The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds †
Abstract
1. Introduction
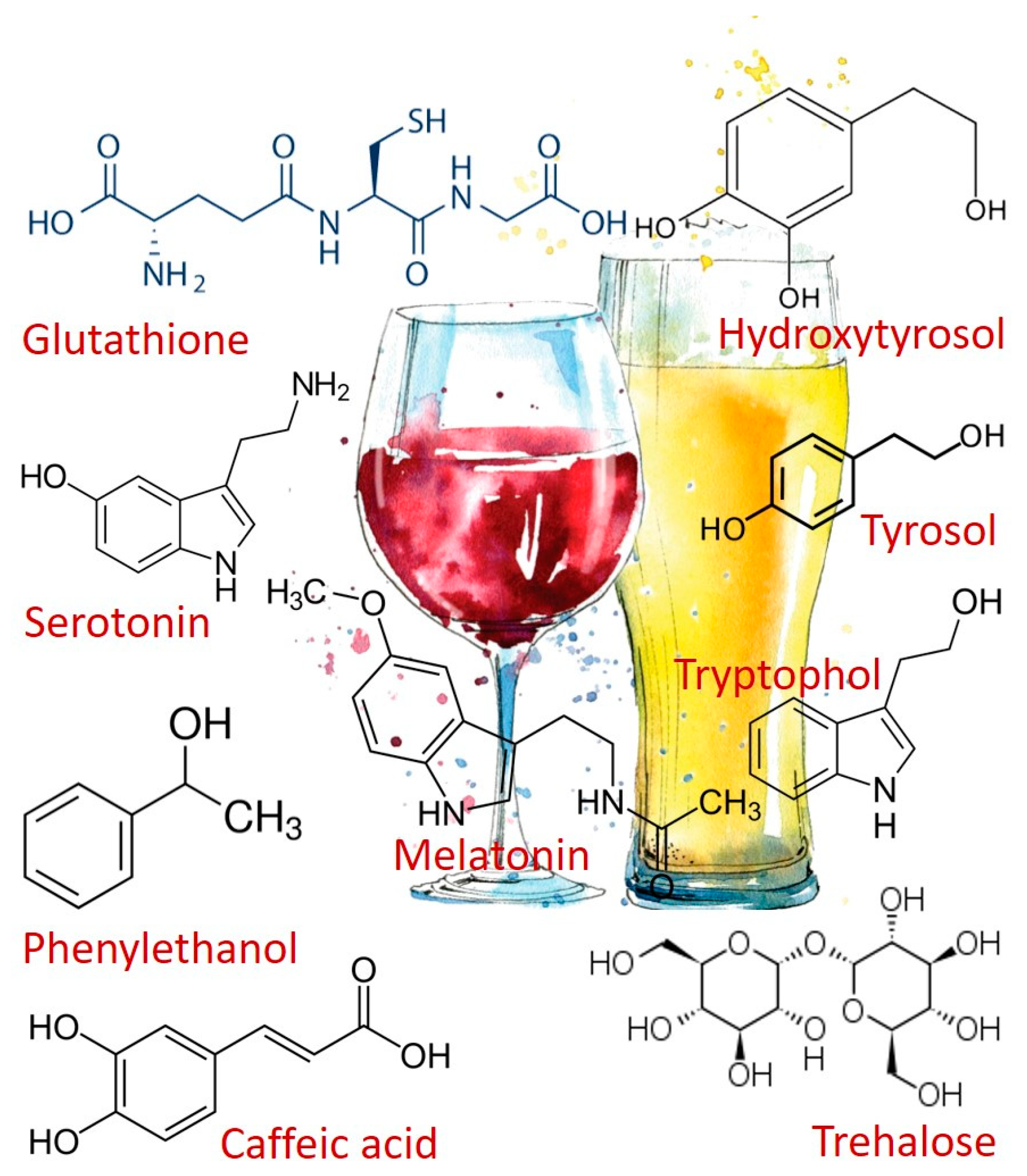
2. Alcoholic Beverages Consumption and Health-Promoting Compounds
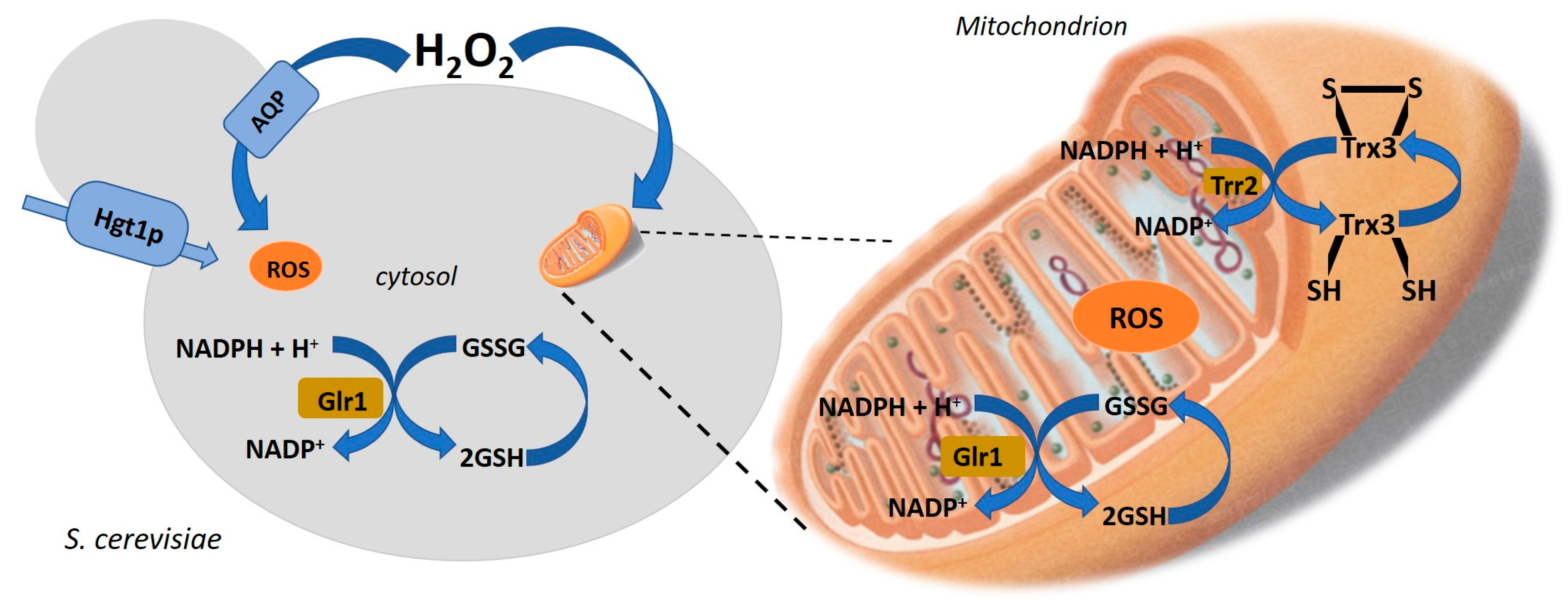
3. Mechanisms of Microbial Resistance to Environment Changes that Produce Health-Promoting Compounds
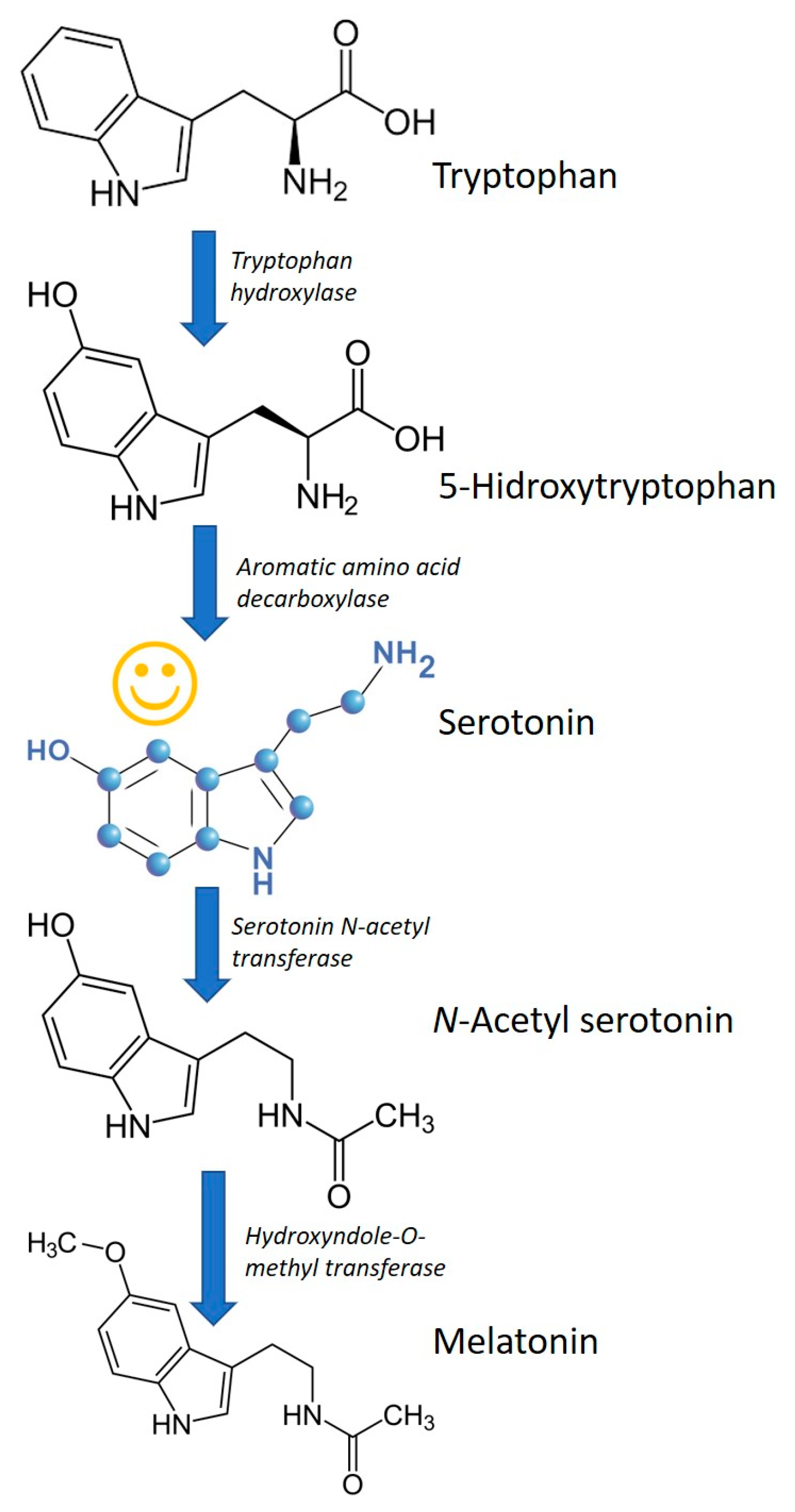
4. Melatonin and Other Tryptophan Metabolites
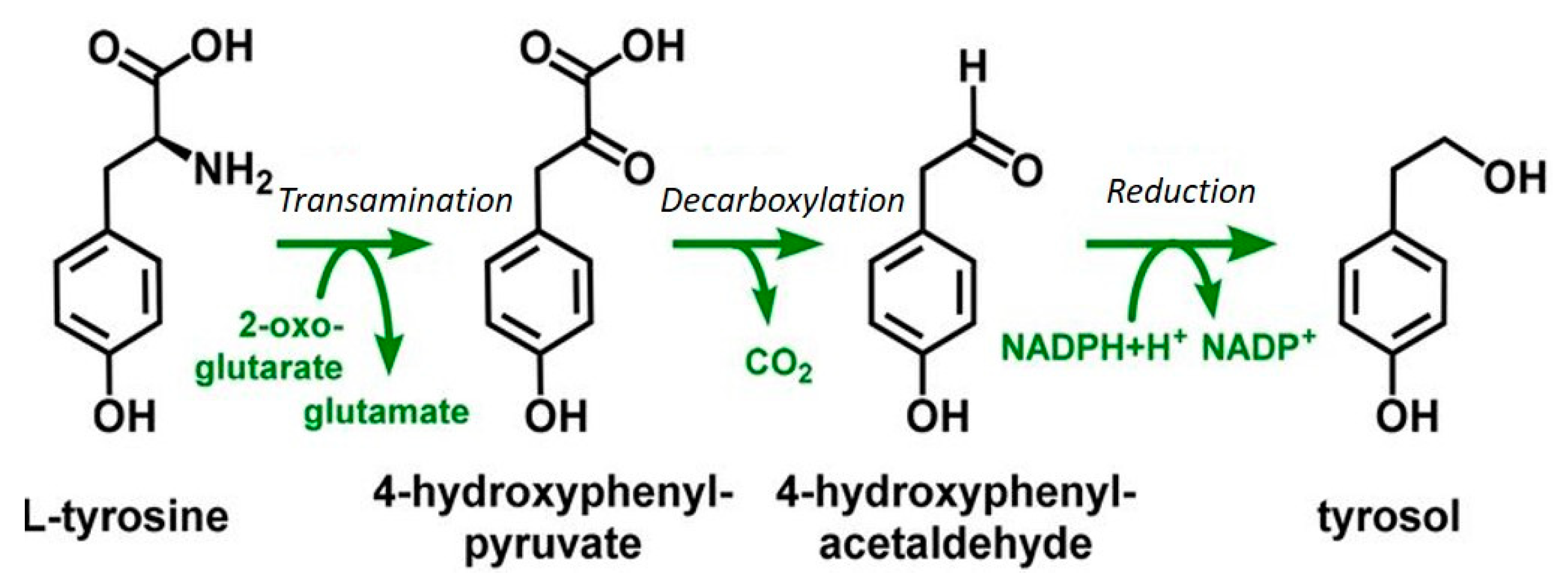
5. Fusel Alcohols Formed Via the Ehrlich Pathway
6. Fermented Beverages Containing Probiotics
7. Final Remarks
Funding
Conflicts of Interest
References
- Manchester, K.L. Louis Pasteur (1822–1895)—chance and the prepared mind. Trends Biotechnol. 1995, 13, 511–515. [Google Scholar] [CrossRef]
- Truninger, M. The historical development of industrial and domestic food technologies. In The Handbook of Food Research; Murcott, A., Belasco, W., Jackson, P., Eds.; Bloomsbury: London, UK, 2013; pp. 82–102. [Google Scholar]
- Mishra, S.S.; Ray, R.C.; Panda, S.K.; Montet, D. Technological Innovations in Processing of Fermented Foods. An Overview. In Fermented Foods, Part II: Technological Intervention, 1st ed.; Ray, R.C., Montet, D., Eds.; Taylors and Francis, CRC Press: London, UK, 2017; p. 525. [Google Scholar]
- Ray, R.C.; Joshi, V.K. Fermented Foods: Past, present, and future scenario. In Microorganisms and Fermentation of Traditional Foods; Ray, R.C., Montet, D., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–36. [Google Scholar]
- Ray, R.C. Fermented foods in health-related issues. Int. J. Food Ferment. Technol. 2013, 3, 1. [Google Scholar]
- Ghosh, J.S. Solid state fermentation and food processing: a short review. J. Nutr. Food Sci. 2015, 6, 453. [Google Scholar] [CrossRef]
- Blaylock, J.; Smallwood, D.; Kassel, K.; Variyam, J.; Aldrich, L. Economics, food choices, and nutrition. Food Policy 1999, 24, 269–286. [Google Scholar] [CrossRef]
- Lähteenmäki, L. Claiming health in food products. Food Qual. Prefer. 2013, 27, 196–201. [Google Scholar] [CrossRef]
- Renaud, S.D.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Guilford, J.M.; Pezzuto, J.M. Wine and health: A review. Am. J. Enol. Vitic. 2011, 62, 471–486. [Google Scholar] [CrossRef]
- Poli, A.; Marangoni, F.; Avogaro, A.; Barba, G.; Bellentani, S.; Bucci, M.; Cambieri, R.; Catapano, A.L.; Costanzo, S.; Cricelli, C.; et al. Moderate alcohol use and health: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 487–504. [Google Scholar] [CrossRef]
- Chakravarthi, S.; Jessop, C.E.; Bulleid, N.J. The role of glutathione in disulfide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep. 2006, 7, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. Glutathione and The Sulfur-Containing Amino Acids: An Overview. In Glutathione and Sulfur Amino Acids in Human Health and Disease; Masella, R., Mazza, G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-0-470-17085-4. [Google Scholar]
- Luyckx, J.; Baudouin, C. Trehalose: An intriguing disaccharide with potential for medical application in ophthalmology. Clin. Ophthalmol. 2011, 5, 577–581. [Google Scholar] [CrossRef]
- Eleutherio, E.; Panek, A.; De Mesquita, J.F.; Trevisol, E.; Magalhães, R. Revisiting. yeast trehalose metabolism. Curr. Genet. 2015, 61, 263–274. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C.; Mas, A. Melatonin and Other Tryptophan Metabolites Produced by Yeasts: Implications in Cardiovascular and Neurodegenerative Diseases. Front. Microbiol. 2016, 6, 1565. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Pilar-Terron, M.; Flores, L.J.; Koppisepi, S. Medical implications of melatonin: receptor-mediated and receptor-independent actions. Adv. Med. Sci. 2007, 52, 11–28. [Google Scholar]
- Mas, A.; Guillamon, J.M.; Torija, M.J.; Beltran, G.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Bioactive compounds derived from the yeast metabolism of aromatic amino acids during alcoholic fermentation. BioMed. Res. Int. 2014, 898045. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Paredes, S.D.; Cubero, J.; Lozano, M.; Toribio-Delgado, A.F.; Muñoz, J.L.; Reiter, R.J.; Barriga, C.; Rodríguez, A.B. Jerte valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65A, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Simonetti, P.; Faoro, F.; Fico, G.; Iriti, M. The presence of melatonin in grapevine (Vitis vinifera L.) berry tissues. J. Pineal Res. 2011, 51, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.D.; Moreno, H.; Calvo, J.R. Melatonin present in beer contributes to increase the levels of melatonin and antioxidant capacity of the human serum. Clin. Nutr. 2009, 28, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Naranjo, M.I.; Torija, M.J.; Mas, A.; Cantos-Villar, E.; Garcia-Parrilla, M.D. Production of melatonin by Saccharomyces strains undergrowth and fermentation conditions. J. Pineal Res. 2012, 53, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Hall, B.A.; Le, C.H.; Saxena, P.K. Changes in the levels of indoleamine phytochemicals during véraison and ripening of wine grapes. J. Pineal Res. 2010, 49, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos, E.C.; Garcia-Parrilla, M.C. Melatonin: A new bioactive compound present in wine. J. Food Compost. Anal. 2011, 24, 603–608. [Google Scholar] [CrossRef]
- Samuel, S.M.; Thirunavukkarasu, M.; Penumathsa, S.V.; Paul, D.; Maulik, N. Akt/FOXO3a/SIRT1-Mediated Cardioprotection by n-Tyrosolagainst Ischemic Stress in Rat in Vivo Model of Myocardial Infarction: Switching Gears toward Survival and Longevity. J. Agric. Food Chem. 2008, 56, 9692–9698. [Google Scholar] [CrossRef]
- Dudley, J.I.; Lekli, I.; Mukherjee, S.; Das, M.; Bertelli, A.A.; Das, D.K. Does white wine qualify for French paradox? Comparison of the cardioprotective effects of red and white wines and their constituents: resveratrol, tyrosol, and hydroxytyrosol. J. Agric. Food Chem. 2008, 56, 9362–9373. [Google Scholar] [CrossRef]
- Willcox, B.J.; Donlon, T.A.; He, Q.; Chen, R.; Grove, J.S.; Yano, K.; Masaki, K.H.; Willcox, D.C.; Rodriguez, B.; Curb, J.D. FOXO3a Genotype Is Strongly Associated with Human Longevity. Proc. Natl. Acad. Sci. USA 2008, 105, 13987–13992. [Google Scholar] [CrossRef]
- Thirunavukkarasu, M.; Penumathsa, S.V.; Samuel, S.M.; Akita, Y.; Zhan, L.; Bertelli, A.A.; Maulik, G.; Maulik, N. White Wine-induced Cardio Protection against Ischemia-Reperfusion Injury is Mediated by Life-Extending Akt/FOXO3a/NFκB Survival Pathway. J. Agric. Food Chem. 2008, 56, 6733–6739. [Google Scholar] [CrossRef]
- Utsunomiya, H. Flavor terminology and reference standards for sensory analysis of sake. J. Brew. Soc. Jpn. 2006, 101, 730–739. [Google Scholar] [CrossRef]
- Luís, R.S.; Paula, B.A.; Patrícia, V.; Rosa, M.S.; Martha, E.T.; Encarna, V. Analysis of non-colored phenolics in red wine: Effect of Dekkera bruxellensis. Yeast 2005, 89, 185–189. [Google Scholar] [CrossRef]
- Gould, B.A.; Mann, S.; Davies, A.B.; Altman, D.G.; Raftery, E.B. α-Adrenoreceptor Blockade with Indoramin in Hypertension. J. Cardiovasc. Pharmacol. 1983, 5, 343–348. [Google Scholar] [CrossRef]
- Kirby, R.S.; Pool, J.L. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: Past, present, and future. Br. J. Urol. 1997, 80, 521–532. [Google Scholar] [CrossRef]
- Lingappa, B.T.; Prasad, M.; Lingappa, Y.; Hunt, D.F.; Biemann, K. Phenethyl Alcohol, and Tryptophol: Auto-antibiotics Produced by the Fungus Candida albicans. Science 1969, 163, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Luna-Solano, G.; Salgado-Cervantes, M.A.; Ramirez-Lepe, M.; Garcia-Alvarado, M.A.; Rodriguez-Jimenes, G.C. Effect of drying type and drying conditions over the fermentative ability of brewer’s yeast. J. Food Process. Eng. 2003, 26, 135–147. [Google Scholar] [CrossRef]
- Rapoport, A. Anhydrobiosis and Dehydration of Yeasts. In Biotechnology of Yeasts and Filamentous Fungi; Sibirny, A.A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 87–116. [Google Scholar]
- Gervais, P.; Beney, L. Osmotic mass transfer in the yeast Saccharomyces cerevisiae. Cell. Mol. Biol. 2001, 47, 831–840. [Google Scholar] [PubMed]
- Dupont, S.; Beney, L.; Ritt, J.-F.; Lherminier, J.; Gervais, P. Lateral reorganization of the plasma membrane is involved in the yeast resistance to severe dehydration. Biochim. Biophys. Acta Biomembr. 2010, 1798, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Garre, E.; Raginel, F.; Palacios, A.; Julien, A.; Matallana, E. Oxidative stress responses, and lipid peroxidation damage are induced during dehydration in the production of dry active wine yeasts. Int. J. Food Microbiol. 2010, 136, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Câmara, A.A., Jr.; Nguyen, T.D.; Jossier, A.; Endrizzi, A.; Saurel, R.; Simonin, H.; Husson, F. Improving total glutathione and trehalose contents in Saccharomyces cerevisiae cells to enhance their resistance to fluidized bed drying. Process. Biochem. 2018, 69, 45–51. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for the quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Bourboulou, A.; Shahi, P.; Chakladar, A.; Delrot, S.; Bachhawat, A.K. Hgt1p, a High-Affinity Glutathione Transporter from the Yeast Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 13259–13265. [Google Scholar] [CrossRef]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen peroxide sensing and signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef]
- Gostimskaya, I.; Grant, C.M. Yeast mitochondrial glutathione is an essential antioxidant with mitochondrial thioredoxin providing a back-up system. Free Radic. Biol. Med. 2016, 94, 55–65. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Thakur, A.; Kaur, J.; Zulkifli, M. Glutathione transporters. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3154–3164. [Google Scholar] [CrossRef]
- Russell, I. Chapter 4—Yeast. In Brewing Materials and Processes; Bamforth, C.W., Ed.; Academic Press: London, UK, 2016; pp. 77–96. [Google Scholar]
- Herdeiro, R.S.; Pereira, M.D.; Panek, A.D.; Eleutherio, E.C.A. Trehalose protects Saccharomyces cerevisiae from lipid peroxidation during oxidative stress. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 340–346. [Google Scholar] [CrossRef]
- Câmara Jr., A.A.; Maréchal, P.-A.; Tourdot-Maréchal, R.; Husson, F. Dehydration stress responses of yeasts Torulaspora delbrueckii, Metschnikowia pulcherrima and Lachancea thermotolerans: Effects of glutathione and trehalose biosynthesis. Food Microbiol. 2019, 79, 137–146. [Google Scholar] [CrossRef]
- Sprenger, J.; Hardeland, R.; Fuhrberg, B.; Han, S.-Z. Melatonin and other 5-methoxylated indoles in yeast: presence in high concentrations and dependence on tryptophan availability. Cytologia 1999, 64, 209–213. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef]
- Germann, S.M.; Jacobsen, S.A.; Schneider, K.; Harrison, S.J.; Jensen, N.B.; Stahlhut, S.G.; Borodina, I.; Luo, H.; Zhu, J.; Maury, J. Glucose-based microbial production of the hormone melatonin in yeast Saccharomyces cerevisiae. Biotech. J. 2016, 11, 717–724. [Google Scholar] [CrossRef]
- Manfroi, L.; Silva, P.H.A.; Rizzon, L.A.; Sabaini, P.S.; Glória, M.B.A. Influence of alcoholic and malolactic starter cultures on bioactive amines in Merlot wines. Food Chem. 2009, 116, 208–213. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Ye, D.O.; Zhu, B.Q.; Wu, G.F.; Duan, C.Q. Rapid HPLC analysis of amino acids and biogenic amines in wines during fermentation and evaluation of matrix effect. Food Chem. 2014, 163, 6–15. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef]
- Fernández-Cruz, E.; Cerezo, A.; Cantos-Villar, E.; Troncoso, A.; García-Parrilla, M. Time course of l-tryptophan metabolites when fermenting natural grape musts: effect of inoculation treatments and cultivar on the occurrence of melatonin and related indolic compounds. Aust. J. Grape Wine R. 2019, 25, 92–100. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Melatonin is synthesised by yeast during alcoholic fermentation in wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef]
- Fernández-Cruz, E.; Alvarez-Fernández, M.A.; Valero, E.; Troncoso, A.M.; García-Parrilla, M.C. Melatonin and derived tryptophan metabolites produced during alcoholic fermentation by different yeast strains. Food Chem. 2017, 217, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A. Use of Nonconventional Yeasts for Modulating Wine Acidity. Fermentation 2019, 5, 27. [Google Scholar] [CrossRef]
- Puertas, B.; Jiménez, M.J.; Cantos-Villar, E.; Cantoral, J.M.; Rodríguez, M.E. Use of Torulaspora delbrueckii and Saccharomyces cerevisiae in semi-industrial sequential inoculation to improve quality of Palomino and Chardonnay wines in warm climates. J. Appl. Microbiol. 2017, 122, 733–746. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.; Kim, K.; Roh, S. The Protective Effect of Indole-3-Acetic Acid (IAA) on H2O2-Damaged Human Dental Pulp Stem Cells Is Mediated by the AKT Pathway and Involves Increased Expression of the Transcription Factor Nuclear Factor-Erythroid 2-Related Factor 2 (Nrf2) and Its Downstream Target Heme Oxygenase 1 (HO-1). Oxid. Med. Cell Longev. 2017, 2017, 8639485. [Google Scholar] [CrossRef]
- Ehrlich, F. Uber Tryptophol (β-Indolyl-Athylalkohol), Ein Neues Gar Produkt der Hefe Aus Aminosäuren. Ber. Dtsch. Chem. Ges. 1912, 45, 883–889. [Google Scholar] [CrossRef]
- Dickinson, J.R. Nitrogen metabolism. In The Metabolism and Molecular Physiology of Saccharomyces cerevisiae, 2nd ed.; Dickinson, J.R., Schweizer, M., Eds.; CRC Press: London, UK, 2004; ISBN 0-415-29900-490000. [Google Scholar]
- Hazelwood, L.A.; Jean-Marc, D.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway fuel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Banach, A.; Ooi, B. Enhancing the Yields of Phenolic Compounds during Fermentation Using Saccharomyces cerevisiae Strain 96581. Food Nut. Sci. 2014, 5, 2063–2070. [Google Scholar] [CrossRef]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P.D. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J. Environ. Res. Public Health. 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Kozyrovska, N.O.; Reva, O.N.; Goginyan, V.B.; De Vera, J.-P. Kombucha microbiome as a probiotic: A view from the perspective of post-genomics and synthetic ecology. Biopolym. Cell. 2012, 28, 103–113. [Google Scholar] [CrossRef]
- Teoh, A.L.; Heard, G.; Cox, J. Yeast ecology of Kombucha fermentation. Int. J. Food Microbiol. 2004, 95, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Gachhui, R. Nitrogen-fixing, and cellulose-producing Gluconacetobacter kombuchae sp. nov., isolated from Kombucha tea. Int. J. Syst. Evol. Microbiol. 2007, 57, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Gachhui, R. Novel nitrogen-fixing Acetobacter nitrogenifigens sp. nov., isolated from Kombucha tea. Int. J. Syst. Evol. Microbiol. 2006, 56, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.L.; Ramos, C.L.; da Costa Souza, P.N.; Cardoso, M.G.B.; Schwan, R.F. Nondairy beverage produced by controlled fermentation with potential probiotic starter cultures of lactic acid bacteria and yeast. Int. J. Food Microbiol. 2017, 248, 39–46. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, A. The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation 2019, 5, 46. https://doi.org/10.3390/fermentation5020046
Vilela A. The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation. 2019; 5(2):46. https://doi.org/10.3390/fermentation5020046
Chicago/Turabian StyleVilela, Alice. 2019. "The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds" Fermentation 5, no. 2: 46. https://doi.org/10.3390/fermentation5020046
APA StyleVilela, A. (2019). The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation, 5(2), 46. https://doi.org/10.3390/fermentation5020046