Limosilactobacillus fermentum SWP-AFFS02 Improves the Growth and Survival Rate of White Shrimp via Regulating Immunity and Intestinal Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of L. fermentum SWP-AFFS02
2.2. The Number of LAB in Feed
2.3. Husbandry Conditions and Feeding Trial
2.4. Detection of Total Bacteria and Vibrio spp. Counts in Seawater Surrounding
2.5. Assay for Immune Activity (Hymolymph Parameters)
2.5.1. Preparation of Hemolymph Solution
2.5.2. Total Hemocyte Count (THC) and Differential Hemocyte Count (DHC)
2.5.3. Measurement of Superoxide Anion Respiratory Burst
2.5.4. Assay for Phenoloxidase (PO) Activity
2.5.5. Reverse Transcription Quantitative PCR (RT-qPCR)
2.6. Microbiota Sequencing
2.7. Statistical Analysis
3. Results
3.1. Variation of LAB Feed-Contained L. fermentum SWP-AFFS02 after Stroage
3.2. Regulations of Growth, Environmental Microbe, and Immunity in L. vannamei Shrimp by LAB Feed-Contained L. fermentum SWP-AFFS02
3.2.1. Growth, FCR, and Survival Rate of Shrimp
3.2.2. Environmental Microbial Population (Total Bacteria Level and Vibrio spp.)
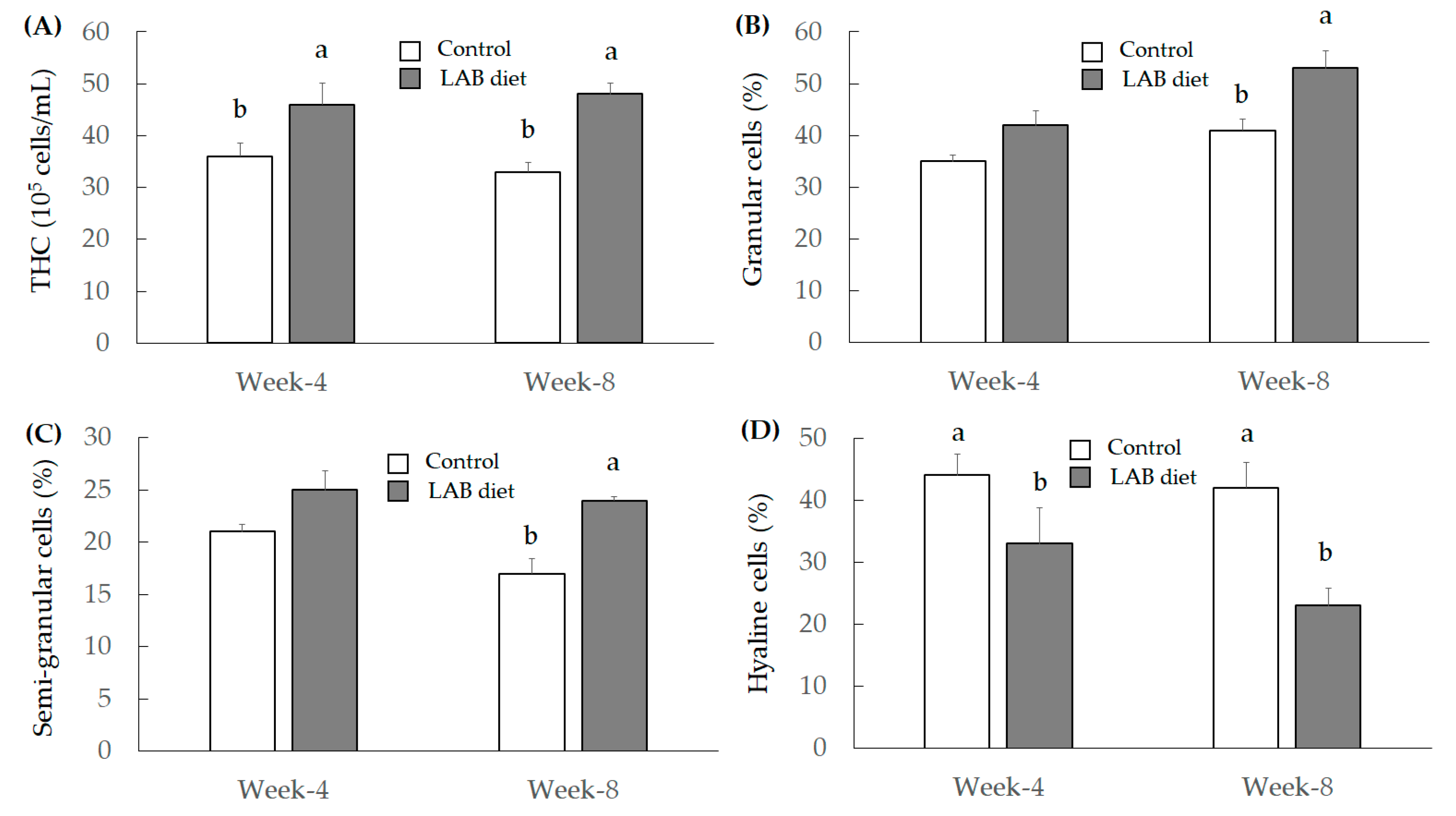
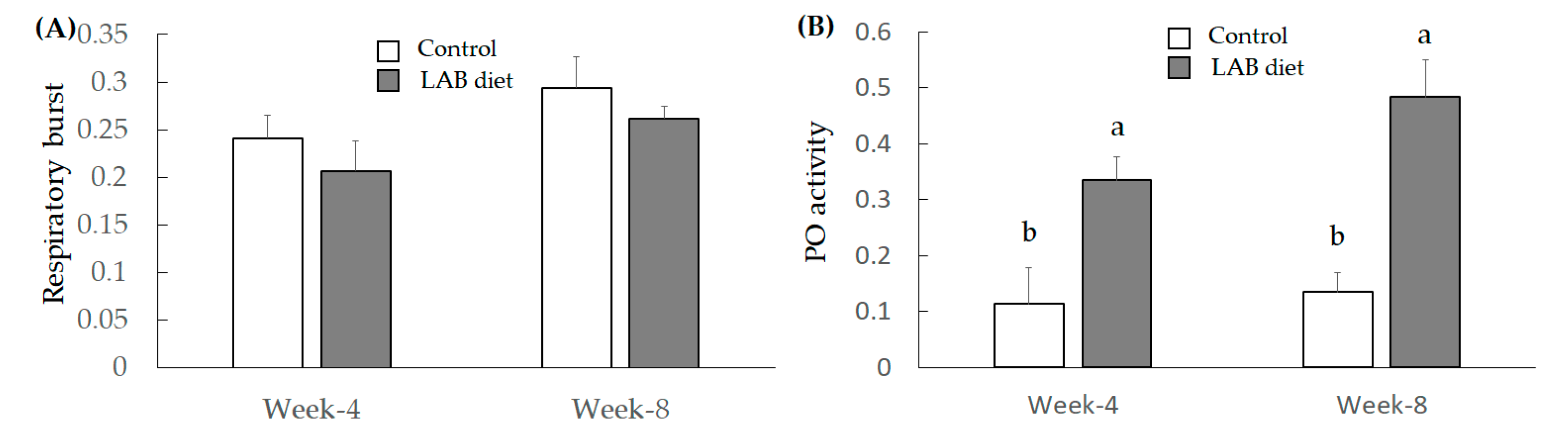
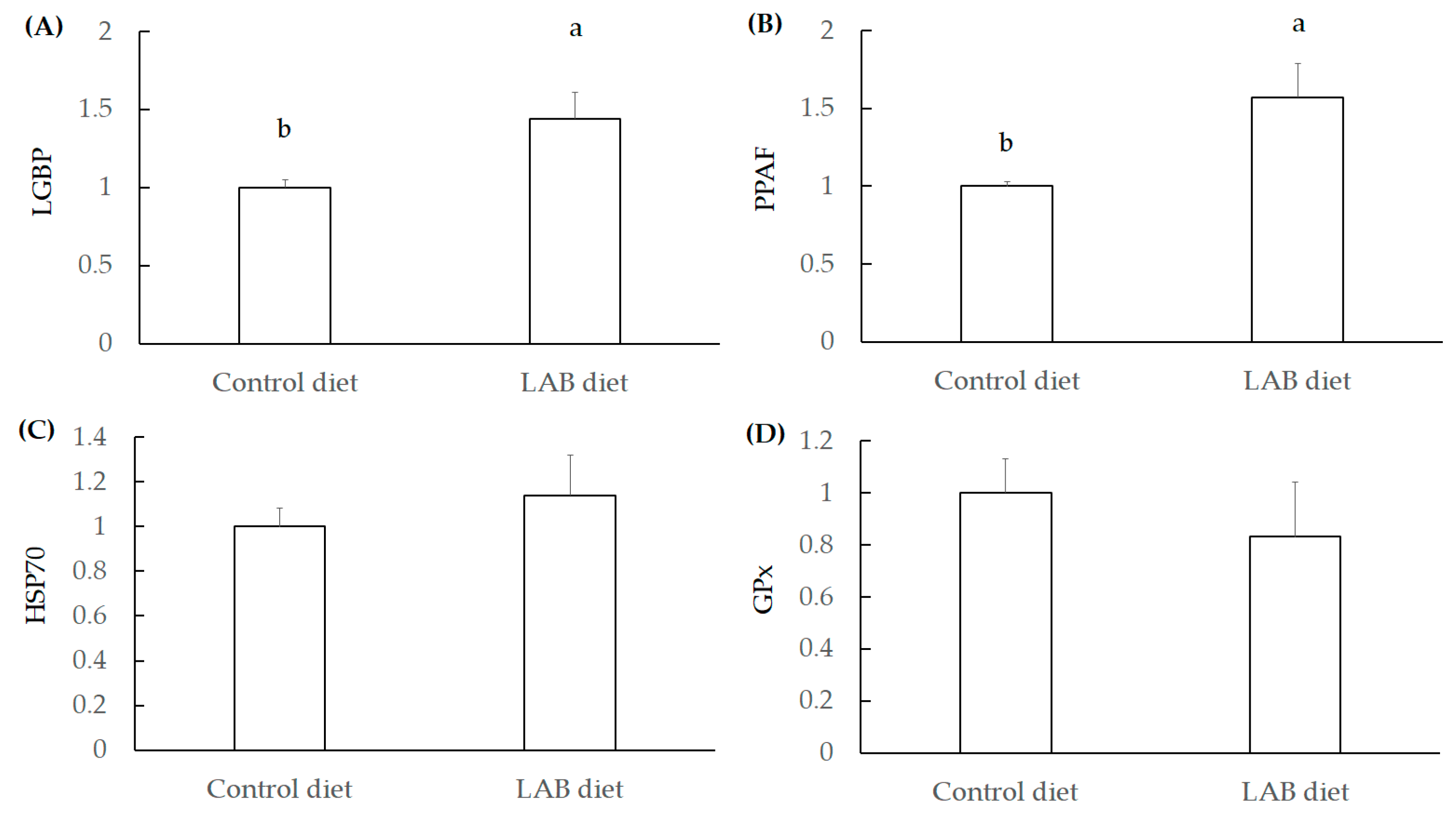
3.2.3. Investigation for Immunity Index
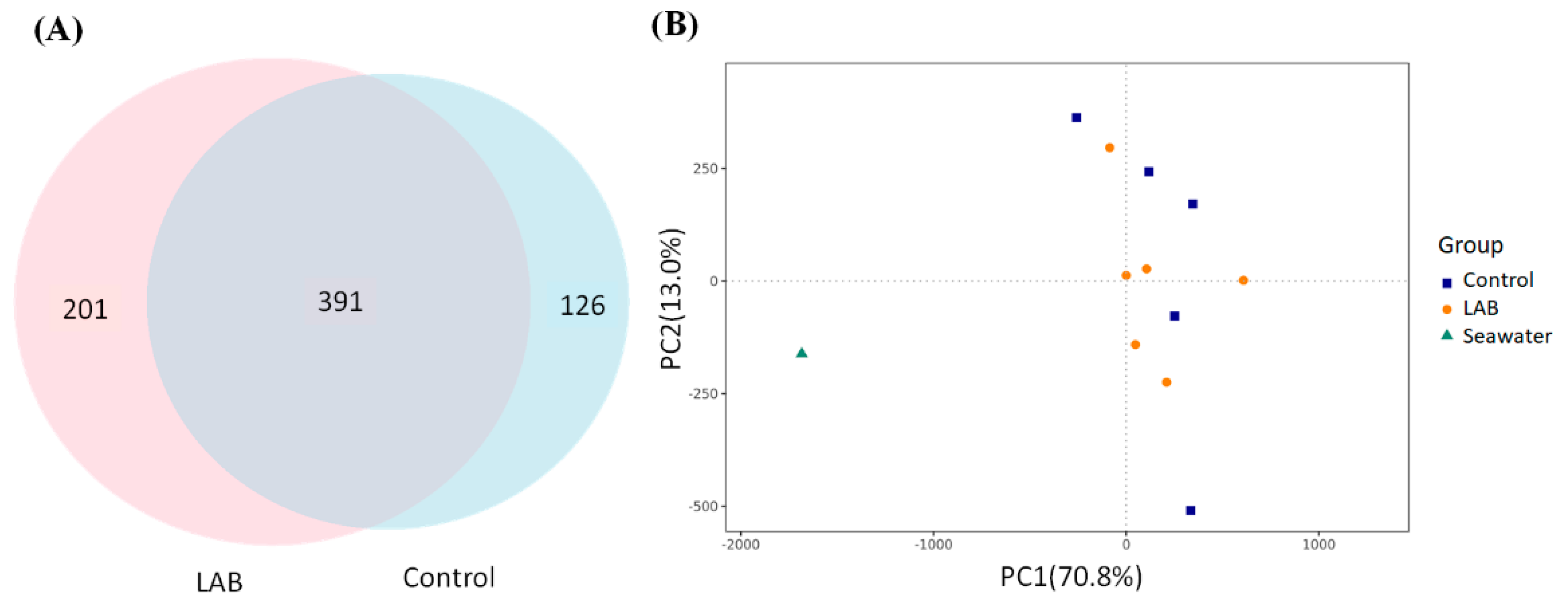
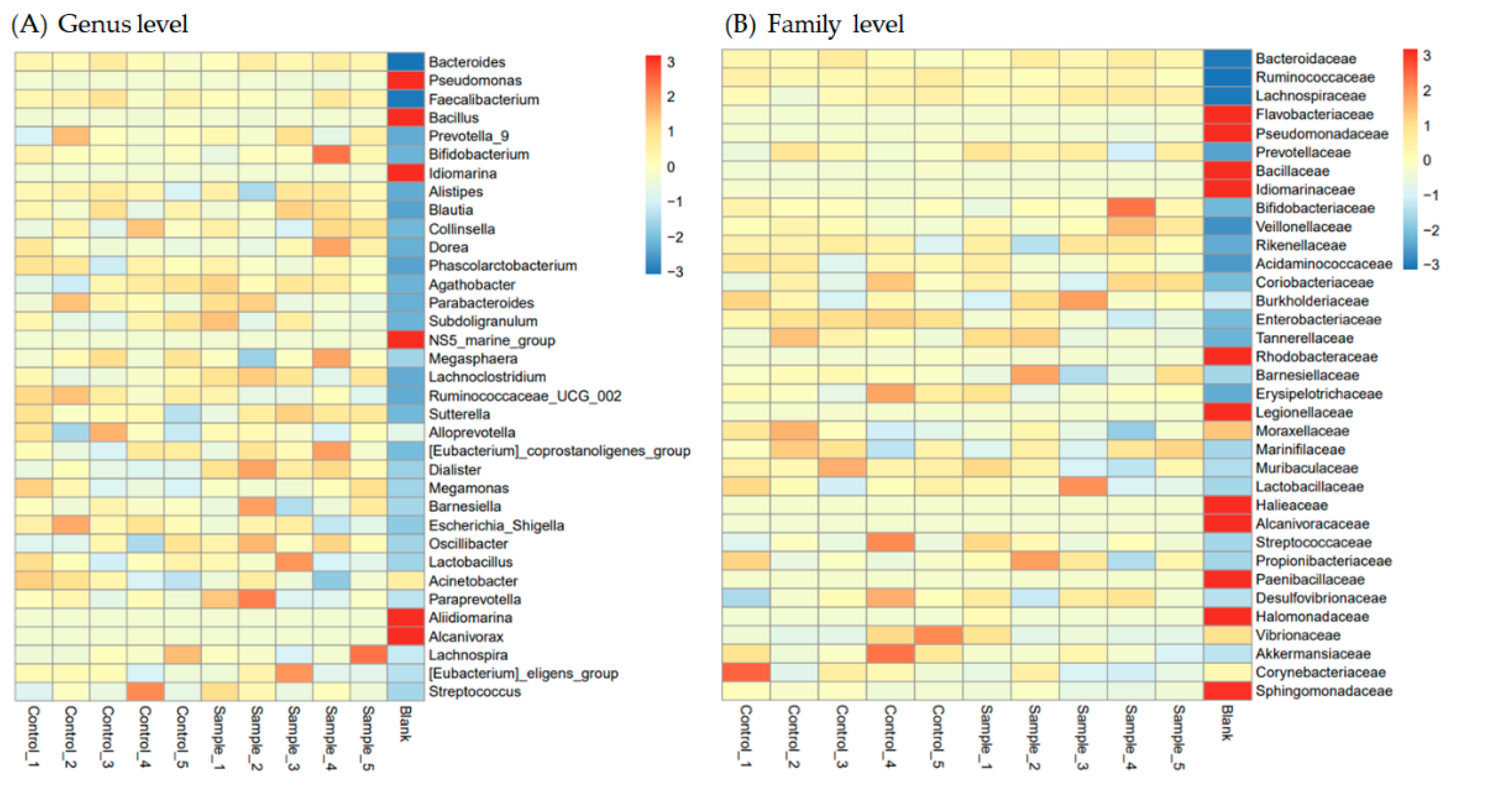
3.3. Protection of Intestinal Microbiota in L. vannamei Shrimp by LAB Feed Containing L. fermentum SWP-AFFS02
4. Discussion
5. Conclusions
Author Contributions
Funding
Ethical Approval
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Rasheeda, M.K.; Rangamaran, V.R.; Srinivasan, S.; Ramaiah, S.K.; Gunasekaran, R.; Jaypal, S.; Gopal, D.; Ramalingam, K. Comparative profiling of microbial community of three economically important fishes reared in sea cages under tropical offshore environment. Mar. Genom. 2017, 34, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Utiswannakul, P.; Sangchai, S.; Rengpipat, S. Enhanced growth of black tiger shrimp Penaeus monodon by dietary supplementation with Bacillus (BP11) as a probiotic. J. Aquac. Res. Dev. 2011, 2, 6. [Google Scholar] [CrossRef]
- Rengpipat, S.; Tunyanun, A.; Fast, A.W.; Piyatiratitivorakul, S.; Menasveta, P. Enhanced growth and resistance to Vibrio challenge in pond-reared black tiger shrimp Penaeus monodon fed a Bacillus probiotic. Dis. Aquat. Org. 2003, 55, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Vaseeharan, B.; Ramasamy, P. Control of pathogenic Vibrio spp. Bacillus subtilis BT23, by a possible probiotic treatment for black tiger shrimp Penaeus monodon. Lett. Appl. Microbiol. 2003, 36, 83–87. [Google Scholar] [CrossRef]
- Zheng, X.; Duan, Y.; Dong, H.; Zhang, J. Effects of dietary Lactobacillus plantarum on growth performance, digestive enzymes and gut morphology of Litopenaeus vannamei. Probiotics Antimicrob Proteins 2018, 10, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, N.M.; Nazar, A.R.; Rajagopal, S.; Khan, S.A. Use of probiotics in water quality management during shrimp culture. J. Aquac. Trop. 1999, 14, 227–236. [Google Scholar]
- Sha, Y.; Liu, M.; Wang, B.; Jiang, K.; Qi, C.; Wang, L. Bacterial population in intestines of Litopenaeus vannamei fed different probiotics or probiotic supernatant. J. Microbiol. Biotechnol. 2016, 26, 1736–1745. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Wang, X.; Pi, X.; Wang, X.; Zhou, H.; Mai, K.; He, G. Improved utilization of soybean meal through fermentation with commensal Shewanella sp. MR-7 in turbot (Scophthalmus maximus L.). Microb. Cell Fact. 2019, 18, 214. [Google Scholar] [CrossRef]
- Dash, G.; Raman, R.P.; Prasad, K.P.; Makesh, M.; Pradeep, M.A.; Sen, S. Evaluation of Lactobacillus plantarum as feed supplement on host associated microflora, growth, feed efficiency, carcass biochemical composition and immune response of giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879). Aquaculture 2014, 432, 225–236. [Google Scholar] [CrossRef]
- Wirunpan, M.; Savedboworn, W.; Wanchaitanawong, P. Survival and shelf life of Lactobacillus lactis 1464 in shrimp feed pellet after fluidized bed drying. Agric. Nat. Resour. 2016, 50, 1–7. [Google Scholar] [CrossRef][Green Version]
- Ngo, H.V.T.; Huang, H.T.; Lee, P.T.; Liao, Z.H.; Chen, H.Y.; Nan, F.H. Effects of Phyllanthus amarus extract on nonspecific immune responses, growth, and resistance to Vibrio alginolyticus in white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 107, 1–8. [Google Scholar] [CrossRef]
- Song, Y.L.; Yu, C.I.; Lien, T.W.; Huang, C.C.; Lin, M.N. Haemolymph parameters of Pacific white shrimp (Litopenaeus vannamei) infected with Taura syndrome virus. Fish Shellfish Immunol. 2003, 14, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Tsing, A.; Arcier, J.M.; Brehelin, M. Hemocytes of penaeid and Palaemonid shrimps: Morphology, cytochemistry and hemegram. J. Invertebr. Pathol. 1989, 53, 64–77. [Google Scholar] [CrossRef]
- Lee, P.T.; Tran, H.T.Q.; Huang, H.T.; Nan, F.H.; Lee, M.C. Sargassum horneri extracts stimulate innate immunity, enhance growth performance, and upregulate immune genes in the white shrimp Litopenaeus vannamei. Fish Shell Immunol. 2020, 102, 276–285. [Google Scholar] [CrossRef]
- Sung, H.H.; Kou, G.H.; Song, Y.L. Vibriosis resistance induced by glucan treatment in tiger shrimp (Penaeus monodon). Fish Pathol. 1994, 29, 11–17. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Söderhäll, K.; Unestam, T. Activation of serum prophenoloxidase in arthropod immunity: The specificity of cell wall glucan activation and activation by purified fungal glycoproteins of crayfish phenoloxidase. Can. J. Microbiol. 1979, 25, 406–414. [Google Scholar] [CrossRef]
- Duan, Y.F.; Wang, Y.; Zhang, J.S.; Sun, X.X.; Wang, J. Dietary effects of succinic acid on the growth, digestive enzymes, immune response and resistance to ammonia stress of Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 8, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.W.; Zheng, L.; Wan, M.G.; Niu, J.; Liu, Y.J.; Tain, L.X. Effect of deoxynivalenol on growth performance, histological morphology, anti-oxidative ability and immune response of juvenile Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 82, 442–452. [Google Scholar] [CrossRef]
- Vanmaele, S.; Defoirdt, T.; Cleenwerck, I.; De Vos, P.; Bossier, P. Characterization of the virulence of Harveyi clade vibrios isolated from a shrimp hatchery in vitro and in vivo, in a brine shrimp (Artemia franciscana) model system. Aquaculture 2015, 435, 28–32. [Google Scholar] [CrossRef]
- Moullac, G.L.; Groumellec, M.L.; Ansquer, D.; Froissard, S.; Levy, P. Haematological and phenoloxidase activity changes in the shrimp Penaeus stylirostris in relation with the moult cycle: Protection against Vibrio. Fish Shellfish Immunol. 1997, 7, 227–234. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, J.C. Effects of environmental factors on the immune responses of freshwater prawn Macrobrachium rosenbergii and other decapod crustaceans. J.-Fisher Soc. Taiwan 2002, 29, 1–20. [Google Scholar]
- Cheng, W.; Chen, J.C. The virulence of Enterococcus to freshwater prawn Macrobrachium rosenbergii and its immune resistance under ammonia stress. Fish Shellfish Immonol. 2002, 12, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Risjani, Y.; Mutmainnah, N.; Manurung, P.; Wulan, S.N.; Yunianta. Exopolysaccharide from Porphyridium cruentum (purpureum) is not toxic and stimulates immune response against Vibriosis: The assessment using zebrafish and white shrimp Litopenaeus vannamei. Mar. Drugs 2021, 19, 133. [Google Scholar] [CrossRef]
- Rodriguez, J.; Le Moullac, G. State of the art of immunological tools and health control of Penaeid shrimp. Aquaculture 2000, 191, 109–119. [Google Scholar] [CrossRef]
- Chase, M.R.; Raina, K.; Bruno, J.M. Purification, characterization and molecular cloning of prophenol oxidases from Sarcophaga bullata. Insect Biochem. Mol. Biol. 2000, 30, 953–967. [Google Scholar] [CrossRef]
- Cerenius, L.; Soderhall, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004, 77, 21–26. [Google Scholar] [CrossRef]
- Guo, H.; Xian, J.A.; Li, B.; Ye, C.X.; Wang, A.L.; Miao, Y.T.; Liao, S.A. Gene expression of apoptosis-related genes, stress protein and antioxidant enzymes in hemocytes of whit shrimp Litopenaeus vannamei under nitrite stress. Comp. Biochem. Phys. C 2013, 157, 366–371. [Google Scholar]
- Cheng, A.C.; Yeh, S.P.; Hu, S.Y.; Lin, H.L.; Liu, C.H. Intestinal microbiota of white shrimp, Litopenaeus vannamei, fed diets containing Bacillus subtilis E20-fermented soybean meal (FSBM) or an antimicrobial peptide derived from B. subtilis E20-FSBM. Aquac. Res. 2019, 51, 41–50. [Google Scholar] [CrossRef]
- Xie, Z.; Hu, L.; Li, Y.; Geng, S.; Cheng, S.; Fu, X.; Zhao, S.; Han, X. Changes of gut microbiota structure and morphology in weaned piglets treated with fresh fermented soybean meal. World J. Microbiol. Biotechnol. 2017, 33, 213. [Google Scholar] [CrossRef]
- Catalán, N.; Villasante, A.; Wacyk, J.; Ramirez, C.; Romero, J. Fermented soybean meal increases lactic acid bacteria in gut microbiota of Atlantic salmon (Salmo salar). Probiotics Antimicrob Proteins 2018, 10, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Santos, H.M.; Hu, S.Y.; Sang, C.Y.; Yanuaria, C.A.S.; Lola, E.N.G.; Tayo, L.L.; Nituram, K.M.A.; Liu, C.H.; Chuang, K.P. LpxD gene knockout elicits protection to Litopenaeus vannamei, white shrimp, against Vibrio parahaemolyticus infection. Aquac. Int. 2019, 27, 1383–1393. [Google Scholar] [CrossRef]
- Huynh, T.G.; Hu, S.Y.; Chiu, C.H.; Truong, Q.P.; Liu, C.H. Bacterial population in intestines of white shrimp, Litopenaeus vannamei fed a synbiotic containing Lactobacillus plantarum and galactooligosaccharide. Aquac. Res. 2019, 50, 807–817. [Google Scholar] [CrossRef]
- Huang, Z.; Li, X.; Wang, L.; Shao, Z. Changes in the intestinal bacterial community during the growth of white shrimp, Litopenaeus vannamei. Aquac. Res. 2016, 47, 1737–1746. [Google Scholar] [CrossRef]
- Huynh, T.G.; Cheng, A.C.; Chi, C.C.; Chiu, K.H.; Liu, C.H. A synbiotic improves the immunity of white shrimp, Litopenaeus vannamei: Metabolomics analysis reveal compelling evidence. Fish Shellfish Immunol. 2018, 79, 284–293. [Google Scholar] [CrossRef]
- Li, E.; Chen, L.Q.; Zeng, C.; Yu, N.; Xiong, Z.Q.; Chen, X.F.; Qin, J.G. Comparison of digestive and antioxidant enzymes activities, haemolymph oxyhemocyanin contents and hepatopancreas histology of white shrimp, Litopenaeus vannamei, at various salinities. Aquaculture 2008, 274, 80–86. [Google Scholar] [CrossRef]
- Lavilla Pitogo, C.R.; Leano, E.M.; Paner, M.G. Mortalities of pond-cultured juvenile shrimp, Penaeus monodon, associated with dominance of luminescent Vibrios in the rearing environment. Aquaculture 1998, 164, 337–349. [Google Scholar] [CrossRef]
- Zhu, X.F.; Guo, H.; Li, G.L.; Zhu, C.H. Effects of dietary hydrolysable tannins on growth performance, antioxidant capacity, intestinal microflora and resistance against Vibrio parahaemolyticus of juvenile Pacific white shrimp, Litopenaeus vannamei (Boone, 1931). Aquac. Rep. 2021, 19, 100601. [Google Scholar] [CrossRef]
- Chen, S.J.; Zhuang, Z.X.; Yin, P.; Chen, X.; Zhang, Y.M.; Tain, L.X.; Niu, J.; Liu, Y.J. Changes in growth performance, haematological parameters, hepatopancreas histopathology and antioxidant status of pacific white shrimp (Litopenaeus vannamei) fed oxidized fish oil: Regulation by dietary myo-inositol. Fish Shellfish Immunol. 2019, 88, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Bachere, E.; Gueguen, Y.; Gonzalez, M.; De Lorgeril, J.; Garnier, J.; Romestand, B. Insights into the anti-microbial defense of marine invertebrates: The penaeid shrimps and the oyster Crassostrea gigas. Immunol. Rev. 2004, 198, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gu, Y.; Zhou, H.; Xu, L.; Cao, H.; Gai, C. Acinetobacter venetianus, a potential pathogen of red leg disease in freshwater-cultured whiteleg shrimp Penaeus vannamei. Aquac. Rep. 2020, 18, 100543. [Google Scholar] [CrossRef]
- Ott, L. Adhesion properties of toxigenic corynebacterial. AIMS Microbiol. 2018, 4, 85–103. [Google Scholar] [CrossRef]
- Shen, F.; Zheng, R.D.; Sun, X.Q.; Ding, W.J.; Wang, X.Y.; Fan, J.G. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobillary Pancreat Dis. Int. 2017, 16, 375–381. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Backhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- de Vadder, F.; Mithieux, G. Gut-brain signaling in energy homeostasis: The unexpected role of microbiota-derived succinate. J. Endocrinol. 2018, 236, R105–R108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.N.J.; Jyothsna, R.; Reddy, M.H.; Sreevani, S.P.R. Effect of Bacillus subtilis and Lactobacillus rhamnosus incorporated probiotic diet on growth pattern and enzymes in Penaeus vannamei. Int. J. Life Sci. Pharm. Res. 2013, 3, 6–11. [Google Scholar]
- Ahmmed, F.; Ahmmed, M.K.; Shah, M.S.; Banu, G.R. Use of indigenous beneficial bacteria (Lactobacillus spp.) as probiotics in shrimp (Penaeus monodon) aquaculture. Res. Agric. Livest. Fish. 2018, 5, 127–135. [Google Scholar] [CrossRef][Green Version]
- Pooljun, C.; Daorueang, S.; Weerachatyanukul, W.; Direkbusarakom, S.; Jariyapong, P. Enhancement of shrimp health and immunity with diets supplemented with combined probiotics: Application to Vibrio parahaemolyticus infections. Dis. Aquac. Org. 2020, 140, 37–46. [Google Scholar] [CrossRef]
| Days | Storage at 25 °C | Storage at 4 °C |
|---|---|---|
| Concentration (CFU/g) | ||
| 0 | 4.47 × 108 ± 0.31 × 107 | 4.47 × 108 ± 0.31 × 107 |
| 1 | 4.01 × 108 ± 0.44 × 107 | 4.56 × 108 ± 0.25 × 107 |
| 2 | 3.77 × 108 ± 0.48 × 107 | 4.11 × 108 ± 0.22 × 107 |
| 3 | 3.52 × 108 ± 0.78 × 107 | 3.96 × 108 ± 0.38 × 107 |
| 4 | 3.21 × 108 ± 1.04 × 107 | 3.78 × 108 ± 0.41 × 107 |
| 5 | 2.84 × 108 ± 1.11 × 107 | 3.55 × 108 ± 0.37 × 107 |
| 6 | 6.45 × 107 ± 0.19 × 107 | 3.32 × 108 ± 0.63 × 107 |
| 7 | 4.08 × 107 ± 0.28 × 107 | 3.18 × 108 ± 0.79 × 107 |
| Groups | Initial Weight (g) | Final Weight (g) | WG (%) | FCR | Survival Rate (%) |
|---|---|---|---|---|---|
| Control feed | 8.22 ± 0.38 | 15.72 ± 2.75 b | 45.95 ± 11.57 b | 7.22 ± 5.05 a | 68.8 ± 10.1 b |
| LAB feed | 8.27 ± 0.53 | 22.09 ± 2.50 a | 61.98 ± 6.09 a | 4.38 ± 1.04 b | 82.2 ± 6.9 a |
| Weeks | Total Bacterial Numbers (CFU/mL) | |
|---|---|---|
| Control Feed | LAB Feed | |
| 0 | 2.10 × 104 ± 0.24 × 103 a | 1.49 × 104 ± 0.12 × 103 b |
| 2 | 1.33 × 104 ± 0.18 × 103 b | 1.94 × 104 ± 0.15 × 103 a |
| 4 | 0.89 × 103 ± 0.71 × 102 b | 1.65 × 104 ± 0.17 × 103 a |
| 6 | 1.42 × 104 ± 0.27 × 103 a | 1.57 × 104 ± 0.08 × 103 a |
| 8 | 1.16 × 103 ± 0.62 × 102 b | 1.61 × 104 ± 0.11 × 103 a |
| Weeks | Vibrio spp. Numbers (CFU/mL) | |
|---|---|---|
| Control Feed | LAB Feed | |
| 0 | 0.70 × 103 ± 0.08 × 102 | 0.79 × 103 ± 0.12 × 102 |
| 2 | 1.03 × 103 ± 0.03 × 102 | 1.14 × 103 ± 0.05 × 102 |
| 4 | 0.89 × 103 ± 0.06 × 102 | 1.08 × 103 ± 0.11 × 102 |
| 6 | 1.82 × 103 ± 0.11 × 102 | 1.77 × 103 ± 0.13 × 102 |
| 8 | 1.76 × 103 ± 0.15 × 102 | 1.91 × 103 ± 0.09 × 102 |
| Short-chain Fatty Acids | Acetic Acid | Propionic Acid | Butyric Acid |
|---|---|---|---|
| Concentration (μM) | |||
| Control feed | 17.1 ± 1.4 b | 11.2 ± 1.3 | 37.6 ± 5.3 b |
| LAB feed | 29.8 ± 0.8 a | 13.6 ± 1.8 | 63.3 ± 8.7 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-H.; Hsu, W.-H.; Chen, Y.-Z.; Hsu, K.-T.; Pan, T.-M. Limosilactobacillus fermentum SWP-AFFS02 Improves the Growth and Survival Rate of White Shrimp via Regulating Immunity and Intestinal Microbiota. Fermentation 2021, 7, 179. https://doi.org/10.3390/fermentation7030179
Lee B-H, Hsu W-H, Chen Y-Z, Hsu K-T, Pan T-M. Limosilactobacillus fermentum SWP-AFFS02 Improves the Growth and Survival Rate of White Shrimp via Regulating Immunity and Intestinal Microbiota. Fermentation. 2021; 7(3):179. https://doi.org/10.3390/fermentation7030179
Chicago/Turabian StyleLee, Bao-Hong, Wei-Hsuan Hsu, You-Zuo Chen, Kung-Ting Hsu, and Tzu-Ming Pan. 2021. "Limosilactobacillus fermentum SWP-AFFS02 Improves the Growth and Survival Rate of White Shrimp via Regulating Immunity and Intestinal Microbiota" Fermentation 7, no. 3: 179. https://doi.org/10.3390/fermentation7030179
APA StyleLee, B.-H., Hsu, W.-H., Chen, Y.-Z., Hsu, K.-T., & Pan, T.-M. (2021). Limosilactobacillus fermentum SWP-AFFS02 Improves the Growth and Survival Rate of White Shrimp via Regulating Immunity and Intestinal Microbiota. Fermentation, 7(3), 179. https://doi.org/10.3390/fermentation7030179









