Food Industrial Production of Monosaccharides Using Microbial, Enzymatic, and Chemical Methods
Abstract
1. Introduction
2. N-Acetyl-d-Glucosamine
3. d-Glucosamine
4. d-Fructose
5. d-Mannose and other Fru-Related Hexoses
6. d-Galactose and d-Tagatose
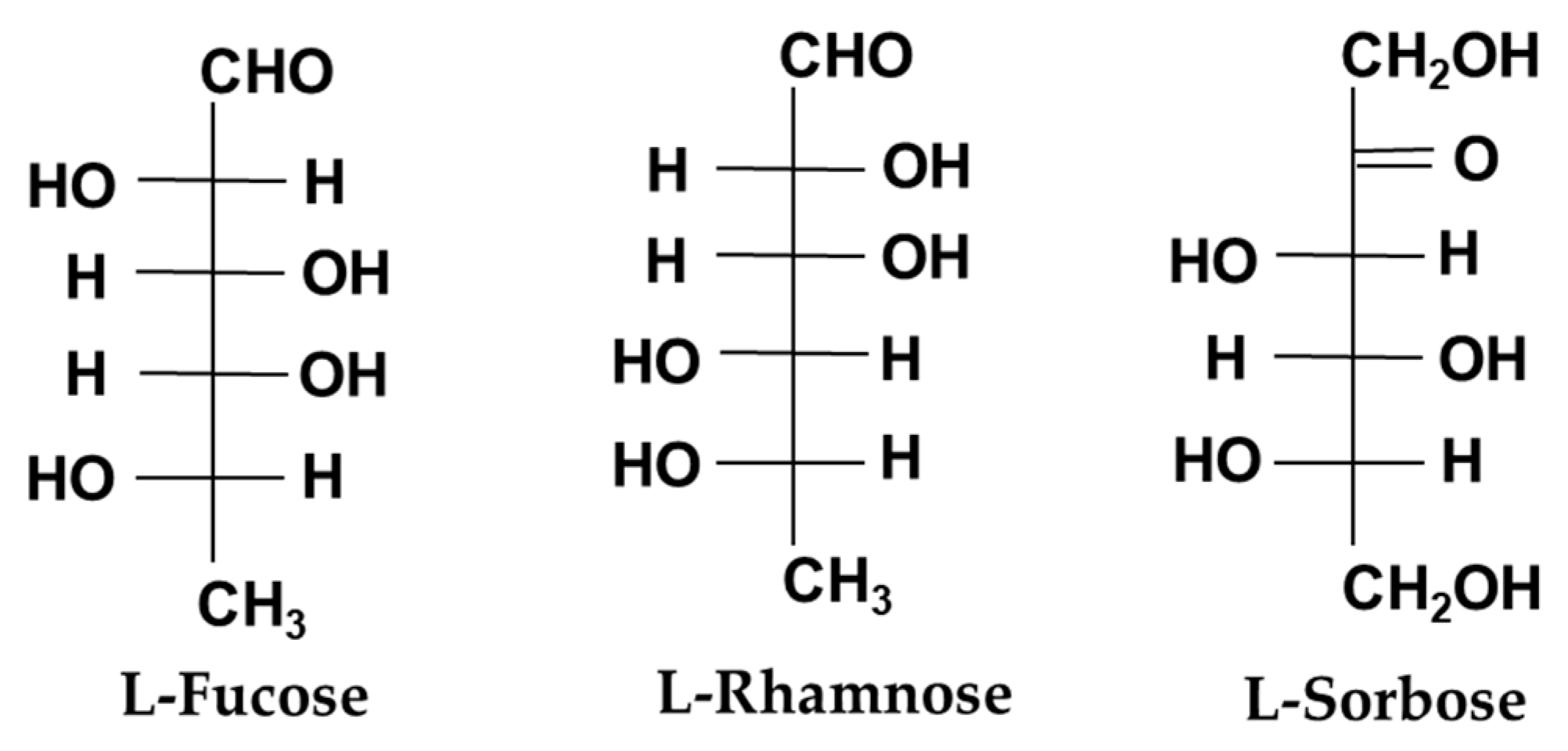
7. l-Hexoses
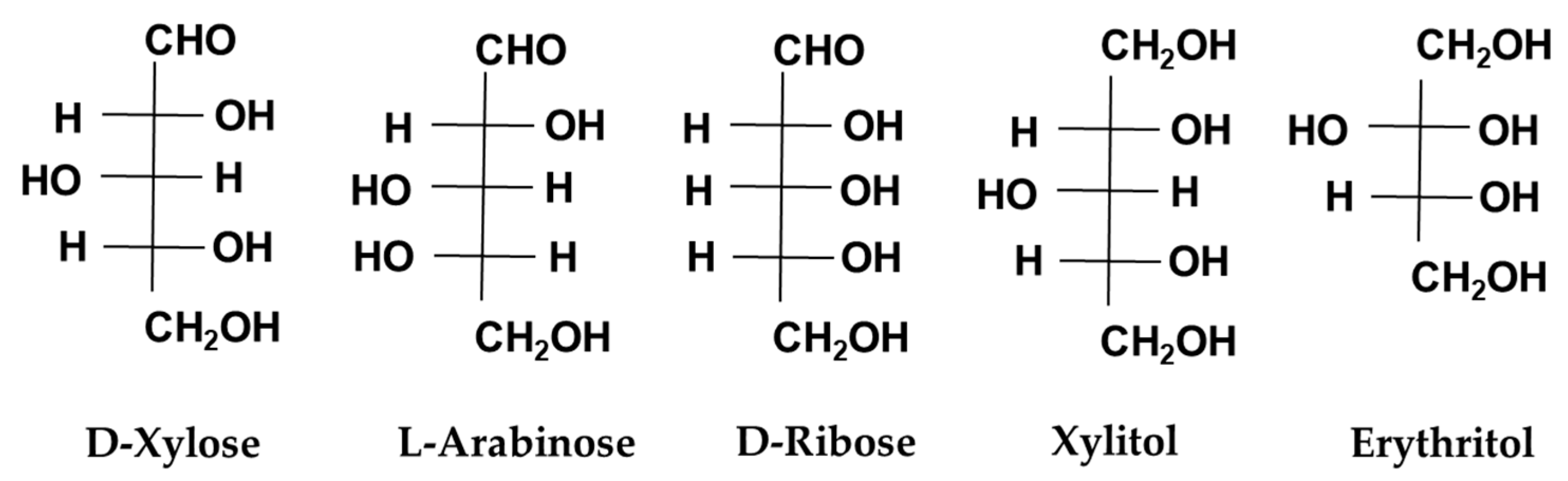
8. Other Monosaccharides
9. Discussion and Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Prydz, K.; Dalen, K.T. Synthesis and sorting of proteoglycans. J. Cell Sci. 2000, 113, 193–205. [Google Scholar] [PubMed]
- Hoff, J.E. Determination of the N-acetylglucosamine-1-phosphate content of cow milk from the difference between the values obtained for total and free N-acetylglucosamine. J. Dairy Sci. 1963, 46, 573–574. [Google Scholar] [CrossRef]
- Gooday, G.W. The Ecology of Chitin Degradation. In Advances in Microbial Ecology; Springer: Boston, MA, USA, 1990; ISBN 0147-4863. [Google Scholar]
- Sakai, K.; Uchiyama, T.; Matahira, Y.; Nanjo, F. Immobilization of chitinolytic enzymes and continuous production of N-acetylglucosamine with the immobilized enzymes. J. Ferment. Bioeng. 1991, 72, 168–172. [Google Scholar] [CrossRef]
- Techikawara, K.; Kobayashi, H.; Fukuoka, A. Conversion of N-acetylglucosamine to protected amino acid over Ru/C catalyst. ACS Sustain. Chem. Eng. 2018, 6, 12411–12418. [Google Scholar] [CrossRef]
- Zhang, A.; Gao, C.; Wang, J.; Chen, K.; Ouyang, P. An efficient enzymatic production of N-acetyl-d-glucosamine from crude chitin powders. Green Chem. 2016, 18, 2147–2154. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Deroisy, R.; Rovati, L.C.; Lee, R.L.; Lejeune, E.; Bruyere, O.; Giacovelli, G.; Henrotin, Y.; Dacre, J.E.; Gossett, C. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomised, placebo-controlled clinical trial. Lancet 2001, 357, 251–256. [Google Scholar] [CrossRef]
- Rege, P.R.; Block, L.H. Chitosan processing: Influence of process parameters during acidic and alkaline hydrolysis and effect of the processing sequence on the resultant chitosan’s properties. Carb. Res. 1999, 235–245. [Google Scholar] [CrossRef]
- KOYO Glucosamine. Available online: http://www.koyochemical.jp/english/glucosamine (accessed on 15 April 2019).
- Park, Y.K.; Yetley, E.A. Intakes and food sources of fructose in the United States. Am. J. Clin. Nutr. 1993, 58, 737S–747S. [Google Scholar] [CrossRef]
- Shintani, T.; Iida, T. Kishoutou no kaihatsu to riyou. Food Ind. Tomorrow 2015, 4, 34–41. [Google Scholar]
- Tosa, T.; Shibatani, T. Industrial application of immobilized biocatalysts in Japan. Ann. N. Y. Acad. Sci. 1995, 750, 364–375. [Google Scholar] [CrossRef]
- Vasic-Racki, D. History of industrial biotransformations—Dreams and realities. In Industrial Biotransformations, 2nd ed.; Wiley: Weinheim, Germany, 2006; pp. 1–36. [Google Scholar]
- Hanover, L.M.; White, J.S. Manufacturing, composition, and applications of fructose. Am. J. Clin. Nutr. 1993, 58, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, Y.; Ohya, T. Mannose Isomerase and Process for Mannose Production Using It. U.S. Patent 5,124,262A, 23 June 1992. [Google Scholar]
- Hirose, J.; Kinoshita, Y.; Fukuyama, S.; Hayashi, S.; Yokoi, H.; Takasaki, Y. Continuous isomerization of d-fructose to d-mannose by immobilized Agrobacterium radiobacter cells. Biotechnol. Lett. 2003, 25, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Kim, J.E.; Choi, J.G.; Oh, D.K. Characterization of a recombinant cellobiose 2-epimerase from Caldicellulosiruptor saccharolyticus and its application in the production of mannose from glucose. Appl. Microbiol. Biotechnol. 2011, 92, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shi, Y.; Zhang, P.; Miao, M.; Zhang, T.; Jiang, B. d-Mannose: Properties, production, and applications: An overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 773–785. [Google Scholar] [CrossRef]
- Takamine, S.; Nakamura, M.; Iida, T.; Okuma, K.; Ken, I. Manufacturing method of rare sugar syrup through alkali isomerization and its inhibitory effect of α-glucosidase. Bull. Appl. Glycosci. 2015, 5, 44–49. [Google Scholar] [CrossRef]
- Shintani, T.; Yamada, T.; Hayashi, N.; Iida, T.; Nagata, Y.; Ozaki, N.; Toyoda, Y. Rare sugar syrup containing d-allulose but not high-fructose corn syrup maintains glucose tolerance and insulin sensitivity partly via hepatic glucokinase translocation in Wistar rats. J. Agric. Food Chem. 2017, 65, 2888–2894. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, A.; Kozakai, T.; Shintani, T.; Matsutani, R.; Ohtani, K.; Iida, T.; Akimitsu, K.; Izumori, K.; Gullapalli, P.K. Purification and characterization of d-allulose 3-epimerase derived from Arthrobacter globiformis M30, a GRAS microorganism. J. Biosci. Bioeng. 2017, 123, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.T.; Park, D.H. Production of sugars and levulinic acid from marine biomass Gelidium amansii. Appl. Biochem. Biotechnol. 2010, 161, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Szczodrak, J. Hydrolysis of lactose in whey permeate by immobilized β-galactosidase from Kluyveromyces fragilis. J. Mol. Catal. B Enzym. 2000, 10, 631–637. [Google Scholar] [CrossRef]
- Izumori, K. Izumoring: A strategy for bioproduction of all hexoses. J. Biotechnol. 2006, 124, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Jayamuthunagai, J.; Gautam, P.; Srisowmeya, G.; Chakravarthy, M. Biocatalytic production of d-tagatose: A potential rare sugar with versatile applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 3430–3437. [Google Scholar] [CrossRef] [PubMed]
- Drabo, P.; Delidovich, I. Catalytic isomerization of galactose into tagatose in the presence of bases and Lewis acids. Catal. Commun. 2018, 107, 24–28. [Google Scholar] [CrossRef]
- Grenby, T.H. Advances in Sweeteners; Blackie Academic & Professional Publisher: Detroit, MI, USA, 2012. [Google Scholar]
- Uchihori, Y.; Sekitani, Y.; Yoshida, I. Process for producing L-sorbose by subculture of seed. U.S. Patent 4,945,048, 31 July 1990. [Google Scholar]
- Vanhooren, P.T.; Vandamme, E.J. l-Fucose: Occurrence, physiological role, chemical, enzymatic and microbial synthesis. J. Chem. Technol. Biotechnol. 1999, 74, 479–497. [Google Scholar] [CrossRef]
- Boehm, G.; Stahl, B. Oligosaccharides from milk. J. Nutr. 2007, 137, 847S–849S. [Google Scholar] [CrossRef] [PubMed]
- Saari, P.; Häkkä, K.; Heikkilä, H.; Jumppanen, J.; Hurme, M. A novel chromatographic production scale separation process for l-fucose. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2050–2064. [Google Scholar] [CrossRef]
- Trummler, K.; Effenberger, F.; Syldatk, C. An integrated microbial/enzymatic process for production of rhamnolipids and l-(+)-rhamnose from rapeseed oil with Pseudomonas sp. DSM 2874. Eur. J. Lipid Sci. Technol. 2003, 105, 563–571. [Google Scholar] [CrossRef]
- Zorbach, W.; Tio, C. A new synthesis of D-rhamnose (6-deoxy-D-mannose). J. Org. Chem. 1961, 26, 3543–3545. [Google Scholar] [CrossRef]
- Kanzaki, N.; Ono, Y.; Shibata, H.; Moritani, T. Glucosamine-containing supplement improves locomotor functions in subjects with knee pain a randomized, double-blind, placebo-controlled study. Clin. Interv. Aging 2015, 10, 1743. [Google Scholar] [CrossRef]
- Gueniche, A.; Castiel-Higounenc, I. Efficacy of glucosamine sulphate in skin ageing: Results from an ex vivo anti-ageing model and a clinical trial. Skin Pharmacol. Physiol. 2017, 30, 36–41. [Google Scholar] [CrossRef]
- Bao, J.; Liu, N.; Zhu, L.; Xu, Q.; Huang, H.; Jiang, L. Programming a biofilm-mediated multienzyme-assembly-cascade system for the biocatalytic production of glucosamine from chitin. J. Agric. Food Chem. 2018, 66, 8061–8068. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D. V Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Lê, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, Y. Studies on sugar-isomerizing enzyme: Production and utilization of glucose isomerase from Streptomyces sp. Agric. Biol. Chem. 1966, 30, 1247–1253. [Google Scholar] [CrossRef]
- Yamanaka, K. Sugar isomerases: Part I. Production of d-glucose isomerase from heterolactic acid bacteria Part II. Purification and properties of d-glucose isomerase from Lactobacillus brevis. Agric. Biol. Chem. 1963, 27, 265–278. [Google Scholar]
- Ryu, D.Y.; Chung, S.H.; Katoh, K. Performance of the continuous glucose isomerase reactor system for the production of fructose syrup. Biotechnol. Bioeng. 1977, 19, 154–184. [Google Scholar] [CrossRef]
- Kainuma, K.; Tadokoro, K.; Suzuki, S. Isomerization of dextrose into fructose. II. Isomerization with various types of alkaline reagents. Nippon Nogei Kagaku Kaishi 1966, 40, 35. [Google Scholar] [CrossRef][Green Version]
- Kooyman, C.; Vellenga, K.; De Wilt, H.G.J. The isomerization of d-glucose into d-fructose in aqueous alkaline solutions. Carbohydr. Res. 1977, 54, 33–44. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Ishiba, H.; Yoshimoto, Y.; Katano, T. Functional research and production technology development of 1,5-anhydro-d-fructose. Bull. Appl. Glycosci. 2011, 75, 70–75. [Google Scholar] [CrossRef][Green Version]
- Kranjčec, B.; Papeš, D.; Altarac, S. d-Mannose powder for prophylaxis of recurrent urinary tract infections in women: A randomized clinical trial. World J. Urol. 2014, 32, 79–84. [Google Scholar] [CrossRef]
- Makkee, M.; Kieboom, A.P.G.; Van Bekkum, H. Production methods of d-mannitol. Starch 1985, 37, 136–141. [Google Scholar] [CrossRef]
- Hossain, A.; Yamaguchi, F.; Matsuo, T.; Tsukamoto, I.; Toyoda, Y.; Ogawa, M.; Nagata, Y.; Tokuda, M. Rare sugar d-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2015, 155, 49–59. [Google Scholar] [CrossRef]
- Takeshita, K.; Suga, A.; Takada, G.; Izumori, K. Mass production of d-psicose from d-fructose by a continuous bioreactor system using immobilized d-tagatose 3-epimerase. J. Biosci. Bioeng. 2000, 90, 453–455. [Google Scholar] [CrossRef]
- Granström, T.B.; Takata, G.; Tokuda, M.; Izumori, K. Izumoring: A novel and complete strategy for bioproduction of rare sugars. J. Biosci. Bioeng. 2004, 97, 89–94. [Google Scholar] [CrossRef]
- Izumori, K. Bioproduction strategies for rare hexose sugars. Naturwissenschaften 2002, 89, 120–124. [Google Scholar] [CrossRef]
- Murata, A.; Sekiya, K.; Watanabe, Y.; Yamaguchi, F.; Hatano, N.; Izumori, K.; Tokuda, M. A novel inhibitory effect of d-allose on production of reactive oxygen species from neutrophils. J. Biosci. Bioeng. 2003, 96, 89–91. [Google Scholar] [CrossRef]
- Bhuiyan, S.H.; Itami, Y.; Rokui, Y.; Katayama, T.; Izumori, K. D-Allose Production from D-Psicose Using Immobilized L-Rhamnose Isomerase. J. Ferment. Bioeng. 1998, 89, 539–541. [Google Scholar] [CrossRef]
- Gullapalli, P.K.; Shintani, T.; Nishimoto, Y.; Harazono, K. Method for Producing d-Allose. JP Patent P6209526, 8 May 2014. [Google Scholar]
- Ammam, M.; Fransaer, J. Two-enzyme lactose biosensor based on β-galactosidase and glucose oxidase deposited by AC-electrophoresis: Characteristics and performance for lactose determination in milk. Sens. Actuators B Chem. 2010, 148, 583–589. [Google Scholar] [CrossRef]
- Yang, S.T.; Silva, E.M. Novel products and new technologies for use of a familiar carbohydrate, milk lactose. J. Dairy Sci. 1995, 78, 2541–2562. [Google Scholar] [CrossRef]
- Kretchmer, N. Lactose and lactase. Sci. Am. 1972, 227, 70–79. [Google Scholar] [CrossRef]
- Heyman, M.B. Lactose intolerance in infants, children, and adolescents. Pediatrics 2006, 118, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.P.; Koenders, D.; Bruins, J.M. Lactose-free dairy products: Market developments, production, nutrition and health benefits. Nutriets 2019, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Hodges, K.J.; Cao, S.; Cladis, P.D.; Weaver, M.C. Lactose intolerance and bone health: The challenge of ensuring adequate calcium intake. Nutriets 2019, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Matsuno, R.; Torii, K.; Yamamoto, K.; Kamikubo, T. Properties of immobilized β-d-galactosidase from Bacillus circulans. Enzym. Microb. Technol. 1983, 5, 115–120. [Google Scholar] [CrossRef]
- Michaëlsson, K.; Wolk, A.; Langenskiöld, S.; Basu, S.; Lemming, E.W.; Melhus, H.; Byberg, L. Milk intake and risk of mortality and fractures in women and men: Cohort studies. BMJ 2014, 349, g6015. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Elsas, L.J.; Wierenga, K.J. Galactose toxicity in animals. IUBMB Life 2009, 61, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Wyss, M.; Agüero, S.D.; Dávila, L.A. d-Tagatose is a promising sweetener to control glycaemia: A new functional food. BioMed Res. Int. 2018, 8718053. [Google Scholar] [CrossRef] [PubMed]
- Levin, G.V. Tagatose, the new GRAS sweetener and health product. J. Med. Food 2002, 5, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Vastenavond, C.M.; Bertelsen, H.; Hansen, S.J.; Laursen, R.S.; Saunders, J.; Eriknauer, K. Tagatose (d-tagatose). In Alternative Sweeteners; CRC Press: Boca Raton, FL, USA, 2012; pp. 197–222. [Google Scholar]
- Xu, S.; Wang, X.; Du, G.; Zhou, J.; Chen, J. Enhanced production of l-sorbose from d-sorbitol by improving the mRNA abundance of sorbitol dehydrogenase in Gluconobacter oxydansWSH-003. Microb. Cell Fact. 2014, 13, 146. [Google Scholar] [CrossRef]
- Schweiger, R.G. Preparation of Alpha-l-Fucosides and l-Fucose from Fucoidan. U.S. Patent 3,240,775A, 15 March 1966. [Google Scholar]
- Lang, S.; Wullbrandt, D. Rhamnose lipids—Biosynthesis, microbial production and application potential. Appl. Microbiol. Biotehcnol. 1999, 1, 22–32. [Google Scholar] [CrossRef]
- Verstraeten, L.M.J. Infrared spectra of some 2-ketoses. Anal. Chem. 1964, 36, 1040–1044. [Google Scholar] [CrossRef]
- Namiki, M. Chemistry of Maillard reactions: Recent studies on the browning reaction mechanism and the development of antioxidants and mutagens. Adv. Food Res. 1988, 32, 115–184. [Google Scholar] [PubMed]
- Inoue, S.; Sanai, K.; Seri, K. Effect of l-arabinose on blood glucose level after ingestion of sucrose-containing food in human. J. Jpn. Soc. Nutr. Food Sci. 2000, 53, 243–247. [Google Scholar] [CrossRef]
- Pauly, D.F.; Pepine, C.J. d-Ribose as a supplement for cardiac energy metabolism. J. Cardiovasc. Pharmacol. Ther. 2000, 5, 249–258. [Google Scholar] [CrossRef] [PubMed]
- De Wulf, P.; Vandamme, E.J. Production of d-ribose by fermentation. Appl. Microbiol. Biotechnol. 1997, 48, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Burt, B.A. The use of sorbitol-and xylitol-sweetened chewing gum in caries control. J. Am. Dent. Assoc. 2006, 137, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Peldyak, J.; Makinen, K.K. Xylitol for caries prevention. J. Dent. Hyg. 2002, 76, 276–285. [Google Scholar]
- Lee, T.; Olcese, G.; Bell, Z.; Roy, G.; Mutilangi, W.; Hirs, R.; Given, P. Use of Erythritol and d-tagatose in Zero-or Low-Calorie Beverages. U.S. Patent 2,009,028,0232A1, 13 September 2007. [Google Scholar]
- Moon, H.J.; Jeya, M.; Kim, I.W.; Lee, J.K. Biotechnological production of erythritol and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 1017–1025. [Google Scholar] [CrossRef]
- Winkelhausen, E.; Kuzmanova, S. Microbial conversion of d-xylose to xylitol. J. Ferment. Bioeng. 1998, 86, 1–14. [Google Scholar] [CrossRef]
- Spencer, J.F.T.; Sallans, H.R. Production of polyhydric alcohols by osmophilic yeasts. Can. J. Microbiol. 1956, 2, 72–79. [Google Scholar] [CrossRef]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic acid: Properties, applications and microbial production. Food Technol. Biotechnol. 2006, 44, 185–195. [Google Scholar]
- Shintani, T.; Okuma, K.; Sakoguchi, H.; Sato, M. Rare sugars d-psicose and d-allose as calorie restriction mimetic-anti-metabolic syndrome effects and anti-aging effects. J. Brew. Soc. Jpn. 2013, 108, 565–574. [Google Scholar] [CrossRef]
- Shintani, H.; Shintani, T.; Ashida, H.; Sato, M. Calorie restriction mimetics: Upstream-type compounds for modulating glucose metabolism. Nutrients 2018, 10, 1821. [Google Scholar] [CrossRef] [PubMed]
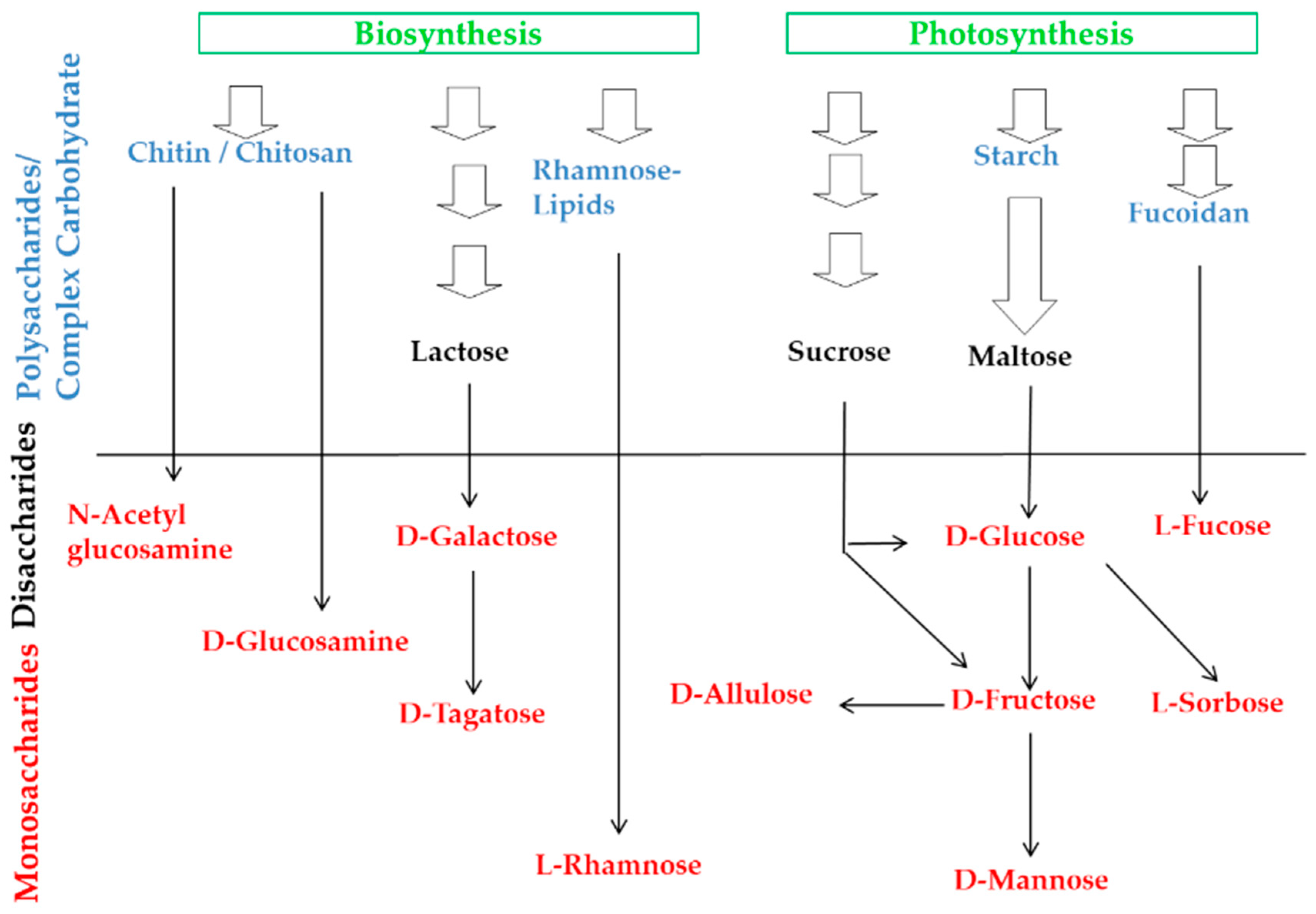
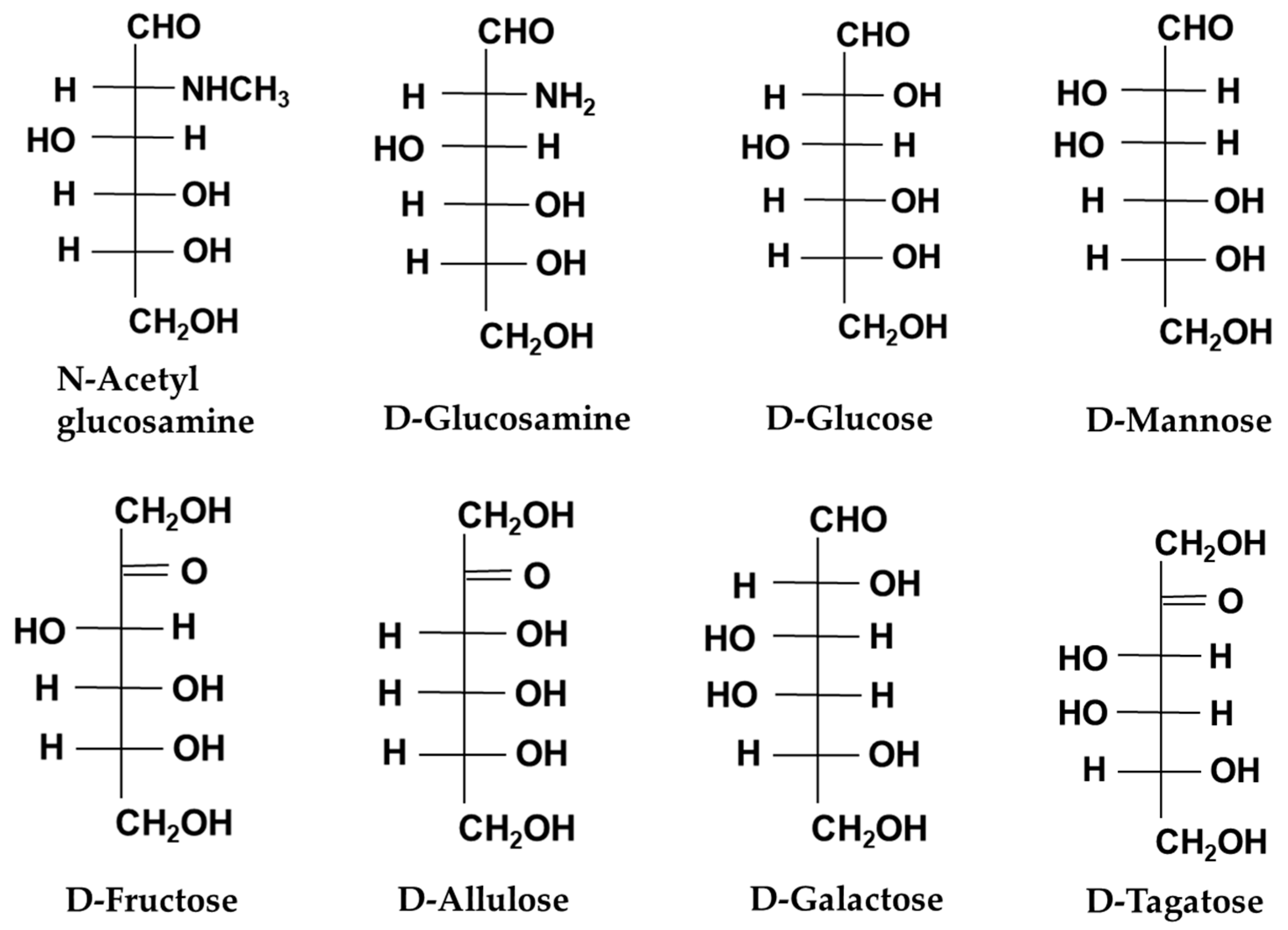
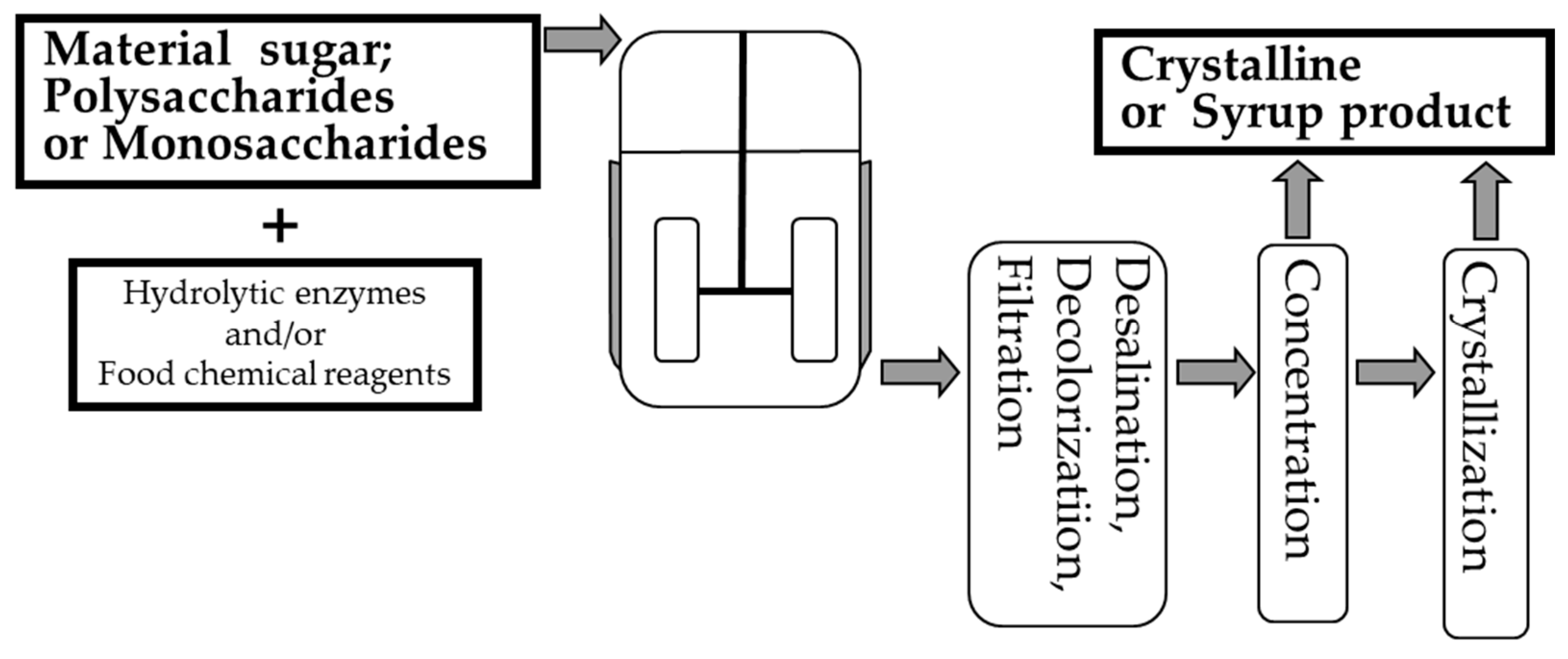
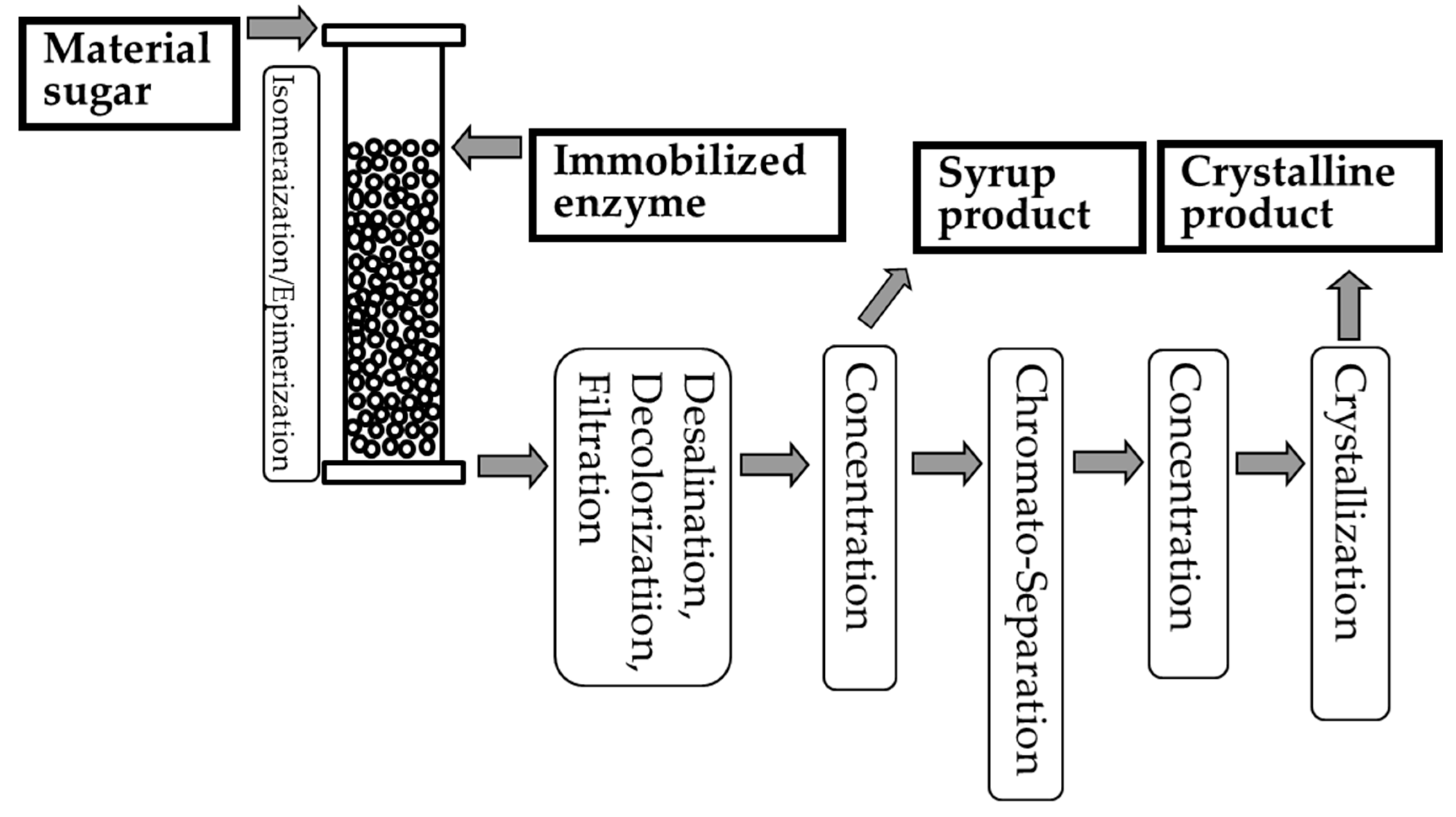
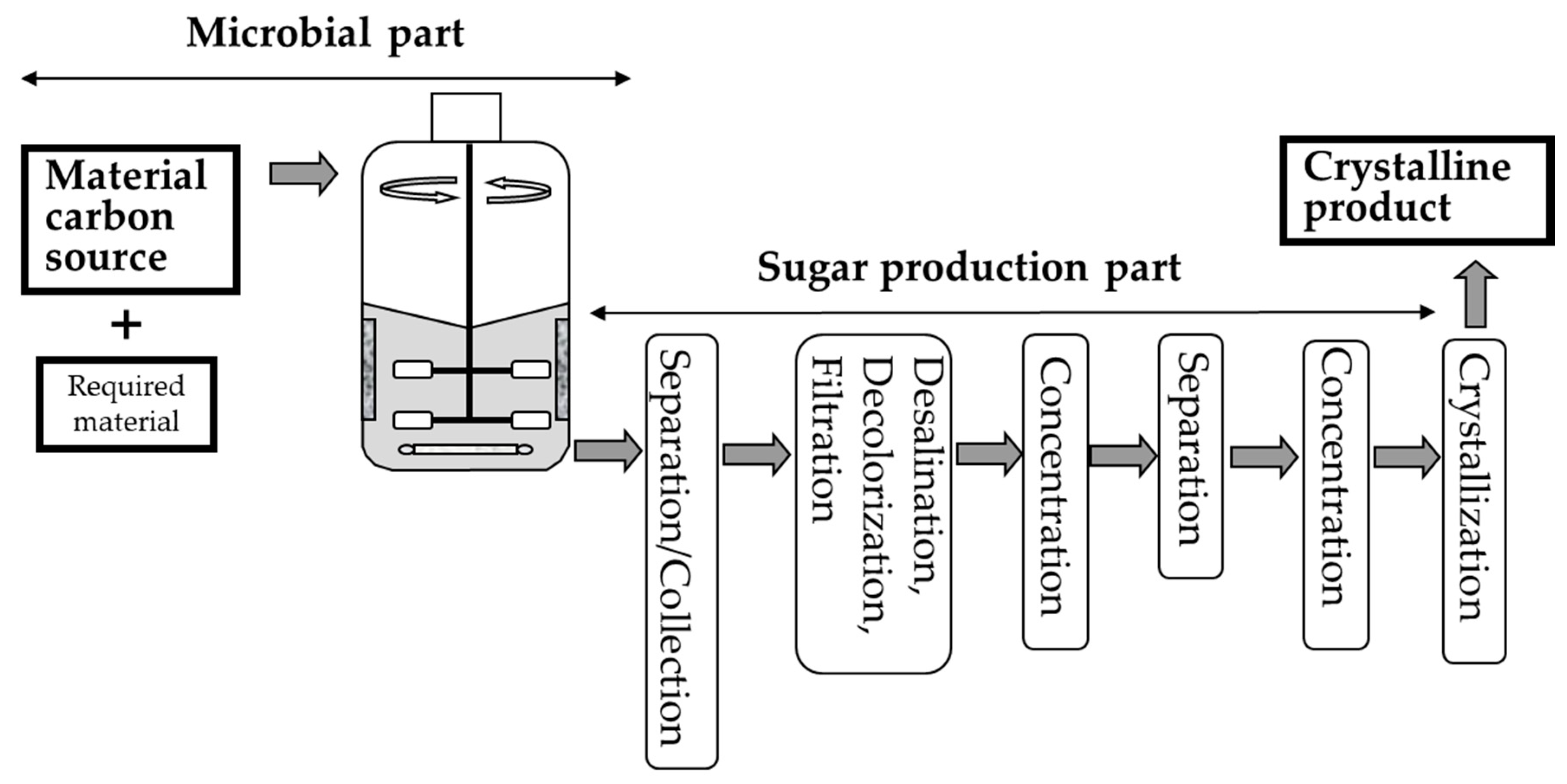
| Sugar | Production Method | Use or Origin Microbe | Raw Material | Use | Health Benefits | References |
|---|---|---|---|---|---|---|
| N-Acetyl-d-glucosamine | Enzymatic/Acidic hydrolysis | Streptomyces species | Chitin | Dietary supplement | Skin health | [3,4,5] |
| d-Glucosamine | Hydrolysis by HCl | - | Chitin/Chitosan | Dietary supplement | Joint health/Anti-aging | [7,8,9] |
| d-Fructose | Immobilized enzyme from hydrolysis | Streptomyces species | Glucose from starch | Bulk sweetener | Low glycemic response | [10,11,12,13,14] |
| d-Mannose | Immobilized enzyme/Hydrolysis by HCl | Agrobacterium radiobacter | Fructose/Mannan | Dietary supplement | Prevention of urinary-tract infection | [15,16,17,18] |
| d-Allulose | Immobilized enzyme/Alkaline reaction by NaOH | Arthrobacter species | Fructose | Food ingredient | Anti-metabolic syndrome | [19,20,21] |
| d-Galactose | Enzymatic hydrolysis | Kluyveromyces species | Lactose | Food ingredient | Immunostimulation | [22,23] |
| d-Tagatose | Immobilized enzyme/Alkaline reaction by CaOH | Thermotoga species | Galactose | Food ingredient | Anti-metabolic syndrome/Tooth friendly | [24,25,26] |
| l-Sorbose | Fermentation | Gluconobacter or Acetobacter species | Sorbitol from glucose | Sweetener | Low calorie | [27,28] |
| l-Fucose | Hydrolysis by HCl | - | Fucoidan | Food ingredient | Immunostimulation | [29,30,31] |
| l-Rhamnose | Fermentation extraction | Acinetobacter species | A Rhamnus or Toxicodendron vernix plant | Cosmetic/Food ingredient | Skin health | [32,33] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shintani, T. Food Industrial Production of Monosaccharides Using Microbial, Enzymatic, and Chemical Methods. Fermentation 2019, 5, 47. https://doi.org/10.3390/fermentation5020047
Shintani T. Food Industrial Production of Monosaccharides Using Microbial, Enzymatic, and Chemical Methods. Fermentation. 2019; 5(2):47. https://doi.org/10.3390/fermentation5020047
Chicago/Turabian StyleShintani, Tomoya. 2019. "Food Industrial Production of Monosaccharides Using Microbial, Enzymatic, and Chemical Methods" Fermentation 5, no. 2: 47. https://doi.org/10.3390/fermentation5020047
APA StyleShintani, T. (2019). Food Industrial Production of Monosaccharides Using Microbial, Enzymatic, and Chemical Methods. Fermentation, 5(2), 47. https://doi.org/10.3390/fermentation5020047





