Effect of Fermentation Parameters on the Anthocyanin Content, Sensory Properties, and Physicochemical Parameters of Potato Blueberry Yogurt
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Pretreatment of Potato and Blueberry
2.3. Production of Potato Blueberry Yogurt
2.4. Microbiological Analysis of Yogurt Samples
2.5. Anthocyanin Content Analysis
2.6. Soluble Solid Content, pH Determination, and Color Analysis
2.7. Determination of Reducing Sugars
2.8. Sensory Evaluation
2.9. One Factor at a Time (OFAT) Experiment
2.10. Electronic Tongue Analysis
2.11. PB Design
2.12. BBD
2.13. Statistical Analysis
3. Results
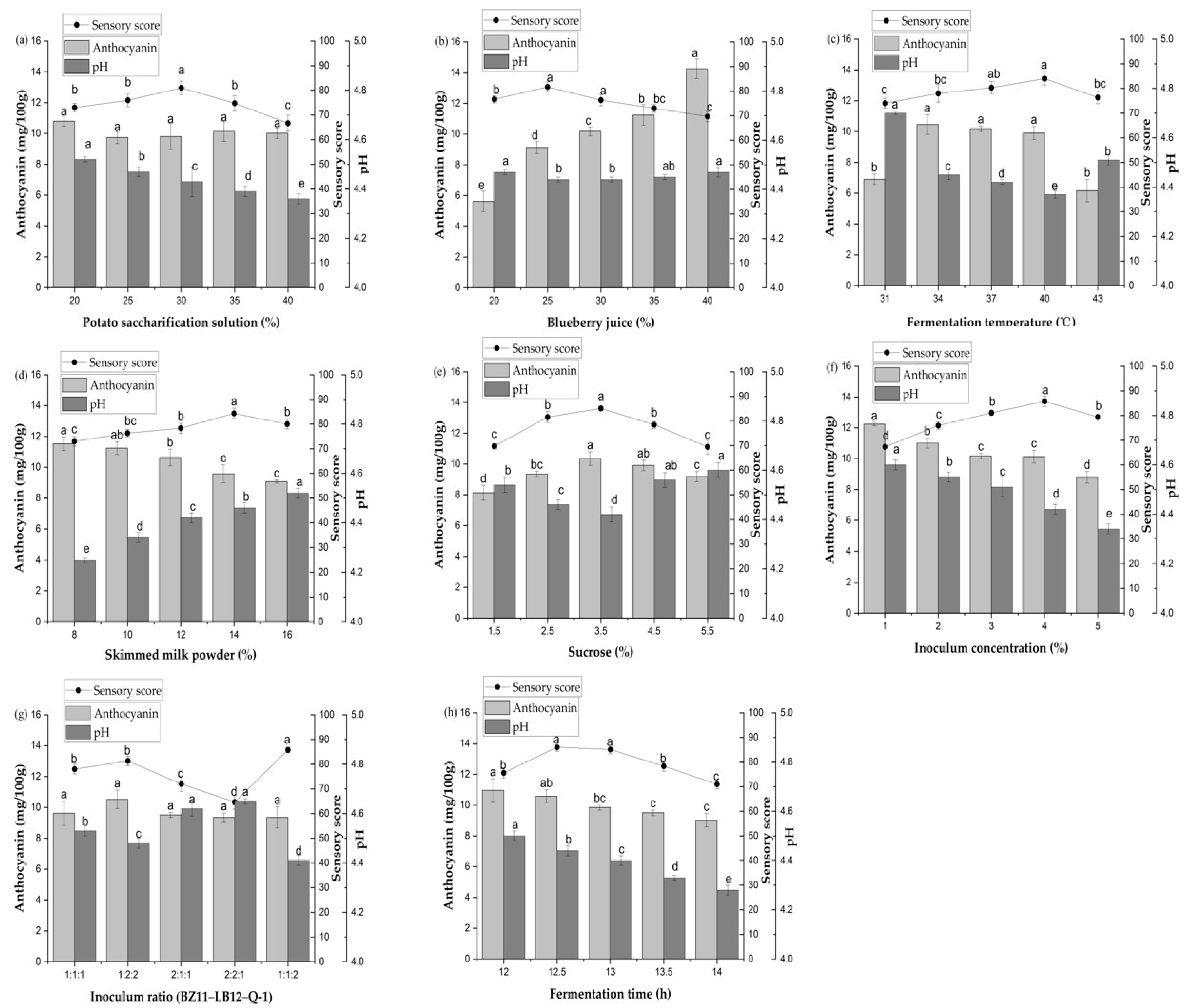
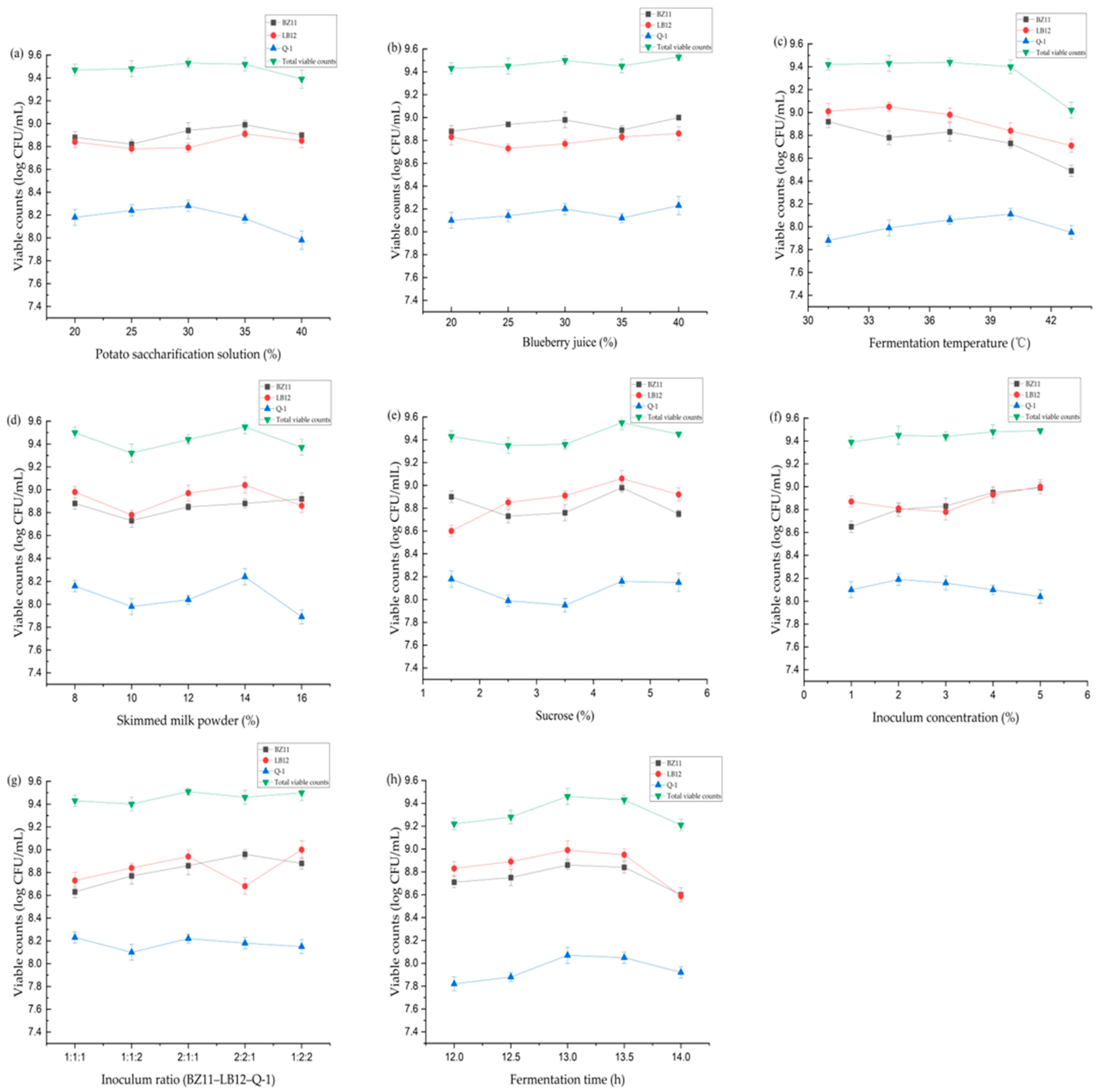
3.1. Effect of Potato Saccharification Solution on Potato Blueberry Yogurt
3.2. Effect of Blueberry Juice on Potato Blueberry Yogurt
3.3. Effect of Fermentation Temperature on Potato Blueberry Yogurt
3.4. Effect of Skimmed Milk Powder on Potato Blueberry Yogurt
3.5. Effect of Sucrose on Potato Blueberry Yogurt
3.6. Effect of Inoculum Concentration on Potato Blueberry Yogurt
3.7. Effect of Inoculum Ratio on Potato Blueberry Yogurt
3.8. Effect of Fermentation Time on Potato Blueberry Yogurt
3.9. Plackett–Burman Design
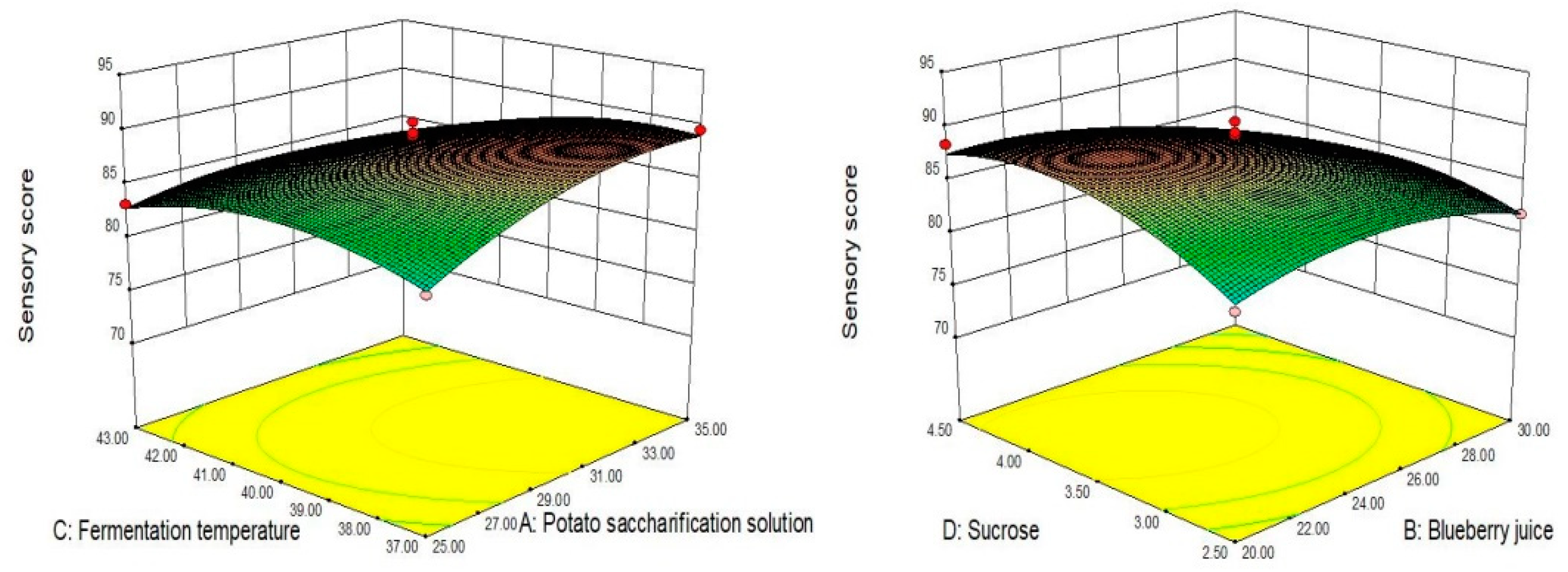
3.10. Response Surface Analysis by Box–Behnken Design
3.11. Determination and Validation of Yogurt Process
3.12. Physicochemical Changes of Potato Blueberry Yogurt during Fermentation
3.13. Correlation Analysis
3.14. Taste Attributes of Potato Blueberry Yogurt
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savaiano, D.A.; Hutkins, R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2021, 79, 599–614. [Google Scholar] [CrossRef]
- Yildiz, E.; Ozcan, T. Functional and textural properties of vegetable-fibre enriched yoghurt. Int. J. Dairy Technol. 2018, 72, 199–207. [Google Scholar] [CrossRef]
- Kiros, E.; Seifu, E.; Bultosa, G.; Solomon, W.K. Effect of carrot juice and stabilizer on the physicochemical and microbiological properties of yoghurt. LWT-Food Sci. Technol. 2016, 69, 191–196. [Google Scholar] [CrossRef]
- Kowaleski, J.; Quast, L.B.; Steffens, J.; Lovato, F.; dos Santos, L.R.; da Silva, S.Z.; de Souza, D.M.; Felicetti, M.A. Functional yogurt with strawberries and chia seeds. Food Biosci. 2020, 37, 100726. [Google Scholar] [CrossRef]
- Zhu, W.; Lyu, F.; Naumovski, N.; Ajlouni, S.; Ranadheera, C.S. Functional efficacy of probiotic Lactobacillus sanfranciscensis in apple, orange and tomato juices with special reference to storage stability and in vitro gastrointestinal survival. Beverages 2020, 6, 13. [Google Scholar] [CrossRef]
- Beals, K.A. Potatoes, nutrition and health. Am. J. Potato Res. 2018, 96, 102–110. [Google Scholar] [CrossRef]
- Shetty, A. Significance of sugar intake in young adults: A review. Int. J. Adolesc. Med. Health 2021, 33, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Torrico, D.D.; Tam, J.; Fuentes, S.; Viejo, C.G.; Dunshea, F.R. Consumer rejection threshold, acceptability rates, physicochemical properties, and shelf-life of strawberry-flavored yogurts with reductions of sugar. J. Sci. Food Agric. 2020, 100, 3024–3035. [Google Scholar] [CrossRef]
- Gomes, E.R.; Carneiro, L.C.M.; Stephani, R.; Carvalho, A.F.d.; Renhe, I.R.T.; Wolfschoon-Pombo, A.F.; Perrone, Í.T. Effect of sugar reduction and addition of corn fibre and polydextrose on pore size and syneresis of yoghurt. Int. Dairy J. 2022, 129, 105298. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive compounds of strawberry and blueberry and their potential health effects based on human intervention studies: A brief overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Wang, X.; Lu, B.; Zhang, J. Preparation of blueberry anthocyanin liposomes and changes of vesicle properties, physicochemical properties, in vitro release, and antioxidant activity before and after chitosan modification. Food Sci. Nutr. 2022, 10, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.M.; Muzs, K.; Spencer, J.P.; Yaqoob, P. Pelargonidin-3-O-glucoside and its metabolites have modest anti-inflammatory effects in human whole blood cultures. Nutr. Res. 2017, 46, 88–95. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Sun, X.-h.; Zhou, T.-T.; Wei, C.-H.; Lan, W.-Q.; Zhao, Y.; Pan, Y.-J.; Wu, V.C.H. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Li, X.; Wu, C.; Liu, C.; Xue, Z.; Kou, X. Investigation on the biological activity of anthocyanins and polyphenols in blueberry. J. Food Sci. 2021, 86, 614–627. [Google Scholar] [CrossRef]
- Anuyahong, T.; Chusak, C.; Adisakwattana, S. Incorporation of anthocyanin-rich riceberry rice in yogurts: Effect on physicochemical properties, antioxidant activity and in vitro gastrointestinal digestion. LWT-Food Sci. Technol. 2020, 129, 109571. [Google Scholar] [CrossRef]
- Jaster, H.; Arend, G.D.; Rezzadori, K.; Chaves, V.C.; Reginatto, F.H.; Petrus, J.C.C. Enhancement of antioxidant activity and physicochemical properties of yogurt enriched with concentrated strawberry pulp obtained by block freeze concentration. Food Res. Int. 2018, 104, 119–125. [Google Scholar] [CrossRef]
- Horiuchi, H.; Inoue, N.; Liu, E.; Fukui, M.; Sasaki, Y.; Sasaki, T. A method for manufacturing superior set yogurt under reduced oxygen conditions. J. Dairy Sci. 2009, 92, 4112–4121. [Google Scholar] [CrossRef] [PubMed]
- Aryana, K.J.; Olson, D.W. A 100-year review: Yogurt and other cultured dairy products. J. Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef] [PubMed]
- Bosnea, L.A.; Kopsahelis, N.; Kokkali, V.; Terpou, A.; Kanellaki, M. Production of a novel probiotic yogurt by incorporation of L. casei enriched fresh apple pieces, dried raisins and wheat grains. Food Bioprod. Process. 2017, 102, 62–71. [Google Scholar] [CrossRef]
- Lang, Y.; Li, B.; Gong, E.; Shu, C.; Si, X.; Gao, N.; Zhang, W.; Cui, H.; Meng, X. Effects of α-casein and β-casein on the stability, antioxidant activity and bioaccessibility of blueberry anthocyanins with an in vitro simulated digestion. Food Chem. 2021, 334, 127526. [Google Scholar] [CrossRef] [PubMed]
- Dorris, M.R.; Voss, D.M.; Bollom, M.A.; Krawiec-Thayer, M.P.; Bolling, B.W. Browning index of anthocyanin-rich fruit juice depends on pH and anthocyanin loss more than the gain of soluble polymeric pigments. J. Food Sci. 2018, 83, 911–921. [Google Scholar] [CrossRef]
- Baba, W.N.; Jan, K.; Punoo, H.A.; Wani, T.A.; Dar, M.M.; Masoodi, F.A. Techno-functional properties of yoghurts fortified with walnut and flaxseed oil emulsions in guar gum. LWT-Food Sci. Technol. 2018, 92, 242–249. [Google Scholar] [CrossRef]
- Liu, J.; Zuo, M.; Low, S.S.; Xu, N.; Chen, Z.; Lv, C.; Cui, Y.; Shi, Y.; Men, H. Fuzzy evaluation output of taste information for liquor using electronic tongue based on cloud model. Sensors 2020, 20, 686. [Google Scholar] [CrossRef]
- Ye, Y.; Li, P.; Zhou, J.; He, J.; Cai, J. The improvement of sensory and bioactive properties of yogurt with the introduction of tartary buckwheat. Foods 2022, 11, 1774. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, H.; Zhao, Y.; Zheng, Y.; Xu, X.; Yue, J. Optimization of high hydrostatic pressure treatments on soybean protein isolate to improve its functionality and evaluation of its application in yogurt. Foods 2021, 10, 667. [Google Scholar] [CrossRef]
- de Campo, C.; Assis, R.Q.; da Silva, M.M.; Costa, T.M.H.; Paese, K.; Guterres, S.S.; de Oliveira Rios, A.; Flores, S.H. Incorporation of zeaxanthin nanoparticles in yogurt: Influence on physicochemical properties, carotenoid stability and sensory analysis. Food Chem. 2019, 301, 125230. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, L.; Xing, G.; Xu, X.; Tu, C.; Dong, M. Effect of co-fermentation with lactic acid bacteria and K. marxianus on physicochemical and sensory properties of goat milk. Foods 2020, 9, 299. [Google Scholar] [CrossRef]
- Erkaya-Kotan, T. In vitro angiotensin converting enzyme (ACE)-inhibitory and antioxidant activity of probiotic yogurt incorporated with orange fibre during storage. J. Food Sci. Technol. 2020, 57, 2343–2353. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Solomakou, N.; Kokkinomagoulos, E.; Kandylis, P. Yogurts supplemented with juices from grapes and berries. Foods 2020, 9, 1158. [Google Scholar] [CrossRef]
- Estrada, J.D.; Boeneke, C.; Bechtel, P.; Sathivel, S. Developing a strawberry yogurt fortified with marine fish oil. J. Dairy Sci. 2011, 94, 5760–5769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Wang, Y.; Kong, B.; Chen, Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. LWT 2021, 140, 110764. [Google Scholar] [CrossRef]
- Ahmad, I.; Xiong, Z.; Hanguo, X.; Khalid, N.; Rasul Suleria, H.A. Effect of enzymatically hydrolyzed potato powder on quality characteristics of stirred yogurt during cold storage. J. Food Process. Preserv. 2021, 45, e15690. [Google Scholar] [CrossRef]
- Ścibisz, I.; Ziarno, M.; Mitek, M.; Zaręba, D. Effect of probiotic cultures on the stability of anthocyanins in blueberry yoghurts. LWT-Food Sci. Technol. 2012, 49, 208–212. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Tao, C.; Liu, M.; Pan, Y.; Lv, Z. Effect of temperature and pH on stability of anthocyanin obtained from blueberry. J. Food Meas. Charact. 2018, 12, 1744–1753. [Google Scholar] [CrossRef]
- Tarrah, A.; Noal, V.; Treu, L.; Giaretta, S.; da Silva Duarte, V.; Corich, V.; Giacomini, A. Short communication: Comparison of growth kinetics at different temperatures of Streptococcus macedonicus and Streptococcus thermophilus strains of dairy origin. J. Dairy Sci. 2018, 101, 7812–7816. [Google Scholar] [CrossRef]
- Cebeci, F.; Sahin-Yesilcubuk, N. The matrix effect of blueberry, oat meal and milk on polyphenols, antioxidant activity and potential bioavailability. Int. J. Food Sci. Nutr. 2014, 65, 69–78. [Google Scholar] [CrossRef]
- Torrico, D.D.; Tam, J.; Fuentes, S.; Viejo, C.G.; Dunshea, F.R. D-tagatose as a sucrose substitute and its effect on the physico-chemical properties and acceptability of strawberry-flavored yogurt. Foods 2019, 8, 256. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yoshimoto, K.; Okada, Y.; Nomura, M. Effect of impregnation using sucrose solution on stability of anthocyanin in strawberry jam. LWT-Food Sci. Technol. 2011, 44, 891–895. [Google Scholar] [CrossRef]
- Bitaraf, M.S.; Khodaiyan, F.; Mohammadifar, M.A.; Mousavi, S.M. Application of response surface methodology to improve fermentation time and rheological properties of probiotic yogurt containing Lactobacillus reuteri. Food Bioprocess Technol. 2010, 5, 1394–1401. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Mujumdar, A.S.; Chang, L. Effect of edible rose (Rosa rugosa cv. Plena) flower extract addition on the physicochemical, rheological, functional and sensory properties of set-type yogurt. Food Biosci. 2021, 43, 101249. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. Adding apple pomace as a functional ingredient in stirred-type yogurt and yogurt drinks. Food Hydrocoll. 2020, 100, 105453. [Google Scholar] [CrossRef]
- Atwaa, E.S.H.; Shahein, M.R.; El-Sattar, E.S.A.; Hijazy, H.H.A.; Albrakati, A.; Elmahallawy, E.K. Bioactivity, physicochemical and sensory properties of probiotic yoghurt made from whole milk powder reconstituted in aqueous fennel extract. Fermentation 2022, 8, 52. [Google Scholar] [CrossRef]
- Peerkhan, N.; Nair, S. Optimization of wheat dextrin yogurt formulation using response surface methodology. J. Food Sci. Technol. 2021, 58, 1740–1749. [Google Scholar] [CrossRef]
- Tudor Kalit, M. Application of electronic nose and electronic tongue in the dairy industry. Mljekarstvo 2014, 64, 228–244. [Google Scholar] [CrossRef]
- Xu, X.; Cui, H.; Xu, J.; Yuan, Z.; Liu, X.; Fan, X.; Li, J.; Zhu, D.; Liu, H. Effects of different probiotic fermentations on the quality, soy isoflavone and equol content of soy protein yogurt made from soy whey and soy embryo powder. LWT 2022, 157, 113096. [Google Scholar] [CrossRef]

| Variables | Units | Levels | |
|---|---|---|---|
| −1 | 1 | ||
| X1—potato saccharification solution | % | 25 | 35 |
| X2—blueberry juice | % | 20 | 30 |
| X4—fermentation temperature | °C | 37 | 43 |
| X5—skimmed milk powder | % | 12 | 16 |
| X7—sucrose | % | 2.5 | 4.5 |
| X8—inoculum concentration | % | 3 | 5 |
| X10—fermentation time | h | 12 | 13 |
| X11—inoculum ratio (BZ11-LB12-Q-1) | 1:1:1 | 1:2:2 | |
| X3, X6, X9, X12 | −1 | 1 | |
| Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Potato saccharification solution (%) | 25 | 30 | 35 |
| Blueberry juice (%) | 20 | 25 | 30 |
| Fermentation temperature (°C) | 37 | 40 | 43 |
| Sucrose (%) | 2.5 | 3.5 | 4.5 |
| Run | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | X10 | X11 | Sensory Score | Anthocyanin Content (mg/100 g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 78.96 ± 0.78 | 7.57 ± 0.24 |
| 2 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 74.67 ± 0.81 | 12.25 ± 025 |
| 3 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 74.00 ± 1.63 | 10.63 ± 0.13 |
| 4 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 70.69 ± 0.49 | 4.17 ± 0.18 |
| 5 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 75.92 ± 1.34 | 7.40 ± 0.24 |
| 6 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 81.42 ± 1.50 | 7.85 ± 0.21 |
| 7 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | 68.17 ± 1.02 | 10.68 ± 0.32 |
| 8 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | 64.57 ± 1.12 | 8.13 ± 0.11 |
| 9 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | 67.00 ± 0.82 | 13.30 ± 0.14 |
| 10 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 65.83 ± 0.87 | 11.46 ± 0.18 |
| 11 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 63.33 ± 0.70 | 10.96 ± 0.11 |
| 12 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 75.07 ± 0.65 | 4.34 ± 0.15 |
| Source | Sum of Squares | df | Mean Square | F Value | p Value | |
|---|---|---|---|---|---|---|
| Sensory | Model | 373.51 | 8 | 46.69 | 17.89 | 0.0186 ** |
| X1 | 97.41 | 1 | 97.41 | 37.33 | 0.0088 ** | |
| X2 | 94.25 | 1 | 94.25 | 36.12 | 0.0092 ** | |
| X4 | 73.06 | 1 | 73.06 | 28 | 0.0132 * | |
| X5 | 25.55 | 1 | 25.55 | 9.79 | 0.0521 | |
| X7 | 53.38 | 1 | 53.38 | 20.46 | 0.0202 * | |
| X8 | 15.89 | 1 | 15.89 | 6.09 | 0.0902 | |
| X10 | 9.38 | 1 | 9.38 | 3.6 | 0.1542 | |
| X11 | 4.58 | 1 | 4.58 | 1.75 | 0.2773 | |
| Residual | 7.83 | 3 | 2.61 | |||
| Cor total | 381.33 | 11 | ||||
| Anthocyanin | Model | 92.43 | 8 | 11.55 | 9.43 | 0.0458 * |
| X1 | 0.065 | 1 | 0.065 | 0.053 | 0.8332 | |
| X2 | 74.1 | 1 | 74.1 | 60.47 | 0.0044 ** | |
| X4 | 0 | 1 | 0 | 0 | 0 | |
| X5 | 1.66 | 1 | 1.66 | 1.35 | 0.3289 | |
| X7 | 1.83 | 1 | 1.83 | 1.49 | 0.3095 | |
| X8 | 6.6 | 1 | 6.6 | 5.39 | 0.103 | |
| X10 | 8.04 | 1 | 8.04 | 6.56 | 0.0831 | |
| X11 | 0.15 | 1 | 0.15 | 0.12 | 0.7534 | |
| Residual | 3.68 | 3 | 1.23 | |||
| Cor total | 96.11 | 11 |
| Run | Potato Saccharification Solution (%) | Blueberry Juice (%) | Temperature (°C) | Sucrose (%) | Sensory Score |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 86.5 ± 1.47 |
| 2 | 0 | 1 | 0 | 1 | 81.9 ± 1.41 |
| 3 | 0 | 0 | 1 | 1 | 80.6 ± 0.81 |
| 4 | 0 | 0 | 0 | 0 | 89.2 ± 0.82 |
| 5 | −1 | 1 | 0 | 0 | 80.6 ± 1.25 |
| 6 | 0 | 0 | −1 | −1 | 83.8 ± 2.18 |
| 7 | 0 | 0 | −1 | 1 | 85.0 ± 0.98 |
| 8 | 1 | 0 | 1 | 0 | 83.0 ± 0.94 |
| 9 | 0 | 0 | 1 | −1 | 81.6 ± 1.24 |
| 10 | 1 | −1 | 0 | 0 | 87.1 ± 1.82 |
| 11 | 0 | 0 | 0 | 0 | 90.5 ± 0.82 |
| 12 | −1 | 0 | 1 | 0 | 83.2 ± 1.18 |
| 13 | 0 | 1 | −1 | 0 | 83.5 ± 0.82 |
| 14 | 0 | −1 | −1 | 0 | 88.4 ± 1.89 |
| 15 | 0 | 0 | 0 | 0 | 86.9 ± 1.23 |
| 16 | 0 | −1 | 0 | −1 | 81.9 ± 2.05 |
| 17 | −1 | 0 | 0 | −1 | 80.6 ± 2.94 |
| 18 | 1 | 0 | −1 | 0 | 89.5 ± 1.25 |
| 19 | 0 | −1 | 0 | 1 | 88.4 ± 1.25 |
| 20 | 0 | 1 | 1 | 0 | 79.3 ± 1.67 |
| 21 | 1 | 0 | 0 | −1 | 84.5 ± 2.49 |
| 22 | −1 | 0 | 0 | 1 | 84.5 ± 2.05 |
| 23 | 0 | −1 | 1 | 0 | 83.2 ± 2.16 |
| 24 | 0 | 0 | 0 | 0 | 89.5 ± 1.63 |
| 25 | −1 | −1 | 0 | 0 | 84.5 ± 2.16 |
| 26 | 1 | 0 | 0 | 1 | 84.1 ± 1.86 |
| 27 | 1 | 1 | 0 | 0 | 83.2 ± 2.94 |
| 28 | −1 | 0 | −1 | 0 | 82.5 ± 2.88 |
| 29 | 0 | 1 | 0 | −1 | 81.9 ± 2.36 |
| Source | Sum of Squares | df | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Model | 246.45 | 14 | 17.6 | 10.64 | <0.0001 ** |
| A-potato saccharification solution | 20.05 | 1 | 20.05 | 12.11 | 0.0037 ** |
| B-blueberry juice | 44.43 | 1 | 44.43 | 26.84 | 0.0001 ** |
| C-temperature | 39.64 | 1 | 39.64 | 23.95 | 0.0002 ** |
| D-sucrose | 8.69 | 1 | 8.69 | 5.25 | 0.038 * |
| AB | 0 | 1 | 0 | 0 | 1 |
| AC | 13 | 1 | 13 | 7.85 | 0.0141 * |
| AD | 4.62 | 1 | 4.62 | 2.79 | 0.1169 |
| BC | 0.25 | 1 | 0.25 | 0.15 | 0.7034 |
| BD | 10.53 | 1 | 10.53 | 6.36 | 0.0244 * |
| CD | 1.21 | 1 | 1.21 | 0.73 | 0.4069 |
| A2 | 25.01 | 1 | 25.01 | 15.11 | 0.0016 ** |
| B2 | 37.01 | 1 | 37.01 | 22.36 | 0.0003 ** |
| C2 | 38.18 | 1 | 38.18 | 23.07 | 0.0003 ** |
| D2 | 59.4 | 1 | 59.4 | 35.89 | <0.0001 ** |
| Residual | 23.17 | 14 | 1.66 | ||
| Lack of fit | 11.12 | 10 | 1.11 | 0.37 | 0.9082 |
| Pure error | 12.05 | 4 | 3.01 | ||
| Cor total | 269.62 | 28 |
| Time (h) | Anthocyanin (mg/100 g) | pH | Soluble Solids (%) | L* | a* | b* | Total Viable Counts log CFU/mL | Q-1 Viable Counts log CFU/mL | LB12 Viable Counts log CFU/mL | BZ11 Viable Counts log CFU/mL | Reducing Sugar (mg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 11.69 ± 0.33 a | 6.33 ± 0.03 a | 19.56 ± 0.35 a | 35.47 ± 0.21 f | 3.33 ± 0.21 d | −5.73 ± 0.40 a | 7.04 ± 0.04 g | 6.88 ± 0.03 g | 6.31 ± 0.01 e | 6.10 ± 0.17 f | 63.69 ± 0.13 a |
| 2 | 11.52 ± 0.59 a | 6.32 ± 0.02 a | 19.23 ± 0.35 ab | 36.57 ± 0.35 e | 3.50 ± 0.36 d | −5.73 ± 0.66 a | 7.14 ± 0.03 f | 6.99 ± 0.09 f | 6.36 ± 0.03 e | 6.10 ± 0.17 f | 60.18 ± 0.25 b |
| 4 | 11.13 ± 0.32 ab | 6.19 ± 0.01 b | 18.93 ± 0.15 b | 37.37 ± 0.63 d | 3.53 ± 0.40 d | −6.43 ± 0.75 a | 7.25 ± 0.07 e | 7.11 ± 0.07 e | 6.42 ± 0.10 e | 6.36 ± 0.10 e | 58.22 ± 0.31 c |
| 6 | 10.74 ± 0.35 bc | 6.07 ± 0.02 c | 18.50 ± 0.10 c | 39.97 ± 0.64 c | 3.80 ± 0.26 d | −5.90 ± 0.10 a | 7.59 ± 0.02 d | 7.27 ± 0.05 d | 7.04 ± 0.04 d | 6.97 ± 0.06 d | 53.61 ± 0.36 f |
| 8 | 10.19 ± 0.17 cd | 5.66 ± 0.03 d | 17.90 ± 0.10 d | 40.47 ± 0.25 c | 5.47 ± 0.29 c | −6.23 ± 0.15 a | 8.12 ± 0.02 c | 7.57 ± 0.03 c | 7.72 ± 0.01 c | 7.64 ± 0.07 c | 56.34 ± 0.18 d |
| 10 | 9.85 ± 0.60 de | 5.02 ± 0.07 e | 17.06 ± 0.12 e | 43.33 ± 0.06 b | 9.10 ± 0.26 b | −6.20 ± 0.26 a | 8.56 ± 0.01 b | 7.76 ± 0.03 b | 8.18 ± 0.02 b | 8.18 ± 0.03 b | 55.29 ± 0.27 e |
| 12.5 | 9.41 ± 0.10 e | 4.47 ± 0.03 f | 16.13 ± 0.31 f | 47.10 ± 0.52 a | 11.90 ± 0.10 a | −6.03 ± 0.40 a | 9.31 ± 0.03 a | 7.95 ± 0.05 a | 8.79 ± 0.08 a | 8.71 ± 0.03 a | 50.56 ± 0.22 g |
| BZ11 Viable Counts | LB12 Viable Counts | Q-1 Viable Counts | Total Viable Counts | Anthocyanin | pH | Soluble Solids | L* | a* | b* | Reducing Sugar | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BZ11 viable counts | 1 | ||||||||||
| LB12 viable counts | 0.997 ** | 1 | |||||||||
| Q-1 viable counts | 0.994 ** | 0.991 ** | 1 | ||||||||
| Total viable counts | 0.988 ** | 0.993 ** | 0.983 ** | 1 | |||||||
| Anthocyanin | −0.992 ** | −0.985 ** | −0.997 ** | −0.976 ** | 1 | ||||||
| pH | −0.969 ** | −0.975 ** | −0.962 ** | −0.990 ** | 0.950 ** | 1 | |||||
| Soluble solids | −0.987 ** | −0.987 ** | −0.988 ** | −0.996 ** | 0.983 ** | 0.987 ** | 1 | ||||
| L* | 0.975 ** | 0.975 ** | 0.972 ** | 0.987 ** | −0.972 ** | −0.973 ** | −0.993 ** | 1 | |||
| a* | 0.934 ** | 0.946 ** | 0.925 ** | 0.970 ** | −0.907 ** | −0.993 ** | −0.965 ** | 0.951 ** | 1 | ||
| b* | −0.364 | −0.317 | −0.426 | −0.314 | 0.450 | 0.305 | 0.362 | −0.305 | −0.236 | 1 | |
| Reducing sugar | −0.840 * | −0.824 * | −0.853 * | −0.833 * | 0.877 ** | 0.781 * | 0.861 * | −0.900 * | −0.733 | 0.354 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; He, Z.; He, L.; Li, C.; Tao, H.; Ye, C.; Liu, L.; Zeng, X.; Ran, G. Effect of Fermentation Parameters on the Anthocyanin Content, Sensory Properties, and Physicochemical Parameters of Potato Blueberry Yogurt. Fermentation 2022, 8, 489. https://doi.org/10.3390/fermentation8100489
Li M, He Z, He L, Li C, Tao H, Ye C, Liu L, Zeng X, Ran G. Effect of Fermentation Parameters on the Anthocyanin Content, Sensory Properties, and Physicochemical Parameters of Potato Blueberry Yogurt. Fermentation. 2022; 8(10):489. https://doi.org/10.3390/fermentation8100489
Chicago/Turabian StyleLi, Meidan, Zuyan He, Laping He, Cuiqin Li, Han Tao, Chun Ye, Lihua Liu, Xuefeng Zeng, and Guangyan Ran. 2022. "Effect of Fermentation Parameters on the Anthocyanin Content, Sensory Properties, and Physicochemical Parameters of Potato Blueberry Yogurt" Fermentation 8, no. 10: 489. https://doi.org/10.3390/fermentation8100489
APA StyleLi, M., He, Z., He, L., Li, C., Tao, H., Ye, C., Liu, L., Zeng, X., & Ran, G. (2022). Effect of Fermentation Parameters on the Anthocyanin Content, Sensory Properties, and Physicochemical Parameters of Potato Blueberry Yogurt. Fermentation, 8(10), 489. https://doi.org/10.3390/fermentation8100489





