Abstract
Malaysia is the second largest palm oil producer and exporter globally. When crude palm oil is produced in both plantations and oil processing mills, a large amount of oil palm empty fruit bunch (OPEFB) is simultaneously produced as a waste product. Here, we describe the preparation of hydrolysate from OPEFB. After OPEFB was hydrothermally treated at 180–200 °C, the resultant liquid phase was subjected to high-performance liquid chromatography analysis, while the solid phase was used for acidic and enzymatic hydrolysis. Hemicellulose yield from the acid-treated solid phase decreased from 153 mg/g-OPEFB to 27.5 mg/g-OPEFB by increasing the hydrothermal treatment temperature from 180 to 200 °C. Glucose yield from the enzyme-treated solid phase obtained after hydrothermal treatment at 200 °C was the highest (234 ± 1.90 mg/g-OPEFB, 61.7% production efficiency). In contrast, xylose, mannose, galactose, and arabinose yields in the hydrolysate prepared from the solid phase hydrothermally treated at 200 °C were the lowest. Thus, we concluded that the optimum temperature for hydrothermal pretreatment was 200 °C, which was caused by the low hemicellulose yield. Based on these results, we have established an effective method for preparing OPEFB hydrolysates with high glucose content.
1. Introduction
Malaysia has a tropical rainforest climate with high temperature and precipitation, which is suitable for growing crops throughout the year. Thus, agriculture is a major industry in Malaysia, with forestry and palm oil, natural rubber, and timber production being the dominant sectors. Malaysia is also the second largest palm oil producer and exporter after Indonesia, producing more than 19.8 million tons of crude palm oil in 2015 [1]. During crude palm oil production, several by-products, such as oil palm empty fruit bunch (OPEFB), oil palm frond fiber (OPFF), and oil palm mesocarp fiber (OPMF) are also generated in both plantations and oil processing mills, and the amount of OPEFB generated is the largest [1]. Due to its high calorific value and low greenhouse gas emission during combustion, OPEFB has great potential for being utilized as a substitute for woody plants. For example, a mixture containing OPEFB, fiber, and shell is used to produce steam for electricity generation [2]. Adding cellulose nanofibers prepared from OPEFB to a hydrogel also enhances the mechanical strength, and decreases the amount of raw material required, which further reduces the production cost [3]. However, OPEFB is not used as a carbon source for the industrial fermentation of valuable compounds.
Fermentation is a multi-step reaction within the cell; thus, it is useful for producing enzymes, antibiotics, organic acids, and alcohols. To reduce dependence on petroleum and establish an environmentally friendly society, lignocellulosic biomass is used as a source material for fermentation for producing useful substances after pretreatment and enzymatic hydrolysis steps [4,5,6]. During pretreatment, lignocellulosic biomass is degraded into cellulose, hemicellulose, and lignin, using chemical or physical methods. During enzymatic hydrolysis, the pretreated biomass is hydrolyzed using several enzymes, such as Acremonium cellulase [7], Trichoderma cellulase [7], and xylanase [8], which generate the hydrolysate containing mixed sugars. Finally, the hydrolysate is used as the carbon source during fermentation. Genetically engineered microorganisms are used for producing valuable compounds, such as acetoin [9], 2,3-butanediol [9], and isobutanol [10] during fermentation. However, microorganisms have carbon catabolite repression, which leads to selective glucose usage in the mixed sugars [9,10,11,12,13,14]. Thus, preparing hydrolysates with high glucose content is an effective method to enhance the production yield of the valuable compounds.
Several methods for preparing hydrolysate from lignocellulosic biomass have been developed. For example, hydrolysates including arabinose, galactose, glucose, xylose are prepared from rye straw and bermudagrass by enzymatic hydrolysis after dilute acid pretreatment [15]. Enzymatic hydrolysis with milling pretreatment is an effective method for preparing hydrolysate from sugarcane bagasse with glucose and xylose as main components [16]. However, those methods have been developed to increase the total sugar yield and show low glucose content. Further investigation is required to enhance glucose content.
In this study, we demonstrated the hydrothermal pretreatment of OPEFB. After the hydrothermal pretreatment, the efficiency was evaluated by determining the sugar concentration in the liquid phase as well as cellulose, hemicellulose, and lignin yields in the residual solid phase. Subsequently, the hydrothermally treated solid phase was hydrolyzed using an enzyme cocktail comprising Acremonium cellulose, Novozyme 188, and Optimash BG to produce the hydrolysate with high glucose content. Finally, the method of hydrolysate preparation from OPEFB was evaluated by comparing with the results of the previous study. Based on these results, we have established an effective method for preparing hydrolysate from OPEFB with high glucose content, and which enables the application of OPEFB to fermentation for producing valuable compounds.
2. Materials and Methods
2.1. Hydrothermal Pretreatment
Hydrothermal pretreatment using hot compressed water was performed in a 1 L stainless steel autoclave system with an external electric heater and stirring motor (Nitto Koatsu; Ibaraki, Japan). Briefly, 50 g OPEFB was placed in an autoclave with 500 mL water for 20 min at 180 ± 3 °C, 190 ± 3 °C, or 200 ± 3 °C [14]. The inner temperature was measured using a thermocouple (Type K). Under these conditions, the severity factors (SFs) were 3.66, 3.94, and 4.25, respectively, and the values were calculated using Equations (1) and (2) [17]:
where T and t are the temperature (°C) and time (min) of pretreatment, respectively. The mixture was stirred at 300 rpm throughout the pretreatment process.
R0 = exp[(T − 100)/14.75] × t
SF = log(R0)
After hydrothermal pretreatment for 20 min at the desired temperature, the electric heater was removed, and the autoclave was immersed in an ice-water bath to quench the hydrothermally pretreated sample. The mixture was separated by centrifugation. The liquid phase was subjected to high-performance liquid chromatography (HPLC) analysis. In addition, the solid phase was used for acidic and enzymatic hydrolysis after rinsing thrice with water.
2.2. HPLC Analysis of Sugar, Aldehyde, and Organic Acid
The sugar (glucose, xylose, mannose, galactose, arabinose, and cellobiose) concentrations were determined using an LC-10AD VP system (Shimadzu; Kyoto, Japan) with an Aminex HPX-87H cationic exchange column (Bio-Rad Laboratories; Richmond, CA, USA). The chromatographic conditions were as follows: mobile phase, ultrapure water; flow rate, 0.6 mL/min; column oven temperature, 80 °C.
The aldehyde [furfural and 5-hydroxymethylfurfural (HMF)] and organic acid (acetic acid, formic acid, and glycolic acid) concentrations were determined using an LC-2000 Plus system (Jasco; Tokyo, Japan) with a Shodex SUGAR SH1821 column (Showa Denko; Tokyo, Japan). The chromatographic conditions were as follows: mobile phase, 2.0 mM H2SO4; flow rate, 0.6 mL/min; column oven temperature, 60 °C.
2.3. Acidic Hydrolysis of the Solid Phase
The solid phase was prepared by centrifugation of the hydrothermally pretreated sample. The monomeric sugars in the solid phase were determined by the analytical procedure of NREL with some modifications [18]. Briefly, 0.05 g vacuum-dried solid phase was placed in a glass tube with 0.6 mL H2SO4 solution (72 wt%). The mixture was stirred in an incubator for 90 min at 30 °C. After diluting the mixture with 16.8 mL water, the sample was heated in an autoclave for 120 min at 120 °C. The autoclaved sample was immediately cooled in an ice-water bath, adjusted to 20 mL by adding water, and rested for 5 min. Using a guard filter (Dionex OnGuard II A, Thermo Fisher Scientific K.K.; Kanagawa, Japan), the resultant supernatant was filtered to remove H2SO4, yielding a solution containing a mixture of monomeric sugars.
2.4. Determination of Cellulose, Hemicellulose, and Lignin Yields
Cellulose and hemicellulose yields were calculated from the corresponding monomeric sugar concentrations using anhydro corrections of 0.88 and 0.90 for C5 (xylose and arabinose) and C6 (glucose, galactose, and mannose) sugars, respectively. Lignin yield was determined as the residue insoluble in 72% H2SO4 solution.
2.5. Enzymatic Hydrolysis
After hydrothermal pretreatment, the sample was hydrolyzed with an enzyme cocktail comprising Acremonium cellulose (40 FPU/mL; Meiji Seika Pharma; Tokyo, Japan), Novozyme 188 (600 U/mL; Novozymes; Bagsværd, Denmark), and Optimash BG (10%; Genencor; Palo Alto, CA, USA) in 50 mM citrate buffer (pH 5.0). After incubating for 72 h at 50 °C, the reaction mixtures were harvested by centrifugation and filtered using a 0.2 μm filter. The resultant supernatant was subjected to HPLC analysis. The production efficiency was determined using Equation (3):
Production efficiency (%) = (weight of sugar after the enzymatic hydrolysis/weight of potential total sugar of the solid phase after hydrolysis using H2SO4) × 100
3. Results and Discussion
3.1. Hydrothermal Pretreatment of OPEFB
We have previously developed effective methods for hydrothermal pretreatment of eucalyptus [19], OPFF [20], or OPMF [21], which may be used to degrade cellulose and hemicellulose in different lignocellulosic biomass. Based on the findings of our previous studies, in this study, we hydrothermally pretreated OPEFB.
To enhance glucose yield, hydrothermal pretreatment requires degrading hemicellulose while leaving cellulose. Hemicellulose hydrolysis is accelerated above 180 °C, and cellulose degrades above 200 °C [19]. Thus, OPEFB was hydrothermally pretreated at 180–200 °C (Figure 1). The glucose yield in the residual solution was the highest at 200 °C (2.29 ± 0.0590 mg/g-OPEFB), which was 2.1-fold higher than that at 180 °C (1.11 ± 0.168 mg/g-OPEFB). A similar trend was observed for xylose, mannose, and cellobiose, with xylose yield at 200 °C (9.41 ± 0.390 mg/g-OPEFB) being 7.3-fold higher than that at 180 °C (1.29 ± 0.427 mg/g-OPEFB). Based on these results, we hypothesized that cellulose and hemicellulose degradation in OPEFB was enhanced by increasing the hydrothermal pretreatment temperature.
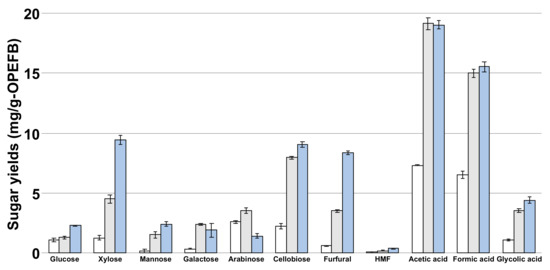
Figure 1.
The sugar, aldehyde, and organic acid yields in the residual liquid phase after hydrothermally pretreating OPEFB. The yields at 180 °C, 190 °C, and 200 °C are indicated in white, gray, and blue bars, respectively. HMF: 5-hydroxymethylfurfural.
3.2. Acidic Hydrolysis of the Residual Solid Phase After Hydrothermal Treatment
To further evaluate hydrothermal pretreatment, the residual solid phase after hydrothermal pretreatment was hydrolyzed by adding H2SO4, and then the monomeric sugar concentrations were determined (Table 1). The cellulose and hemicellulose yields in the residue were calculated based on the monomeric sugar concentrations.

Table 1.
Sugar yields from the acid-treated residual solid phase after hydrothermal treatment.
The xylose, galactose, arabinose, and mannose concentrations were the maximum at 180 °C (Table 1). Moreover, cellulose and hemicellulose yields decreased in proportion to the pretreatment temperature (Figure 2). In particular, the hemicellulose yield decreased from 153 to 27.5 mg/g-OPEFB by increasing the treatment temperature from 180 °C to 200 °C. These results showed that the hydrothermal pretreatment of OPEFB at 200 °C was the most efficient, which was consistent with the sugar yields calculated from the residual solution (Figure 1).
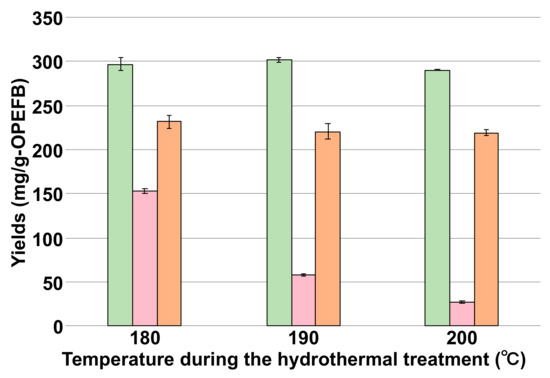
Figure 2.
Effect of hydrothermal pretreatment temperature on cellulose, hemicellulose, and lignin yields. Cellulose, hemicellulose, and lignin yields are indicated in green, pink, and orange bars, respectively.
We also determined the lignin yield, which is one of the main components of lignocellulosic feedstocks. Interestingly, the lignin yield in the residual solid phase did not decrease compared to the hemicellulose yield (Figure 2). This result showed that the developed hydrothermal method requires further improvement to degrade lignin. Lignin is composed of heterogeneous aromatic acids and its degradation requires ligninolytic enzymes such as laccase, lignin peroxidase, and manganese peroxidase [22,23]. To use lignin as a carbon source for fermentation, it is necessary to add ligninolytic enzymes while preparing the hydrolysate or use lignin-degrading bacteria as a host for fermentation.
3.3. Enzymatic Hydrolysis of the Hydrothermally Treated Solid Phase
To enhance the glucose productivity and prevent the by-production of other sugars, the samples hydrothermally treated at 180–200 °C were enzymatically hydrolyzed using an enzyme cocktail comprising Acremonium cellulose, Novozyme 188, and Optimash BG (Figure 3). When the sample hydrothermally treated at 180 °C was used as the source material, glucose, xylose, mannose, galactose, arabinose, and cellobiose were produced, respectively. In contrast, the concentrations of arabinose produced from the samples hydrothermally treated at 190 °C and 200 °C were below the detection limit. Glucose was obtained as the main sugar, and the maximum yield (234 ± 1.90 mg/g-OPEFB) was observed using the solid phase hydrothermally treated at 200 °C with 61.7% production efficiency (Table 2). On the contrary, the xylose (21.7 ± 0.201 mg/g-OPEFB) and other sugar yields were the lowest using the solid phase hydrothermally treated at 200 °C. These results demonstrated that enzymatic hydrolysis combined with the previously developed hydrothermal pretreatment method was useful for glucose production from OPEFB.
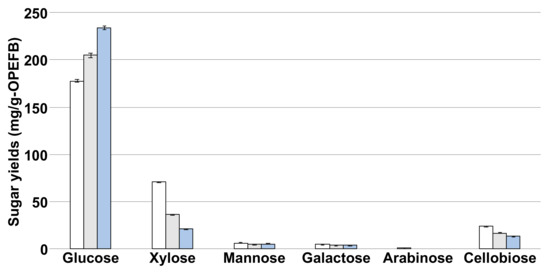
Figure 3.
Sugar yields from the hydrolysate after the enzymatic hydrolysis. The yields after hydrothermal treatment at 180, 190, and 200 °C are indicated as white, gray, and blue bars, respectively.

Table 2.
Production efficiency of glucose and xylose after the enzymatic hydrolysis.
The production efficiency of glucose increased and that of xylose decreased in proportion to the increase in hydrothermal treatment temperature (Table 2). We hypothesized that these production efficiencies were due to the combination of hydrothermal pretreatment and enzymatic characteristics. Acremonium cellulase and Novozyme 188 are commercial enzymes widely applied in biofuel production from lignocellulosic feedstocks. Both enzymes have β-glucosidase activity and catalyze cellobiose hydrolysis to produce bimolecular glucose. Optimash BG is a mixture of β-glucanase and xylanase, which hydrolyzes cellobiose, hemicellulose, and xylose. When the sample hydrothermally treated at 200 °C was hydrolyzed by the enzyme cocktail, the glucose yield was maximum (Figure 3). This result indicated that OPEFB degradation was facilitated by the increase in hydrothermal pretreatment temperature, and then the resultant cellulose and hemicellulose were used as the substrate by the enzyme cocktail, resulting in efficient glucose production.
3.4. Comparison of the Sugar, Aldehyde, and Organic Acid Yields
We have previously prepared the hydrolysates from eucalyptus [19], OPFF [20], and OPMF [24], respectively. To confirm the usefulness of the hydrolysate preparation method developed in this study, we compared the sugar yield with that reported in the previous study (Table 3).

Table 3.
Comparison of the sugar yields in the hydrolysate.
When the yield of glucose was compared, the yield of hydrolysate from OPEFB was approximately 1.7-fold higher than that of hydrolysate from OPMF (Table 3). The xylose and galactose contents in enzyme-treated eucalyptus hydrothermally pretreated at 200 °C were 33% and 7.4%, respectively, of the glucose content (Table 3). In contrast, the xylose and galactose contents in OPEFB hydrolysate were 9.3% and 1.8%, respectively, of the glucose content, which were more than 3-fold lower than those of the eucalyptus hydrolysate (Table 3). The hydrolysate from OPEFB showed a similar glucose yield to that of hydrolysates from eucalyptus and OPFF, while the glucose content was highest than those of the other hydrolysates. Thus, hydrolysate from OPEFB is preferable for fermentation, considering control by carbon catabolite repression to the engineered microorganisms. We hypothesized that the high glucose content in OPEFB hydrolysate was due to the low hemicellulose yield in the hydrothermally treated solid phase. The hydrothermal hydrolysis of hemicellulose produces xylose, furfural, and HMF, and the subsequent degradation produces organic acids, such as acetic acid. Moreover, hemicellulose degradation is facilitated by the acetic acid generated [15]. When OPEFB was hydrothermally treated at 200 °C, furfural, HMF, and acetic acid concentrations in the liquid phase were the highest (Figure 1), but hemicellulose yield was the lowest (Figure 2). However, furfural and HMF disrupt the cell membrane, inhibit the housekeeping enzymes, and damage the DNA structure. Thus, to use the OPEFB hydrolysate for fermentation, it might be better to provide enzymatic detoxification capability into the host cells [25,26]. Fermentation using the hydrolysate from OPEFB will be presented in our next study.
4. Conclusions
In this study, we hydrothermally pretreated OPEFB. Subsequently, the liquid phase was subjected to HPLC analysis to determine the sugar, aldehyde, and organic acid concentrations. To further evaluate the hydrothermal pretreatment, the solid phase was acid-hydrolyzed to determine the cellulose, hemicellulose, and lignin yields. The hydrothermally treated OPEFB was also enzymatically hydrolyzed to prepare the hydrolysate. Glucose yield and content in the hydrolysate were the highest using the solid phase hydrothermally treated at 200 °C, which was consistent with the results of liquid phase HPLC analysis and the solid phase after acid hydrolysis. The xylose and galactose contents reported in this study were more than 3-fold lower compared with those reported in previous studies, which showed that the glucose content in OPEFB hydrolysis was higher than that in the eucalyptus hydrolysate. Based on these results, we have established an effective method for preparing OPEFB hydrolysate suitable for fermentation. In the future, it will be necessary to study the improvement of glucose yield in the hydrolysate and the actual fermentation production using the hydrolysate from OPEFB.
Author Contributions
Conceptualization, H.A.; methodology, M.Z.M.Y., S.F.; validation, H.A., S.F.; formal analysis, M.Z.M.Y., S.F.; investigation, H.A., S.F.; resources, S.F.; data curation, H.A., M.Z.M.Y.; writing—original draft preparation, H.A.; writing—review and editing, H.A., M.Z.M.Y., S.F.; visualization, H.A.; supervision, H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to all members of the Bio-conversion Research Group at our Institute (Research Institute for Sustainable Chemistry, National Institute of Advanced Industrial Science and Technology (AIST)) for their technical assistance and valuable discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mahlia, T.M.I.; Ismail, N.; Hossain, N.; Silitonga, A.S.; Shamsuddin, A.H. Palm oil and its wastes as bioenergy sources: A comprehensive review. Environ. Sci. Pollut. Res. Int. 2019, 26, 14849–14866. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.; Jawaid, M.; Sultan, M.T.H. Thermal properties of oil palm biomass based composites. In Lignocellulosic Fibre and Biomass-Based Composite Materials, 1st ed.; Jawaid, M., Paridah, M.T., Saba, N., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2017; Volume 6, pp. 95–122. [Google Scholar]
- Padzil, F.N.M.; Lee, S.H.; Ainun, Z.M.A.; Lee, C.H.; Abdullah, L.C. Potential of oil palm empty fruit bunch resources in nanocellulose hydrogel production for versatile applications: A review. Materials 2020, 13, 1245. [Google Scholar] [CrossRef] [PubMed]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potentialissues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of second generation bioethanol production from residual biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef]
- Fujii, T.; Fang, X.; Inoue, H.; Murakami, K.; Sawayama, S. Enzymatic hydrolyzing performance of Acremonium cellulolyticus and Trichoderma reesei against three lignocellulosic materials. Biotechnol. Biofuels 2009, 2, 24. [Google Scholar] [CrossRef]
- Mathibe, B.N.; Malgas, S.; Radosavljevic, L.; Kumar, V.; Shukla, P.; Pletschke, B.I. Lignocellulosic pretreatment-mediated phenolic by-products generation and their effect on the inhibition of an endo-1,4-β-xylanase from Thermomyces lanuginosus VAPS-24. 3 Biotech. 2020, 10, 349. [Google Scholar] [CrossRef]
- Nakashima, N.; Akita, H.; Hoshino, T. Establishment of a novel gene expression method, BICES (biomass-inducible chromosome-based expression system), and its application to the production of 2,3-butanediol and acetoin. Metab. Eng. 2014, 25, 204–214. [Google Scholar] [CrossRef]
- Akita, H.; Nakashima, N.; Hoshino, T. Bacterial production of isobutanol without expensive reagents. Appl. Microbiol. Biotechnol. 2015, 99, 991–999. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Hassan, S.E.D.; Alrefaey, H.M.A.; Elsakhawy, T. Efficient co-utilization of biomass-derived mixed sugars for lactic acid production by Bacillus coagulans Azu-10. Fermentation 2021, 7, 28. [Google Scholar] [CrossRef]
- Kayikci, Ö.; Nielsen, J. Glucose repression in Saccharomyces cerevisiae. FEMS Yeast Res. 2015, 15, fov068. [Google Scholar] [CrossRef]
- Simpson-Lavy, K.; Kupiec, M. Carbon catabolite repression in yeast is not limited to glucose. Sci. Rep. 2019, 9, 6491. [Google Scholar] [CrossRef] [PubMed]
- Mohd, Y.M.Z.; Akita, H.; Hassan, M.A.; Fujimoto, S.; Yoshida, M.; Nakashima, N.; Hoshino, T. Production of acetoin from hydrothermally pretreated oil mesocarp fiber using metabolically engineered Escherichia coli in a bioreactor system. Bioresour. Technol. 2017, 245, 1040–1048. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J.J. Dilute acid pretreatment of rye straw and bermudagrass for ethanol production. Bioresour. Technol. 2005, 96, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.S.; Inoue, H.; Endo, T.; Yano, S.; Bon, E.P. Milling pretreatment of sugarcane bagasse and straw for enzymatic hydrolysis and ethanol fermentation. Bioresour. Technol. 2010, 101, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- Overend, R.P.; Chornet, E.; Gascoigne, J.A. Fractionation of lignocellulosics by steam–aqueous pretreatments. Philos. Trans. R. Soc. A 1987, 321, 523–536. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. NREL Rep. 2008, TP–510–42618. [Google Scholar]
- Fujimoto, S.; Inoue, S.; Yoshida, M. High solid concentrations during the hydrothermal pretreatment of eucalyptus accelerate hemicellulose decomposition and subsequent enzymatic glucose production. Bioresour. Technol. Rep. 2018, 4, 16–20. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Hirata, S.; Fujimoto, S.; Hassan, M.A. Combined pretreatment with hot compressed water and wet disk milling opened up oil palm biomass structure resulting in enhanced enzymatic digestibility. Bioresour. Technol. 2015, 193, 128–134. [Google Scholar] [CrossRef]
- Ahmad, N.; Zakaria, M.R.; Mohd, Y.M.Z.; Fujimoto, S.; Inoue, H.; Ariffin, H.; Hassan, M.A.; Shirai, Y. Subcritical water-carbon dioxide pretreatment of oil palm mesocarp fiber for xylooligosaccharide and glucose production. Molecules 2018, 23, 1310. [Google Scholar] [CrossRef]
- Dashtban, M.; Schraft, H.; Syed, T.A.; Qin, W. Fungal biodegradation and enzymatic modification of lignin. Int. J. Biochem. Mol. Biol. 2010, 1, 36–50. [Google Scholar] [PubMed]
- Plácido, J.; Capareda, S. Ligninolytic enzymes: A biotechnological alternative for bioethanol production. Bioresour. Bioprocess. 2015, 2, 23. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Hirata, S.; Hassan, M.A. Combined pretreatment using alkaline hydrothermal and ball milling to enhance enzymatic hydrolysis of oil palm mesocarp fiber. Bioresour. Technol. 2014, 169, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Akita, H.; Watanabe, M.; Suzuki, T.; Nakashima, N.; Hoshino, T. Characterization of the Kluyveromyces marxianus strain DMB1 YGL157w gene product as a broad specificity NADPH-dependent aldehyde reductase. AMB Express 2015, 5, 17. [Google Scholar] [CrossRef]
- Akita, H.; Watanabe, M.; Suzuki, T.; Nakashima, N.; Hoshino, T. Molecular cloning and characterization of two YGL039w genes encoding broad specificity NADPH-dependent aldehyde reductases from Kluyveromyces marxianus strain DMB1. FEMS Microbiol. Lett. 2015, 362, fnv116. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).