Abstract
Biosurfactants exhibit antioxidant, antibacterial, antifungal, and antiviral activities. They can be used as therapeutic agents and in the fight against infectious diseases. Moreover, the anti-adhesive properties against several pathogens point to the possibility that they might serve as an anti-adhesive coating agent for medical inserts and prevent nosocomial infections, without using synthetic substances. In this study, the antimicrobial, antibiofilm, cell surface hydrophobicity, and antioxidative activities of biosurfactant extracted from Bacillus sp., against four pathogenic strains of Staphylococcus spp. associated with vaginal infection, were studied. Our results have shown that the tested biosurfactant possesses a promising antioxidant potential, and an antibacterial potency against multidrug clinical isolates of Staphylococcus, with an inhibitory diameter ranging between 27 and 37 mm, and a bacterial growth inhibition at an MIC of 1 mg/ mL, obtained. The BioSa3 was highly effective on the biofilm formation of different tested pathogenic strains. Following their treatment by BioSa3, a significant decrease in bacterial attachment (p < 0.05) was justified by the reduction in the optical (from 0.709 to 0.111) following their treatment by BioSa3. The antibiofilm effect can be attributed to its ability to alter the membrane physiology of the tested pathogens to cause a significant decrease (p < 0.05) of over 50% of the surface hydrophobicity. Based on the obtained result of the bioactivities in the current study, BioSa3 is a good candidate in new therapeutics to better control multidrug-resistant bacteria and overcome bacterial biofilm-associated infections by protecting surfaces from microbial contamination.
1. Introduction
A miscarriage is one of the most common and understudied pregnancy outcomes. Some viral and bacterial infections can increase the risk of pregnancy loss [1]. There are, however, many microorganisms involved in the inflammation of the pelvis. Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma genitalium are the most common causes of pelvic inflammatory disease [2]. Several cases are associated with bacteria, such as Trichomonas vaginalis. Different pathogenic bacteria, such as Enterobacteriaceae, Staphylococcus spp., Streptococcus spp., and anaerobes, may be present in the case of complicated pelvic inflammatory disease or as a result of vaginal carriage after delivery, abortion, or intrauterine procedure [2]. There is evidence that aerobic vaginosis is associated with miscarriage [3]. A variety of studies have reported prevalence rates of aerobic vaginosis ranging from 5% to 27.6% [4]. Thus, identifying aerobic vaginosis is important to develop standardized treatment methods. Indeed, Escherichia coli, Streptococcus spp., S. aureus, coagulase-negative staphylococci, S. epidermidis, and Enterococcus faecalis colonize aerobic vaginosis patients in relatively high numbers [5,6]. Therefore, the vaginal epithelium usually forms a biofilm when a patient has bacterial vaginosis. Hence, it is widely believed that the formation of protective biofilms plays a crucial role in the pathogenesis of bacterial vaginosis [7]. A biofilm also exhibits a high resistance to antibiotic treatment. In fact, the exopolysaccharide (EPS) matrix, providing anchorage and support, which makes the bacteria less sensitive to these drugs [8] and antimicrobial treatment, fails due to the presence of a significant amount of drug-resistant bacteria in the biofilm. Tenke et al. [9] have observed that sessional bacteria that do not carry any genetic determinants of antibiotic resistance become resistant to antibiotics when embedded in a biofilm. Among the leading pathogens responsible for chronic biofilm infections is S. aureus. It is becoming more difficult to treat these infections due to drug resistance, especially since biofilms created by S. aureus can limit the efficiency of antibiotics, causing severe morbidity and mortality [10]. This ability of Staphylococcus spp. to adhere and interact with surfaces gives them persistence in the host. In 2003, the National Institutes of Health [11] reported that biofilm-related pathogens cause approximately 80% of bacterial infections in humans. It is frequently difficult to eradicate biofilms from the infected host due to their recalcitrant nature, by which they grow on self-produced extracellular polymeric materials [12]. Furthermore, antibiotic resistance among staphylococci further complicates treatment, as biofilms are difficult to inhibit with conventional antibiotics [10]. A very important, clinically significant characteristic of biofilm development is its high resistance to antimicrobial agents. Biofilms typically resist antibiotics by a factor of 1000 times more than their counterparts living in water [13]. Biofilms provide several specific defense mechanisms controlling bacteria embedded in them, including barriers by extracellular polymers from inactivating antimicrobial agents, which make them intrinsically resistant to antibiotics [10].
Biological approaches have been studied over the past decade to avoid this phenomenon and control the spread of bacteria. By the discovery of this antimicrobial resistance, recent reports suggest that the use biosurfactant (BS) might provide an alternative strategy that may provide a solution to this problem [14]. BS is an emulsifier molecule with properties to reduce the tension of liquid interfaces [15]. In this sense, microbial surfactants include several different types of molecules, including glycolipids, lipopeptides, polysaccharide–protein complexes, protein-like substances, phospholipids, fatty acids, and neutral lipids [16]. As well as its physiological functions, BS also has some interesting properties, such as the ability to increase the surface area and bioavailability of hydrophobic water-insoluble substrates, the ability to bind to heavy metals, and the ability to detect quorums [17]. In order for biofilms to form, bacteria must adhere to surfaces [18], which can be reduced and controlled by BSs [19,20]. Additionally, BSs produced by bacteria has been shown to interfere with biofilm development and cell-to-cell communication [21]. Additionally, they have antibacterial properties and anti-stick and anti-biofilm capabilities against multidrug-resistant bacteria strains, such as Acinetobacter baumannii, E. coli, and methicillin-resistant S. aureus (MRSA) [22].
These observations, in turn, prompted us to carry out the present study. In vitro antimicrobial tests, such as antibacterial and antibiofilm assays, were performed against multidrug-resistant staphylococci, considered as one among the most important pathogens associated with vaginal infection, to assess the effectiveness of BS in controlling infectious diseases. To gain a deeper understanding of the mechanism of the antibiofilm effect, we studied cell surface hydrophobicity, which was previously identified as a key factor in pathogenic bacteria’s adhesion to surfaces. Needed bioactivity, such as antioxidant capacity, was also evaluated.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
In this study, Bacillus sp. “HM117834” was used, which was isolated from the hypersaline environment and previously investigated for its probiotic properties [23]. Four pathogenic multidrug-resistant bacteria (S1—S. aureus; S2—S. aureus; S3—S. haemolyticus; S4—S. haemolyticus) used in this study were identified using the sequencing of the 16sRNA gene. The obtained sequence of the 16sRNA gene for the isolates S1, S2, S3, and S4 were deposited, respectively, in GenBank under the following references: MZ475010; MZ475016; MZ474996; MZ475011. All the tested strains are involved in vaginal infections. The isolation was carried out from cervicovaginal samples from women who had two or more abortions in the external consultation service for maternity and neonatal care in Monastir. The potential probiotic strain was grown in nutrient broth medium (NB) for 5 days at 37 °C with vigorous shaking. Filtered olive oil through a 0.45 mm filter was added to the culture medium as a carbon source. Pathogenic strains, however, were grown for 24 h at 37 °C on nutrient broth agar (Difco).
2.2. Production of BS
In a 1 L falcon, 500 mL of minimal medium containing sterile olive oil (4%) as a source of carbon were inoculated with a 20 mL of an overnight culture of Bacillus sp. “HM117834” strain (106 CFU/mL) followed by incubation for 48 h at 33 °C and 180 rpm. Afterwards, the cells were centrifuged at 5000 rpm for 20 min to remove bacteria. The culture supernatant was filtered through a 0.45 mm filter and an acidification to pH 2 with 6 M HCl was performed before incubating overnight at 25 °C. Extractions were performed three times using ethyl acetate in equal parts v/v. Anhydrous sodium sulfate (Na2SO4) was applied at a concentration of 10 g/100 mL and the organic phase was dried over and evaporated. To remove traces of solvent, crude BS “BioSa3” was incubated overnight at 37 °C. BS production was expressed in grams per liter [24].
2.3. Oil Displacement Test
The production of BS was carried out by applying the most commonly used test in the literature—the oil spread test, which is used to screen bacterial isolates [25,26]. The ability of BS to reduce surface tension is used as an endpoint [27]. The occurrence of the clear zone indicated the BS production [24,28]. We used a Petri dish (85 mm diameter) containing 20 mL of distilled water. Then, 300 µL of the oil sample was added to the surface of the distilled water until a thin oil layer was established. Then, an equal volume of the solution, containing the crude BioSa3, was deposited on the surface of the oil. The halo diameter was measured and compared to positive (containing 0.1% sodium dodecyl sulfate (SDS)) and negative (containing distilled water) controls. All the experiments were performed in triplicate.
2.4. Antimicrobial Susceptibility Assay
Following the CA SFM/ EUCAST 2021, the four pathogenic strains were tested for susceptibility to different antimicrobials using the disk–agar diffusion technique. The antimicrobial disks used were methicillin (5 µg), penicillin (1U), erythromycin (15 µg), ampicillin (10 µg), kanamycin (30 µg), and gentamicin (10 µg). After 18–24 h of incubation at 37 °C, the results of these tests were recorded.
2.5. Diffusion Agar Assay
A diffusion agar assay, using direct contact between the biosurfactant and pathogens, was used to test the antibacterial activity of the extracted BioSa3 against multidrug-resistant Staphylococcus strains. Pathogenic bacterial strains were cultivated for 24 h on nutrient agar at 37 °C. Then, a bacterial suspension (106 UFC/mL) of 10 mL of physiological medium from pure culture was prepared. Agar plates were covered with 1 mL of the suspension and 10 µL of the BioSa3 at a concentration of 25 mg/mg [29] were deposited on the Mueller–Hinton (MH) agar and left incubating at 37 °C for 24 h. By measuring the diameter (mm) of the clear zone formed around the well, antibacterial activity was determined [30].
2.6. Antibacterial Activity
The minimum inhibitory concentration (MIC) measurement was used to determine the antimicrobial activity of BioSa3 against the pathogenic bacterial strains studied in this work using 96-well plates. Briefly, the samples were inoculated into 3 mL of MH broth and incubated at 37 °C overnight with shaking. Subsequently, 1/100 dilutions in 3 mL of MH were made and incubated for 4 h at 37 °C. A measure of 0.1 mL of MH broth was placed in the first column to serve as a negative control. A volume of 0.05 mL of BioSa3 at different concentrations, ranging from 16 up to 0.0625 mg/mL, was filled in the following columns. The inoculum was adjusted to a number of 106 CFU/mL, corresponding to a final optical density (OD) of 0.01 at a wavelength of 600 nm (Infinite F200 PRO, TECAN, Lyon, France). The last column of the plate contained only 0.05 mL of inoculum and 0.05 mL of MH broth, which served as a positive control (without BioSa3). The MIC was determined, after 24 h of incubation at 37 °C, by measuring the OD at 600 nm in an ELISA reader (Infinite F200 PRO, TECAN). The MIC is defined as the minimum inhibitory concentration of BioSa3 necessary to prevent bacterial growth as seen visually (no growth seen from the back of the plate against a dark background illuminated by reflected light) [31].
2.7. Antibiofilm Assay
The effectiveness of the BioSa3 concentrations that represent MIC/2 in avoiding bactericidal activity was assessed using a semi-quantitative adherence assay for their potential anti-biofilm effect. During the assays, 96-well polystyrene plates with flat bottoms were used. Strains of staphylococci were grown in 5 mL tryptic soy broth supplemented with yeast extract (TSB-YE) for 24 h at 37 °C. An aliquot of 10 µL (106 UFC) was deposed into each well of 96-well plates (Nunc, Roskilde, Denmark) containing 100 µL of BioSa3 at MIC/2 supplemented with 2% glucose (w/v). Control wells contained only TSB-YE/glucose (2% w/v). Then, the plates were incubated at 37 °C for 24 h to allow biofilm to form. Using the method of Djordjevic et al. [32], the crystal violet test was performed to measure biofilm formation. Results were expressed as percentage of biofilm inhibition (BI): BI = [(OD negative control − OD Experimental)/OD negative control] × 100.
2.8. Cell Surface Hydrophobicity
To determine the hydrophobicity of the staphylococci before and after treatment with BioSa3, the solvent microbial adhesion test (MATS) was used accordingly to Bellon et al. [33]. This experiment evaluated the affinity of cells to a polar solvent (hexadecane). After centrifugation at 7000× g for 5 min, the bacteria cells were re-suspended in buffered saline (pH 7.0) to a final density of 109 CFU/ ml (OD = 0.4 at 600 nm). Each cell suspension was equilibrated for 10 min with 1 milliliter of hexadecane (Sigma). The suspensions were then re-incubated at 37 °C for 30 min. After removing the aqueous layer and aerating it, all traces of hexadecane were removed, and absorbance (OD600) was measured with a hexadecane-extracted PBS blank. To test the effect of BioSa3 on the cell surface hydrophobicity, a concentration that represent CMI/2 to avoid bactericidal activity was used. For the treatment with BioSa3, bacterial cultures in nutrient broth were incubated with the BS for 24 h at 37 °C before the evaluation of the cell surface hydrophobicity percentage. The hydrophobicity index was calculated by comparing the hexadecane-extracted sample to the sample before extraction [33,34].
2.9. Antioxidant Activity: DPPH Radical Scavenging Assay
Using the method described by Shimada and colleagues [35], the BioSa3 scavenging ability was determined. A solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH, 300 µM) was mixed with 1 mL of BioSa3 (0.5, 1, 2, 5, 10 mg/mL). After mixing the reaction mixture, incubation was carried out at room temperature for 30 min. The solution was measured at 517 nm for absorbance. The standard used was ascorbic acid at a concentration of 250 µg/mL. Equation (1) was used to calculate the inhibitory percentage of DPPH, as follows:
DPPH Scavenging effect (%) = [1 − (Abssample/Abscontrol)] × 100
2.10. Statistical Analysis
The different assays were compared using ANOVA and Duncan’s test. Statistica (version 10.0) was used to perform all statistical analyses at a 0.05 level of probability.
3. Results
3.1. Oil Displacement
Due to its surfactant property, the oil displacement test is the most used method for detecting BS production. The results summarized in Table 1 showed a significant oil clearance zone (average clear zone of 4.53 cm ± 0.11), demonstrating positive oil displacement for the extracted BioSa3. Comparatively to the SDS-treated positive control, a significant difference occurred (average clear zone of 2.56 cm ± 0.15) (p < 0.05).

Table 1.
Test results of oil displacement of the two BioSa3 producing strains.
3.2. Antibiotic Resistance Profile, Diffusion Agar Assays, and MIC Determination
All the tested staphylococci strains were multiresistant to the tested antibiotic. As shown in the first part of Table 2 Antibiotics, the isolates demonstrated a resistance to erythromycin (15 µg), kanamycin (30 µg), methicillin (5 µg), and gentamycin (10 µg). The tested BioSa3 was highly effective on the used pathogenic Staphylococcus strains with a diameter of inhibition ranging between 27 and 37 mm. BioSa3 also demonstrated an antibacterial activity against all the multidrug-resistant strains of staphylococci with a MIC value of 1 mg/mL (Table 2).

Table 2.
Antibiotic resistance assay and antibacterial activity of BioSa3.
3.3. Hydrophobicity of the Cell Surface and Antibiofilm Activity
BioSa3 had high effectiveness in preventing biofilm forming in the tested multidrug-resistant staphylococci isolates (Figure 1). The anti-adhesive effect of the tested BioSa3 was a strain-dependent effect. This variation is due to the sensitivity of the biofilm created towards the molecules of the BioSa3 and the concentration tested (MIC/2). A significant decrease (p < 0.05) between biofilm formation (without BioSa3) and biofilm inhibition (with BioSa3) was noticed.
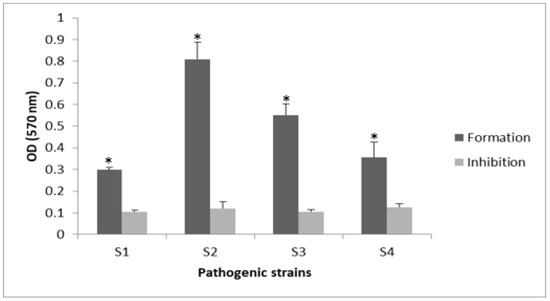
Figure 1.
Antibiofilm effect of BioSa3 on S.aureus and S. haemolyticus strains. Formation “without BioSa3” and inhibition “with BioSa3”. S1—MZ475010 (S. aureus); S2—MZ475016 (S. aureus); S3—MZ474996 (S. haemolyticus); S4—MZ475011 (S. haemolyticus). * p < 0.05.
To evaluate the effect of BioSa3 on the surface properties of the pathogens studied in this work, we studied the affinity of bacteria with and without BioSa3 with hexadecane. In Figure 2, the BioSa3 treatment significantly reduced the surface hydrophobicity of various staphylococci. Hydrophobicity is determined based on the percentage of cells bound to hexadecane. According to Chae et al. [36], a cell can be strongly hydrophobic when the percentage of hydrophobicity is more than 55%, moderately hydrophobic at 30–54%, moderately hydrophilic at 10–29%, and strongly hydrophilic <10%. Then, S3 has a medium affinity with hexadecane, and they were classified as moderately hydrophobic. However, S1, S2, and S4 shown a moderately hydrophilic characteristic. After treatment with BioSa3, all strains became more hydrophilic with an expected decrease in hexadecane affinity. However, all strains except S3, which became moderately hydrophilic, presented a strongly hydrophilic character.
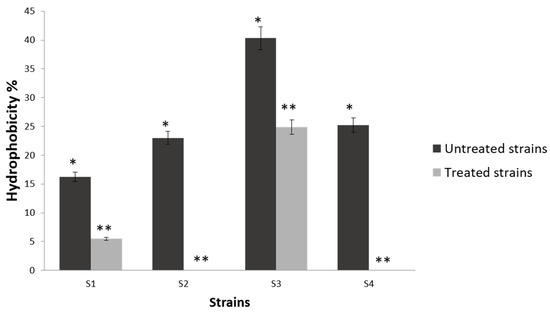
Figure 2.
Affinity of treated and non-treated bacterial cells with BioSa3 to hexadecane used in MATS test. S1—MZ475010 (S. aureus); S2—MZ475016 (S. aureus); S3—MZ474996 (S. haemolyticus); S4—MZ475011 (S. haemolyticus). The stars (*–**) indicate a significant difference in the same line between the different treated and non-treated cells according to Duncan’s test (p < 0.05).
3.4. Antioxidant Properties
The observed in vitro results confirm that the BS tested represent an important source of molecules with antioxidant potency (Figure 3). We find that BioSa3 has a greater capacity to inhibit the DPPH radical with a percentage inhibition that reaches 63%. In vitro, BioSa3 has been compared with ascorbic acid (AA) for its ability to scavenge DPPH radicals. In Figure 3, the scavenging activity of BioSa3 and AA on DPPH radicals was a concentration-dependent activity. The antioxidant potential of BioSa3 was quite high (63% at 10 mg/1 mL), but it was still lower than that of AA (95% at 1 mg/mL).
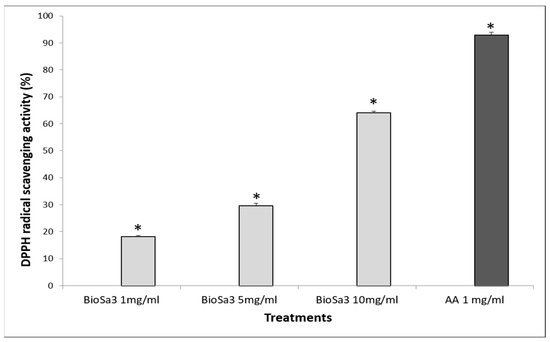
Figure 3.
Antioxidant activity of the tested BioSa3. AA— ascorbic acid. (* p < 0.05).
4. Discussion
Since staphylococci is one of the most prevalent pathogens in infections in nosocomical infections, it has developed resistance to antimicrobials, and causes often recalcitrant infections. This pathogen has gained more interest in scientific researchers as a significant health problem [37]. Indeed, by producing toxins or by penetrating into the human or animal body, Staphylococcus can cause a large range of diseases and infections [38,39]. These infections may be related to biofilm formation by pathogenic strains, such as S. aureus [10]. As a result of the entanglement of bacteria in a biofilm, the protection from innate and adaptive immunity and can persist despite antibiotics [8]. Likewise, biofilm formation on the vaginal epithelium is known to occur in cases of bacterial vaginosis. Hence, there is a hypothesis that establishing a biofilm is a critical element of bacterial vaginosis pathogenesis [7]. A healthy vaginal epithelium is dominated by Lactobacilli, which act as protective microorganism-producing surfactants. These surfactants inhibit the adhesion and growth of pathogens [40,41,42]. In addition to Lactobacilli, different microorganisms, including fungi and bacteria, such as Pseudomonas, Burkholderia, and Bacillus species, produce BS molecules that are used in industrial and medical applications [43,44]. Two strains that belong to the genera Bacillus and Lactobacillus were used as producers of BS, which had previously been widely reported to have potential applications in industries involving pharmaceuticals and food [45,46,47,48].
In the present study, BioSa3, produced by Bacillus sp. “HM117834”, previously examined for its probiotic properties [23], was used to identify the biofilm inhibition of four multidrug-resistant pathogenic strains of S. aureus and S. haemolyticus. Additionally, the antibacterial and antioxidant activity of biosurfactant has been studied. A preliminary test using oil displacement to determine the production of BioSa3 from Bacillus sp was applied. It has been found that when BS is present in the free supernatant, a clear zone and displacement zone are formed. These clearing zones are related to BS activity. In the case of pure BS, the diameter of the clearing zone correlates linearly with the quantity of the used biosurfactant [49,50]. BSs derived from the Lactobacillus genus are mostly composed of polysaccharides, phosphate, and protein, and they are mainly classified as glycolipids or glycolipoproteins [51]. These molecules have antimicrobial properties against several common pathogenic bacteria, including Neisseria gonorrhoeae, Escherichia coli, S.saprophyticus, Enterobacteraerogenes, and Klebsiellapneumoniae, and antifungal properties against Candida albicans [29,46]. There are many different types of BSs, including fatty acids, lipopeptides, neutral lipids, and glycolipids [52]. Bacillus sp. produces different lipopeptide, which consist of fatty acids of variable length (hydrophobic portion), bound to peptide chains of seven or ten amino acids (hydrophilic portion). As an example, we can mention surfactin that exhibits high emulsifying properties, in addition to its properties such as antiviral, antimicrobial, and antitumor [52]. Indeed, the Bacillus genus is known for its ability to produce antimicrobial molecules, such as bacteriocin [53]. Similarly, in a recent study, a number of strains of L. plantarum, L. inners, L. reuteri, and L. brevis were shown to produce surfactin, which has many similarities with the surfactin biosynthesis pathway derived from Bacillus species [54]. Due to their complex chemical structures, biological surfactants have hydrophobic and hydrophilic regions, which are not separated as in chemical surfactants. It is rather a mosaic distribution of polarity, with branched or circular structures [55], allowing surfactin to form spherical structures at the interfaces and present a greater complexity. The surface-active properties of glycolipid, such as rhamnolipids and sophorolipids, vary depending on the size and saturation of the hydrophobic region, the presence of sugar groups, and the degree of acetylation [55]. The amphiphilic nature of rhamnolipids facilitates insertion into membranes at concentrations below the critical micelle concentration (CMC), resulting in modulation of membrane structure and removal of lipopolysaccharide, often in association with hydrophobic precursors [55]. Defining surfactant surface activity and self-assembled aggregation, CMC is a key chemical–physical parameter [56]. Accordingly, the inhibitory effect of BS appears to be dependent on the type of BS, surface properties and microorganisms [57]. In this study, all evaluated strains have shown a significant decrease in adhesion following to the BioSa3 treatment. Concerning the attachment to hexadecane, an expected decrease in the affinity to the solvent was obtained. All strains became more hydrophilic with an expected decrease in hexadecane affinity, as the adhesion is between 0 and 25%. However, all strains except one strain, that became moderately hydrophilic, presented a strongly hydrophilic character. This is justified by the optical density in all the tested strains of S. aureus or S. haemolyticus (from 0.709 to 0.111). Antibiofilm potency may result from BioSa3’s ability to modify the membrane of bacteria. BSs have been studied with metal surfaces, and they demonstrated that their lipophilic tails are oriented toward corrosion-inducing external environments, and their lipophobic heads toward metal surfaces [58]. BSs are also antimicrobial, reducing the biomass of sulfate-reducing bacteria (SRBs) and inhibiting biofilm growth, both of which potentially cause corrosion [59,60]. Researchers conducted a study in which it was found that BS reduced hydrophobic interactions, thereby decreasing bacteria adhesion. As a result, microorganisms are more likely to colonize hydrophobic surfaces, since microbes can better establish close contact with the substratum, thus eliminating interfacial moisture [61]. Thus, a surface conditioned by BS could decrease the attachment of microorganisms, since it has a more hydrophilic surface [62]. Our results are in concordance with these findings. In addition to these factors, other factors affect this process as well, including the surface structures of the microorganisms and the substrates, the presence of fimbriae and flagella, as well as surface proteins, and bacteria producing extracellular polymeric substances (EPS) [63,64]. Furthermore, the functional groups present on a cell’s surface also play an important role in its interaction with its environment. Multiple reports suggest that rhamnolipids can modify the functional groups on cellular surfaces, but the effect will vary depending on the microbe. Lipopolysaccharides, saturated alcohols, carboxyl, phosphoryl, and amine groups on cell surfaces have been altered [65,66,67].
Gram-negative bacteria’s wall structure can be altered by BSs, but Gram-positive bacteria can also be altered by modifying one of their structural components. This may be because of the fact that BS penetration and adsorption at the surface of the solid surface inhibit the growth of lichens in biofilms, reducing the interfacial tension and promoting bacterial separation [68].
Finally, these substances also prevented oxidative chain reactions, suggesting they may possess antioxidant properties [69]. Assays, based on the DPPH method, have been used to determine whether compounds are proton scavengers or donor compounds [70]. In the DPPH assays, BioSa3 at different concentrations (1–5 and 10 mg/mL) and AA (1 mg/mL) were evaluated to compare their ability to scavenge DPPH radicals. BioSa3 have shown a high antioxidant potential (63% at 10 mg/1 mL), but still less than that of AA (95% at 1 mg/mL). Based on the current study, the results of the DPPH assay for BioSa3 were dose-dependent results. The DPPH tests for rhamnolipids and surfactin revealed dose-dependent results, as demonstrated by Abdollahi et al. [69]. In fact, by transferring electrons or protons, these BSs neutralize free radicals [71]. This may be due to the presence of some active residues within surfactin’s peptide ring, among them a proline residue within its pyrrolidine ring and a tyrosine residue via its phenolic hydroxyl group [69]. While hydrocarbon chains appeared to enhance radical scavenging activity, their chain length variability did not appear to play a role. In turn, surfactants with low molecular mass peptides are more effective at scavenging DPPH radicals [72].
5. Conclusions
In conclusion, the present study demonstrated that BioSa3, produced from Bacillus sp. “HM117834”, possesses significant in vitro antimicrobial, antibiofilm, and antioxidant activities against four clinical multidrug-resistant strains of S. aureus and S. haemolyticus. Therefore, BioSa3 could be useful in preventing the formation of biofilms as well as eliminating those already established by pathogenic strains. Probiotics are increasingly receiving scientific interest due to their therapeutic potential. A growing number of studies have been conducted in this area. Additionally, a significant demand for new antimicrobial and antifungal agents exists because pathogenic organisms are becoming increasingly resistant to existing antimicrobials and antifungals. Thereby, BSs produced by probiotics may provide new sources of antimicrobial and antifungal drugs. These molecules are amphiphilic and they have beneficial effects when administered in the proper amount.
Author Contributions
Conceptualization, N.H. and A.M.; data curation, N.B.; formal analysis, O.B.; funding acquisition, N.H.; investigation, N.H.; methodology, K.N. and M.G.; project administration, N.H. and A.M.; software, W.B.; supervision, R.M. and A.M.; validation, A.M.; visualization, O.B.; writing—original draft, N.H.; writing—review and editing, M.G., N.L. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by the Scientific research Deanship at the University of Ha’il—Saudi Arabia, grant number RG-191248.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research has been funded by the Scientific research Deanship at the University of Ha’il–Saudi Arabia through project number RG-191248.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Giakoumelou, S.; Wheelhouse, N.; Cuschieri, K.; Entrican, G.; Howie, S.E.; Horne, A.W. The role of infection in miscarriage. Hum. Reprod. Update 2016, 22, 116–133. [Google Scholar] [CrossRef]
- Quentin, R.; Verdon, R. Les infections génitales hautes: Bases microbiologiques du diagnostic et du traitement. J. Gynécologie Obs. Biol. Reprod. 2012, 41, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Zhang, L.; Zhang, Q.; Lv, T.; Chen, R.; Wang, L.; Huang, Z.; Hu, L.; Liao, Q. The pathogenesis of streptococcus anginosus in aerobic vaginitis. Infect. Drug Resist. 2019, 12, 3745. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Baptista, P.; Lima-Silva, J.; Pinto, C.; Saldanha, C.; Beires, J.; Martinez-de-Oliveira, J.; Donders, G. Bacterial vaginosis, aerobic vaginitis, vaginal inflammation and major Pap smear abnormalities. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Rumyantseva, T.; Bellen, G.; Savochkina, Y.; Guschin, A.; Donders, G. Diagnosis of aerobic vaginitis by quantitative real-time PCR. Arch. Gynecol. Obstet. 2016, 294, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.; Yue, Y.; Geng, N.; Zhang, H.; Wang, Y.; Xue, F. Aerobic vaginitis and mixed infections: Comparison of clinical and laboratory findings. Arch. Gynecol. Obstet. 2013, 287, 329–335. [Google Scholar] [CrossRef]
- Machado, A.; Cerca, N. Influence of biofilm formation by Gardnerella vaginalis and other anaerobes on bacterial vaginosis. J. Infect. Dis. 2015, 212, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, V.; Chittaranjan, S.; Kurian, V.M.; Doble, M. Characteristics of bacterial biofilm associated with implant material in clinical practice. Polym. J. 2013, 45, 137–152. [Google Scholar] [CrossRef]
- Tenke, P.; Riedl, C.R.; Jones, G.L.; Williams, G.J.; Stickler, D.; Nagy, E. Bacterial biofilm formation on urologic devices and heparin coating as preventive strategy. Int. J. Antimicrob. Agents 2004, 23, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-G.; Lee, S.-Y.; Lee, S.-M.; Lim, K.-H.; Ha, E.-J.; Eom, Y.-B. Activity of novel inhibitors of Staphylococcus aureus biofilms. Folia Microbiol. 2017, 62, 157–167. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health. Research on Microbial Biofilms; PA Number: PA-03-047; National Institute of Health: Bethesda, MD, USA, 2003. [Google Scholar]
- Hassan, A.; Usman, J.; Kaleem, F.; Omair, M.; Khalid, A.; Iqbal, M. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz. J. Infect. Dis. 2011, 15, 305–311. [Google Scholar] [CrossRef]
- Rice, S.A.; McDougald, D.; Kumar, N.; Kjelleberg, S. The use of quorum-sensing blockers as therapeutic agents for the control of biofilm-associated infections. Curr. Opin. Investig. Drugs (Lond. Engl. 2000) 2005, 6, 178–184. [Google Scholar]
- Sumathy, V.; Parveen, J.; Zahangir, A.; Mohammed, S.J.; Noor Bin, S.; Wan Mohd Fazli, W.N. Biosurfactant as the next antimicrobial agents in pharmaceutical applications. Biomed. J. Sci. Tech. Res. 2019, 13, 9950–9952. [Google Scholar]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Fayaz, F.; Alara, O.R. Biosurfactants—A new frontier for social and environmental safety: A mini review. Biotechnol. Res. Innov. 2018, 2, 81–90. [Google Scholar] [CrossRef]
- Van Hamme, J.D.; Singh, A.; Ward, O.P. Physiological aspects: Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol. Adv. 2006, 24, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Cameotra, S.S. Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol. 2004, 22, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Dusane, D.H.; Nancharaiah, Y.V.; Zinjarde, S.S.; Venugopalan, V.P. Rhamnolipid mediated disruption of marine Bacillus pumilus biofilms. Colloids Surf. B Biointerfaces 2010, 81, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Rivardo, F.; Turner, R.; Allegrone, G.; Ceri, H.; Martinotti, M. Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl. Microbiol. Biotechnol. 2009, 83, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Da Re, S.; Henry, N.; Fontaine, T.; Balestrino, D.; Latour-Lambert, P.; Ghigo, J.-M. Broad-spectrum biofilm inhibition by a secreted bacterial polysaccharide. Proc. Natl. Acad. Sci. USA 2006, 103, 12558–12563. [Google Scholar] [CrossRef] [PubMed]
- Sambanthamoorthy, K.; Feng, X.; Patel, R.; Patel, S.; Paranavitana, C. Antimicrobial and antibiofilm potential of biosurfactants isolated from lactobacilli against multi-drug-resistant pathogens. BMC Microbiol. 2014, 14, 197. [Google Scholar] [CrossRef] [PubMed]
- Mahdhi, A.; Hmila, Z.; Chaieb, K.; Kamoun, F.; Bakhrouf, A. Probiotic properties of halophilic Bacillus strains enhance protection of Artemia culture against pathogenic Vibrio. Aquat. Biol. 2011, 13, 225–231. [Google Scholar] [CrossRef][Green Version]
- Sharma, A.; Soni, J.; Kaur, G.; Kaur, J. A study on biosurfactant production in Lactobacillus and Bacillus sp. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 723–733. [Google Scholar]
- Habib, S.; Ahmad, S.A.; Wan Johari, W.L.; Abd Shukor, M.Y.; Alias, S.A.; Smykla, J.; Saruni, N.H.; Abdul Razak, N.S.; Yasid, N.A. Production of lipopeptide biosurfactant by a hydrocarbon-degrading Antarctic Rhodococcus. Int. J. Mol. Sci. 2020, 21, 6138. [Google Scholar] [CrossRef] [PubMed]
- Sriram, M.I.; Gayathiri, S.; Gnanaselvi, U.; Jenifer, P.S.; Raj, S.M.; Gurunathan, S. Novel lipopeptide biosurfactant produced by hydrocarbon degrading and heavy metal tolerant bacterium Escherichia fergusonii KLU01 as a potential tool for bioremediation. Bioresour. Technol. 2011, 102, 9291–9295. [Google Scholar] [CrossRef] [PubMed]
- Mnif, S.; Chamkha, M.; Labat, M.; Sayadi, S. Simultaneous hydrocarbon biodegradation and biosurfactant production by oilfield-selected bacteria. J. Appl. Microbiol. 2011, 111, 525–536. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Cooper, D.G.; Neufeld, R.J. Selection of microbes producing biosurfactants in media without hydrocarbons. J. Ferment. Technol. 1984, 62, 311–314. [Google Scholar]
- Gudina, E.J.; Teixeira, J.A.; Rodrigues, L.R. Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloids Surf. B Biointerfaces 2010, 76, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Vaseeharan, B.; Ramasamy, P. Abundance of potentially pathogenic micro-organisms in Penaeus monodon larvae rearing systems in India. Microbiol. Res. 2003, 158, 299–308. [Google Scholar] [CrossRef]
- Mahdhi, A.; Leban, N.; Chakroun, I.; Chaouch, M.A.; Hafsa, J.; Fdhila, K.; Mahdouani, K.; Majdoub, H. Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microb. Pathog. 2017, 109, 214–220. [Google Scholar] [CrossRef]
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Bellon-Fontaine, M.-N.; Rault, J.; Van Oss, C. Microbial adhesion to solvents: A novel method to determine the electron-donor/electron-acceptor or Lewis acid-base properties of microbial cells. Colloids Surf. B Biointerfaces 1996, 7, 47–53. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Han, J.-Z. The role of probiotic cell wall hydrophobicity in bioremediation of aquaculture. Aquaculture 2007, 269, 349–354. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Chae, M.S.; Schraft, H.; Hansen, L.T.; Mackereth, R. Effects of physicochemical surface characteristics of Listeria monocytogenes strains on attachment to glass. Food Microbiol. 2006, 23, 250–259. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Coetzer, J.A.W.; Thomson, G.R.; Tustin, R.C. Infectious Diseases of Livestock with Special Reference to Southern Africa; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Kloos, W.E. Natural populations of the genus Staphylococcus. Annu. Rev. Microbiol. 1980, 34, 559–592. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Rusch, K.; Rusch, V. Throwing the dice for the diagnosis of vaginal complaints? Ann. Clin. Microbiol. Antimicrob. 2006, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Terraf, M.L.; Juárez Tomás, M.; Nader-Macías, M.; Silva, C. Screening of biofilm formation by beneficial vaginal lactobacilli and influence of culture media components. J. Appl. Microbiol. 2012, 113, 1517–1529. [Google Scholar] [CrossRef] [PubMed]
- Petricevic, L.; Domig, K.J.; Nierscher, F.J.; Sandhofer, M.J.; Fidesser, M.; Krondorfer, I.; Husslein, P.; Kneifel, W.; Kiss, H. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci. Rep. 2014, 4, 5136. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, J.; Arguelles-Arias, A.; Dhondt-Cordelier, S.; Cordelier, S.; Pršić, J.; Hoff, G.; Mazeyrat-Gourbeyre, F.; Baillieul, F.; Clément, C.; Ongena, M. Biosurfactants in plant protection against diseases: Rhamnolipids and lipopeptides case study. Front. Bioeng. Biotechnol. 2020, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Satpute, S.K.; Kulkarni, G.R.; Banpurkar, A.G.; Banat, I.M.; Mone, N.S.; Patil, R.H.; Cameotra, S.S. Biosurfactant/s from Lactobacilli species: Properties, challenges and potential biomedical applications. J. Basic Microbiol. 2016, 56, 1140–1158. [Google Scholar] [CrossRef]
- Morais, I.; Cordeiro, A.; Teixeira, G.; Domingues, V.; Nardi, R.; Monteiro, A.; Alves, R.; Siqueira, E.; Santos, V. Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P 6A and Lactobacillus gasseri P 65. Microb. Cell Factories 2017, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Moosavi-Nasab, M.; Setoodeh, P.; Mesbahi, G.; Yousefi, G. Biosurfactant production by lactic acid bacterium Pediococcus dextrinicus SHU1593 grown on different carbon sources: Strain screening followed by product characterization. Sci. Rep. 2019, 9, 5287. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant-producing lactobacilli: Screening, production profiles, and effect of medium composition. Appl. Environ. Soil Sci. 2011, 2011, 201254. [Google Scholar] [CrossRef]
- Sari, M.; Kusharyoto, W.; Artika, I.M. Screening for biosurfactant-producing yeast: Confirmation of biosurfactant production. Biotechnology 2014, 13, 106. [Google Scholar] [CrossRef]
- Walter, V.; Syldatk, C.; Hausmann, R. Screening concepts for the isolation of biosurfactant producing microorganisms. In Biosurfactants; Springer: New York, NY, USA, 2010. [Google Scholar]
- Foschi, C.; Salvo, M.; Cevenini, R.; Parolin, C.; Vitali, B.; Marangoni, A. Vaginal lactobacilli reduce Neisseria gonorrhoeae viability through multiple strategies: An in vitro study. Front. Cell. Infect. Microbiol. 2017, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Perez, K.J.; Viana, J.d.S.; Lopes, F.C.; Pereira, J.Q.; Dos Santos, D.M.; Oliveira, J.S.; Velho, R.V.; Crispim, S.M.; Nicoli, J.R.; Brandelli, A. Bacillus spp. isolated from puba as a source of biosurfactants and antimicrobial lipopeptides. Front. Microbiol. 2017, 8, 61. [Google Scholar] [CrossRef]
- De Giani, A.; Zampolli, J.; Di Gennaro, P. Recent trends on biosurfactants with antimicrobial activity produced by bacteria associated with human health: Different perspectives on their properties, challenges, and potential applications. Front. Microbiol. 2021, 12, 678. [Google Scholar] [CrossRef] [PubMed]
- Otzen, D.E. Biosurfactants and surfactants interacting with membranes and proteins: Same but different? Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 639–649. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Lorusso, N.; Palmieri, G.F.; Bonacucina, G.; Blasi, P. Surfactant self-assembling and critical micelle concentration: One approach fits all? Langmuir 2020, 36, 5745–5753. [Google Scholar] [CrossRef] [PubMed]
- Walencka, E.; Różalska, S.; Sadowska, B.; Różalska, B. The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol. 2008, 53, 61. [Google Scholar] [CrossRef] [PubMed]
- Fenibo, E.O.; Ijoma, G.N.; Selvarajan, R.; Chikere, C.B. Microbial surfactants: The next generation multifunctional biomolecules for applications in the petroleum industry and its associated environmental remediation. Microorganisms 2019, 7, 581. [Google Scholar] [CrossRef]
- Astuti, D.; Purwasena, I.A.; Putri, F.Z. Potential of biosurfactant as an alternative biocide to control biofilm associated biocorrosion. J. Environ. Sci. Technol. 2018, 11, 104–111. [Google Scholar] [CrossRef]
- Basafa, M.; Hawboldt, K. Reservoir souring: Sulfur chemistry in offshore oil and gas reservoir fluids. J. Pet. Explor. Prod. Technol. 2019, 9, 1105–1118. [Google Scholar] [CrossRef]
- Rodrigues, L.; Banat, I.M.; Van der Mei, H.; Teixeira, J.; Oliveira, R. Interference in adhesion of bacteria and yeasts isolated from explanted voice prostheses to silicone rubber by rhamnolipid biosurfactants. J. Appl. Microbiol. 2006, 100, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Zeraik, A.E.; Nitschke, M. Biosurfactants as agents to reduce adhesion of pathogenic bacteria to polystyrene surfaces: Effect of temperature and hydrophobicity. Curr. Microbiol. 2010, 61, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front. Bioeng. Biotechnol. 2021, 9, 82. [Google Scholar] [CrossRef]
- Jin, X.; Marshall, J.S. Mechanics of biofilms formed of bacteria with fimbriae appendages. PLoS ONE 2020, 15, e0243280. [Google Scholar]
- Bai, N.; Wang, S.; Abuduaini, R.; Zhang, M.; Zhu, X.; Zhao, Y. Rhamnolipid-aided biodegradation of carbendazim by Rhodococcus sp. D-1: Characteristics, products, and phytotoxicity. Sci. Total Environ. 2017, 590, 343–351. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Dick, R.P.; Li, H.; Shen, D.; Gao, Y.; Waigi, M.G.; Ling, W. Rhamnolipid influences biosorption and biodegradation of phenanthrene by phenanthrene-degrading strain Pseudomonas sp. Ph6. Environ. Pollut. 2018, 240, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Mukherji, S. Surfactant aided biodegradation of NAPLs by Burkholderia multivorans: Comparison between Triton X-100 and rhamnolipid JBR-515. Colloids Surf. B Biointerfaces 2013, 102, 644–652. [Google Scholar] [CrossRef]
- Coronel-León, J.; Marqués, A.; Bastida, J.; Manresa, A. Optimizing the production of the biosurfactant lichenysin and its application in biofilm control. J. Appl. Microbiol. 2016, 120, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, S.; Tofighi, Z.; Babaee, T.; Shamsi, M.; Rahimzadeh, G.; Rezvanifar, H.; Saeidi, E.; Amiri, M.M.; Ashtiani, Y.S.; Samadi, N. Evaluation of anti-oxidant and anti-biofilm activities of biogenic surfactants derived from bacillus amyloliquefaciens and Pseudomonas aeruginosa. Iran. J. Pharm. Res. 2020, 19, 115. [Google Scholar]
- Ayed, H.B.; Bardaa, S.; Moalla, D.; Jridi, M.; Maalej, H.; Sahnoun, Z.; Rebai, T.; Jacques, P.; Nasri, M.; Hmidet, N. Wound healing and in vitro antioxidant activities of lipopeptides mixture produced by Bacillus mojavensis A21. Process Biochem. 2015, 50, 1023–1030. [Google Scholar] [CrossRef]
- Tofani, D.; Balducci, V.; Gasperi, T.; Incerpi, S.; Gambacorta, A. Fatty acid hydroxytyrosyl esters: Structure/antioxidant activity relationship by ABTS and in cell-culture DCF assays. J. Agric. Food Chem. 2010, 58, 5292–5299. [Google Scholar] [CrossRef] [PubMed]
- Tabbene, O.; Gharbi, D.; Slimene, I.B.; Elkahoui, S.; Alfeddy, M.N.; Cosette, P.; Mangoni, M.L.; Jouenne, T.; Limam, F. Antioxidative and DNA protective effects of bacillomycin D-like lipopeptides produced by B38 strain. Appl. Biochem. Biotechnol. 2012, 168, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).