Co-Production of Isobutanol and Ethanol from Prairie Grain Starch Using Engineered Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strain
2.2. Grain Samples
2.3. Starch Hydrolysis Enzymes
2.4. Starch Feedstock Preparation
2.5. Fermentation Reactions
2.6. Mycological Analysis
2.7. Analytical Methods
3. Results
3.1. SSF Treatment of Grain Starch
3.2. Isobutanol and Ethanol Production from Starch
3.3. Isobutanol and Ethanol Production Using SSF in a Fed-Batch Fermentation
3.4. Influence of the Nitrogen Source
3.5. Fermentation of Mycotoxin Contaminated Grains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Energy Information Administration. Short-Term Energy Outlook; US EIA: Washington, DC, USA, 2014.
- Christensen, E.; Yanowitz, J.; Ratcliff, M.; McCormick, R.L. Renewable oxygenate blending effects on gasoline properties. Energy Fuels 2011, 25, 4723–4733. [Google Scholar] [CrossRef]
- Dürre, P. Biobutanol: An attractive biofuel. Biotechnol. J. 2007, 2, 1525–1534. [Google Scholar] [CrossRef]
- Jones, D.T.; Woods, D.R. Acetone-butanol fermentation revisited. Microbiol. Rev. 1986, 50, 484–524. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 2008, 451, 86–99. [Google Scholar] [CrossRef]
- Varman, A.M.; Xiao, Y.; Pakrasi, H.B.; Tang, Y.J. Metabolic engineering of Synechocystis sp. strain PCC 6803 for isobutanol production. Appl. Environ. Microbiol. 2013, 79, 908–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ofuonye, E.J.; Kutin, K.; Stuart, D.T. Engineering Saccharomyces cerevisiae fermentative pathways for the production of isobutanol. Biofuels 2013, 4, 185–201. [Google Scholar] [CrossRef]
- Chen, X.; Nielsen, K.F.; Borodina, I.; Kielland-Brandt, M.C.; Karhumaa, K. Increased isobutanol production in Saccharomyces cerevisiae by overexpression of genes in valine metabolism. Biotechnol. Biofuels 2011, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Lane, S.; Zhang, Y.; Yun, E.J.; Ziolkowski, L.; Zhang, G.; Jin, Y.S.; Avalos, J.L. Xylose assimilation enhances the production of isobutanol in engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2020, 117, 372–381. [Google Scholar] [CrossRef]
- Higashide, W.; Yongchao, L.; Yang, Y.; Liao, J.C. Metabolic engineering of Clostridium cellulolyticum for production of isobutanol from cellulose. Appl. Environ. Microbiol. 2011, 77, 2727–2733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.X.; Zhang, M.; Zhang, C.T.; Zhang, H.; Mo, X.H.; Xing, X.H.; Yang, S. Metabolomic analysis improves bioconversion of methanol to isobutanol in Methylorubrum extorquens AM1. Biotechnol. J. 2021, 16, e2000413. [Google Scholar] [CrossRef]
- Novak, K.; Baar, J.; Freitag, P.; Pflügl, S. Metabolic engineering of Escherichia coli W for isobutanol production on chemically defined medium and cheese whey as alternative raw material. J. Ind. Microbiol. Biotechnol. 2020, 47, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Seo, H.M.; Jeon, J.M.; Moon, Y.M.; Hong, J.W.; Hong, Y.G.; Bhatia, S.K.; Ahn, J.; Lee, H.; Kim, W.; et al. Enhanced isobutanol production from acetate by combinatorial overexpression of acetyl-CoA synthetase and anaplerotic enzymes in engineered Escherichia coli. Biotechnol. Bioeng. 2018, 115, 1971–1978. [Google Scholar] [CrossRef]
- Adom, F.; Dunn, J.B.; Han, J.; Sather, N. Life-cycle fossil energy consumption and greenhouse gas emissions of bioderived chemicals and their conventional counterparts. Environ. Sci. Technol. 2014, 48, 14624–14631. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, W.; Zhang, A.; Shao, W. Biorefinery: The production of isobutanol from biomass feedstocks. Appl. Sci. 2020, 10, 8222. [Google Scholar] [CrossRef]
- Cheng, J.J.; Timilsina, G.R. Status and barriers of advanced biofuel technologies: A review. Renew. Energy 2011, 36, 3541–3549. [Google Scholar] [CrossRef]
- Ryan, C. GEVO White Paper: An Overview of GEVO’s Biobased Isobutanol Production Process; Gevo: Englewood, CO, USA, 2019. [Google Scholar]
- Weightman, R.M.; Kindred, D.R.; Clarke, S. Cereals for Bioethanol: Quantifying the Alcohol Yield of UK Hard Wheats and the Grain Yields and N Requirements of Triticale in the Second Cereal Position; HGCA Agriculture and Horticulture Development Board: Warwickshire, UK, 2011. [Google Scholar]
- Fioj, R.; Heidari, B.; Dadkhodaie, A. Investigation of triticale and wheat perfromance under dry land conditions on the basis of variations in agronomic and morphological traits. J. Adv. Biol. Biotech. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Ayalew, H.; Kumssa, T.T.; Butler, T.J.; Ma, X.F. Triticale improvement for forage and cover crop uses in the southern great plains of the United States. Front. Plant Sci. 2018, 9, 1130. [Google Scholar] [CrossRef] [Green Version]
- Puligundla, P.; Smogrovicova, D.; Obulam, V.S.; Ko, S. Very high gravity (VHG) ethanolic brewing and fermentation: A research update. J. Ind. Microbiol. Biotechnol. 2011, 38, 1133–1144. [Google Scholar] [CrossRef]
- Patni, N.; Pillai, S.G.; Dwivedi, A.H. Wheat as a promising substitute of corn for bioethanol production. Procedia Eng. 2013, 51, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Davis-Knight, H.R.; Weightman, R.M. The Potential of Triticale As a Low Input Cereal for Bioethanol Production; HGCA Agriculture and Horticulture Development Board: Warwikshire, UK, 2008. [Google Scholar]
- Montalbo-Lomboy, M.; Khanal, S.K.; van Leeuwen, J.; Raj Raman, D.; Grewell, D. Simultaneous saccharification and fermentation and economic evaluation of ultrasonic and jet cooking pretreatment of corn slurry. Biotechnol. Prog. 2011, 27, 1561–1569. [Google Scholar] [CrossRef]
- Olofsson, K.; Bertilsson, M.; Liden, G. A short review on SSF—An interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Cheng, J.Y. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Tang, Y.; Zhu, L.; Zhang, W.; Shang, X.; Jiang, J. Integrated process of starch ethanol and cellulosic lactic acid for ethanol and lactic acid production. Appl. Microbiol. Biotechnol. 2013, 97, 1923–1932. [Google Scholar] [CrossRef]
- Mendes, C.V.T.; dos Santos Rocha, J.M.; de Menezes, F.F.; da Graça Videira Sousa Carvalho, M. Batch and fed-batch simultaneous saccharification and fermentation of primary sludge from pulp and paper mills. Environ. Technol. 2017, 38, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, D.; Wlodzimierz, G. Fed-batch simultaneous saccharification and ethanol fermentation of native corn starch. Acta Sci. Pol. Technol. Aliment. 2009, 8, 5–16. [Google Scholar]
- Thomas, B.J.; Rothstein, R. Elevated recombination rates in transcriptionally active DNA. Cell 1989, 56, 619–630. [Google Scholar] [CrossRef]
- Gietz, D.R.; Woods, R.A. Transformation of Yeast by Lithium Acetate/Single-Stranded Carrier DNA/Polyethylene Glycol Method. In Methods in Enzymology; Guthrie, C., Fink, G.R., Eds.; Academic Press: Cambridge, MA, USA, 2002; pp. 87–96. [Google Scholar]
- Sherman, F. Getting Started with Yeast. In Methods in Enzymology; Guthrie, C., Fink, G.R., Eds.; Academic Press: Cambridge, MA, USA, 2002; pp. 3–41. [Google Scholar]
- Rabie, C.J.; Lübben, A.; Marais, G.J.; Jansen van Vuuren, H. Enumeration of fungi in barley. Int. J. Food Microbiol. 1997, 35, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Ramos, D.; van den Broek, M.; van Maris, A.J.; Pronk, J.T.; Daran, J.M. Genome-scale analyses of butanol tolerance in Saccharomyces cerevisiae reveal an essential role of protein degradation. Biotechnol. Biofuels 2013, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Seguinot, P.; Sanchez, I.; Ortiz-Julien, A.; Heras, J.M.; Querol, A.; Camarasa, C.; Guillamón, J.M. Nitrogen sources preferences of non-Saccharomyces yeasts to sustain growth and fermentation under winemaking conditions. Food Microbiol. 2020, 85, 103287. [Google Scholar] [CrossRef]
- Nganje, W.E.; Kaitibie, S.; Wilson, W.W.; Leistritz, F.L.; Bangsund, D.A. Economic Impacts of Fusarium Head Blight in Wheat and Barley: 1993–2001; Agribusiness and Applied Economics Rep. No. 528; North Dakota State University: Grand Forks, ND, USA, 2004. [Google Scholar] [CrossRef]
- Osborne, L.E.; Stein, J.M. Epidemiology of Fusarium head blight on small-grain cereals. Int. J. Food Microbiol. 2007, 119, 103–108. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Kugler, K.G.; Jandric, Z.; Beyer, R.; Klopf, E.; Glaser, W.; Lemmens, M.; Shams, M.; Mayer, K.; Adam, G.; Schüller, C. Ribosome quality control is a central protection mechanism for yeast exposed to deoxynivalenol and trichothecin. BMC Genom. 2016, 17, 417. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Iwahashi, Y. Low toxicity of deoxynivalenol-3-glucoside in microbial cells. Toxins 2015, 7, 187–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böswald, C.; Engelhardt, G.; Vogel, H.; Wallnöfer, P.R. Metabolism of the Fusarium mycotoxins zearalenone and deoxynivalenol by yeast strains of technological relevance. Nat. Toxins 1995, 3, 138–144. [Google Scholar] [CrossRef]
- Brat, D.; Boles, E. Isobutanol production from D-xylose by recombinant Saccharomyces cerevisiae. FEMS Yeast Res. 2013, 13, 241–244. [Google Scholar] [CrossRef] [Green Version]
- Promdonkoy, P.; Mhuantong, W.; Champreda, V.; Tanapongpipat, S.; Runguphan, W. Improvement in D-xylose utilization and isobutanol production in S. cerevisiae by adaptive laboratory evolution and rational engineering. J. Ind. Microbiol. Biotechnol. 2020, 47, 497–510. [Google Scholar] [CrossRef]
- Dickinson, J.R.; Harrison, S.J.; Hewlins, M.J. An investigation of the metabolism of valine to isobutyl alcohol in Saccharomyces cerevisiae. J. Biol. Chem. 1998, 273, 25751–25756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, F.; Ishii, J.; Kondo, T.; Ida, K.; Tezuka, H.; Kondo, A. Increased isobutanol production in Saccharomyces cerevisiae by eliminating competing pathways and resolving cofactor imbalance. Microb. Cell Fact. 2013, 12, 119. [Google Scholar] [CrossRef] [Green Version]
- Wess, J.; Brinek, M.; Boles, E. Improving isobutanol production with the yeast Saccharomyces cerevisiae by successively blocking competing metabolic pathways as well as ethanol and glycerol formation. Biotechnol. Biofuels 2019, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Brat, D.; Weber, C.; Lorenzen, W.; Bode, H.B.; Boles, E. Cytosolic re-localization and optimization of valine synthesis and catabolism enables inseased isobutanol production with the yeast Saccharomyces cerevisiae. Biotechnol. Biofuels 2012, 5, 65. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-H.; Seo, S.-O.; Bae, Y.-H.; Nan, H.; Jin, Y.-S.; Seo, J.-H. Isobutanol production in engineered Saccharomyces cerevisiae by overexpression of 2-ketoisovalerate decarboxylase and valine biosynthetic enzymes. Bioprocess Biosyst. Eng. 2012, 35, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.M.; Zhang, Y.; Mehl, J.; Park, H.; Lalwani, M.A.; Toettcher, J.E.; Avalos, J.L. Optogenetic regulation of engineered cellular metabolism for microbial chemical production. Nature 2018, 555, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Oud, B.; Flores, C.-L.; Gancedo, C.; Zhang, X.; Trueheart, J.; Daran, J.-M.; Pronk, J.T.; van Maris, A.J.A. An internal deletion in MTH1 enables growth on glucose of pyruvate-decarboxylase negative, non-fermentative Saccharomyces cerevisiae. Microb. Cell Factories 2012, 11, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, R.M.R.; Gunawardena, U.; Urano, J.; Maeinhold, P.; Aristidou, A.A.; Dundon, C.A.; Smith, C. Yeast Organism Producing Isobutanol at High Yield. U.S. Patent US8017375B2, 13 September 2011. [Google Scholar]
- Oettler, G. The fortune of a botanical curiosity—Triticale: Past, present and future. J. Agric. Sci. 2005, 143, 329–346. [Google Scholar] [CrossRef]
- Graham, R.; Geytenbeek, P.; Radcliffe, B. Response of triticale, wheat, rye and barley to nitrogen fertilizer. Aust. J. Exp. Agric. 1983, 23, 73–79. [Google Scholar] [CrossRef]
- Brock, P.; Madden, P.; Schwenke, G.; Herridge, D. Greenhouse gas emissions profile for 1 tonne of wheat produced in Central Zone (East) New South Wales: A life cycle assessment approach. Crop Pasture Sci. 2012, 63, 319–329. [Google Scholar] [CrossRef]
- Myer, R.; Lozano del Rio, A.J. Triticale as Animal Feed. In Triticale Improvement and Production; Mergoun, M., Gomez-Macpherson, H., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; pp. 49–58. [Google Scholar]
- Khan, M.K.; Pandey, A.; Athar, T.; Choudhary, S.; Deval, R.; Gezgin, S.; Hamurcu, M.; Topal, A.; Atmaca, E.; Santos, P.A.; et al. Fusarium head blight in wheat: Contemporary status and molecular approaches. 3 Biotech 2020, 10, 172. [Google Scholar] [CrossRef]
- U.S. FDA. Guidance for Industry and FDA: Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain By-Products Used for Animal Feed. 2010. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-and-fda-advisory-levels-deoxynivalenol-don-finished-wheat-products-human (accessed on 19 February 2021).
- Elmekawy, A.; Diels, L.; De Wever, H.; Pant, D. Valorization of cereal based biorefinery byproducts: Reality and expectations. Environ. Sci. Technol. 2013, 47, 9014–9027. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Eksteen, J.M.; Van Rensburg, P.; Cordero Otero, R.R.; Pretorius, I.S. Starch fermentation by recombinant Saccharomyces cerevisiae strains expressing the alpha-amylase and glucoamylase genes from Lipomyces kononenkoae and Saccharomycopsis fibuligera. Biotechnol. Bioeng. 2003, 84, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Li, Z.; Jia, C.; Zhang, W.; Zhang, Y.; Yi, C.; Xie, S. Recent advances on bio-based isobutanol separation. Energy Convers. Manag. X 2021, 10, 100059. [Google Scholar]
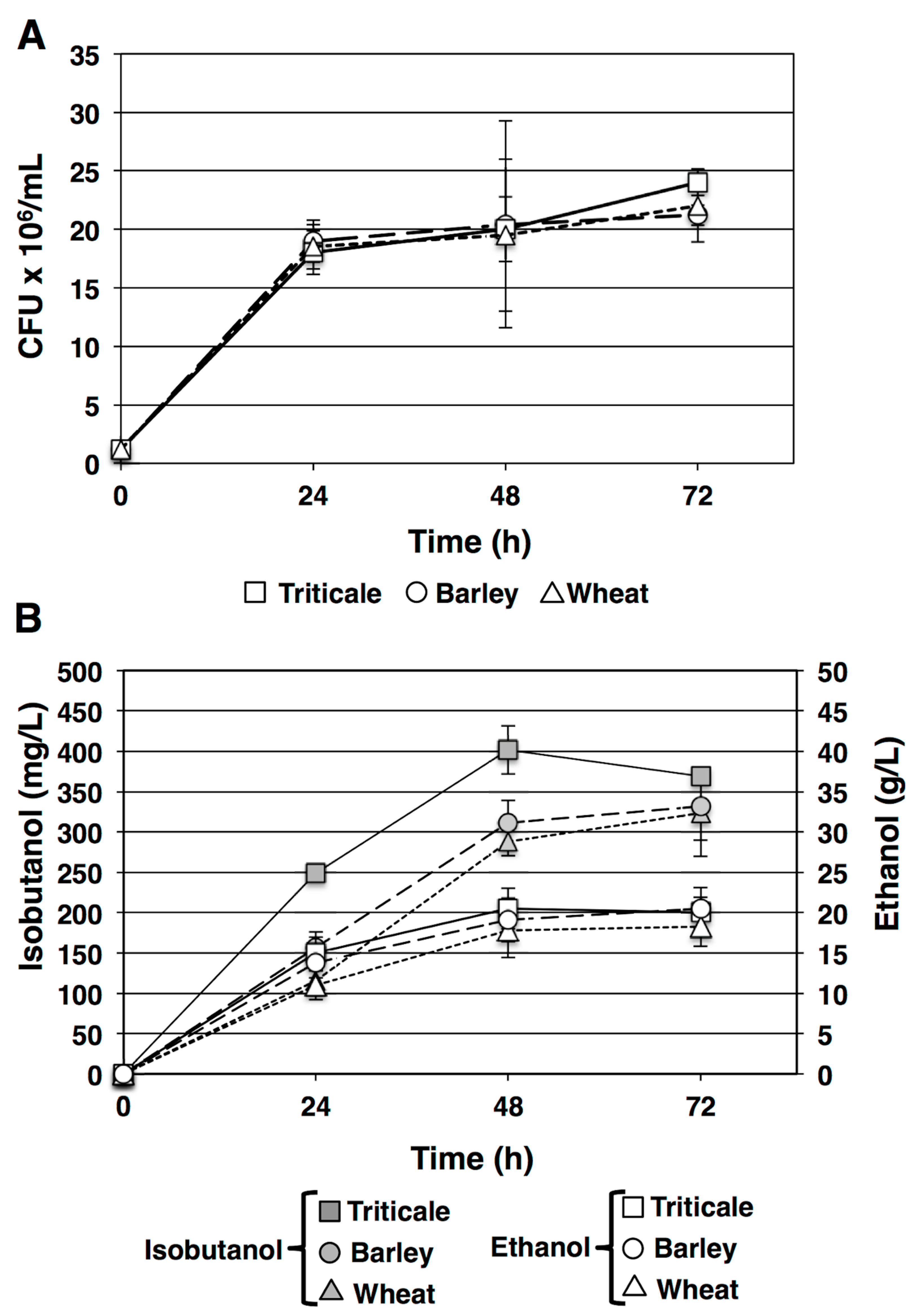

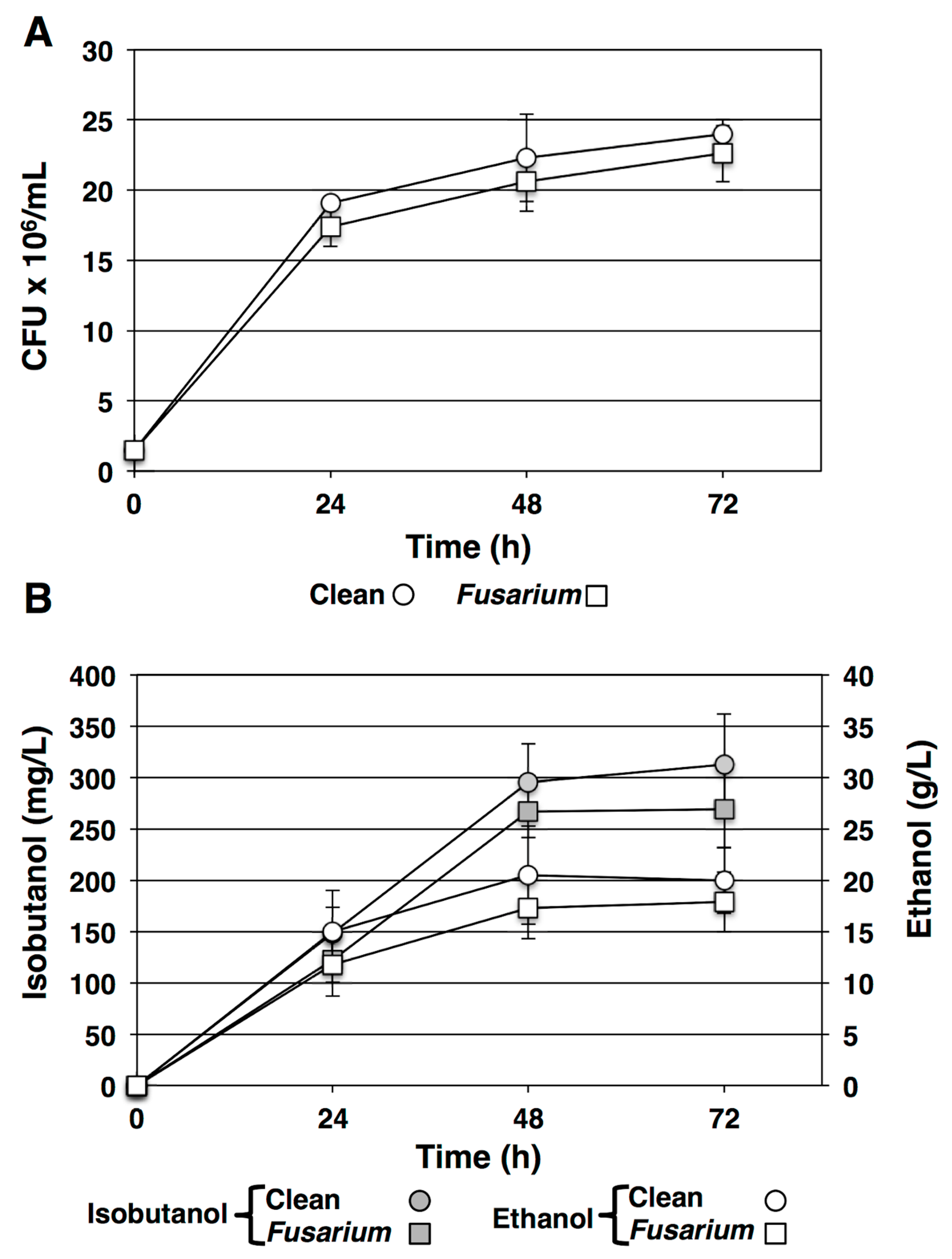
| Grain | Sugar | 0 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|---|
| Triticale | Glucose | 147.99 ± 6.42 | 7.11 ± 0.49 | 6.38 ± 0.22 | 6.81 ± 0.83 |
| Xylose | 1.9 ± 0.01 | 2.4 ± 0.03 | 2.7 ± 0.01 | 2.8 ± 0.1 | |
| Wheat | Glucose | 107.66 ± 14.23 | 3.49 ± 0.17 | 3.11 ± 0.22 | 6.02 ± 1.62 |
| Xylose | 2.4 ± 0.06 | 2.7 ± 0.1 | 2.9 ± 0.03 | 2.9 ± 0.08 | |
| Barley | Glucose | 129.65 ± 8.4 | 5.82 ± 1.2 | 4.21 ± 0.3 | 5.91 ± 1.5 |
| Xylose | 3.1 ± 0.1 | 3.5 ± 0.03 | 3.3 ± 0.08 | 3.3 ± 0.2 |
| Nitrogen Source | Isobutanol mg/L b | Ethanol g/L b |
|---|---|---|
| None added | 62.31 ± 18.11 | 4.83 ± 0.37 |
| 10 mM Urea | 398.01 ± 25.06 | 20.33 ± 0.08 |
| 20 mM Urea | 397.82 ± 12.46 | 18.34 ± 0.11 |
| 10 mM (NH4)2SO4 | 396.83 ± 25.77 | 19.38 ± 0.10 |
| 20 mM (NH4)2SO4 | 389.12 ± 17.87 | ND |
| 20 mM NH4Cl | 390.57 ± 34.80 | 18.77 ± 0.18 |
| 40 mM NH4Cl | 387.82 ± 27.89 | 17.34 ± 0.41 |
| Glucose (mg/100 mL) | Xylose (mg/100 mL) | DON (µg/100 mL) | |
|---|---|---|---|
| Barley (Fusarium) | 123 ± 7.9 | 2.9 ± 0.4 | 150.4 ± 12.5 |
| Barley (clean) | 138 ± 6.1 | 4.3 ± 0.6 | 0.08 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Unaegbunam, E.; Stuart, D.T. Co-Production of Isobutanol and Ethanol from Prairie Grain Starch Using Engineered Saccharomyces cerevisiae. Fermentation 2021, 7, 150. https://doi.org/10.3390/fermentation7030150
Liu X, Unaegbunam E, Stuart DT. Co-Production of Isobutanol and Ethanol from Prairie Grain Starch Using Engineered Saccharomyces cerevisiae. Fermentation. 2021; 7(3):150. https://doi.org/10.3390/fermentation7030150
Chicago/Turabian StyleLiu, Xiaodong, Ebele Unaegbunam, and David T. Stuart. 2021. "Co-Production of Isobutanol and Ethanol from Prairie Grain Starch Using Engineered Saccharomyces cerevisiae" Fermentation 7, no. 3: 150. https://doi.org/10.3390/fermentation7030150
APA StyleLiu, X., Unaegbunam, E., & Stuart, D. T. (2021). Co-Production of Isobutanol and Ethanol from Prairie Grain Starch Using Engineered Saccharomyces cerevisiae. Fermentation, 7(3), 150. https://doi.org/10.3390/fermentation7030150





