Abstract
In this study, biogas residue fermented by biogas engineering was used as compost raw material, and different quality biochar was added in the composting process to explore the effect of biochar on the transformation of heavy metals in the composting process. The composting process was comprehensively analyzed with the potential ecological risk assessment of heavy metals and redundancy analysis. The addition of 10.0% biochar during composting had a strong passivation effect on exchangeable Cu and Cd, with passivation rates of 11.75 and 63.89%, respectively; the addition of 2.5 and 7.5% biochar had strong passivation ability for exchangeable Zn and Pb, and the passivation rates were 15.26 and 45.02%, respectively. At the end of composting, the potential ecological risk indexes of each treatment were T4 (10.0% biochar) > T3 (7.5% biochar) > T2 (5.0% biochar) > T1 (2.5% biochar) > CK (no biochar added). The risk of heavy metal pollution during the aerobic composting of biogas residue was low, which significantly reduced secondary pollution during the composting process.
1. Introduction
With the large-scale developments in the livestock and poultry breeding industries, the emission of livestock and poultry manure increases year by year [,]. To improve the economic benefits of livestock and poultry breeding and shorten the production cycle, Cu, As, Fe, Zn, and other elements, as well as antibiotics, hormones, and other substances are usually added to the feed. As a result, the biogas residue generated by the biogas project, with livestock and poultry manure as the main raw material, will not only increase the potential risks of soil salinity, but also NO3−-N content in groundwater and secondary salinization when it is directly returned to the field. Long-term and large-scale applications of biogas residue will also cause soil pollution due to the enrichment of heavy metals and accumulation of polycyclic aromatic hydrocarbons (PAHs), thereby adversely affecting the quality and yield of crops [].
The treatment of biogas residue after anaerobic fermentation by aerobic composting can effectively improve the shortcomings of direct digestion, and improve the quality of compost products, thereby increasing the added value of fertilizer utilization of biogas residue []. However, due to the use of additives in livestock and poultry breeding, an accumulation of heavy metals may exist in biogas residue, and studies have shown that the biological activities of Cu and Zn in biogas residue after composting treatment increases, and long-term return to farmland will cause potential pollution in the soil. Studies have shown that aerobic composting of biogas residue cannot eliminate and reduce the toxic effect of heavy metals [,]. After composting, heavy metal levels have not been effectively decreased, so it is urgent to add additives to adsorb and passivate heavy metals. Therefore, the distribution characteristics and adverse and passivation effects of heavy metals in biogas residue during aerobic composting need to be further explored.
Biochar is a stable and highly aromatic carbon-rich substance produced by the slow pyrolysis of animal and plant residues at high temperature under anoxic conditions []. Due to its unique properties, such as chemical composition, refractoriness, high surface area, micropore type, and adsorption capacity, biomass shows great potential as a modifier for various types of waste composting. Biochar improves nutrient cycling, adsorbs heavy metals, and promotes crop growth. For example, the addition of biochar and green waste compost in contaminated soil significantly increased (>30 times) the concentration of unstable arsenic, and decreased concentrations of Cd and Zn []. Studies have shown that the biological activity of Cu and Zn increased after composting, and long-term use of biogas residue cause potential pollution hazards to soil []. Therefore, the distribution characteristics and adverse and passivation effects of heavy metals in biogas residue during aerobic composting need to be further explored.
In a previous experiment, we determined the physical and chemical properties of biochar and concluded that the addition of biochar shortened the time to reach the high temperature cycle (3 days), increased the composting temperature (63.8 °C) and germination index (GI), reduced electrical conductivity (EC), reduced the loss of C and N elements, and increased the number of microorganisms in the composting process. These results indicate that biochar can improve the maturity and fertility of compost products, significantly regulate the structure and function of microbial communities during composting, promote the degradation of lignocellulose, and improve the humification of compost, which may lead to passivation effects of heavy metals.
Therefore, the passivation effects of biochar addition on heavy metal elements Cu, Zn, Cd, and Pb were explored, and the migration and transformation mechanisms of heavy metals in exchangeable, reduced, oxidized, and residual states during biogas residue composting were clarified. The purpose of this study was: (1) To determine the bio carbon effect on composting and its combination efficiency, so as to reduce the bioavailability of heavy metals; (2) To investigate the effect of biochar addition on the transformation of heavy metals in biogas residue compost; (3) To study physical and chemical factors, the relationship between heavy metals and microbial and organic degradation and mineralization, and analyze and evaluate the effect of biochar on the improvement of heavy metal pollution in sediment or soil.
2. Materials and Methods
2.1. Composting Process
The biogas residue obtained from the solid–liquid separation of the mixed residues of pig manure and corn stalk after 30 days of dry anaerobic fermentation was used as the main composting raw material. The pig manure samples were taken from a pig farm of Sanyuan Animal Production Co., Ltd., Harbin, and the corn stalk and corn cob were taken from the Acheng Experimental practice base of Northeast Agricultural University. Biochar is a compost additive that is made of dried corncob in a N2 tubular carbonization furnace (600 °C, 1 h) under low oxygen conditions The composting bacterial agent was obtained by the high-value utilization team of agricultural and animal husbandry waste resources of Northeast Agricultural University. The initial basic physical and chemical properties of each composting raw material are shown in Table 1.

Table 1.
Initial physical and chemical properties of composting materials.
In the experiment, biogas residue after solid–liquid separation of residues from anaerobic fermentation of pig manure and corn stalk was used as the main compost raw material, and biochar was used as a compost additive, with the determined addition ratio of 0–10% [,]. The auxiliary materials included a small amount of crushed corn stalk and pig manure. To adjust the C/N ratio and water content of the compost materials, they were made to be 25:1 and 60–65%, respectively. The five treatment settings were as follows: T1: composting raw material +2.5% biochar; T2: composting material +5.0% biochar; T3: composting raw material +7.5% biochar; T4: composting raw material +10.0% biochar; CK (control): no biochar added. The amount of added biochar was calculated according to the dry basis quality of compost raw materials, and the added amount of compost raw materials for each treatment is shown in Table 2. Each treatment was carried out in triplicate.

Table 2.
Amount of composting materials added in each treatment.
An aerobic composting reactor was used in this experiment. The reactor is made of stainless steel. The outer diameter, height, and wall thickness of the equipment are 35, 70, and 5 cm, respectively, and the effective volume is 25 L. After the composting materials were fully mixed, they were put into the composting reactor for aerobic composting of biogas residue for 40 days. A stirring device was used to stir the reactor every 2 days. In the process of composting, the initial and room temperature (19–21 °C) of each treatment were kept the same, to eliminate the impact of external environmental temperature differences on the composting system. In this experiment, the temperature of the reactor body increased through the microbial reactions in the system. There was no external temperature control device, and the double-layer insulated stainless steel of the reactor could effectively prevent the loss of heat from the reactor body. The ventilation volume of the reactor was determined based on temperature control, and the calculation formula is shown in Formula (1) []:
where Q(θ)is the ventilation demand during composting, m3·; mc is the dry weight of stock, kg; mo is the initial dry weight of stacking material, kg; me is the dry weight of non-degradable material in the pile, kg; θ is composting time, d; ρ0 is the air density, taking 1.18 kg·m3; hc is the burning heat of the reactor material, which is −20,000 kJ·; k is the disappearance rate of dry matter in the stock, d−1; ΔH is the enthalpy difference between inlet and outlet air, kJ·; and β0 is the biochemical degradation constant, 0.5.
The maximum ventilation demand was calculated to be 0.05 m3/(min·m3). The aeration ventilation device was connected to the bottom of the compost reactor for ventilation. The aeration duration was set at 5 min and the interval at 40 min through the reactor control system. During the experiment, the temperature of the center of the pile was measured every day, and 200 g compost samples at 20 cm of the pile were collected on Days 1, 2, 4, 8, 16, 24, 32, and 40. Each sample was divided into two parts on average, which were used for the detection of physical and chemical indices and stored in a −4 °C refrigerator. Each index was repeated 3 times, in parallel.
2.2. Analytical Methods
The moisture content was measured by the drying method (105 °C, 12 h). The pH was determined by the pH potentiometer method (PHSJ-3F); EC was determined by a conductivity meter; and specific surface area was determined by a specific surface and pore size analyzer (Bei Shi De, Beijing, China) [,]. Total nitrogen (TN) content was determined using an EA 3000 elemental analyzer (Euro Vector, Italy). Total organic carbon (TOC) content was determined by a potassium dichromate external heating method and organic carbon analyzer (Elementar, Germany). The content of organic matter (OM) was determined by Muffle furnace burning. The contents of total phosphorus (TP) and total potassium (TK) were determined by ammonium molybdate spectrophotometry and flame atomic absorption spectrometry.
2.3. Determination of Heavy Metals
The compost samples were pretreated with an improved BCR continuous extraction method, and the contents of heavy metals, Cu, Zn, Cd, and Pb, were determined according to the four forms of exchangeable state, reduced state, oxidized state, and residual state [].
Exchangeable heavy metals are toxic to plant growth. Therefore, in this study, the passivation effect of exchangeable heavy metals was selected to measure the passivation of their activity. The distribution rate of various forms of heavy metals and the passivation effect were determined according to Formula (2) [,].
where is the distribution rate of various forms of heavy metals, %; is the amount of different forms of heavy metals, kg; is the total mass of the heavy metal, kg; is the passivation effect of heavy metals, %; is the distribution rate of heavy metals before composting, %;and is the distribution rate of heavy metals after composting, %.
2.4. Data Processing
Canoco 5 was used for redundancy analysis (RDA) between heavy metals and physicochemical properties after composting. To evaluate the impact of compost products on environmental capacity, the ecological risk assessment index of heavy metals was used to evaluate the potential pollution risk of heavy metals in compost products [].
2.5. Statistical Analysis
The experimental data were expressed in the form of “mean ± standard error”. The data were sorted using Excel 2013 software, and the drawing was made by Origin 9.0 software. One-way analysis of variance (ANOVA), Duncan’s multiple comparisons, correlation analysis, PCA, and CA were analyzed using SPSS 25.0 software, and p < 0.05 was considered to be a significant difference.
3. Results
3.1. Activity Changes of Heavy Metals Cu, Zn, Cd, and Pb
The bioavailability of the exchangeable forms of heavy metals is high, whereas the bioavailability of other forms is low. Therefore, reducing levels of exchangeable heavy metals will reduce their toxicity to crops and reduce toxicity entering crops. The distribution rate of heavy metal forms is an important index to evaluate the impact of compost on the environment.
The distribution rates of exchangeable Cu in each treatment decreased after composting (Table 3). The passivation effect on exchangeable Cu increased with the increase of biochar addition, and T4 had the best passivation effect (Figure 1). Some researchers have used biochar to passivate Cu during composting, and their results were similar to our findings []. Among the treatments, only the reduced content of T2 increased after composting. This may be due to the continuous degradation of compost, with the dry matter mass of compost decreasing slowly and the wet matter mass significantly decreasing, which can lead to an increase in the concentration of dry matter in the total compost, resulting in the concentration effect (dry matter quality: the quality of the remaining material after drying water). Based on the analysis, we found that biochar has a strong ability to transform substances due to its rich porous structure and high specific surface area. At the same time, biochar contains functional groups such as phenolic hydroxyl and carboxyl on its surface, and the structural characteristics of these aromatic compounds enable biochar to adsorb heavy metal ions in the reactor body through ion exchange, electrostatic interaction, and diffusion into the internal surface of micropores, effectively promoting the passivation of Cu bioavailability in the reactor body. We conclude that the addition of biochar during biogas residue composting can reduce the activity and mobility of Cu, thus effectively inhibiting its bioavailability.

Table 3.
Effect of biochar application on speciation of Cu during biogas residue composting.
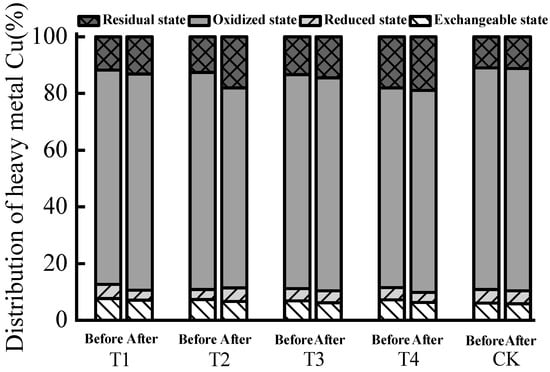
Figure 1.
Distribution rate of various forms of Cu before and after biogas residue composting.
The relationship between the content of exchangeable Zn in each treatment after composting was CK > T2 > T3 > T1 > T4. Compared with no addition (CK), T1–T4 decreased by 12.07, 4.50, 10.54, and 13.60%, respectively (Table 4). The passivation effect of T4 on heavy metal Zn was significantly higher than that of other treatments, whereas the passivation effect of T2, T3, and T4 were not significantly different. Each treatment with biochar addition effectively reduced the distribution rate of exchangeable Zn (Table 4). In addition, the relationship between the four forms and content of Zn in each treatment was in the order of reduced state > oxidized state > residual state > exchangeable state (Figure 2). The results showed that extractable Zn rapidly decreased when biochar addition was 2–7%. With the addition of 10–13% biochar, the reduction effect was not obvious [], which is similar to the trends found in this study. The analysis showed that the passivation effect of the heavy metal Zn was related to the amount of added biochar. The concentration of heavy metals passivated by biochar had a lower limit. When the content of heavy metals in the reactor was low, the increase in biochar addition was not obvious in its passivation effect.

Table 4.
Effect of biochar application on speciation of Zn during biogas residue composting.
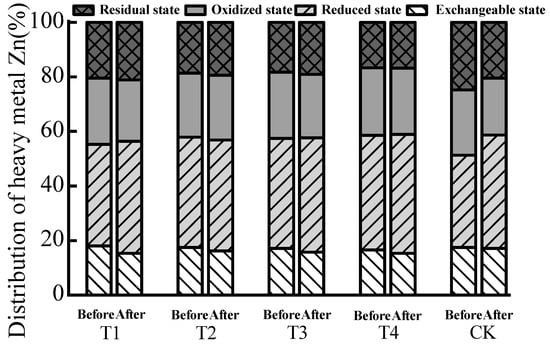
Figure 2.
Distribution rate of various forms of Zn before and after biogas residue composting.
The relationship between the four forms and content of Cd in each treatment was oxidized state > residual state > exchangeable state > reduced state (Table 5). The content of exchangeable Cd decreased after composting, and the content of reduced Cd and residual Cd increased (Figure 3), indicating that the addition of biochar promoted the transformation of Cd from an active to weak form. Compared with CK, the distribution rate of exchangeable Cd in each treatment decreased more with the addition of biochar. The passivation effect of biochar on Cd increased as more biochar was added, and there was no significant difference among T1, T2, and T3 treatments (Table 5). Comprehensive analysis showed that the chemical inertness and aromatization of biochar determined that they could adsorb Cd2+ through non-electrostatic physical adsorption with relatively weak adsorption affinity []. Studies have shown that biochar, humic acid, and peat were selected as passivation agents during composting, and biochar had the strongest passivation effect on the heavy metal Cd []. Combined with the results of this study, this indicates that biochar’s smaller particle size and larger specific surface area makes it better at adsorbing Cd2+.

Table 5.
Effect of biochar application on speciation of Cd during biogas residue composting.
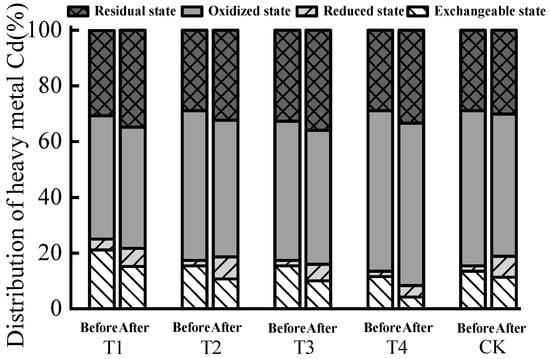
Figure 3.
Distribution rate of various forms of Cd before and after biogas residue composting.
After composting, the content of reduced, oxidized, and residual forms of heavy metal Pb increased, and the content of exchangeable forms decreased (Figure 4), indicating that the addition of biochar during composting effectively promoted the transformation of exchangeable Pb to less toxic oxidized and residual forms. Similar results were obtained in related studies []. The content of exchangeable Pb in each treatment after composting decreased by 8.47, 16.67, 37.50, 35.19, and 3.03%, respectively (Table 6), and the decrease was more obvious when biochar addition was 7.5%. The distribution rate of exchangeable Pb decreased after composting. In terms of the passivation effect of biochar on Pb, the passivation effect was the best when the biochar addition was 7.5%, which was significantly higher than in other treatments. The passivation effect of biochar treatments were significantly higher than CK, whereas the differences between T1, T2, and T4 treatments were nonsignificant. The comprehensive analysis showed that the biochar had a strong passivation ability to the heavy metal Pb in the pile. The main reason is that, during the preparation of biochar, the organic components are cracked into organic C, and the remaining inorganic mineral components exist in the form of ash; the inorganic mineral components show strong adsorption affinity and capacity for Pb2+, imparting significant passivation effects for Pb []:
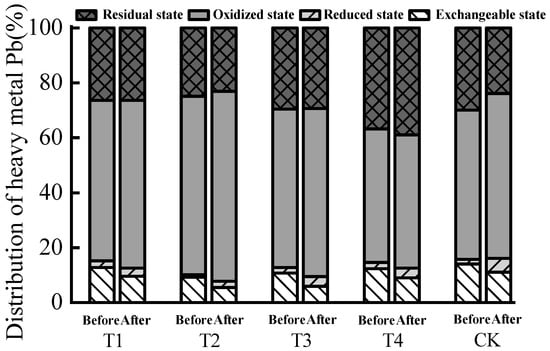
Figure 4.
Distribution rate of various forms of Pb before and after biogas residue composting.

Table 6.
Effect of biochar application on speciation of Pb during biogas residue composting.
3.2. Environmental Risk Assessment of Heavy Metals
The potential ecological hazard index method is based on the characteristics of heavy metals themselves and their impact on environmental behavior, combined with the principles of ecology, biotoxicology, environmental science, and sedimentology. It quantitatively evaluates the degree of heavy metal pollution in sediment or soil and their potential environmental risks []. During the entire composting process, the relationship between the potential ecological risk coefficients of the heavy metals in each treatment was Cd > Cu > Pb > Zn. After composting, the potential ecological risk coefficients of heavy metals, Cu and Cd, decreased in each treatment, indicating that biochar had a significant passivation effect on these elements (Table 7), which was confirmed by previous findings. The potential ecological risk index in the biochar treatments gradually decreased with the increase in added biochar; the potential ecological risk of each treatment was in the lowest rank of “slight degree”. The analysis showed that biochar played an effective role in reducing the bioavailability and passivation of various heavy metals during biogas residue composting, and the risk of heavy metal pollution during aerobic composting was low, which significantly reduced the secondary pollution during composting.

Table 7.
Potential ecological risk assessment of heavy metals in biogas residue compost products.
3.3. Formatting of Mathematical Components
Using RDA can more accurately describe the correlation between heavy metal speciation and physical and chemical properties of compost (Figure 5) [,]. The 20 physicochemical properties of the selected compost explained 100% of all the eigenvalues, indicating that the above factors had a significant impact on the content and morphological distribution of heavy metals in compost. C/N, moisture content, organic matter, and fungal content were the main factors affecting the content and morphological distribution of heavy metals (Table 8). Wang et al. [] used RDA to show that heavy metals were correlated with the humification and degradation of organic matter. In this study, heavy metals were associated with compost physicochemical properties, microorganism and organic matter degradation, and mineralization. In addition, the organic matter content in the stack was significantly positively correlated with the exchangeable states of the heavy metals, Cu, Zn, Cd, and Pb (Figure 5). Due to the continuous degradation of organic matter during the composting process, the content of ash substances, such as P, K, and other elements, increases in the compost. The addition of biochar can effectively promote the combination of P and K with heavy metals, resulting in precipitation and gradual conversion of exchangeable heavy metals, thereby reducing the bioavailability of heavy metals [].
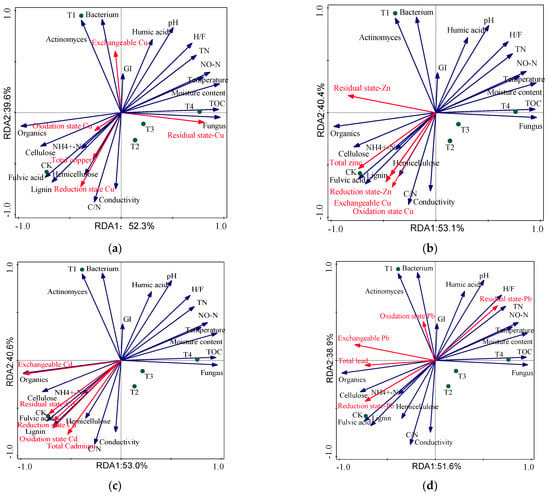
Figure 5.
Redundancy analysis (RDA) of heavy metal speciation and physicochemical properties in compost with biochar addition: (a) Cu, (b) Zn, (c) Cd, and (d) Pb.

Table 8.
Redundancy analysis (RDA) results of heavy metals and physicochemical property factors in compost.
4. Effect Mechanism of Biochar on Composting of Biogas Residue
There were significant differences in the effects of different quality biochar on the aerobic composting of biogas residue. Compared with CK, adding biochar increased the organic matter degradation rate by 7.75–17.55% by increasing the material porosity of the pile body, improving internal air circulation, improving microbial activity, and shortening the time at peak temperature of the pile body, which increased the heap body temperature, resulting in high temperature compost for longer periods and promoting organic matter decomposition and humification. There was a large amount of lignocellulose in the compost biogas residue. The degree of lignocellulose degradation plays a key role in promoting the formation of humus and improving the maturity of compost. The results of this study showed that, compared with CK, the degradation rate of lignocellulose increased in all treatments with biochar. Therefore, biochar can improve the conversion and degradation efficiency of lignocellulose in the heap by optimizing the heap structure, which plays an important role in the humification process of biogas residue composting. In the process of aerobic composting, the decrease in exchangeable heavy metal content in the heap and increase in residual heavy metal content can represent a decrease in the bioavailability of heavy metals. The addition of biochar significantly reduced the secondary pollution in the composting process by reducing the bioavailability of heavy metals in the composting process. The results of this study showed that, by the end of composting, the exchangeable Cu, Zn, Cd, and Pb contents of the biochar-added treatments decreased by 9.87–16.97, 3.48–13.56, 31.25–66.67, and 8.47–37.50%, respectively. This means that the Cu, Zn, Cd, and Pb levels were decreased 2.93, −2.15, 14.29, and 3.03% faster in biochar treatments than in CK. The contents of Cu, Zn, Cd, and Pb in the residue increased by 5.22–11.45, −1.74–8.66, 10.12–15.56, and −7.48–6.03%, respectively, which were higher than those of CK by 1.57, −17.23, 4.65, and −20.11%, respectively.
Biochar not only optimizes the pores of the pile, but also accelerates the humification process of biogas slag compost by improving the physical and chemical properties of the pile. Biochar contains a certain amount of OH− ions to neutralize the H+ in the reactor. In addition, the ash content is relatively high, among which salt ions such as Na+, K+, Mg2+, and Ca2+ can effectively exchange H+ in the reactor, and regulate the pH of the reactor. The ions mentioned above can also exchange with heavy metal ions in the pile. At the same time, the humic acid generated in the composting process can be complexed with heavy metals. The -COOH, -OH, and -C=O in organic matter can promote the conversion of heavy metals from an exchangeable state to residue state through a complexation reaction, thus reducing the bioavailability of heavy metals in biogas slag compost.
The exchangeable forms of Cu, Zn, Pb, and Cd have the highest mobility and bioavailability among the extractable parts. pH change and adsorption strongly influences the morphology of heavy metals. On the one hand, the pH value after composting showed an increasing trend; on the other hand, the addition of biochar reduced the activity of heavy metals in the fertilizer through ion exchange, surface adsorption, and co-precipitation. The main phase of Cu was the oxidized state. There was no significant increase in Cu residue because humic acid was the main factor affecting Cu inactivation during composting, and Cu mainly combined with humic acid to become its unavailable form. The main phase of Zn was the reduced state, and Zn has a high adsorption affinity for hydroxides. The aerobic alkaline condition of compost was conducive to the formation of hydroxides, although compost can produce a large amount of humus, making it possible to form a stable complex with Zn. The well-known high affinity of Cu for dissolved organic matter may explain why Cu release in compost is mainly driven by complexation with dissolved organic matter, depending on the concentration and aromaticity of dissolved organic matter and despite the enhanced solid-relative Cu binding capacity resulting from simultaneous composting of pH. In contrast, Zn had a lower affinity for dissolved organic matter compared with Cu, which could explain why Zn was not passivated as well as Cu with increasing compost pH []. The main phase of Cd was the oxidized state, and the exchangeable fraction of Cd decreased before and after composting. The reduced and residual state increased. Cd can exist in the form of divalent organic complexes [,], chelates, and complex ions, and can bind humus. However, unlike Cu, which is closely bound to humus, Cd mainly binds to aliphatic regions of humus in digestion. Therefore, Cd is less stable than Cu []. The main phase of Pb was the oxidized state, and biochar is usually alkaline, which increases the pH of the compost. Heavy metal ions can also be converted to hydroxides and deposited on the surface of biochar. Pb formed complex compounds with functional groups on the surface of biochar. In the preparation of biochar, the organic component is decomposed into organic carbon, and the remaining inorganic mineral component exists in the form of ash. Inorganic mineral fractions showed strong adsorption affinity and capacity for Pb2+.
5. Conclusions
This study revealed that adding different quality biochar during aerobic composting of biogas residue could change the bioavailability of heavy metals, decreasing the content of exchangeable heavy metals in compost, increasing the content of residual heavy metals, and decreasing heavy metal bioavailability. The passivation rates of exchangeable heavy metals, Cu, Zn, Cd, and Pb, by biochar were 7.41–11.75, 7.41–15.26, 28.06–63.89, and 25.16–45.02%, respectively. The order of the potential ecological risk coefficients of single heavy metals was Cd > Cu > Pb > Zn. The potential ecological risk indices of heavy metals in each biochar treatment were lower at the end of composting than in the treatment without biochar(CK), and the lowest value was obtained when 0.38 kg (T4) was added. The results of redundancy analysis showed that C/N, water content, organic matter, and fungal contents were the main factors affecting the content and speciation distribution of heavy metals in the physical and chemical properties of the heap.
Author Contributions
Conceptualization, Y.Q., J.Q., and Y.S.; methodology, Y.S. and W.Y. (Wencong Yan); validation, W.Y. (Wencong Yan), Y.S., and W.Y. (Weiming Yi); formal analysis, W.Y. (Wencong Yan) and T.Y.; investigation, W.Y. (Wencong Yan); resources, Q.Z., W.Y. (Wencong Yan), and X.L.; data curation, W.Y. (Wencong Yan), J.Q. and Y.S.; writing—original draft preparation, W.Y. (Wencong Yan), J.Q., and Y.S.; writing—review and editing, Y.Q., T.Y., and X.L.; supervision, Y.S. and W.Y. (Wencong Yan); project administration, J.Q. and Y.S.; funding acquisition, J.Q.,Y.S., Q.Z., W.Y. (Weiming Yi), and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China, Grant Number 2019YFD1100603 and Special Project of Heilongjiang Provincial Academy of Science and Technology Cooperation, China, Grant Number YS20B01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the Academic Backbone Project of Northeast Agricultural University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xiao, X.; Xi, B.D.; He, X.S. Hydrophobicity-dependent electron transfer capacities of dissolved organic matter derived from chicken manure compost. Chemosphere 2019, 222, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Tan, W.B. Biowaste-source-dependent synthetic pathways of redox functional groups within humic acids favoring pentachlorophenol dechlorination in composting process. Environ. Int. 2020, 135, 105380. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Q.; Ma, J. Fungal community composition change and heavy metal accumulation in response to the long-term application of anaerobically digested slurry in a paddy soil. Ecotoxicol. Environ. Saf. 2020, 196, 110453. [Google Scholar] [CrossRef]
- Meng, X.; Yan, J.; Zuo, B.; Wang, Y.; Yuan, X.; Cui, Z. Full-scale of composting process of biogas residues from corn stover anaerobic digestion: Physical-chemical, biology parameters and maturity indexes during whole process. Bioresour. Technol. 2020, 302, 122742. [Google Scholar] [CrossRef]
- Lei, C.; Yongcui, W.; Bin, H.; Jian, M.; Xin, C. Dissipation Dynamics of Doxycycline and Gatifloxacin and Accumulation of Heavy Metals during Broiler Manure Aerobic Composting. Molecules 2021, 26, 5225. [Google Scholar] [CrossRef]
- Knoop, C.; Tietze, M.; Dornack, C.; Raab, T. Fate of nutrients and heavy metals during two-stage digestion and aerobic post-treatment of municipal organic waste. Bioresour. Technol. 2018, 251, 238–248. [Google Scholar] [CrossRef]
- Pareek, A.; Dhankher, O.P.; Foyer, C.H. Mitigating the impact of climate change on plant productivity and ecosystem sustainability. J. Exp. Bot. 2020, 71, 451–456. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 58, 2282–2287. [Google Scholar] [CrossRef]
- Chang, Y.; Zhao, H.; Sun, L.; Cui, J.; Liu, J.; Tang, Q.; Du, F.; Liu, X.; Yao, D. Resource Utilization of Biogas Waste as Fertilizer in China Needs More Inspections Due to the Risk of Heavy Metals. Agriculture 2022, 12, 72. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, H.; Fu, Q. Effects of four additives in pig manure composting on greenhouse gas emission reduction and bacterial community change. Bioresour. Technol. 2019, 292, 121896. [Google Scholar] [CrossRef]
- He, X.; Yin, H.; Han, L. Effects of biochar size and type on gaseous emissions during pig manure/wheat stalk aerobic composting: Insights into multivariate-microscale characterization and microbial mechanism. Bioresour. Technol. 2019, 271, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, B.; Damasceno, F.A.; Andrade, R.R.; Saraz, J.A.O.; Barbari, M.; Vega, F.A.O.; Nascimento, J.A.C. Comparison of airflow homogeneity in Compost Dairy Barns with different ventilation systems using the CFD model. Agron. Res. 2020, 18, 788–796. [Google Scholar] [CrossRef]
- Qu, J.; Liu, Y.; Xu, X.; Liu, Z.; Meng, X. Evaluation of the effects of different fertilization modes on black soil fertility in China based on principal component and cluster analysis. Agrochimica 2020, 64, 149–166. [Google Scholar] [CrossRef]
- Qu, J.; Sun, Y.; Awasthi, M.K.; Liu, Y.; Xu, X.; Meng, X.; Zhang, H. Effect of different aerobic hydrolysis time on the anaerobic digestion characteristics and energy consumption analysis. Bioresour. Technol. 2021, 320, 124332. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Xiong, T.; Wang, H.; Wu, Z.; Jiang, L.; Zeng, G.; Li, Y. Immobilization of heavy metals in two contaminated soils using a modified magnesium silicate stabilizer. Environ. Sci. Pollut. Res. 2018, 25, 32562–32571. [Google Scholar] [CrossRef]
- Lan, J.; Zhang, S.; Dong, Y. Stabilization and passivation of multiple heavy metals in soil facilitating by pinecone-based biochar: Mechanisms and microbial community evolution. J. Hazard. Mater. 2021, 420, 126588. [Google Scholar] [CrossRef]
- Zhang, L.; Shang, Z.; Guo, K. Speciation analysis and speciation transformation of heavy metal ions in passivation process with thiol-functionalized nano-silica. Chem. Eng. J. 2019, 369, 979–987. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, H.; Zhao, L.; Shen, Y.; Hou, Y.; Cheng, H.; Song, L. Effect of biochar and humic acid on the copper, lead, and cadmium passivation during composting. Bioresour. Technol. 2018, 258, 279–286. [Google Scholar] [CrossRef]
- Kong, Y.; Ma, R.; Li, G. Impact of biochar, calcium magnesium phosphate fertilizer and spent mushroom substrate on humification and heavy metal passivation during composting. Sci. Total Environ. 2022, 824, 153755. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, Z.M.; Li, X.; Liu, H.; Wei, S. Mitigation of rice cadmium (Cd) accumulation by joint application of organic amendments and selenium (Se) in high-Cd-contaminated soils. Chemosphere 2020, 241, 125106. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Qiu, L.; Zheng, S.A. Kinetic approach for assessing the effect of flooding on the redistribution of heavy metals in paddy soil. Fresenius Environ. Bull. 2014, 23, 113–121. [Google Scholar]
- Shen, Z.; Hou, D.; Jin, F. Effect of production temperature on lead removal mechanisms by rice stalk biochars. Sci. Total Environ. 2019, 655, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Z.; Lu, X. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Cai, Q.; Mo, C.; Wu, Q. Concentration and speciation of heavy metals in six different sewage sludge-composts. J. Hazard. Mater. 2007, 147, 1063–1072. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Zeng, G.; Dong, H.; Chen, Y.; Huang, C. Multivariate relationships between microbial communities and environmental variables during co-composting of sewage sludge and agricultural waste in the presence of PVP-AgNPs. Bioresour. Technol. 2018, 261, 10–18. [Google Scholar] [CrossRef]
- Wang, Q.; Awasthi, M.K.; Ren, X.; Zhao, J.; Li, R.; Wang, Z.; Chen, H.; Wang, M.; Zhang, Z. Comparison of biochar, zeolite and their mixture amendment for aiding organic matter transformation and nitrogen conservation during pig manure composting. Bioresour. Technol. 2017, 245, 300–308. [Google Scholar] [CrossRef]
- Meng, J.; Wang, L.; Liu, X.; Wu, J.; Brookes, P.C.; Xu, J. Physicochemical properties of biochar produced from aerobically composted swine manure and its potential use as an environmental amendment. Bioresour. Technol. 2013, 142, 641–646. [Google Scholar] [CrossRef]
- Laurent, C.; Bravin, M.N.; Crouzet, O.; Pelosi, C.; Tillard, E.; Lecomte, P.; Lamy, I. Increased soil pH and dissolved organic matter after a decade of organic fertilizer application mitigates copper and zinc availability despite contamination. Sci. Total Environ. 2020, 709, 135927. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zou, X.; Shu, R.; Ding, L.; Yao, K. Impact of humin on soil adsorption and remediation of Cd (II), Pb (II), and Cu (II). Soil Sediment Contam. Int. J. 2016, 25, 700–715. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Sun, Y.; Yang, G. One-pot pyrolysis route to Fe−N-Doped carbon nanosheets with outstanding electrochemical performance as cathode materials for microbial fuel cell. Int. J. Agric. Biol. Eng. 2020, 13, 207–214. [Google Scholar] [CrossRef]
- Barančíková, G.; Makovníková, J. The influence of humic acid quality on the sorption and mobility of heavy metals. Plant Soil Environ. 2003, 49, 565–571. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).