–201. [Google Scholar] [CrossRef]
- Tang, W.-Y.; Wang, D.-P.; Tian, Y.; Fan, X.; Wang, C.; Lu, X.-Y.; Li, P.-W.; Ji, X.-J.; Liu, H.-H. Metabolic engineering of Yarrowia lipolytica for improving squalene production. Bioresour. Technol. 2021, 323, 124652. [Google Scholar] [CrossRef]
- Shaikh, K.M.; Odaneth, A.A. Metabolic engineering of Yarrowia lipolytica for the production of isoprene. Biotechnol. Prog. 2021, 37, e3201. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liwei, M.; Park, J.-B.; Jeong, S.-H.; Wei, G.; Wang, Y.; Kim, S.-W. Microbial Platform for Terpenoid Production, Escherichia coli and Yeast. Front. Microbiol. 2018, 9, 2460. [Google Scholar] [CrossRef] [PubMed]
- Qing, Q.; Guo, Q.; Wang, P.; Qian, H.; Gao, X.; Zhang, Y. Kinetics study of levulinic acid production from corncobs by tin tetrachloride as catalyst. Bioresour. Technol. 2018, 260, 150–156. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Ma, L.; Dong, S.; Liu, L. Corn stalk as starting material to prepare a novel adsorbent via SET-LRP and its adsorption performance for Pb(II) and Cu(II). R Soc. Open Sci. 2020, 7, 191811. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Gao, F.; Yin, D.M.; Luo, Q.; Fu, Z.Q.; Zhou, Y.G. Processing of Superfine Grinding Corn Straw Fiber-Reinforced Starch Film and the Enhancement on Its Mechanical Properties. Polymers 2018, 10, 855. [Google Scholar] [CrossRef]
- Xu, W.; Fu, S.; Yang, Z.; Lu, J.; Guo, R. Improved methane production from corn straw by microaerobic pretreatment with a pure bacteria system. Bioresour. Technol. 2018, 259, 18–23. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, L.; Yin, C.; Lebailly, P.; Azadi, H. Urban residents’ willingness to pay for corn straw burning ban in Henan, China, Application of payment card. J. Clean. Prod. 2018, 193, 471–478. [Google Scholar] [CrossRef]
- Jia, F.; Liu, H.-J.; Zhang, G.-G. Preparation of carboxymethyl cellulose from corncob. Procedia Environ. Sci. 2016, 31, 98–102. [Google Scholar] [CrossRef]
- Fujiwara, R.; Noda, S.; Tanaka, T.; Kondo, A. Metabolic engineering of Escherichia coli for shikimate pathway derivative production from glucose-xylose co-substrate. Nat. Commun. 2020, 11, 279. [Google Scholar] [CrossRef]
- Shen, L.; Kohlhaas, M.; Enoki, J.; Meier, R.; Schönenberger, B.; Wohlgemuth, R.; Kourist, R.; Niemeyer, F.; van Niekerk, D.; Bräsen, C.; et al. A combined experimental and modelling approach for the Weimberg pathway optimization. Nat. Commun. 2020, 11, 1098. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, L. Bioconversion of corn stover hydrolysate to ethanol by a recombinant yeast strain. Fuel Process. Technol. 2010, 91, 1807–1811. [Google Scholar] [CrossRef]
- Qureshi, N.; Cotta, M.A.; Saha, B.C. Bioconversion of barley straw and corn stover to butanol (a biofuel) in integrated fermentation and simultaneous product recovery bioreactors. Food Bioprod. Process. 2014, 92, 298–308. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Kenealy, W.R.; Jeffries, T.W. Xylitol production from DEO hydrolysate of corn stover by Pichia stipitis YS-30. J. Ind. Microbiol. Biotechnol. 2011, 38, 1649–1655. [Google Scholar] [CrossRef]
- Cao, G.; Ren, N.; Wang, A.; Lee, D.-J.; Guo, W.; Liu, B.; Feng, Y.; Zhao, Q. Acid hydrolysis of corn stover for biohydrogen production using Thermoanaerobacterium thermosaccharolyticum W16. Int. J. Hydrogen Energy 2009, 34, 7182–7188. [Google Scholar] [CrossRef]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A.; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Holkenbrink, C.; Dam, M.I.; Kildegaard, K.R.; Beder, J.; Dahlin, J.; Doménech Belda, D.; Borodina, I. EasyCloneYALI, CRISPR/Cas9-based synthetic toolbox for engineering of the yeast Yarrowia lipolytica. Biotechnol. J. 2018, 13, 1700543. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Xu, G.; Chu, J.; Zhuang, Y.; Zhang, S. Influence of High Solid Concentration on Enzymatic Hydrolysis and Fermentation of Steam-Exploded Corn Stover Biomass. Appl. Biochem. Biotech. 2010, 160, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; Frogue, K.; Ramesh, A.; Misa, J.; Wheeldon, I. CRISPRi repression of nonhomologous end-joining for enhanced genome engineering via homologous recombination in Yarrowia lipolytica. Biotechnol. Bioeng. 2017, 114, 2896–2906. [Google Scholar] [CrossRef]
- Tai, M.; Stephanopoulos, G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab. Eng. 2013, 15, 1–9. [Google Scholar] [CrossRef]
- Blazeck, J.; Liu, L.; Redden, H.; Alper, H. Tuning gene expression in Yarrowia lipolytica by a hybrid promoter approach. Appl. Environ. Microbiol. 2011, 77, 7905–7914. [Google Scholar] [CrossRef]
- Maruyama, T.; Ito, M.; Honda, G. Molecular Cloning, Functional Expression and Characterization of (E)-β-Farnesene Synthase from Citrus junos. Biol. Pharm. Bull. 2001, 24, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ching, C.B. Mitochondrial acetyl-CoA utilization pathway for terpenoid productions. Metab. Eng. 2016, 38, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Ye, L.; Lv, X.; Xu, H.; Yu, H. Sequential control of biosynthetic pathways for balanced utilization of metabolic intermediates in Saccharomyces cerevisiae. Metab. Eng. 2015, 28, 8–18. [Google Scholar] [CrossRef]
- Chandran, S.S.; Kealey, J.T.; Reeves, C.D. Microbial production of isoprenoids. Process Biochem. 2011, 46, 1703–1710. [Google Scholar] [CrossRef]
- Kwak, S.; Kim, S.R.; Xu, H.; Zhang, G.-C.; Lane, S.; Kim, H.; Jin, Y.-S. Enhanced isoprenoid production from xylose by engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017, 114, 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Xiao, F.; Gao, J.; Ye, C.; Jiang, L.; Dong, C.; Lian, J. Establishing Komagataella phaffii as a Cell Factory for Efficient Production of Sesquiterpenoid α-Santalene. J. Agric. Food Chem. 2022, 70, 8024–8031. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Qiao, K.; Stephanopoulos, G. (13)C Metabolic Flux Analysis of acetate conversion to lipids by Yarrowia lipolytica. Metab. Eng. 2016, 38, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Wasylenko, T.M.; Ahn, W.S.; Stephanopoulos, G. The oxidative pentose phosphate pathway is the primary source of NADPH for lipid overproduction from glucose in Yarrowia lipolytica. Metab. Eng. 2015, 30, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nielsen, J.; Liu, Z. Engineering yeast metabolism for production of terpenoids for use as perfume ingredients, pharmaceuticals and biofuels. FEMS Yeast Res. 2017, 17, fox080. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Triantaphyllidou, I.-E.; Mizerakis, P.; Aggelis, G. High lipid accumulation in Yarrowia lipolytica cultivated under double limitation of nitrogen and magnesium. J. Biotechnol. 2016, 234, 116–126. [Google Scholar] [CrossRef]
- van Rossum, H.M.; Kozak, B.U.; Niemeijer, M.S.; Dykstra, J.C.; Luttik, M.A.; Daran, J.M.G.; Van Maris, A.J.; Pronk, J.T. Requirements for Carnitine Shuttle-Mediated Translocation of Mitochondrial Acetyl Moieties to the Yeast Cytosol. Mbio 2016, 7, e00520-16. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.M.; Hussain, M.S.; Gambill, L.; Gao, D.; Yaguchi, A.; Blenner, M. Engineering xylose utilization in Yarrowia lipolytica by understanding its cryptic xylose pathway. Biotechnol. Biofuels 2016, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-K.; Bordes, F.; Letisse, F.; Nicaud, J.-M. Engineering precursor pools for increasing production of odd-chain fatty acids in Yarrowia lipolytica. Metab. Eng. Commun. 2021, 12, e00158. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, S.; Zhang, Y.; Lu, M.; Sha, Y.; Zhai, R.; Xu, Z.; Jin, M. A novel fermentation strategy for efficient xylose utilization and microbial lipid production in lignocellulosic hydrolysate. Bioresour. Technol. 2022, 361, 127624. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yu, Y.; Wang, K.; Ledesma-Amaro, R.; Ji, X.-J. Engineering Yarrowia lipolytica to produce fuels and chemicals from xylose: A review. Bioresour. Technol. 2021, 337, 125484. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.; Cui, Z.; Qi, Q.; Hou, J. α-Farnesene production from lipid by engineered Yarrowia lipolytica. Bioresour. Bioprocess 2021, 8, 78. [Google Scholar] [CrossRef]
- Picaud, S.; Brodelius, M.; Brodelius, P. Expression, purification and characterization of recombinant (E)-β-farnesene synthase from Artemisia annua. Phytochemistry 2005, 66, 961–967. [Google Scholar] [CrossRef] [PubMed]

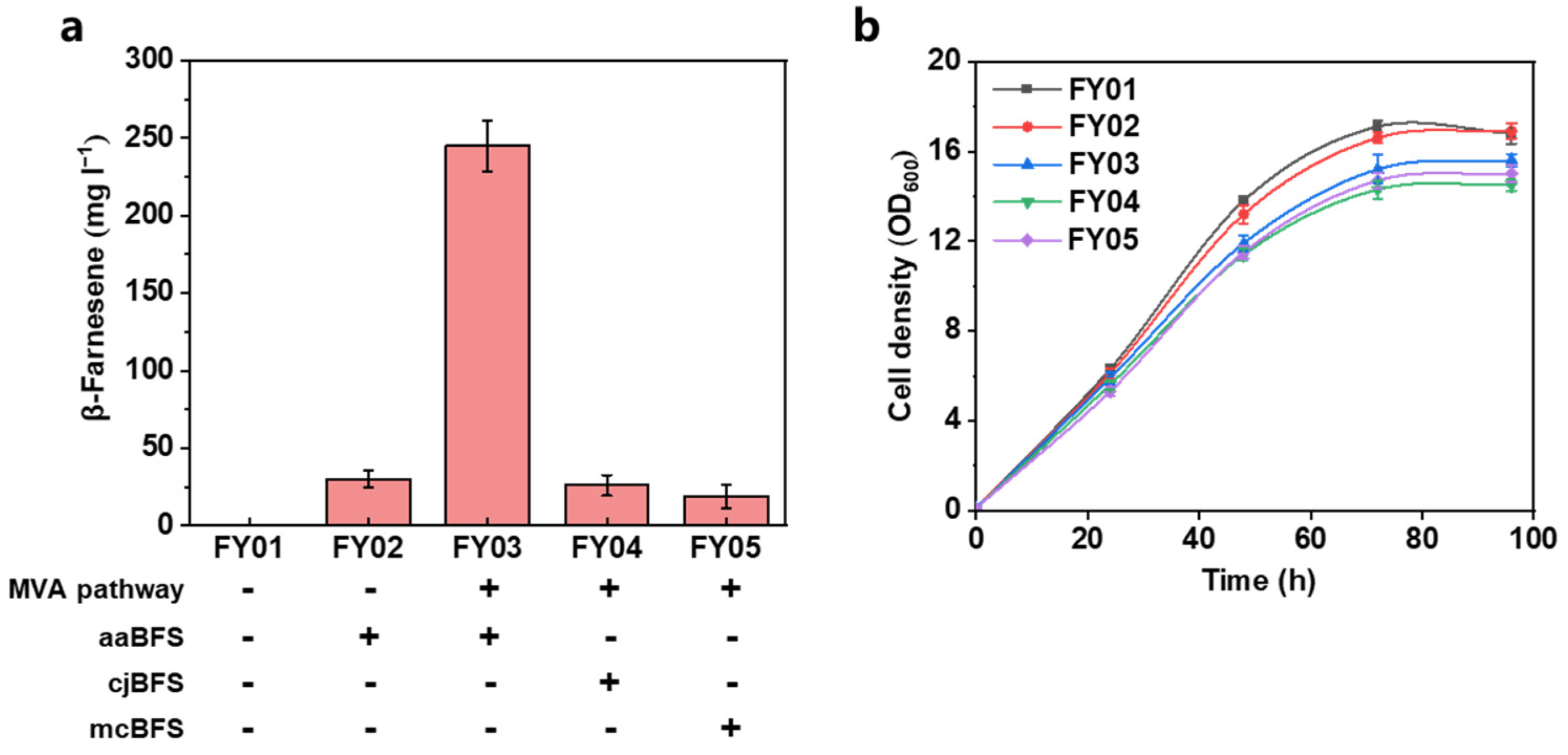
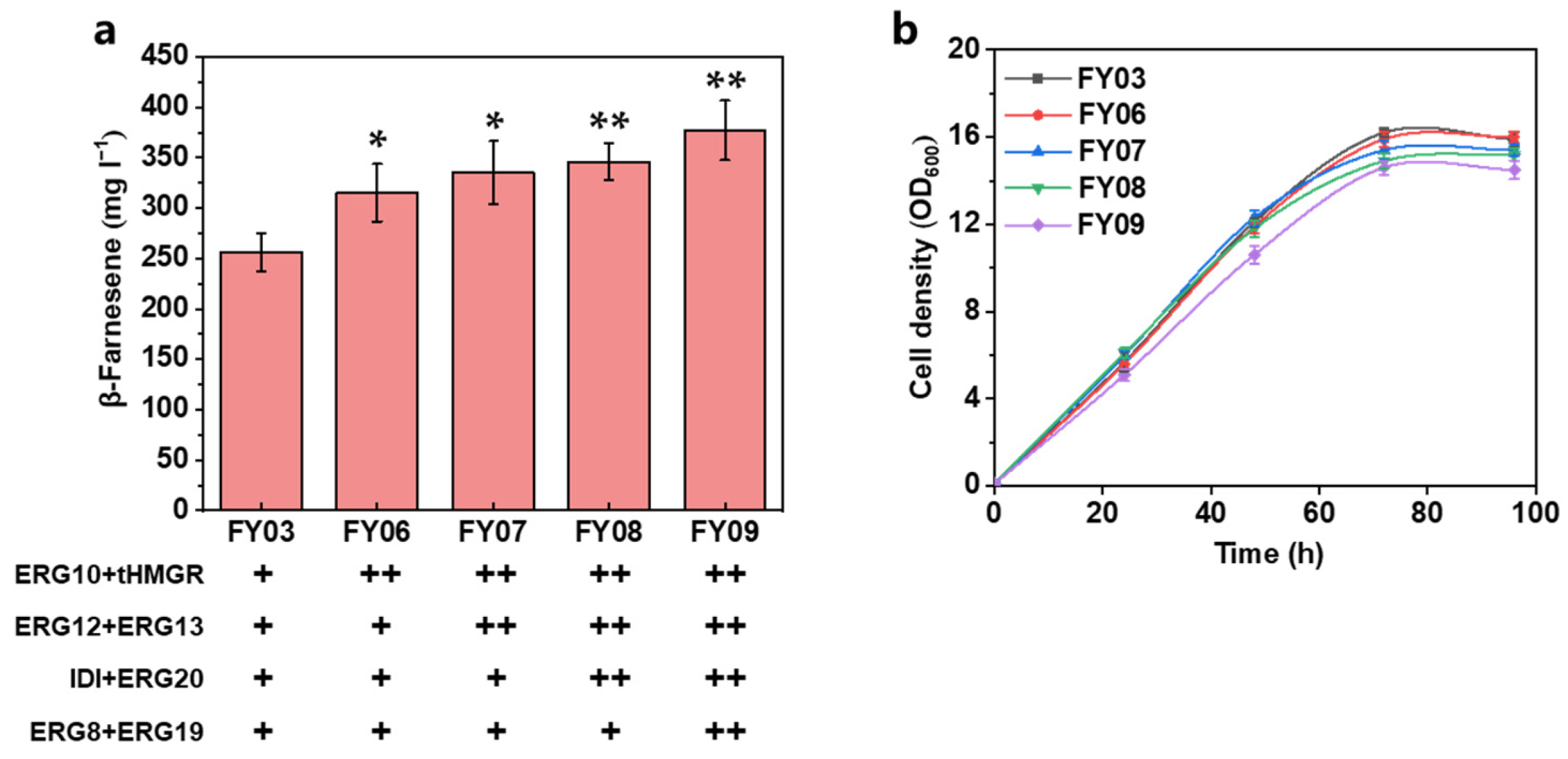

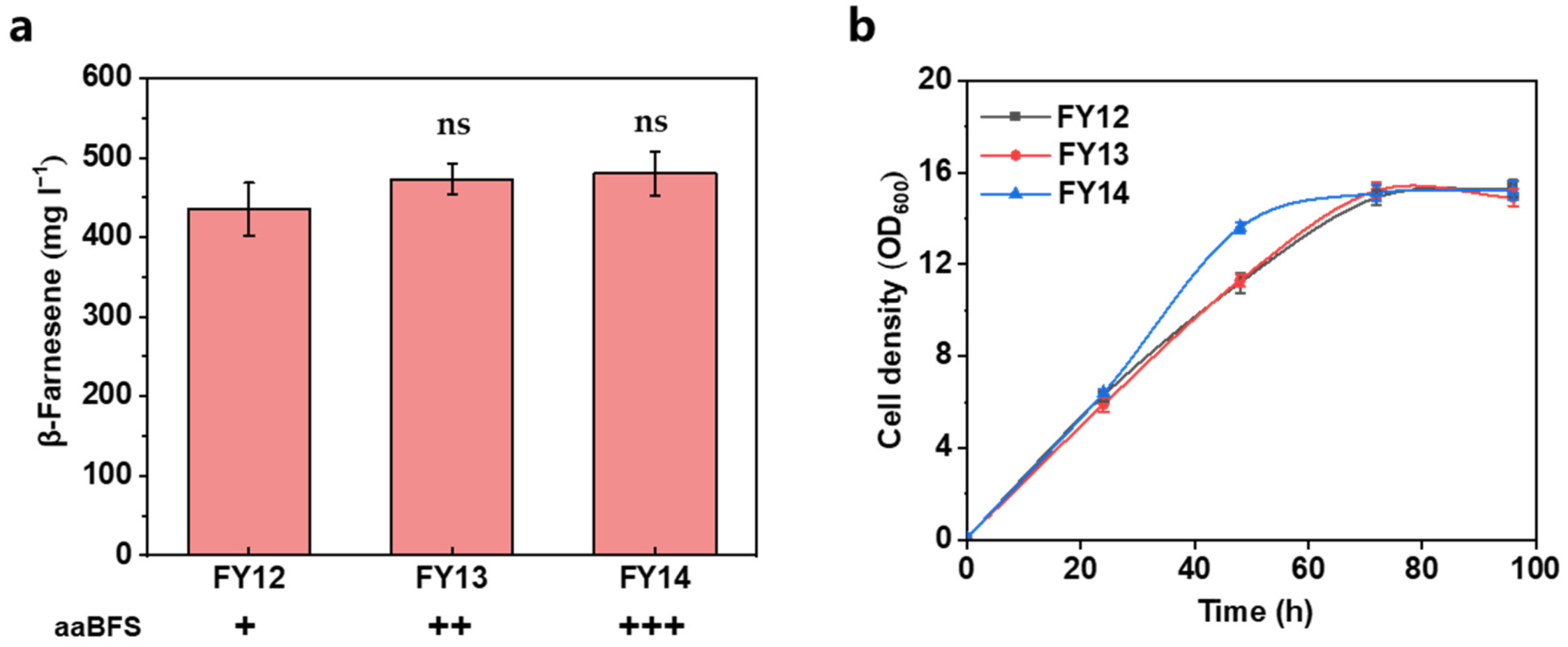
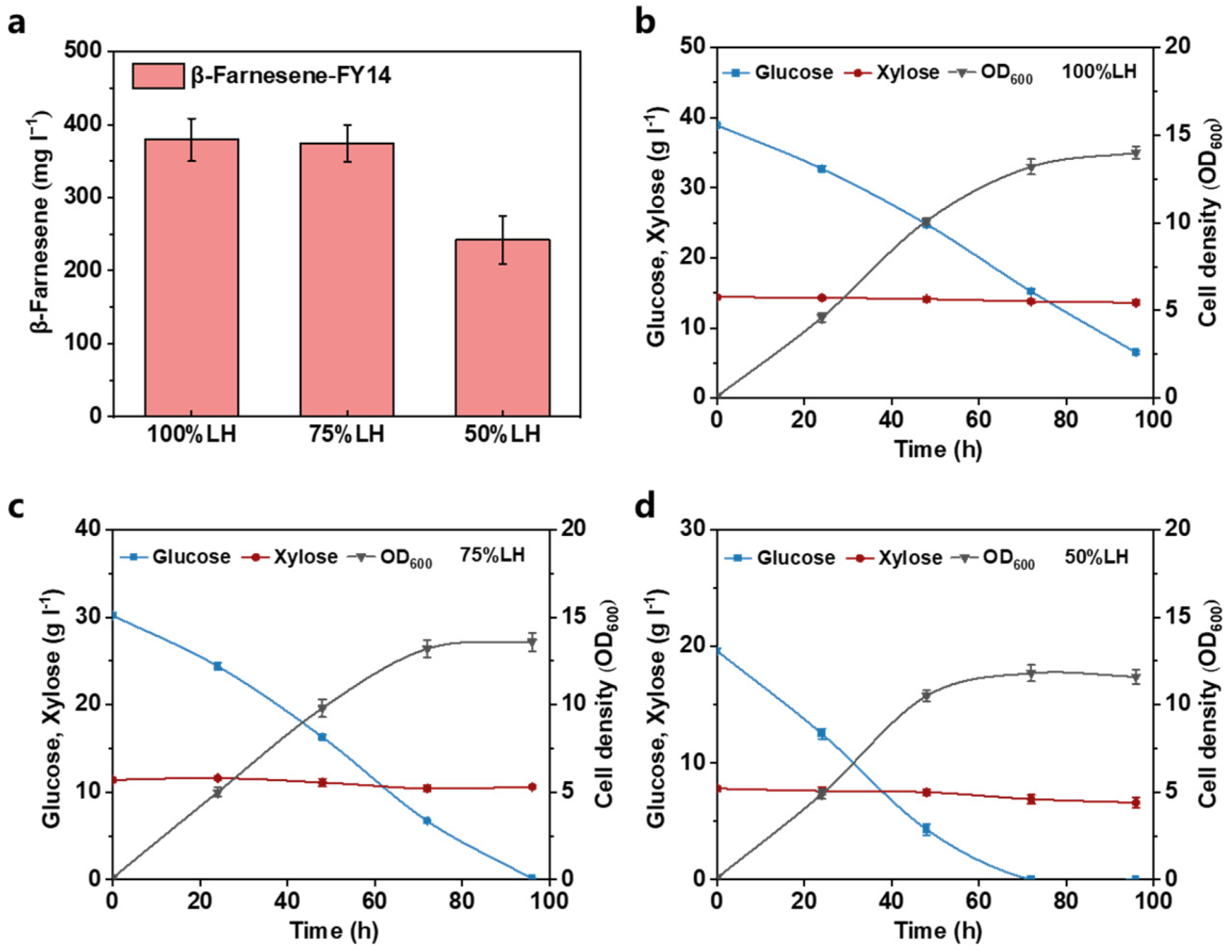

| Strain | Characteristics | Source |
|---|---|---|
| MYA2613 | matA, Ura3-302, Leu2-270, XPR2-322, Axp2-delta NU49,XPR2::SUC2 | ATCC |
| FY01 | MYA2613 Δku70, Δku80:: Leu2 | Lab storage |
| FY02 | FY01 intA1:: aaBFS | This work |
| FY03 | FY02 intC1:: ERG10, tHMGR, intC2:: ERG12, ERG13, intC3:: IDI, ERG20, intE1:: ERG8, ERG19 | This work |
| FY04 | FY03 ΔaaBFS:: cjBFS | This work |
| FY05 | FY03 ΔaaBFS:: mcBFS | This work |
| FY06 | FY03 intE2:: ERG10, tHMGR | This work |
| FY07 | FY03 intE3:: ERG12, ERG13 | This work |
| FY08 | FY03 intE4:: IDI, ERG20 | This work |
| FY09 | FY03 intF1:: ERG8, ERG19 | This work |
| FY10 | FY09 intF2:: tHMGR, tHMGR | This work |
| FY11 | FY09 intF2:: nadh-HMGR, nadh-HMGR | This work |
| FY12 | FY09 intF2:: nadh-HMGR, tHMGR | This work |
| FY13 | FY12 intF2:: aaBFS | This work |
| FY14 | FY12 intF2:: aaBFS, aaBFS | This work |
| Sample | Glucose (g L−1) | Xylose (g L−1) | Acetic Acid (g L−1) | Furfural (g L−1) | 5-Hydroxymethylfurfurals (g L−1) | Soluble Lignin (g L−1) |
|---|---|---|---|---|---|---|
| Hydrolysate | 40.3 ± 0.4 | 14.7 ± 0.3 | 1.4 ± 0.04 | 1.30 ± 0.07 | 0.70 ± 0.02 | 0.31 ± 0.02 |
| Concentrated hydrolysate | 107.4 ± 0.6 | 39.17 ± 0.5 | 3.73 ± 0.06 | 3.46 ± 0.09 | 1.86 ± 0.03 | 0.83 ± 0.01 |
| Detoxified hydrolysate | 102.3 ± 0.6 | 37.6 ± 0.3 | 3.62 ± 0.05 | 0 | 0 | 0.12 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, H.; Xv, C.; Su, C.; Feng, P.; Zhang, C.; Wang, M.; Fang, Y.; Tan, T. β-Farnesene Production from Low-Cost Glucose in Lignocellulosic Hydrolysate by Engineered Yarrowia lipolytica. Fermentation 2022, 8, 532. https://doi.org/10.3390/fermentation8100532
Bi H, Xv C, Su C, Feng P, Zhang C, Wang M, Fang Y, Tan T. β-Farnesene Production from Low-Cost Glucose in Lignocellulosic Hydrolysate by Engineered Yarrowia lipolytica. Fermentation. 2022; 8(10):532. https://doi.org/10.3390/fermentation8100532
Chicago/Turabian StyleBi, Haoran, Chenchen Xv, Changsheng Su, Pan Feng, Changwei Zhang, Meng Wang, Yunming Fang, and Tianwei Tan. 2022. "β-Farnesene Production from Low-Cost Glucose in Lignocellulosic Hydrolysate by Engineered Yarrowia lipolytica" Fermentation 8, no. 10: 532. https://doi.org/10.3390/fermentation8100532
APA StyleBi, H., Xv, C., Su, C., Feng, P., Zhang, C., Wang, M., Fang, Y., & Tan, T. (2022). β-Farnesene Production from Low-Cost Glucose in Lignocellulosic Hydrolysate by Engineered Yarrowia lipolytica. Fermentation, 8(10), 532. https://doi.org/10.3390/fermentation8100532





