The Effect of Non-Saccharomyces and Saccharomyces Non-Cerevisiae Yeasts on Ethanol and Glycerol Levels in Wine
Abstract
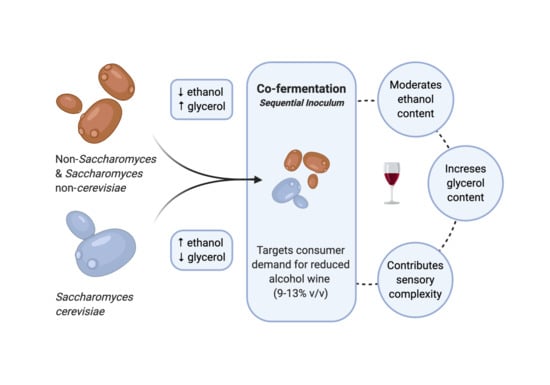
1. Introduction
2. Ethanol Reduction
3. Glycerol
4. Schizosaccharomyces Pombe
5. Metschnikowia Pulcherrima
6. Lachancea Thermotolerans
7. Candida Stellata/Starmerella Bombicola
8. Torulaspora Delbrueckii
9. Other Non-Saccharomyces and Saccharomyces Non-cerevisiae Yeasts
| Grape Variety | Wine Style | Ethanol Reduction % (v/v) | Inoculation | Reference |
|---|---|---|---|---|
| Schizosaccharomyces pombe | ||||
| Airén | White still | 0.4 | Sequential inoculation with | [87] |
| S. cerevisiae | ||||
| Airén | White still | 0.65 | Pure inoculation | [87] |
| Schizosaccharomyces japonicus | ||||
| Trebbiano | White still | 2.4 | Sequential inoculation | [132] |
| S. japonicus (immobilised)+ | ||||
| S. cerevisiae | ||||
| Trebbiano | White still | 1.7 | Co-inoculation | [132] |
| S. japonicus (immobilised)+ | ||||
| S. cerevisiae | ||||
| Metschnikowia pulcherrima | ||||
| Malvasia/Viura | White still | 0.8 | Sequential inoculation with | [99] |
| S. cerevisiae (aeration) | ||||
| Chardonnay | White still | 0.9 | Sequential inoculation with | [49] |
| S. cerevisiae | ||||
| Shiraz | Red still | 0.9 | Sequential inoculation with | [93] |
| S. cerevisiae | ||||
| Merlot | Red still | 1 | Co-inoculation with | [50] |
| S. cerevisiae | ||||
| Synthetic grape juice | – | 1.1–1.3 | Sequential inoculation | [14] |
| M. pulcherrima (immobilised)+ | ||||
| S. cerevisiae | ||||
| Verdicchio | White still | 1.2–1.6 | Sequential inoculation | [14] |
| M. pulcherrima (immobilised)+ | ||||
| S. cerevisiae | ||||
| Chardonnay | White still | 0.7–1.6 | Sequential inoculation | [43] |
| (aeration) | ||||
| Shiraz | Red still | 1.6 | Sequential inoculation with | [49] |
| S. cerevisiae | ||||
| Malvasia/Viura | White still | 3.7 | Sequential inoculation with | [57] |
| S. cerevisiae (aeration) | ||||
| Riesling | White still | 3.8 | Sequential inoculation | [47] |
| (aeration) | ||||
| Lachancea thermotolerans | ||||
| Shiraz | Red still | 0.4 | Sequential inoculation with | [71] |
| S. cerevisiae | ||||
| Airén | White still | 0.4 | Sequential inoculation with | [80] |
| S. cerevisiae | ||||
| Sangiovese | Red still | 0.7 | Sequential inoculation with | [102] |
| S. cerevisiae | ||||
| Tempranillo | Red still | 1.2 | Sequential inoculation with | [89] |
| S. cerevisiae | ||||
| Candida stellata/Starmerella bombicola | ||||
| Trebbiano | White still | 0.6 | Sequential inoculation | [111] |
| C. stellata (immobilised) + | ||||
| S. cerevisiae | ||||
| Chardonnay | White still | 0.7 | Sequential inoculation with | [72] |
| S. cerevisiae | ||||
| Verdicchio | White still | 1.6 | Sequential inoculation with S. | [14] |
| bombicola (immobilised) + | ||||
| S. cerevisiae | ||||
| Candida zemplinina/Starmerella bacillaris | ||||
| Nero d’Avola | Rosé still | 0.3 | Sequential inoculation with | [116] |
| S. cerevisiae | ||||
| Barbera | Red still | 0.3 | Sequential inoculation with | [117] |
| S. cerevisiae | ||||
| Barbera | Red still | 0.7 | Sequential inoculation with | [133] |
| S. cerevisiae | ||||
| Riesling | White still | 0.8 | Sequential inoculation with | [47] |
| S. cerevisiae (aeration) | ||||
| Torulaspora delbrueckii | ||||
| Airén | White still | 0.3 | Sequential inoculation with | [134] |
| S. cerevisiae | ||||
| Corvina, Rondinella, Corvinone | Red still | 0.45 | Sequential inoculation with | [135] |
| S. cerevisiae (aeration) | ||||
| Tempranillo | Red still | 0.5 | Sequential inoculation with | [130] |
| S. cerevisiae | ||||
| Chardonnay | White still | 0.9–1.0 | Sequential inoculation with | [43] |
| S. cerevisiae (aeration) | ||||
| Chemically defined grape juice medium | – | 1.5 | Sequential inoculation with | [56] |
| S. cerevisiae (aeration) | ||||
| Malvasia/Viura | White still | 0.5 | Sequential inoculation with | [99] |
| S. cerevisiae (aeration) | ||||
| Zygosaccharomyces bailii | ||||
| Chardonnay | White still | 1.0 | Sequential inoculation with | [43] |
| S. cerevisiae (aeration) | ||||
| Chemically defined grape juice medium | – | 2.0 | Sequential inoculation with | [56] |
| S. cerevisiae (aeration) | ||||
| Pichia kluvyeri | ||||
| Riesling | White still | 0.25 | Sequential inoculation with | [136] |
| S. cerevisiae | ||||
| Riesling | White still | 3.0 | Sequential inoculation with | [47] |
| S. cerevisiae (aeration) | ||||
| Pichia guilliermodii | ||||
| Riesling | White still | 2.0 | Sequential inoculation with | [47] |
| S. cerevisiae (aeration) | ||||
| Hanseniaspora uvarum | ||||
| Pinotage | Red still | 0.8 | Sequential inoculation with | [137] |
| S. cerevisiae | ||||
| Synthetic grape juice | – | 0.8–1.0 | Sequential inoculation | [14] |
| H. osmophila (immobilised)+ | ||||
| S. cerevisiae | ||||
| Verdicchio | White still | 1.0–1.2 | Sequential inoculation | [14] |
| H. osmophila (immobilised)+ | ||||
| S. cerevisiae | ||||
| Sauvignon blanc | White still | 1.3 | Sequential inoculation with | [137] |
| S. cerevisiae | ||||
| Hanseniaspora opuntiae | ||||
| Pinotage | Red still | 0.6 | Sequential inoculation with | [137] |
| S. cerevisiae | ||||
| Sauvignon blanc | White still | 1.3 | Sequential inoculation with | [137] |
| S. cerevisiae | ||||
| Hanseniaspora osmophila | ||||
| Synthetic grape juice | – | 0.8–1.3 | Sequential inoculation | [14] |
| H. osmophila (immobilised)+ | ||||
| S. cerevisiae | ||||
| Verdicchio | White still | 1 | Sequential inoculation | [14] |
| H. osmophila (immobilised)+ | ||||
| S. cerevisiae | ||||
| Saccharomyces uvarum | ||||
| Shiraz | Red still | 0.8 | Sequential inoculation with | [93] |
| S. cerevisiae | ||||
| Merlot | Red still | 1.7 | Pure inoculation | [50] |
| Grape Variety | Wine Style | Glycerol Concentration (g/L) | Method of Detection | Reference |
|---|---|---|---|---|
| Torulaspora delbrueckii | ||||
| Tempranillo | Red still | * 8.6–8.9 | Enzymatically with MIURA One oenological analyser | [129] |
| Chemically defined grape juice medium | N/A | 9.3 | HPLC | [56] |
| Tempranillo | Red still | 6.7 | Y15 enzymatic analyser | [125] |
| Viura/Macabeo | White still | 4.1 | Enzymatically with MIURA One oenological analyser | [128] |
| Hanseniaspora uvarum | ||||
| Negromaro | Red still | 5.5 | HPLC | [144] |
| Chemically defined grape juice medium | N/A | 3.5 | HPLC | [56] |
| Metschnikowia pulcherrima | ||||
| Tempranillo | Red still | * 8.2–8.6 | Enzymatically with MIURA One oenological analyser | [129] |
| Viura/Macabeo | White still | 4.8 | Enzymatically with MIURA One oenological analyser | [128] |
| Chardonnay | White still | 5.5–7.8 | HPLC | [43] |
| Schizosaccharomyces pombe‡ | ||||
| Airén | Sparkling wine | 4.7 | Y15 enzymatic analyser | [145] |
| Tempranillo | Red sparkling wine | 5.0 | Y15 enzymatic analyser | [145] |
| Schizosaccharomyces japonicus | ||||
| Trebbiano | White still | 15.9 | HPLC | [132] |
| Saccharomycodes ludwigii‡ | ||||
| Airén | Sparkling wine | 5.0 | Y15 enzymatic analyser | [145] |
| Tempranillo | Red sparkling wine | 5.1 | Y15 enzymatic analyser | [145] |
| Saccharomyces uvarum‡ | ||||
| Synthetic grape must | N/A | 5.2 | HPLC | [12] |
| Cabernet franc | Red wine | 10–12 | Enzymatically using Megazyme International assay kit | [146] |
| Lachancea thermotolerans | ||||
| Tempranillo | Red still | * 8.2–8.3 | Enzymatically with MIURA One oenological analyser | [129] |
| Viura/Macabeo | White still | 4.7 | Enzymatically with MIURA One oenological analyser | [128] |
| Starmerella bacillaris | ||||
| Synthetic grape must | N/A | * 7.7–8.2 | HPLC | [128] |
| Williopsis pratensis | ||||
| Tempranillo | Red still | 8.0 | Enzymatically with MIURA One oenological analyser | [129] |
| Zygosaccharomyces bailii | ||||
| Tempranillo | Red still | 7.8 | Enzymatically with MIURA One oenological analyser | [129] |
| Viura/Macabeo | White still | 5.6 | Enzymatically with MIURA One oenological analyser | [128] |
| Candida vini | ||||
| Tempranillo | Red still | 7.9 | Enzymatically with MIURA One oenological analyser | [129] |
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scanes, K.; Hohrnann, S.; Prior, B. Glycerol production by the yeast Saccharomyces cerevisiae and its relevance to wine: A review. S. Afr. J. Enol. Vitic. 1998, 19, 17–24. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Francesca, N.; Gaglio, R.; Alfonzo, A.; Settanni, L.; Corona, O.; Mazzei, P.; Romano, R.; Piccolo, A.; Moschetti, G. The wine: Typicality or mere diversity? The effect of spontaneous fermentations and biotic factors on the characteristics of wine. Agric. Agric. Sci. Procedia 2016, 8, 769–773. [Google Scholar] [CrossRef][Green Version]
- Vigentini, I.; Barrera Cardenas, S.; Valdetara, F.; Faccincani, M.; Panont, C.A.; Picozzi, C.; Foschino, R. Use of native yeast strains for in-bottle fermentation to face the uniformity in sparkling wine production. Front. Microbiol. 2017, 8, 1225. [Google Scholar] [CrossRef]
- Ciani, M.; Canonico, L.; Oro, L.; Comitini, F. Footprint of Nonconventional Yeasts and Their Contribution in Alcoholic Fermentations. In Biotechnological Progress and Beverage Consumption; Academic Press: Cambridge, MA, USA, 2020; pp. 435–465. [Google Scholar]
- Ciani, M.; Capece, A.; Comitini, F.; Canonico, L.; Siesto, G.; Romano, P. Yeast interactions in inoculated wine fermentation. Front. Microbiol. 2016, 7, 555. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Cabellos, J.M.; Arroyo, T. Sequential non-Saccharomyces and Saccharomyces cerevisiae fermentations to reduce the alcohol content in wine. Fermentation 2020, 6, 60. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial resources and enological significance: Opportunities and benefits. Front. Microbiol. 2017, 8, 995. [Google Scholar] [CrossRef]
- Maturano, Y.P.; Mestre, M.V.; Kuchen, B.; Toro, M.E.; Mercado, L.A.; Vazquez, F.; Combina, M. Optimization of fermentation-relevant factors: A strategy to reduce ethanol in red wine by sequential culture of native yeasts. Int. J. Food Microbiol. 2019, 289, 40–48. [Google Scholar] [CrossRef]
- Alonso-del-Real, J.; Lairón-Peris, M.; Barrio, E.; Querol, A. Effect of temperature on the prevalence of Saccharomyces non-cerevisiae species against a S. cerevisiae wine strain in wine fermentation: Competition, physiological fitness, and influence in final wine composition. Front. Microbiol. 2017, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Oro, L.; Ciani, M. Sequential fermentation with selected immobilized non-Saccharomyces yeast for reduction of ethanol content in wine. Front. Microbiol. 2016, 7, 278. [Google Scholar] [CrossRef]
- Pretorius, I.S. Conducting wine symphonics with the aid of yeast genomics. Beverages 2016, 2, 36. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. The influence of non-Saccharomyces species on wine fermentation quality parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef]
- Pérez-Torrado, R.; Barrio, E.; Querol, A. Alternative yeasts for winemaking: Saccharomyces non-cerevisiae and its hybrids. Crit. Rev. Food Sci. Nutr. 2018, 58, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A. Use of nonconventional yeasts for modulating wine acidity. Fermentation 2019, 5, 27. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of non-Saccharomyces yeasts to wine freshness. A review. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef]
- Ivit, N.N.; Kemp, B. The impact of non-Saccharomyces yeast on traditional method sparkling wine. Fermentation 2018, 4, 73. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Loira, I.; Del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Influence of Saccharomyces and non-Saccharomyces yeasts in the formation of pyranoanthocyanins and polymeric pigments during red wine making. Molecules 2019, 24, 4490. [Google Scholar] [CrossRef]
- Jackson, R. Fermentation. In Wine Science: Principles and Applications (Food Science and Technology); Academic Press: Amsterdam, The Netherlands, 2014; Chapter 7; p. 493. [Google Scholar]
- Ickes, C.M.; Cadwallader, K.R. Effects of ethanol on flavor perception in alcoholic beverages. Chemosens. Percept. 2017, 10, 119–134. [Google Scholar] [CrossRef]
- King, E.S.; Dunn, R.L.; Heymann, H. The influence of alcohol on the sensory perception of red wines. Food Qual. Pref. 2013, 28, 235–243. [Google Scholar] [CrossRef]
- Nurgel, C.; Pickering, G. Modeling of sweet, bitter and irritant sensations and their interactions elicited by model ice wines. J. Sens. Stud. 2006, 21, 505–519. [Google Scholar] [CrossRef]
- Fischer, U.; Noble, C.A. The effect of ethanol, catechin concentration, and ph on sourness and bitterness of wine. Am. J. Enol. Vitic. 1994, 45, 6–10. [Google Scholar]
- Gawel, R.; Sluyter, S.V.; Waters, E.J. The effects of ethanol and glycerol on the body and other sensory characteristics of Riesling wines. Aust. J. Grape Wine Res. 2007, 13, 38–45. [Google Scholar] [CrossRef]
- Saha, B.; Longo, R.; Torley, P.; Saliba, A.; Schmidtke, L. SPME method optimized by Box-Behnken design for impact odorants in reduced alcohol wines. Foods 2018, 7, 127. [Google Scholar] [CrossRef]
- OIV. Oiv International Standard for the Labelling of Wines 2015; OIV: Paris, France, 2015. [Google Scholar]
- Godden, P.; Wilkes, E.; Johnson, D. Trends in the composition of Australian wine 1984–2014. Aust. J. Grape Wine Res. 2015, 21, 741–753. [Google Scholar] [CrossRef]
- Alston, J.M.; Fuller, K.B.; Lapsley, J.T.; Soleas, G.; Tumber, K.P. Splendide mendax: False label claims about high and rising alcohol content of wine. J. Wine Econ. 2015, 10, 275–313. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Darriet, P. The impact of climate change on viticulture and wine quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef]
- Williamson, P.O.; Robichaud, J.; Francis, I.L. Comparison of Chinese and Australian consumers’ liking responses for red wines. Aust. J. Grape Wine Res. 2012, 18, 256–267. [Google Scholar] [CrossRef]
- Bindon, K.; Varela, C.; Kennedy, J.; Holt, H.; Herderich, M. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet sauvignon 1. Grape and wine chemistry. Food Chem 2013, 138, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Jordão, A.; Vilela, A.; Cosme, F. From sugar of grape to alcohol of wine: Sensorial impact of alcohol in wine. Beverages 2015, 1, 292–310. [Google Scholar] [CrossRef]
- Bucher, T.; Deroover, K.; Stockley, C. Low-alcohol wine: A narrative review on consumer perception and behaviour. Beverages 2018, 4, 82. [Google Scholar] [CrossRef]
- Saliba, A.; Ovington, L.; Moran, C. Consumer demand for low-alcohol wine in an Australian sample. Int. J. Wine Res. 2013, 5, 1–8. [Google Scholar] [CrossRef]
- Sharma, A.; Sinha, K.; Vandenberg, B. Pricing as a means of controlling alcohol consumption. Br. Med Bull. 2017, 123, 149–158. [Google Scholar] [CrossRef]
- Annunziata, A.; Pomarici, E.; Vecchio, R.; Mariani, A. Do consumers want more nutritional and health information on wine labels? Insights from the eu and USA. Nutrients 2016, 8, 416. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Conditions of Yeast Development. In Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 79–114. [Google Scholar]
- Zapparoli, G.; Tosi, E.; Azzolini, M.; Vagnoli, P.; Krieger, S. Bacterial inoculation strategies for the achievement of malolactic fermentation in high-alcohol wines. S. Afr. J. Enol. Vitic. 2009, 30, 49–55. [Google Scholar] [CrossRef]
- Goold, H.D.; Kroukamp, H.; Williams, T.C.; Paulsen, I.T.; Varela, C.; Pretorius, I.S. Yeast’s balancing act between ethanol and glycerol production in low-alcohol wines. Microb. Biotechnol. 2017, 10, 264–278. [Google Scholar] [CrossRef]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef]
- Longo, R.; Blackman, J.W.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Changes in volatile composition and sensory attributes of wines during alcohol content reduction. J. Sci. Food Agric. 2017, 97, 8–16. [Google Scholar] [CrossRef]
- Longo, R.; Blackman, J.W.; Antalick, G.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. A comparative study of partial dealcoholisation versus early harvest: Effects on wine volatile and sensory profiles. Food Chem. 2018, 261, 21–29. [Google Scholar] [CrossRef]
- Varela, C.; Dry, P.; Kutyna, D.; Francis, I.; Henschke, P.; Curtin, C.; Chambers, P. Strategies for reducing alcohol concentration in wine. Aust. J. Grape Wine Res. 2015, 21, 670–679. [Google Scholar] [CrossRef]
- Röcker, J.; Strub, S.; Ebert, K.; Grossmann, M. Usage of different aerobic non-Saccharomyces yeasts and experimental conditions as a tool for reducing the potential ethanol content in wines. Eur. Food Res. Technol. 2016, 242, 2051–2070. [Google Scholar] [CrossRef]
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues, A.J.; Gonzalez, R. Non-conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 2016, 7, 642. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Barker, A.; Tran, T.; Borneman, A.; Curtin, C. Sensory profile and volatile aroma composition of reduced alcohol Merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. J. Food Microbiol. 2017, 252, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F. Use of non-Saccharomyces yeasts in red winemaking. In Red Wine Technology; Antonio, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 51–68. [Google Scholar]
- Ciani, M.; Comitini, F. Influence of temperature and oxygen concentration on the fermentation behaviour of Candida stellata in mixed fermentation with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2006, 22, 619–623. [Google Scholar] [CrossRef]
- Alexander, M.; Jeffries, T. Respiratory efficiency and metabolite partitioning as regulatory phenomena in yeasts. Enzym. Microb. Technol. 1990, 12, 2–19. [Google Scholar] [CrossRef]
- De Deken, R. The crabtree effect: A regulatory system in yeast. Microbiology 1966, 44, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Galli, E.; Ciani, E.; Comitini, F.; Ciani, M. Exploitation of three non-conventional yeast species in the brewing process. Microorganisms 2019, 7, 11. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.A.; Curtin, C.; Varela, C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015, 205, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Rojas, V.; Quirós, M.; Gonzalez, R. The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl. Microbiol. Biotechnol. 2015, 99, 3993–4003. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Quirós, M.; Morales, P. Yeast respiration of sugars by non-Saccharomyces yeast species: A promising and barely explored approach to lowering alcohol content of wines. Trends Food Sci. Technol. 2013, 29, 55–61. [Google Scholar] [CrossRef]
- Quirós, M.; Rojas, V.; Gonzalez, R.; Morales, P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014, 181, 85–91. [Google Scholar] [CrossRef]
- Mestre Furlani, M.V.; Maturano, Y.P.; Combina, M.; Mercado, L.A.; Toro, M.E.; Vazquez, F. Selection of non-Saccharomyces yeasts to be used in grape musts with high alcoholic potential: A strategy to obtain wines with reduced ethanol content. FEMS Yeast Res. 2017, 17. [Google Scholar] [CrossRef]
- Ballester-Tomás, L.; Prieto, J.A.; Gil, J.V.; Baeza, M.; Randez-Gil, F. The Antarctic yeast Candida sake: Understanding cold metabolism impact on wine. Int. J. Food Microbiol. 2017, 245, 59–65. [Google Scholar] [CrossRef]
- Mbuyane, L.L.; de Kock, M.; Bauer, F.F.; Divol, B. Torulaspora delbrueckii produces high levels of c5 and c6 polyols during wine fermentations. FEMS Yeast Res. 2018, 18, foy084. [Google Scholar] [CrossRef]
- Puškaš, V.S.; Miljić, U.D.; Djuran, J.J.; Vučurović, V.M. The aptitude of commercial yeast strains for lowering the ethanol content of wine. Food Sci. Nutr. 2020, 8, 1489–1498. [Google Scholar] [CrossRef]
- Nieuwoudt, H.; Prior, B.; Pretorius, L.; Bauer, F. Glycerol in South African table wines: An assessment of its relationship to wine quality. S. Afr. J. Enol. Vitic. 2002, 23, 22–30. [Google Scholar] [CrossRef]
- Nissen, T.L.; Hamann, C.W.; Kielland-Brandt, M.C.; Nielsen, J.; Villadsen, J. Anaerobic and aerobic batch cultivations of Saccharomyces cerevisiae mutants impaired in glycerol synthesis. Yeast 2000, 16, 463–474. [Google Scholar] [CrossRef]
- Roustan, J.; Sablayrolles, J. Impact of the addition of electron acceptors on the by-products of alcoholic fermentation. Enzym. Microb. Technol. 2002, 31, 142–152. [Google Scholar] [CrossRef]
- Rantsiou, K.; Dolci, P.; Giacosa, S.; Torchio, F.; Tofalo, R.; Torriani, S.; Suzzi, G.; Rolle, L.; Cocolin, L. Candida zemplinina can reduce acetic acid produced by Saccharomyces cerevisiae in sweet wine fermentations. Appl. Environ. Microbiol. 2012, 78, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Domizio, P.; Liu, Y.; Bisson, L.; Barile, D. Cell wall polysaccharides released during the alcoholic fermentation by Schizosaccharomyces pombe and S. Japonicus: Quantification and characterization. Food Microbiol. 2017, 61, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Di Gianvito, P.; Perpetuini, G.; Tittarelli, F.; Schirone, M.; Arfelli, G.; Piva, A.; Patrignani, F.; Lanciotti, R.; Olivastri, L.; Suzzi, G. Impact of Saccharomyces cerevisiae strains on traditional sparkling wines production. Food Res. Int. 2018, 109, 552–560. [Google Scholar] [CrossRef]
- Longo, R.; Blackman, J.W.; Antalick, G.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Volatile and sensory profiling of Shiraz wine in response to alcohol management: Comparison of harvest timing versus technological approaches. Food Res. Int. 2018, 109, 561–571. [Google Scholar] [CrossRef]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Van der Westhuizen, T.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co-fermented with commercial non-Saccharomyces inocula. Aust. J. Grape Wine Res. 2018, 24, 166–180. [Google Scholar] [CrossRef]
- Soden, A.; Francis, I.; Oakey, H.; Henschke, P. Effects of co-fermentation with Candida stellata and Saccharomyces cerevisiae on the aroma and composition of chardonnay wine. Aust. J. Grape Wine Res. 2000, 6, 21–30. [Google Scholar] [CrossRef]
- Eglinton, J.M.; Heinrich, A.J.; Pollnitz, A.P.; Langridge, P.; Henschke, P.A.; de Barros Lopes, M. Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ald6 aldehyde dehydrogenase gene. Yeast 2002, 19, 295–301. [Google Scholar] [CrossRef]
- Li, H.; Du, G.; Li, H.-L.; Wang, H.-L.; Yan, G.-L.; Zhan, J.-C.; Huang, W.-D. Physiological response of different wine yeasts to hyperosmotic stress. Am. J. Enol. Vitic. 2010, 61, 529–535. [Google Scholar] [CrossRef]
- Pérez-Nevado, F.; Albergaria, H.; Hogg, T.; Girio, F. Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 2006, 108, 336–345. [Google Scholar]
- Shekhawat, K.; Bauer, F.F.; Setati, M.E. Impact of oxygenation on the performance of three non-Saccharomyces yeasts in co-fermentation with Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 2479–2491. [Google Scholar] [CrossRef]
- Prior, B.; Toh, T.; Jolly, N.; Baccari, C.; Mortimer, R. Impact of yeast breeding for elevated glycerol production on fermentative activity and metabolite formation in Chardonnay wine. S. Afr. J. Enol. Vitic. 2000, 21, 92–99. [Google Scholar] [CrossRef]
- Silva, S.; Ramón-Portugal, F.; Andrade, P.; Abreu, S.; de Fatima Texeira, M.; Strehaiano, P. Malic acid consumption by dry immobilized cells of Schizosaccharomyces pombe. Am. J. Enol. Vitic. 2003, 54, 50–55. [Google Scholar]
- Benito, Á.; Calderón, F.; Benito, S. The combined use of Schizosaccharomyces pombe and Lachancea thermotolerans—Effect on the anthocyanin wine composition. Molecules 2017, 22, 739. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality and composition of Airén wines fermented by sequential inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, F.; Morassut, M.; Moruno, E.G.; Di Stefano, R. Influence of yeast strain on Ochratoxin A content during fermentation of white and red must. Food Microbiol. 2006, 23, 411–417. [Google Scholar] [CrossRef]
- Escott, C.; Del Fresno, J.M.; Loira, I.; Morata, A.; Suárez-Lepe, J.A. Zygosaccharomyces rouxii: Control strategies and applications in food and winemaking. Fermentation 2018, 4, 69. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Palomero, F.; González, C.; Suárez-Lepe, J.A. Schizosaccharomyces pombe: A promising biotechnology for modulating wine composition. Fermentation 2018, 4, 70. [Google Scholar] [CrossRef]
- Benito, S. The impacts of Schizosaccharomyces on winemaking. Appl. Microbiol. Biotechnol. 2019, 103, 4291–4312. [Google Scholar] [CrossRef]
- Suárez-Lepe, J.; Palomero, F.; Benito, S.; Calderón, F.; Morata, A. Oenological versatility of Schizosaccharomyces spp. Eur. Food Res. Technol. 2012, 235, 375–383. [Google Scholar] [CrossRef]
- Palomero, F.; Morata, A.; Benito, S.; Calderón, F.; Suárez-Lepe, J. New genera of yeasts for over-lees aging of red wine. Food Chem. 2009, 112, 432–441. [Google Scholar] [CrossRef]
- Benito, S.; Palomero, F.; Morata, A.; Calderon, F.; Palmero, D.; Suárez-Lepe, J. Physiological features of Schizosaccharomyces pombe of interest in making of white wines. Eur. Food Res. Technol. 2013, 236, 29–36. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Morata, A.; Loira, I.; Bañuelos, M.A.; Escott, C.; Benito, S.; Chamorro, C.G.; Suárez-Lepe, J.A. Use of non-Saccharomyces in single-culture, mixed and sequential fermentation to improve red wine quality. Eur. Food Res. Technol. 2017, 243, 2175–2185. [Google Scholar] [CrossRef]
- Miljić, U.; Puškaš, V.; Vučurović, V.; Muzalevski, A. Fermentation characteristics and aromatic profile of plum wines produced with indigenous microbiota and pure cultures of selected yeast. J. Food Sci. 2017, 82, 1443–1450. [Google Scholar] [CrossRef]
- Minnaar, P.; Jolly, N.; Paulsen, V.; Du Plessis, H.; Van Der Rijst, M. Schizosaccharomyces pombe and Saccharomyces cerevisiae yeasts in sequential fermentations: Effect on phenolic acids of fermented kei-apple (Dovyalis caffra L.) juice. Int. J. Food Microbiol. 2017, 257, 232–237. [Google Scholar] [CrossRef]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.-J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast–yeast interactions revealed by aromatic profile analysis of sauvignon blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Contreras, A.; Curtin, C.; Varela, C. Yeast population dynamics reveal a potential collaboration between Metschnikowia pulcherrima and Saccharomyces uvarum for the production of reduced alcohol wines during Shiraz fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 1885–1895. [Google Scholar] [CrossRef]
- Duarte, F.L.; Egipto, R.; Baleiras-Couto, M.M. Mixed fermentation with Metschnikowia pulcherrima using different grape varieties. Fermentation 2019, 5, 59. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in wine biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef]
- Combina, M.; Elía, A.; Mercado, L.; Catania, C.; Ganga, A.; Martinez, C. Dynamics of indigenous yeast populations during spontaneous fermentation of wines from Mendoza, Argentina. Int. J. Food Microbiol. 2005, 99, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and phenotypic characterization of Metschnikowia pulcherrima strains from douro wine region. Fermentation 2018, 4, 8. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Metschnikowia pulcherrima Selected Strain for Ethanol Reduction in Wine: Influence of Cell Immobilization and Aeration Condition. Foods 2019, 8, 378. [Google Scholar] [CrossRef]
- Tronchoni, J.; Curiel, J.A.; Sáenz-Navajas, M.P.; Morales, P.; De-La-Fuente-Blanco, A.; Fernández-Zurbano, P.; Ferreira, V.; Gonzalez, R. Aroma profiling of an aerated fermentation of natural grape must with selected yeast strains at pilot scale. Food Microbiol. 2018, 70, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Alcohols and Other Volatile Compounds. In Handbook of Enology, Volume 2: The Chemistry of Wine Stabilization and Treatments, 2nd ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2006; pp. 51–64. [Google Scholar]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Balikci, E.K.; Tanguler, H.; Jolly, N.P.; Erten, H. Influence of Lachancea thermotolerans on cv. Emir wine fermentation. Yeast 2016, 33, 313–321. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans applications in wine technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Porter, T.J.; Divol, B.; Setati, M.E. Lachancea yeast species: Origin, biochemical characteristics and oenological significance. Food Res. Int. 2019, 119, 378–389. [Google Scholar] [CrossRef]
- Vilela, A. Lachancea thermotolerans, the Non-Saccharomyces Yeast that Reduces the Volatile Acidity of Wines. Fermentation 2018, 4, 56. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological acidification during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Cabellos, J.M.; Arroyo, T. Advances in the study of Candida stellata. Fermentation 2018, 4, 74. [Google Scholar] [CrossRef]
- Csoma, H.; Sipiczki, M. Taxonomic reclassification of Candida stellata strains reveals frequent occurrence of Candida zemplinina in wine fermentation. FEMS Yeast Res. 2008, 8, 328–336. [Google Scholar] [CrossRef]
- Gao, C.; Fleet, G. The effects of temperature and pH on the ethanol tolerance of the wine yeasts, Saccharomyces cerevisiae, Candida stellata and Kloeckera apiculata. J. Appl. Bacteriol. 1988, 65, 405–409. [Google Scholar] [CrossRef]
- Ferraro, L.; Fatichenti, F.; Ciani, M. Pilot scale vinification process using immobilized Candida stellata cells and Saccharomyces cerevisiae. Process Biochem. 2000, 35, 1125–1129. [Google Scholar] [CrossRef]
- Ciani, M.; Ferraro, L. Enhanced glycerol content in wines made with immobilized Candida stellata cells. Appl. Environ. Microbiol. 1996, 62, 128–132. [Google Scholar] [CrossRef]
- Wang, C.; Esteve-Zarzoso, B.; Cocolin, L.; Mas, A.; Rantsiou, K. Viable and culturable populations of Saccharomyces cerevisiae, Hanseniaspora uvarum and Starmerella bacillaris (synonym Candida zemplinina) during Barbera must fermentation. Food Res. Int. 2015, 78, 195–200. [Google Scholar] [CrossRef]
- Magyar, I.; Tóth, T. Comparative evaluation of some oenological properties in wine strains of Candida stellata, Candida zemplinina, Saccharomyces uvarum and Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 94–100. [Google Scholar] [CrossRef]
- Englezos, V.; Giacosa, S.; Rantsiou, K.; Rolle, L.; Cocolin, L. Starmerella bacillaris in winemaking: Opportunities and risks. Curr. Opin. Food Sci. 2017, 17, 30–35. [Google Scholar] [CrossRef]
- Di Maio, S.; Genna, G.; Gandolfo, V.; Amore, G.; Ciaccio, M.; Oliva, D. Presence of Candida zemplinina in Sicilian musts and selection of a strain for wine mixed fermentations. S. Afr. J. Enol. Vitic. 2012, 33, 80–87. [Google Scholar] [CrossRef]
- Rolle, L.; Englezos, V.; Torchio, F.; Cravero, F.; Río Segade, S.; Rantsiou, K.; Giacosa, S.; Gambuti, A.; Gerbi, V.; Cocolin, L. Alcohol reduction in red wines by technological and microbiological approaches: A comparative study. Aust. J. Grape Wine Res. 2017, 24, 62–74. [Google Scholar] [CrossRef]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef] [PubMed]
- Loira, I.; Vejarano, R.; Bañuelos, M.; Morata, A.; Tesfaye, W.; Uthurry, C.; Villa, A.; Cintora, I.; Suárez-Lepe, J. Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. LWT-Food Sci. Technol. 2014, 59, 915–922. [Google Scholar] [CrossRef]
- Arslan, E.; Çelik, Z.D.; Cabaroğlu, T. Effects of pure and mixed autochthonous Torulaspora delbrueckii and Saccharomyces cerevisiae on fermentation and volatile compounds of narince wines. Foods 2018, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii for secondary fermentation in sparkling wine production. Food Microbiol. 2018, 74, 100–106. [Google Scholar] [CrossRef]
- Ramírez, M.; Velázquez, R. The yeast Torulaspora delbrueckii: An interesting but difficult-to-use tool for winemaking. Fermentation 2018, 4, 94. [Google Scholar] [CrossRef]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef]
- Taillandier, P.; Lai, Q.P.; Julien-Ortiz, A.; Brandam, C. Interactions between Torulaspora delbrueckii and Saccharomyces cerevisiae in wine fermentation: Influence of inoculation and nitrogen content. World J. Microbiol. Biotechnol. 2014, 30, 1959–1967. [Google Scholar] [CrossRef]
- Mecca, D.; Benito, S.; Beisert, B.; Brezina, S.; Fritsch, S.; Semmler, H.; Rauhut, D. Influence of nutrient supplementation on Torulaspora delbrueckii wine fermentation aroma. Fermentation 2020, 6, 35. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A. Wine aromatic compound production and fermentative behaviour within different non-Saccharomyces species and clones. J. Appl. Microbiol. 2018, 124, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Viana, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aroma evolution throughout alcoholic fermentation sequentially inoculated with non-Saccharomyces/Saccharomyces yeasts. Food Res. Int. 2018, 112, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in varietal thiol (3-SH and 4-MSP) release in wine sequential fermentations. Int. J. Food Microbiol. 2017, 257, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.J.; Raimbourg, T.; Gonzalez, R.; Morales, P. Environmental factors influencing the efficacy of different yeast strains for alcohol level reduction in wine by respiration. LWT-Food Sci. Technol. 2016, 65, 1038–1043. [Google Scholar] [CrossRef]
- Domizio, P.; Lencioni, L.; Calamai, L.; Portaro, L.; Bisson, L.F. Evaluation of the yeast Schizosaccharomyces japonicus for use in wine production. Am. J. Enol. Vitic. 2018, 69, 266–277. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Starmerella bacillaris and Saccharomyces cerevisiae mixed fermentations to reduce ethanol content in wine. Appl. Microbiol. Biotechnol. 2016, 12, 5515–5526. [Google Scholar] [CrossRef]
- Izquierdo Canas, P.M.; Palacios Garcia, A.T.; Garcia Romero, E. Enhancement of flavour properties in wines using sequential inoculations of non-Saccharomyces (Hansenula and Torulaspora) and Saccharomyces yeast starter. VITIS-J. Grapevine Res. 2011, 50, 177–182. [Google Scholar]
- Azzolini, M.; Fedrizzi, B.; Tosi, E.; Finato, F.; Vagnoli, P.; Scrinzi, C.; Zapparoli, G. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur. Food Res. Technol. 2012, 235, 303–313. [Google Scholar] [CrossRef]
- Benito, A.; Hofmann, T.; Laier, M.; Lochbüler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Rossouw, D.; Bauer, F.F. Exploring the phenotypic space of non-Saccharomyces wine yeast biodiversity. Food Microbiol. 2016, 55, 32–46. [Google Scholar] [CrossRef]
- Benito, S. Combined use of Lachancea thermotolerans and Schizosaccharomyces pombe in winemaking: A review. Microorganisms 2020, 8, 655. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. Combined use of S. pombe and L. thermotolerans in winemaking. Beneficial effects determined through the study of wines’ analytical characteristics. Molecules 2016, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Benito, S. Mixed alcoholic fermentation of Schizosaccharomyces pombe and Lachancea thermotolerans and its influence on mannose-containing polysaccharides wine composition. AMB Express 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016, 209, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.; Alexandre, H.; Robillard, B.; Marchal, R. Effect of production phase on bottle-fermented sparkling wine quality. J. Agric. Food Chem. 2015, 63, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-López, F.N.; Pérez-Torrado, R.; Querol, A.; Barrio, E. Modulation of the glycerol and ethanol syntheses in the yeast Saccharomyces kudriavzevii differs from that exhibited by Saccharomyces cerevisiae and their hybrid. Food Microbiol. 2010, 27, 628–637. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, M.; Bleve, G.; Grieco, F.; Tristezza, M.; Tufariello, M. An optimized procedure for the enological selection of non-Saccharomyces starter cultures. Antonie Van Leeuwenhoek 2011, 99, 189–200. [Google Scholar] [CrossRef]
- Ivit, N.N.; Loira, I.; Morata, A.; Benito, S.; Palomero, F.; Suárez-Lepe, J.A. Making natural sparkling wines with non-Saccharomyces yeasts. Eur. Food Res. Technol. 2018, 244, 925–935. [Google Scholar] [CrossRef]
- Kelly, J.M.; van Dyk, S.A.; Dowling, L.K.; Pickering, G.J.; Kemp, B.; Inglis, D.L. Saccharomyces uvarum yeast isolate consumes acetic acid during fermentation of high sugar juice and juice with high starting volatile acidity. OENO ONE Int. J. Vine Wine Sci. 2020, 54, 199–211. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivit, N.N.; Longo, R.; Kemp, B. The Effect of Non-Saccharomyces and Saccharomyces Non-Cerevisiae Yeasts on Ethanol and Glycerol Levels in Wine. Fermentation 2020, 6, 77. https://doi.org/10.3390/fermentation6030077
Ivit NN, Longo R, Kemp B. The Effect of Non-Saccharomyces and Saccharomyces Non-Cerevisiae Yeasts on Ethanol and Glycerol Levels in Wine. Fermentation. 2020; 6(3):77. https://doi.org/10.3390/fermentation6030077
Chicago/Turabian StyleIvit, Nedret Neslihan, Rocco Longo, and Belinda Kemp. 2020. "The Effect of Non-Saccharomyces and Saccharomyces Non-Cerevisiae Yeasts on Ethanol and Glycerol Levels in Wine" Fermentation 6, no. 3: 77. https://doi.org/10.3390/fermentation6030077
APA StyleIvit, N. N., Longo, R., & Kemp, B. (2020). The Effect of Non-Saccharomyces and Saccharomyces Non-Cerevisiae Yeasts on Ethanol and Glycerol Levels in Wine. Fermentation, 6(3), 77. https://doi.org/10.3390/fermentation6030077