Abstract
Camel milk and dates are well-known for their great nutritional and therapeutical benefits. Therefore, the study aimed to combine the benefits of fermented camel milk (FCM) and Sukkari date (SKD) in a naturally sweetened FCM. Six treatments of FCM using ABT-5 cultures with 0, 5, 7.5, 10, 12.5, and 15% SKD were carried out. Chemical, physicochemical, rheological properties were studied, while organoleptical attributes and probiotic strains viability were monitored during cold storage (4 °C) up to 15 days. Results showed that fortification with SKD increased total solids (TS), ash, dietary fiber, and carbohydrate content compared to plain FCM. Water holding capacity (WHC) values increased with low and medium SKD levels then decreased with high SKD levels. Minerals such as K, P, Mg, Zn, Fe, and Cu were significantly increased, while Na was significantly decreased. Increased SKD levels in FCM resulted in significant increases in total phenolic content (TPC), total flavonoids (TF), total flavonols (TFL), and antioxidant activity (AOA). Instrumental color analysis exhibited a significant change in L*, b*, BI, and ∆E due to adding SKD in a dose-dependent manner. The viability of Streptococcus thermophiles, Lactobacillus acidophilus, and Bifidobacterium bifidum was increased by adding low and medium SKD levels, resulting in a higher number than the accepted threshold for a probiotic effect. Adding 10 and 12.5% SKD recorded the best-balanced flavor score at the beginning and after up to 15 days of storage, respectively. Conclusively, the current study revealed that fortification with SKD at 7.5–12.5% improved the nutritional quality without adverse effects on the technological, organoleptic characteristics, and probiotics viability and provided acceptable, nutritious, and healthy benefits to FCM.
1. Introduction
Interestingly, the camel (Camelus dromedarius) is regarded as a target animal for the 21st century due to its ability to produce high-quality milk in the face of extreme temperatures, a scarcity of grass, and a scarcity of water. The camel population contributes more than 0.4% of milk produced, and its global market share is growing [1]. Camel milk (CM) is well-known throughout the world for its distinct flavor, high vitamin C content, and numerous medicinal benefits [1,2]. In addition, it has a number of significant advantages, including the treatment of autism, the control of diabetes and allergies [3], the prevention of liver cirrhosis, and providing a substitution to cow milk to avoid an allergic reaction in infants [4]. CM contains protein (2.5–4.5%), fat (2.9–5.5%), solid not fat (8.9–14.3%), ash (0.35–0.95%), lactose (2.9–5.8%), and water (86.3–88.5%) [1].
Lactic acid bacteria, found in probiotic-enriched dairy products such as fermented milk, have high nutritional [5,6], antiviral [6,7,8], anti-obesity [8], antioxidant, and nutraceutical properties [9] that distinguish them medically [10] and even provide protection during COVID-19 infection [6,11]. Surprisingly, regular consumption of probiotic-enriched dairy products has been linked to improved health and longevity due to their nutritional and therapeutic properties [12]. Because of its bioactive components and microbial diversity, most studies supported its ability to provide superior benefits and potential therapeutical uses [2,13,14,15,16,17,18]. Ayyash et al. [19] investigated the biological usefulness of the bioaccessible fraction produced in fermented camel milk (FCM) versus fermented bovine milk (FBM) after in vitro digestion. They confirmed that the bioaccessible components of FCM had higher antiproliferative activity and biological functionality than fermented bovine milk (FBM).
The date palm (Phoenix dactylifera L.) is the most important tree in the Middle East and North Africa regarding economic and food security [20,21,22]. A number of studies [21,22,23] have demonstrated the nutritional value of common date fruits. Dates are thought to be a good source of vitamins and minerals, a good source of sugars, and a necessary base for producing date pastes and syrups for confectionery and fermentation [24]. Dates contain trace amounts of thiamine, riboflavin, nicotinic acid, vitamin A, and vitamin C [20,25], and research has shown that they have significant antioxidants [26,27], anticancer [28], and antiviral [29] properties. Dates have a high sugar content (71.2–81.4% dry weight) and ash content (1.68–3.94%), but a low fat and protein content (0.12–0.72% and 1.72–4.73%, respectively) [23,30]. The edible flesh of dates contains a lot of sugar, mainly fructose, glucose, sucrose, dietary fiber (5–8.5%), and polyphenols [31,32]. Sukkari dates (SKD) contain 69.93–78.50 g 100−1 dw carbohydrates, 2.76–2.84 g protein, and 0.52–2.54 g fats, fibers, and minerals. Furthermore, potassium was the most abundant mineral, with a high concentration of essential amino acids in its protein [33]. It also contained significant amounts of phytochemicals [33] and carotenoids [34], both of which had high nutritional and therapeutic properties [26,27,28,29].
A healthier lifestyle encourages people to consume more fruit daily by providing nutritious milk–fruit drinks made from various fruits [21,35]. Milk–fruit beverages appear to be a fantastic fast food containing all the necessary macro-, micro-, dietary fibers, and bioactive substances [36]. The findings confirmed that milk–fruit drinks might be needed to meet the needs of health-conscious customers, and they are expected to gain increased international marketability [37]. Adding 4% avocado or 6% kiwi paste to FCM improved its quality and acceptability [38]. Consumers praised the fermented-probiotic drinks’ organoleptic qualities, as well as their ability to preserve and improve nutritional quantity value. These drinks can be consumed as a nutritious and valuable beverage [39].
Dates and CM have long been used to prevent and treat illness in Arab countries. Due to the high phenolic and flavonoid content of the mixture, feeding a combination of CM and dates appears to improve the body’s defense against free radicals produced by various bio-oxidation processes [40]. Because customers are concerned about manufactured products’ sensory and health aspects, we set out to investigate the physicochemical, bioactivity, and organoleptic qualities of a new probiotic CM supplemented with SKD. To that end, probiotic-enriched FCM supplemented with SKD at different doses was developed and tested for chemical, physicochemical, and microbiological properties. TPC and antioxidant activity (AOA) were also studied. During cold storage, the organoleptic properties and survivability of provided probiotic bacteria from ABT-5 culture were investigated.
2. Materials and Methods
2.1. Ingredients
The CM was obtained from the College of Agriculture and Veterinary Medicine Farm, Qassim University, during February–March 2021. Date palm (P. dactylifera L.), Sukkari variety was purchased from the local market at Qassim region as a yield of season 2020. The chemical and physicochemical characteristics and antioxidant activity of CM and SKD were determined; data are shown in Table 1. The Chr. Hansen ABT-5 starter consists of Streptococcus thermophiles, Lactobacillus acidophilus + Bifidobacterium bifidum in freeze-dried direct-to-vat set form (DVS) kept at –20 ± 1 °C, and it was obtained from Chr. Hansen, Copenhagen, Denmark.

Table 1.
Chemical composition and physicochemical properties of camel milk and SKD (mean ± SE), n = 6.
2.2. Preparation of Probiotic-Enriched FCM and FCM-SKD
Sukkari dates were washed under running water to remove dust, microscopic contaminants and kernels were manually removed, then the dates’ flesh was transferred into date paste using a scraw grinder (Panasonic, MK-ZG1500, Osaka, Japan). Fresh CM was fortified with 5, 7.5, 10, 12.5, or 15% SKD pasta (v:w) and vigorously mixed using a bench-top food processor (Santos, VITA-MAX CORP-Light Industrial Food Preparing Machine Model, VM0122E, Santos Houston, TX, USA) at high speed (speed 6) for 2 min before further processing. Both plain CM or fortified CM with 5, 7.5, 10, 12.5, and 15% of SKD (v:w) were pasteurized at 85 °C for 15 min and then cooled to the inoculation temperature (42 °C) [41]. The Chr. Hansen ABT-5 DVS starter in a ratio of 0.1 g per 100 mL of CM was aseptically added. Subsequently, samples were incubated at 42 °C for ~4 h to reach pH 4.6–4.7, then were cooled down for 12 to 18 h. From each treatment, 50 mL from each treatment was taken in sterile bags aseptically for microbiological and biochemical analysis; hence, the experiment was repeated three times. For microbiological and organoleptical examinations, samples after 1, 8, and 15 days were taken and subsequently analyzed.
2.3. Chemical, Physicochemical and Rheological Properties
Moisture, total solids, total nitrogen (TN), fat, ash, minerals, lactose, crude fiber, available carbohydrates, pH, and titratable acidity were determined according to the methods of AOAC [41]. Water holding capacity (WHC) was determined according to Barakat and Hassan [42]. The apparent viscosity (AV) of FCM and FCM-SKD was measured Fungilab viscometer (Fungilab V100002 Alpha Series L, Fungilab Inc., Barcelona, Spain) with stainless steel spindle R2 with rotation speed 100 rpm and expressed by cP. Measurements were made for 1 min at 10 °C [43].
2.4. Determination of Total Phenolic Content (TPC), Total Flavonoids (TF), and Total Flavonols (TFL)
CM, FCM, and FCM-SKD samples were freeze-dried for 96 h at −52 °C using (CHRIST, Alpha 1–2 LD plus, Osterode am Harz, Germany) and 0.032 mbar. Freeze-dried samples were pulverized using a porcelain morsel to prepare homogeneous powder. The TPC of CM, SKD, FCM, and FCM-SKD was determined by using Folin–Ciocalteu reagent, according to Yawadio Nsimba et al. [44]. The measurements were compared to the standard curve of Gallic acid (GA) solution (R2 = 0.99), and TPC content was expressed as milligrams of Gallic acid equivalents (GAE) per 100 g (mg of GAE g−1 DW). The TF and TFL contents of CM, SKD, FCM, and FCM-SKD were determined according to Mohdaly et al. [45] at 440 and 420 nm, respectively, then expressed as mg quercetin equivalent (QE) per g−1 (mg QE g−1 DW).
2.5. Free Radical Scavenging Ability against DPPH and ABTS
Radical scavenging activity was measured spectrophotometrically based on the bleaching of DPPH radicals’ purple solution according to Yawadio Nsimba et al. [44]. The DPPH radical scavenging activity percentage was used to plot the Trolox calibration curve. The antiradical activity was expressed as micromoles of Trolox Equivalents (TE) per gram (µmol TE g−1). The radical scavenging activity (RSA) SKD palm against the stable ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) radical cation was measured using the method of Barakat and Rohn [46]. A Trolox calibration curve was plotted as a function of the ABTS radical cation scavenging activity percentage. The final results were expressed as micromoles of Trolox Equivalents (TE) per gram (mol of TE g−1 DW).
2.6. Instrumental Color Measurements of FCM and FCM-SKD
The color of each sample was measured via a Chromameter (ColorFlex, Reston, AV, USA) applying the CIELAB-scale (L*, a*, and b*) adjusted with typical white, green, and black tiles. The hue angle (H°), chroma (C), color changes (∆E), and browning index (BI) values, compared with those of the control, were then calculated according to Lavelli et al. [47] using the continuity equations (Equations (1)–(4)):
2.7. Microbiological Examination
Str. Thermophilus and L. acidophilus viable counts were examined by the standard plate count method to determine viable cells as a typical method. Firstly, mL of each sample was suspended in 90 mL of sterile peptone water (Merck, (Darmstadt, Germany), 0.1%), then it was serially diluted. The diluted sample (1 mL) was inoculated on a selective medium for each targeted bacterial strain. M17 agar (pH, 6.8 ± 0.2 at 25 °C, Merck) was applied for the streptococci viable count (Str. thermophilus) in samples and was incubated at 37 °C for 48 h under aerobic conditions [48]. MRS agar was applied to the count of L. acidophilus was incubated at 37 °C for 72 h in anaerobic jars (2.5 mL) with GasPak (GasPak System-Oxoid, Basingstoke, Hampshire, UK) according to standard methods [48]. B. bifidum was selectively counted on Lithium Propionate MRS (LP-MRS) media incubated at 37 °C for 72 h under anaerobic conditions as descriptively mentioned [48]. Data were expressed as logarithm colony forming units per mL (log10 CFU mL−1) for FCM or FCM-SKD samples.
2.8. Organoleptical Attributes
Organoleptical attributes of the different formulas were carried out. Twelve panelists from staff and post-graduate members of the Food Science and Human Nutrition Department, Faculty of Agriculture and veterinary medicine, Qassim University, were asked to evaluate the prepared FCM and FCM-SKD. Certain parameters, including flavor (30), body and texture (30), color and appearance (20), consistency (10), mouth feel (10), and overall acceptability (100), were judged. Results were statistically analyzed, and mean values of mentioned attributes from triplicates experiments and standard error (SE) were presented [42].
2.9. Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS 22.0, IBM, USA) for Windows. Experimental results were expressed as mean ± SE. Statistical significance was tested with one-way ANOVA followed by a post hoc test, and p-values < 0.05 were applied according to Steel et al. [49].
3. Results
3.1. Effect of SKD Addition on Chemical Composition and Physicochemical Properties of FCM
The chemical composition and physicochemical properties of FCM and fortified FCM with SKD at various levels were determined; data are shown in Table 2. Regarding chemical composition, adding SKD to FCM increased the total solids and solid not-fats with increasing SKD levels, significantly. The moisture decreased from 90.16% in FCM + 0% SKD to 80.25% FCM + 15% SKD, which increased TS from 9.84% in FCM + 0% SKD to 19.75% in FCM + 15% SKD when calculated on wet base. No significant change in protein and fat contents was noted. Obviously, adding SKD increased the ash, fiber, and available carbohydrates contents, significantly. The pH and titratable acidity exhibited an opposite relationship, as shown in Table 2. The pH decreased considerably, and titratable acidity was significantly increased when SKD was added. Incorporating SKD improved the WHC, as 5–12.5% SKD could bind more water than plain FCM or FCM + 15% SKD, as shown in Table 2. Adding high SKD levels increased the WHC in a dose-dependent matter, but it decreased again with the highest SKD level. The best binding capacity was recorded when 10% SKD was added, compared with plain FCM.

Table 2.
Chemical composition and physicochemical properties of fermented camels’ milk (FCM) and FCM fortified with Sukkari date (SKD), (mean ± SE), n = 6.
3.2. Effect of SKD Addition on the Mineral Content of FCM
The effect of fortifying FCM with SKD at various levels on mineral content was investigated, and the results are shown in Table 3. Mineral analysis of FCM and FCM-SKD showed significant decreases in Na and P contents and significant increases in K and Mg contents when SKD was added at high levels. The Ca content was not affected significantly with increasing SKD levels. Microelements such as Zn, Fe, and Cu showed a higher content of FCM-SKD containing higher SKD levels than in FCM. The addition of SKD significantly increased the content of K, Mg, Zn, Fe, and Cu and significantly decreased Na and P in a level-dependent manner. Comparing FCM + 0% SKD with FCM + 15% SKD, the increasing rates of K, Mg, Zn, Fe, and Cu were 56, 107, 219, 76, 136%, respectively, while the reduction rates of Na and P were 14 and 13%, respectively.

Table 3.
Mineral contents (mg 100 g−1) of fermented camel milk (FCM) and FCM fortified with Sukkari date (SKD), (mean ± SE), n = 6.
3.3. Effect of SKD Addition on AV of FCM
The effect of adding SKD on the AV of probiotic-enriched FCM is shown in Figure 1. The AV increased with increasing SKD levels, compared with FCM + 0% SKD. Because adding SKD raised the TS (Table 2), FCM-SKD had higher AV values than FCM + 0% SKD. During cold storage up to 15 days, AV values increased during storage from day one until the eighth day then decreased to the end of the storage period.
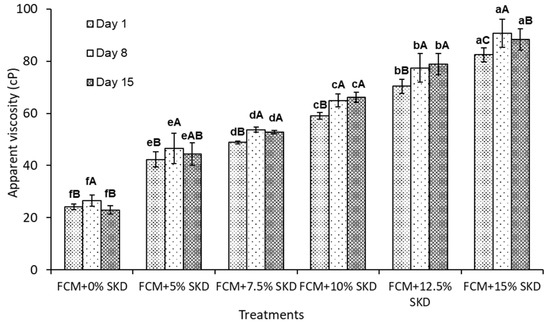
Figure 1.
The effect of adding SKD at 5, 7.5, 10, 12.5, and 15% to FCM on the AV of probiotic-enriched FCM over 15 days at 5 °C, the AV was measured using Fungilab apparatus (Barcelona, Spain) with spindle R2, rotation speed 100 rpm and expressed as cP, measurements were made for 1 min at 10 °C. a,b,c,…: Bars within the same storage day not sharing a common letter are significantly different (p < 0.05). A,B,C: Bars not sharing a common letter during the storage periods are significantly different (p < 0.05).
3.4. Effect of SKD Addition on TPC, TF, TFL, and AOA of FCM
The TPC, TF, TFL, and relative AOA using DPPH and ABTS assays of all formulated FCM were investigated; data are presented in Table 4. The TPC was not detected in FCM. However, AOA was significantly lower in FCM than in FCM-SKD. It is likely that increasing the substitution level up to 15% significantly increased TPC. The TPC was 2.71, 4.06, 5.23, 6.42 and 7.56 mg GAE g−1 for substituting levels of 5, 7.5, 10, 12.5 and 15%, respectively. The TF and TFL were significantly increased with increasing SKD levels. Accordingly, the AOA increased when the substitution level was increased, and the highest values were recorded in FCM + 15% SKD for both DPPH and ABTS radicals. Incorporating the SKD into FCM formulas improved the AOA significantly.

Table 4.
Total phenolic, total flavonoids, total flavonols contents, and relative antioxidant capacity of fermented camel milk (FCM) and FCM fortified with Sukkari date (SKD), (mean ± SE), n = 6.
3.5. Effect of SKD Addition on the Color Parameters of FCM
The results of the color measurement of FCM and FCM-SKD are presented in Table 5. It was observed that the color of FCM fortified with SKD became beige and dim as the contents of SKD were increased gradually from 5 to 15%, as shown in the presented photos. The value of L* (as an indicator for light vs. dark) decreased significantly (p < 0.05) with increasing contents of SKD. The mean a* values (as an indicator for red vs. green) were not significantly affected with increasing SKD levels. Furthermore, FCM enriched with SKD exhibited a higher significance b* value (as an indicator for yellow vs. blue) when the SKD level was increased. The H° had presented a significant difference between FCM and all FCM-SKD, whereas a non-significant difference was recorded among all FCM-SKD treatments. Most importantly, C, BI, and ∆E increased by increasing SKD levels compared with plain FCM.

Table 5.
Instrumental and visual color analysis of fermented camel milk (FCM) and FCM fortified with Sukkari date (SKD) palm, (mean ± SE), n = 6.
3.6. Effect of SKD Addition on the Microbiological Characterization of FCM during Cold Storage for 15 Days
Data showed in Table 6 illustrated the Str. thermophilus, L. acidophilus, and B. bifidum [Log10 CFU mL−1] of FCM and FCM-SKD as affected by adding SKD for up to 15 days during cold storage. Results indicated that Str. thermophilus, L. acidophilus, and B. bifidum viable counts increased significantly with increasing SKD compared to FCM-SKD treatments with FCM + 0% SKD. Obviously, the viable counts of mentioned strains were slightly decreased when 15% SKD was added compared with both 10 and 12.5% of SKD, which may indicate that adding SKD more than 15% could affect the viable microbial counts of Str. thermophilus, L. acidophilus, and B. bifidum increased gradually in most treatments during up to 8 days of storage and then decreased in all treatments until the end of the storage period. Interestingly, the addition of SKD improved the viability and accelerated the growth of Str. thermophilus, L. acidophilus and B. bifidum. On day 1, FCM + 7.5% SKD exhibited the highest Str. thermophilus, L. acidophilus, and B. bifidum count, while on day 8, FCM + 12.5% SKD showed the highest count of Str. thermophilus, L. acidophilus, and B. bifidum. On day 15, FCM + 10% SKD exhibited the highest viable count of Str. thermophilus, L. acidophilus, and B. bifidum.

Table 6.
Microbiological analysis of fermented camel milk (FCM) and FCM fortified with Sukkari date (SKD) palm during storage at 4 °C ± 1 °C for 15 days (mean ± SE), n = 6.
3.7. Effect of SKD Addition on the Organoleptical Attributes of FCM during Cold Storage for 15 Days
The impact of substituted CM with SKD used to make FCM-SKD on organoleptic properties was evaluated, and data are shown in Table 7. The panelists were asked to judge FCM and FCM-SKD parameters including flavor, body and texture, color and appearance, consistency, mouthfeel, and overall acceptability. Significant differences (p < 0.05) were observed between FCM and FCM-SKD in all organoleptic characteristics when SKD levels were increased, giving curved acceptability, which increased with the addition of low levels of SKD, then decreased with high SKD levels. Increasing SKD up to 15% presented more date taste, with a high sweet taste feeling, but the panelists still considered the organoleptic characteristics acceptable. Adding 10 and 12.5% SKD resulted in the best-balanced flavor score at the beginning and after up to 15 days of storage, respectively. Body and texture were affected significantly; incorporation of 7.5–12.5% exhibited good features, and the panelists gave it an improved score. Comparing FCM with FCM-SKD, color and appearance were dramatically affected by increasing SKD levels; results were compatible with color results presented previously. Regarding consistency and mouthfeel, increasing SKD exuded a curved trend based on SKD levels. Interestingly, regarding overall acceptability, it was shown that 10% of SKD added to FCM was significantly favored by the panelists.

Table 7.
Organoleptical attributes of fermented camel milk (FCM) and FCM fortified with date palm (DP) during storage at 4 °C ± 1 °C for 15 days (mean ± SE), n = 12.
4. Discussion
Fermented milk is currently the most popular commercial functional beverage, and it has nutritional and therapeutic properties [2,5,15,16]. According to WHO recommendations, it contains probiotics, which positively improve human health when consumed regularly [8,11]. Because CM and FCM have different sensory properties compared to cow’s milk, efforts to improve its acceptance are concerned in the current study. CM is consumed mainly by the older generation and has lost its popularity among the younger generations in both fresh and fermented forms [50]. Interestingly, adding SKD to FCM could be an excellent way to improve nutritional, physicochemical, and organoleptical characteristics. SKD could provide nutrients and prebiotics to fortify nutritional value, enhance probiotics and human natural microflora [37]. Adding SKD increased TS, solid not-fat, ash, fiber, and available carbohydrates regarding the chemical composition. This may be due to the high solids and nutrient content in SKD (Tawfek et al. [37] and Almosawi and Hassan [51]). The dates are rich in sugar (71.2–81.4% dry weight) and ash (1.68–3.94%), while suppressing small levels of lipid and protein (0.12–0.72% and 1.72–4.73%, respectively [23,30]). The increases in titratable acidity may result from the activation of ABT-5 starter culture when SKD is added [52]. In the current study, increases in the logarithmic numbers of used starter cultures were noted. Almosawi and Hassan [51] stated that adding date extract to fermented CM provided monosaccharides enrichment and approximately equal proportions of glucose and fructose, promoting bacterial growth and lowering pH values.
Moreover, dates contain vitamins, minerals, and sugars that accelerate bacterial growth and metabolic activities, increasing acidity and decreasing pH [37,52,53]. FCM fortified with SKD had high WHC with a rising level of SKD, then WHC later reduced with high SKD levels. This probably could be attributed to the increase of TS by adding SKD. The reduction in whey separation and increase in WHC was presented when the total solids were increased by adding fruits [37,40,43,52].
In the current study, fortifying FCM with SKD to improve its organoleptical attributes increased the macro- and microelements [37,52]. Tawfek et al. [37] and Assirey [23] confirmed that SKD has good macro and micronutrient content; its utilization can effectively fortify FCM to improve its nutritional and organoleptical properties. Table 1 demonstrated that K and Ca were the most abundant macroelements in SKD regarding the mineral analysis. At the same time, Na and Mg presented low content in SKD; results were previously agreed [54,55]. The results demonstrated that SKD represents modified macro- and microelements profiles of FCM with significant increases in K, Mg, Zn, Fe, and Cu and a considerable decrease in Na content as similarly indicated [37,40,52]. Indeed, mineral contents in raw CM and SKD are varied and depend on many factors such as treatments, season, variety, cultivation and breeding conditions, and so on. [1,33,40].
Many attempts have been made to tackle the problems associated with the poor texture of fermented camel milk since texture and viscosity are essential characteristics that determine its quality and consumer acceptance [37,38,41,56]. In the current investigation, plain fermented camel milk produced a watery and fragile consistency, resulting in poor structure. This is most likely due to the large casein micelles, the relative distribution of casein fractions, and the absence of β-LG in camel milk versus bovine milk [14]. The small size of the camel fat globule might be another cause for the poor structure of fermented camel milk [55]. For example, fortifying camel milk with skimmed milk powder [56], and adding hydrocolloids and stabilizers [57], as well as bonding enzymes [55], were successful in improving texture and viscosity. Our findings show that AV increased with increasing SKD levels compared to FCM + 0% SKD. This might be explained by the fact that adding SKD raised the TS (Table 2); FCM-SKD had higher AV values than FCM without SKD. Similar observations for viscosity regarding date milk beverages with different date and date syrup levels were confirmed [37,40]. Nouri et al. (2011) noted that reduction in whey separation and increase in WHC of FCM was presented when the total solids were increased by adding fruits [38]. Values of viscosity, WHC, and syneresis were affected significantly (p < 0.05) by storage time. The AV values increased during storage from the first day until the eighth day, and after, they decreased to the end of the storage period in the lowest and highest levels of SKD. The present study results are in accordance with Tawfek et al. [37], and Al-Humaid et al. [40], who reported a decrease in viscosity of FCM throughout the storage period.
Interestingly, a remarkable incremental trend in TPC, TF, TFL, and relative AOA in FCM-SKD was observed (Table 4). This may be due to the rich content of bioactive compounds and phytochemicals in FCM-SKD, which was increased with increasing SKD levels. Incorporation of SKD into FCM formulas improved the AOA by more than 75 and 45% when 5% SKD was used, assessed by DPPH and ABTS scavenging assays, respectively [1,2,20,25]. These ratios were remarkably increased with increasing SKD levels. The antioxidant capacity was probably due to the presence of phenolic compounds and ascorbic acid in both CM and SKD [25,26]. AlFaris et al. [58] reported that ripe date fruits are a rich source of polyphenols with potent natural antioxidants. As we added ripe SKD, the potential to be used as a nutraceutical or functional food ingredient in the production of FCM as natural antioxidant-rich dairy products is confirmed [59].
Visual assessment plays a vital role in evaluating the quality of milk. Measuring the color of FCM and FCM-SKD, we observed a significant change in a positive relationship with increasing SKD levels. With increasing SKD levels, the L* (as an indicator for light vs. dark) decreased and the b* value (for yellow vs. blue) significantly increased. This may be due to an increase in the pigments and phenolic compounds as SKD levels increased. Hachani et al. [60] stated that colors are primarily attributable to pigments produced through the condensation or browning reactions of phenolic compounds during ripening and storage. Increasing H°, BI, and ∆E with increasing SKD levels reflects the rise in phenolic and pigment contents in formulated FCM. However, Gross et al. [61] identified several pigments in dates, including carotenoids (lycopene, carotenes, flavoxanthin, and lutein), anthocyanins, flavones, and flavonols. At the same time, colors are caused by pigments produced by different enzymatic and nonenzymatic reactions. Such pigments include melanoidins and caramels that result from the Maillard reaction and enzymatic reactions catalyzed by polyphenol oxidase (e.g., melanins) or during milk pasteurization [61,62].
Recently, probiotics contained beneficial bacteria that benefit human health, combined with CM formulating FCM due to their synergic effects, which are highly desirable [63,64]. In the development of probiotic drinks, particular strains based on functional criteria and basal environment should be considered. The present study produced a probiotic-enriched fermented camel milk drink incorporating different SKD levels using an ABT-5 starter containing three bacterial strains. Str. thermophilus, L. acidophilus, and B. bifidum viable counts increased significantly with increasing SKD compared to FCM-SKD treatments with FCM + 0% SKD. Adding SKD at lower and medium levels to FCM enhanced the viability of Str. thermophilus, L. acidophilus, and B. bifidum count in (log CFU g−1) during storage. On day 1, FCM + 7.5% SKD exhibited the highest Str. thermophilus, L. acidophilus, and B. bifidum count, while on day 8, FCM + 12.5% SKD showed the highest count of Str. thermophilus, L. acidophilus, and B. bifidum. On day 15, FCM + 10% SKD exhibited the highest viable count of Str. thermophilus, L. acidophilus, and B. bifidum. This enhancement can be attributed to the presence of soluble dietary fiber, sugars, vitamins, and minerals which are similarly agreed [37,40,52]. Obviously, the viable counts of the mentioned strains were slightly reduced when 15% SKD was added, compared to both 10 and 12.5% SKD, which may indicate that adding SKD in a greater percentage than 15% may affect the viable microbial counts of Str. thermophilus, L. acidophilus, and B. bifidum, which increased gradually in most treatments after up to 8 days of storage and then decreased in all treatments until the end of the storage period. The high sugar content in FCM + 15% might increase the osmotic pressure and affect the cell viability [37,51,65]
Organoleptical attributes are essential indications of potential consumer preferences. As mentioned, CM and FCM as dairy products were not favored among many people because of their different sensory characteristics [51]. Incorporating different flavors into dairy products has been found to increase options for consumers and helps in marketing and retaining consumer interest in camel products [21,35,37,51]. Therefore, the current study carefully investigated the impact of ABT-5 and the incorporation of SKD on organoleptical attributes of FCM. Significant differences (p < 0.05) were observed between FCM and FCM-SKD in all organoleptic characteristics when SKD levels were increased, giving a curved acceptability trend, which increased when low levels of SKD were added, then decreased with high SKD levels. Increasing SKD up to 15% presented more date taste with a high sweet taste, but the panelists still considered the organoleptic characteristics acceptable. Adding 10 and 12.5% SKD recorded the best-balanced flavor score at the beginning and up to 15 days of storage, respectively. It could be concluded that adding SKD in the 7.5–12.5% range could produce highly nutritious and acceptable FCM. These results were in harmony with recent studies [37,41,51], which concluded that adding different date varieties in the 10–15% range could be acceptable and could provide considerable nutritional value.
It is not unexpected that the flavor of FCM-SKD incorporated different SKD levels received the highest results due to the sweet taste of dates, which panelists preferred. Noticeably, the addition of SKD improved flavor and body and texture, consistency, and mouthfeel. However, the addition of SKD resulted in lower color and appearance scores resulting from considerable pigment content in date [60,61]. According to color and appearance observations, consumers favor light-colored dairy products, while dark-colored items have low acceptability. Almosawi and Hassan [50] and Tawfek et al. [37] showed that panelists found the sensory attributes of probiotic fermented milk flavored with date palm to be very acceptable. Our findings showed that FCM containing SKD in the range of 7.5–12.5 averaged with 10% SKD had a significantly higher sensory evaluation, taste, and sweetness than FCM. The improved taste was related to the added date that contains a high amount of carbohydrates, providing a sweet taste. These results were in agreement with those that were previously mentioned [37,40,51].
5. Conclusions
In general, incorporating different flavors into dairy products has been found to increase options for consumers and helps in marketing and retaining consumer interest in camel products. Probiotic-enriched fermented camel milk was produced using ABT-5 culture fortified with SKD and evaluated for chemical, physicochemical, rheological, microbiological, and sensory qualities. The addition of SKD increased the TS and mineral contents of the product, thereby increasing its nutritional value. Bifidobacteria were considerably enhanced when SKD was mixed with FCM at low and medium levels, which could be utilized as a sweetener and prebiotic in probiotic-enriched fermented camel milk production. The organoleptic attributes showed that the incorporation of SKD improved flavor and body and texture, consistency, and mouthfeel. Noticeably, the addition of SKD resulted in lower color and appearance scores resulting from considerable pigment content in date. Our findings showed that FCM containing SKD in the range of 7.5–12.5 averaged with 10% SKD improved the nutritional quality without adverse effects on the technological and organoleptic characteristics and probiotics viability, and provided acceptable, nutritious, and additional health benefits to FCM.
Author Contributions
Conceptualization, methodology, investigation, T.A., H.B., M.R.; project administration, T.A.; validation, data curation, M.R., M.M.A.M., Data visualization, T.A., M.M.A.M.; writing—original draft preparation, writing—review and editing, and visualization, T.A., H.B. All authors have read and agreed to the published version of the manuscript.
Funding
Qassim University, Deanship of Scientific Research, project (10199-CAVM-2020-1-3-I), the academic year 1441 AH/2020 AD.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge Qassim University, represented by the Deanship of Scientific Research, on the financial support for this research under the number (10199-CAVM-2020-1-3-I) during the academic year 1441 A/2020 AD.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
∆E: Color changes; ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); AOA, Antioxidants activity; AV, Apparent viscosity; BI, Browning index; C, Chroma; CM, Camel milk; DPPH, 2,2-diphenyl-1-picrylhydrazyl; DVS, direct-to-vat set; FBM, Fermented bovine milk; FCM, Fermented camel milk; GAE, Gallic acid equivalents; H°, Hue angle; QE, quercetin equivalent; SKD, Sukkari date; TE, Trolox Equivalents; TF, Total flavonoids; TFL, Total flavonols; TN, total nitrogen; TPC, Total phenolic compounds; WHC; Water holding capacity.
References
- Ali, W.; Akyol, E.; Ceyhan, A.; Dilawar, S.; Firdous, A.; ul Qasim, M.Z.; Ahmad, M.M. Milk production and composition in camel and its beneficial uses: A review. Turk. J. Agric.-Food Sci. Technol. 2019, 7, 2142–2147. [Google Scholar]
- Hamed, H.; Chaari, F.; Ghannoudi, Z.; ElFeki, A.; Ellouz, S.C.; Gargouri, A. Beneficial effects of fermented camel milk by lactococcus lactis subsp cremoris on cardiotoxicity induced by carbon tetrachloride in mice. Biomed. Pharmacother. 2018, 97, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.R.; Santos, R.; Cardoso, C.; Carvalho, M. Consumption of camel’s milk by patients intolerant to lactose. A preliminary study. Rev. Alerg. Mex. 2010, 57, 26–32. [Google Scholar] [PubMed]
- Shabo, Y.; Barzel, R.; Margoulis, M.; Yagil, R. Camel milk for food allergies in children. Isr. Med. Assoc. J. 2005, 7, 796. [Google Scholar]
- Mishra, S.S.; Behera, P.K.; Kar, B.; Ray, R.C. Advances in Probiotics, Prebiotics and Nutraceuticals. In Innovations in Technologies for Fermented Food and Beverage Industries; Panda, S.K., Shetty, P.H., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 121–141. [Google Scholar]
- Sundararaman, A.; Ray, M.; Ravindra, P.V.; Halami, P.M. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 8089–8104. [Google Scholar] [CrossRef] [PubMed]
- Al Kassaa, I. The antiviral activity of probiotic metabolites. In New Insights on Antiviral Probiotics; Springer: Berlin/Heidelberg, Germany, 2017; pp. 83–97. [Google Scholar]
- Kobyliak, N.; Falalyeyeva, T.; Boyko, N.; Tsyryuk, O.; Beregova, T.; Ostapchenko, L. Probiotics and nutraceuticals as a new frontier in obesity prevention and management. Diabetes Res. Clin. Pract. 2018, 141, 190–199. [Google Scholar] [CrossRef] [PubMed]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid.-Based Complement. Alternat. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.A.; Santos-Junior, V.A.; Filho, E.R.T.; Silva, H.L.A.; Ferreira, M.V.S.; Graça, J.S.; Esmerino, E.A.; Lollo, P.C.B.; Freitas, M.Q.; Sant’Ana, A.S.; et al. Probiotic Prato cheese consumption attenuates development of renal calculi in animal model of urolithiasis. J. Funct. Foods 2018, 49, 378–383. [Google Scholar] [CrossRef]
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G.M.; et al. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1718. [Google Scholar] [CrossRef]
- Meybodi, N.M.; Mortazavian, A.M.; Arab, M.; Nematollahi, A. Probiotic viability in yoghurt: A review of influential factors. Int. Dairy J. 2020, 109, 104793. [Google Scholar] [CrossRef]
- Nagyzbekkyzy, E.; Sembayeva, D.; Sarsenova, A.; Mansurov, N.; Moldabayeva, A.; Moldagulova, N. Data on the diversity of lactic acid bacteria isolated from raw and fermented camel milk. Data Br. 2020, 31, 105956. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Almazrouei, N.; Galiwango, E.; Esposito, G.; Hunashal, Y.; Hamed, F.; Najjar, Z. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: Structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020, 333, 127418. [Google Scholar] [CrossRef] [PubMed]
- Hamed, H.; Bellassoued, K.; El Feki, A.; Gargouri, A. Evaluation of the hepatoprotective effect of combination between fermented camel milk and Rosmarinus officinalis leaves extract against CCl4 induced liver toxicity in mice. J. Food Sci. Technol. 2019, 56, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Hamed, H.; Gargouri, M.; Boulila, S.; Chaari, F.; Ghrab, F.; Kallel, R.; Ghannoudi, Z.; Boudawara, T.; Chaabouni, S.; Feki, A.E.; et al. Fermented camel milk prevents carbon tetrachloride induced acute injury in kidney of mice. J. Dairy Res. 2018, 85, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Fallah, Z.; Feizi, A.; Hashemipour, M.; Kelishadi, R. Effect of fermented camel milk on glucose metabolism, insulin resistance, and inflammatory biomarkers of adolescents with metabolic syndrome: A double-blind, randomized, crossover trial. J. Res. Med. Sci. 2018, 23, 32. [Google Scholar]
- Fallah, Z.; Feizi, A.; Hashemipour, M.; Kelishadi, R. Positive Effect of Fermented Camel Milk on Liver Enzymes of Adolescents with Metabolic Syndrome: A Double-Blind, Randomized, Cross-over Trial. Mater. Sociomed. 2018, 30, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Ayyash, M.; Abdalla, A.; Alhammadi, A.; Senaka Ranadheera, C.; Affan Baig, M.; Al-Ramadi, B.; Chen, G.; Kamal-Eldin, A.; Huppertz, T. Probiotic survival, biological functionality and untargeted metabolomics of the bioaccessible compounds in fermented camel and bovine milk after in vitro digestion. Food Chem. 2021, 363, 130243. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahib, W.; Marshall, R.J. The fruit of the date palm: Its possible use as the best food for the future? Int. J. Food Sci. Nutr. 2003, 54, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Parn, O.J.; Bhat, R.; Yeoh, T.K.; Al-Hassan, A.A. Development of novel fruit bars by utilizing date paste. Food Biosci. 2015, 9, 20–27. [Google Scholar] [CrossRef]
- Tang, Z.X.; Shi, L.E.; Aleid, S.M. Date fruit: Chemical composition, nutritional and medicinal values, products. J. Sci. Food Agric. 2013, 93, 2351–2361. [Google Scholar] [CrossRef]
- Assirey, E.A.R. Nutritional composition of fruit of 10 date palm (Phoenix dactylifera L.) cultivars grown in Saudi Arabia. J. Taibah Univ. Sci. 2015, 9, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Samarawira, I. Date palm, potential source for refined sugar. Econ. Bot. 1983, 37, 181–186. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef] [PubMed]
- Benmeddour, Z.; Mehinagic, E.; Meurlay, D.L.; Louaileche, H. Phenolic composition and antioxidant capacities of ten Algerian date (Phoenix dactylifera L.) cultivars: A comparative study. J. Funct. Foods 2013, 5, 346–354. [Google Scholar] [CrossRef]
- Suresh, S.; Guizani, N.; Al-Ruzeiki, M.; Al-Hadhrami, A.; Al-Dohani, H.; Al-Kindi, I.; Rahman, M.S. Thermal characteristics, chemical composition and polyphenol contents of date-pits powder. J. Food Eng. 2013, 119, 668–679. [Google Scholar] [CrossRef]
- Ishurd, O.; Kennedy, J.F. The anticancer activity of polysaccharide prepared from Libyan dates (Phoenix dactylifera L.). Carbohydr. Polym. 2005, 59, 531–535. [Google Scholar] [CrossRef]
- Vayalil, P.K. Antioxidant and antimutagenic properties of aqueous extract of date fruit (Phoenix dactylifera L. Arecaceae). J. Agric. Food Chem. 2002, 50, 610–617. [Google Scholar] [CrossRef]
- Amira, E.A.; Guido, F.; Behija, S.E.; Manel, I.; Nesrine, Z.; Ali, F.; Mohamed, H.; Noureddine, H.A.; Lotfi, A. Chemical and aroma volatile compositions of date palm (Phoenix dactylifera L.) fruits at three maturation stages. Food Chem. 2011, 127, 1744–1754. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Alasalvar, C.; Al-Abid, M.; Al-Shoaily, K.; Al-Amry, M.; Al-Rawahy, F. Compositional and functional characteristics of dates, syrups, and their by-products. Food Chem. 2007, 104, 943–947. [Google Scholar] [CrossRef]
- Elleuch, M.; Besbes, S.; Roiseux, O.; Blecker, C.; Deroanne, C.; Drira, N.-E.; Attia, H. Date flesh: Chemical composition and characteristics of the dietary fibre. Food Chem. 2008, 111, 676–682. [Google Scholar] [CrossRef]
- Vayalil, P.K. Date fruits (Phoenix dactylifera Linn): An emerging medicinal food. Crit. Rev. Food 2012, 52, 249–271. [Google Scholar] [CrossRef]
- Boudries, H.; Kefalas, P.; Hornero-Méndez, D. Carotenoid composition of Algerian date varieties (Phoenix dactylifera) at different edible maturation stages. Food Chem. 2007, 101, 1372–1377. [Google Scholar] [CrossRef]
- Vijayanand, P.; Yadav, A.R.; Balasubramanyam, N.; Narasimham, P. Storage Stability of Guava Fruit Bar Prepared Using a New Process. Food Sci. Technol. 2000, 33, 132–137. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Teoh, A.; Massarotto, C.; Wibisono, R.; Wadhwa, S. Comparative analysis of fruit-based functional snack bars. Food Chem. 2010, 119, 1369–1379. [Google Scholar] [CrossRef]
- Tawfek, M.A.; Baker, E.A.; El-Sayed, H.A. Study Properties of Fermented Camels’ and Goats’ Milk Beverages Fortified with Date Palm (Phoenix dactylifera L.). Food Nutr. Sci. 2021, 12, 418–428. [Google Scholar]
- Soliman, T.N.; Shehata, S.H. Characteristics of fermented camel’s milk fortified with kiwi or avocado fruits. Acta Sci. Pol. Technol. Aliment. 2019, 18, 53–63. [Google Scholar] [PubMed]
- Saljooghi, S.; Mansouri-Najand, L.; Ebrahimnejad, H.; Doostan, F.; Askari, N. Microbiological, biochemical and organoleptic properties of fermented-probiotic drink produced from camel milk. Vet. Res. Forum 2017, 8, 313. [Google Scholar] [PubMed]
- Al-Humaid, A.; Mousa, H.; El-Mergawi, R.; Abdel-Salam, A. Chemical composition and antioxidant activity of dates and dates-camel-milk mixtures as a protective meal against lipid peroxidation in rats. Am. J. Food Technol. 2010, 5, 22–30. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis of the AOAC, 17th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2000. [Google Scholar]
- Barakat, H.; Hassan, M.F. Chemical, nutritional, rheological, and organoleptical characterizations of stirred pumpkin-yoghurt. Food Nutr. Sci. 2017, 8, 746. [Google Scholar] [CrossRef] [Green Version]
- Cartashev, A.; Rudic, V. The effect of starter culture producing exopolysaccharide on physicochemical properties of yoghurt. Chem. J. Mold. 2017, 12, 7–12. [Google Scholar] [CrossRef]
- Yawadio Nsimba, R.; Kikuzaki, H.; Konishi, Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008, 106, 760–766. [Google Scholar] [CrossRef]
- Mohdaly, A.A.A.; Hassanien, M.F.R.; Mahmoud, A.; Sarhan, M.A.; Smetanska, I. Phenolics Extracted from Potato, Sugar Beet, and Sesame Processing By-Products. Int. J. Food Prop. 2012, 16, 1148–1168. [Google Scholar] [CrossRef]
- Barakat, H.; Rohn, S. Effect of different cooking methods on bioactive compounds in vegetarian, broccoli-based bars. J. Funct. Foods 2014, 11, 407–416. [Google Scholar] [CrossRef]
- Lavelli, V.; Corey, M.; Kerr, W.; Vantaggi, C. Stability and anti-glycation properties of intermediate moisture apple products fortified with green tea. Food Chem. 2011, 127, 589–595. [Google Scholar] [CrossRef]
- Vinderola, C.G.; Reinheimer, J.A. Culture media for the enumeration of Bifidobacterium bifidum and Lactobacillus acidophilus in the presence of yoghurt bacteria. Int. Dairy J. 1999, 9, 497–505. [Google Scholar] [CrossRef]
- Steel, R.G. Pinciples and Procedures of Statistics a Biometrical Approach, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1997. [Google Scholar]
- Hashim, I.B.; Khalil, A.H.; Habib, H. Quality and acceptability of a set-type yogurt made from camel milk. J. Dairy Sci. 2009, 92, 857–862. [Google Scholar] [CrossRef] [Green Version]
- Almosawi, B.N.E.; Hassan, T.A. Influence of Fortification with Extracts of Three Varieties of Iraqi Dates on the Viability of Lactobacillus plantarum in Probiotic Fermented Milk Products. Iraqi J. Sci. 2019, 18, 216–223. [Google Scholar]
- Almosawi, B.N.; Al-Hamdani, H.M.; Dubaish, A.N. Study of qualification and Sensation properties by using date extraction and date syrup in yoghurt processing. Adv. Life Sci. Technol. 2015, 32, 49–58. [Google Scholar]
- Siddeeg, A.; Zeng, X.-A.; Ammar, A.-F.; Han, Z. Sugar profile, volatile compounds, composition and antioxidant activity of Sukkari date palm fruit. J. Food Sci. Technol. 2019, 56, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Trabzuni, D.M.; Ahmed, S.E.B.; Abu-Tarboush, H.M. Chemical composition, minerals and antioxidants of the heart of Date Palm from three Saudi cultivars. Food Nutr. Sci. 2014, 5, 1379. [Google Scholar] [CrossRef] [Green Version]
- Abou-Soliman, N.H.I.; Sakr, S.S.; Awad, S. Physico-chemical, microstructural and rheological properties of camel-milk yogurt as enhanced by microbial transglutaminase. J. Food Sci. Technol. 2017, 54, 1616–1627. [Google Scholar] [CrossRef] [Green Version]
- Salih, M.M.; Hamid, O.A. Effect of fortifying camel’s milk with skim milk powder on the physicochemical, microbiological and sensory characteristics of set yoghurt. Adv. J. Food Sci. Technol. 2013, 5, 765–770. [Google Scholar] [CrossRef]
- Al-Zoreky, N.S.; Al-Otaibi, M.M. Suitability of camel milk for making yogurt. Food Sci. Biotechnol. 2015, 24, 601–606. [Google Scholar] [CrossRef]
- AlFaris, N.A.; AlTamimi, J.Z.; AlGhamdi, F.A.; Albaridi, N.A.; Alzaheb, R.A.; Aljabryn, D.H.; Aljahani, A.H.; AlMousa, L.A. Total phenolic content in ripe date fruits (Phoenix dactylifera L.): A systematic review and meta-analysis. Saudi J. Biol. Sci. 2021, 28, 3566–3577. [Google Scholar] [CrossRef] [PubMed]
- Zihad, S.M.N.K.; Uddin, S.J.; Sifat, N.; Lovely, F.; Rouf, R.; Shilpi, J.A.; Sheikh, B.Y.; Göransson, U. Antioxidant properties and phenolic profiling by UPLC-QTOF-MS of Ajwah, Safawy and Sukkari cultivars of date palm. Biochem. Biophys. 2021, 25, 100909. [Google Scholar] [CrossRef] [PubMed]
- Hachani, S.; Hamia, C.; Boukhalkhal, S.; Silva, A.M.S.; Djeridane, A.; Yousfi, M. Morphological, physico-chemical characteristics and effects of extraction solvents on UHPLC-DAD-ESI-MSn profiling of phenolic contents and antioxidant activities of five date cultivars (Phoenix dactylifera L.) growing in Algeria. NFS J. 2018, 13, 10–22. [Google Scholar] [CrossRef]
- Gross, J.; Haber, O.; Ikan, R. The carotenoid pigments of the date. Sci. Hortic. 1983, 20, 251–257. [Google Scholar] [CrossRef]
- Serratosa, M.P.; Lopez-Toledano, A.; Merida, J.; Medina, M. Changes in Color and Phenolic Compounds during the Raisining of Grape Cv. Pedro Ximenez. J. Agric. Food Chem. 2008, 56, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Mohamed, A.; Gemiel, D.G.; Atallah, A.A. Microstructural, volatile compounds, microbiological and organoleptical characteristics of low-fat buffalo milk yogurt enriched with whey protein concentrate and ca-caseinate during cold storage. Fermentation 2021, 7, 250. [Google Scholar] [CrossRef]
- Aljutaily, T.; Huarte, E.; Martinez-Monteagudo, S.; Gonzalez-Hernandez, J.L.; Rovai, M.; Sergeev, I.N. Probiotic-enriched milk and dairy products increase gut microbiota diversity: A comparative study. Nut. Res. 2020, 82, 25–33. [Google Scholar] [CrossRef]
- Malik, A.; Yayan, M.; Irwan Zakir, S.D.; Syarif Djaya, M. Effects of Addition of Juice Date Palm to the Extender on the Semen Qualities of Frozen Thawed in Bull Spermatozoa. Glob. Vet. 2016, 16, 100–104. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





