Techno-Economic Bottlenecks of the Fungal Pretreatment of Lignocellulosic Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Process Modeling
2.1.1. Data Collection
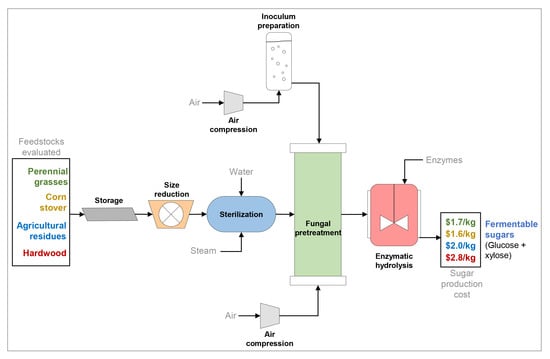
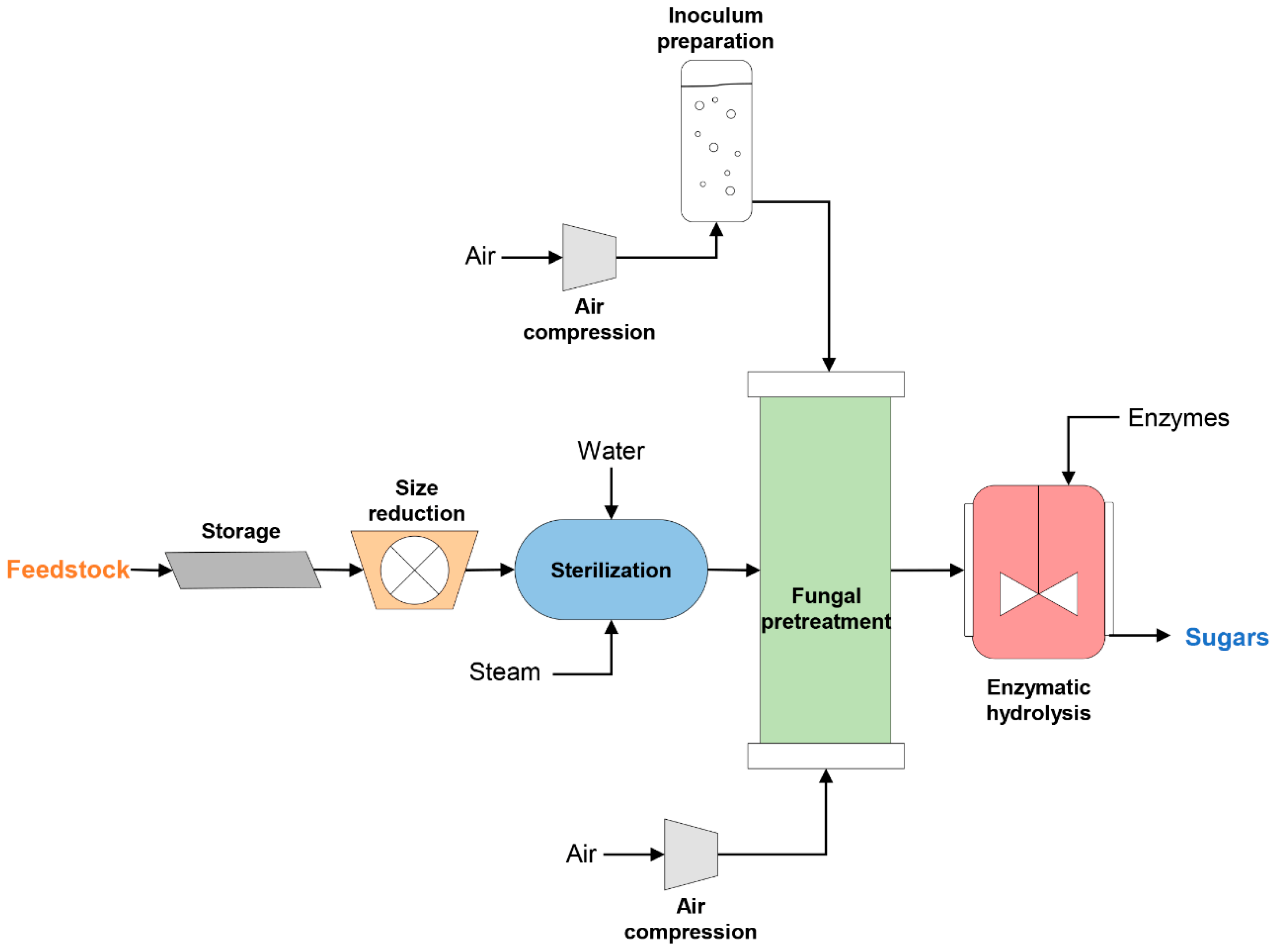
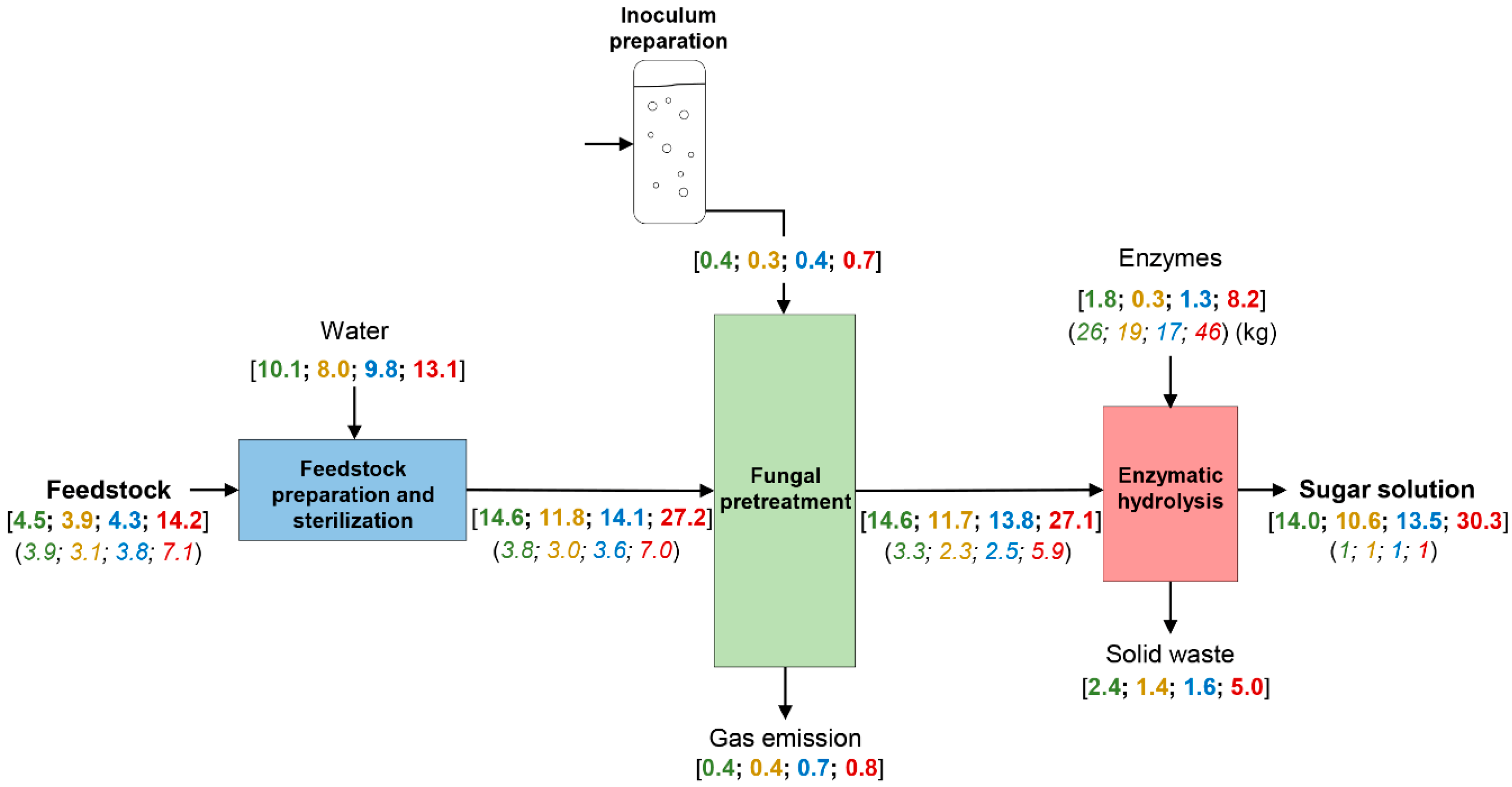
2.1.2. Feedstock Preparation and Preprocessing
2.1.3. Fungal Pretreatment Unit
2.1.4. Enzymatic Hydrolysis Unit
2.2. Economic Analysis
2.3. Sensitivity Analysis
3. Results and Discussion
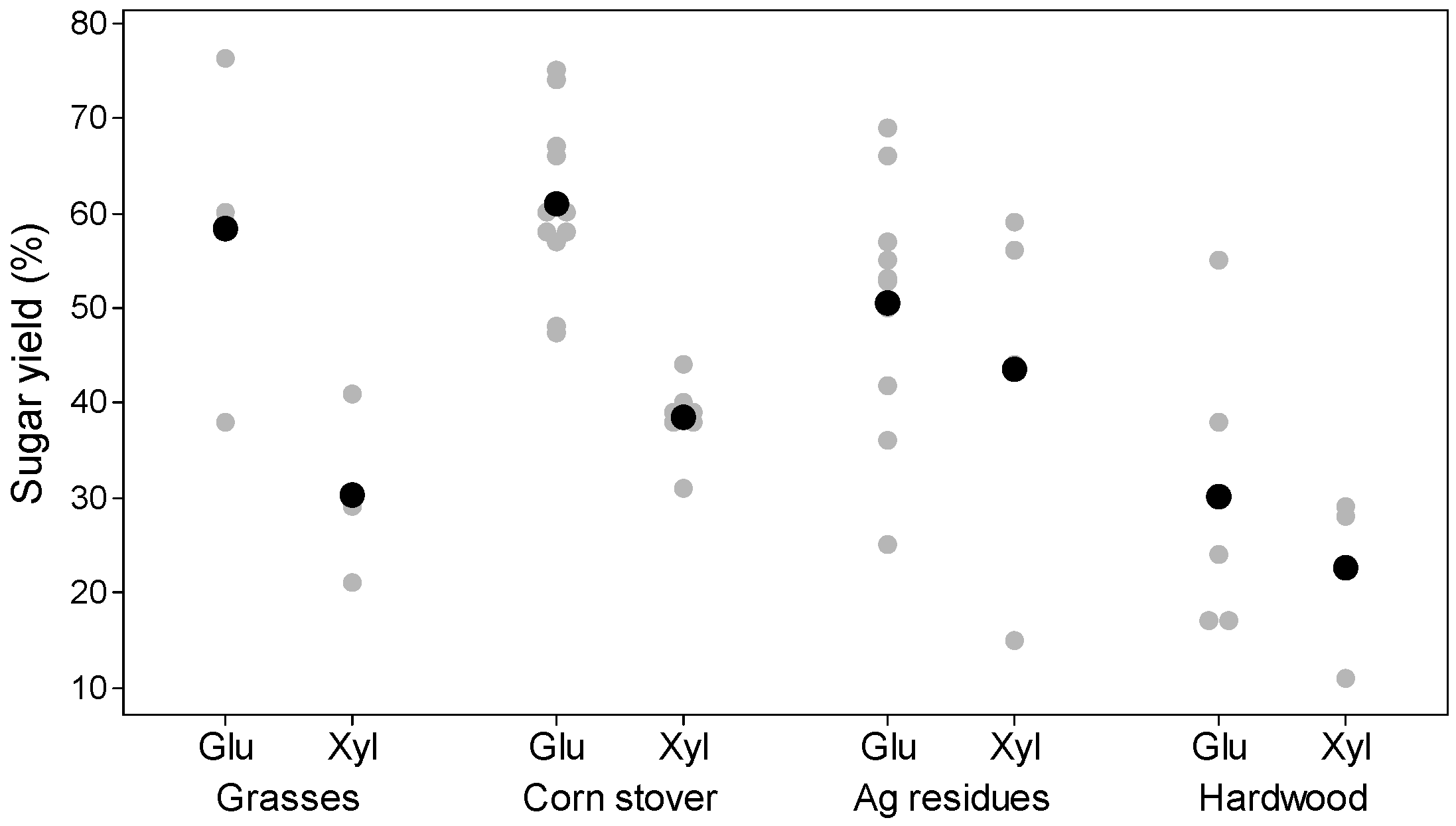
3.1. Material and Energy Balances
3.2. Process Economics
3.3. Sensitivity Analysis
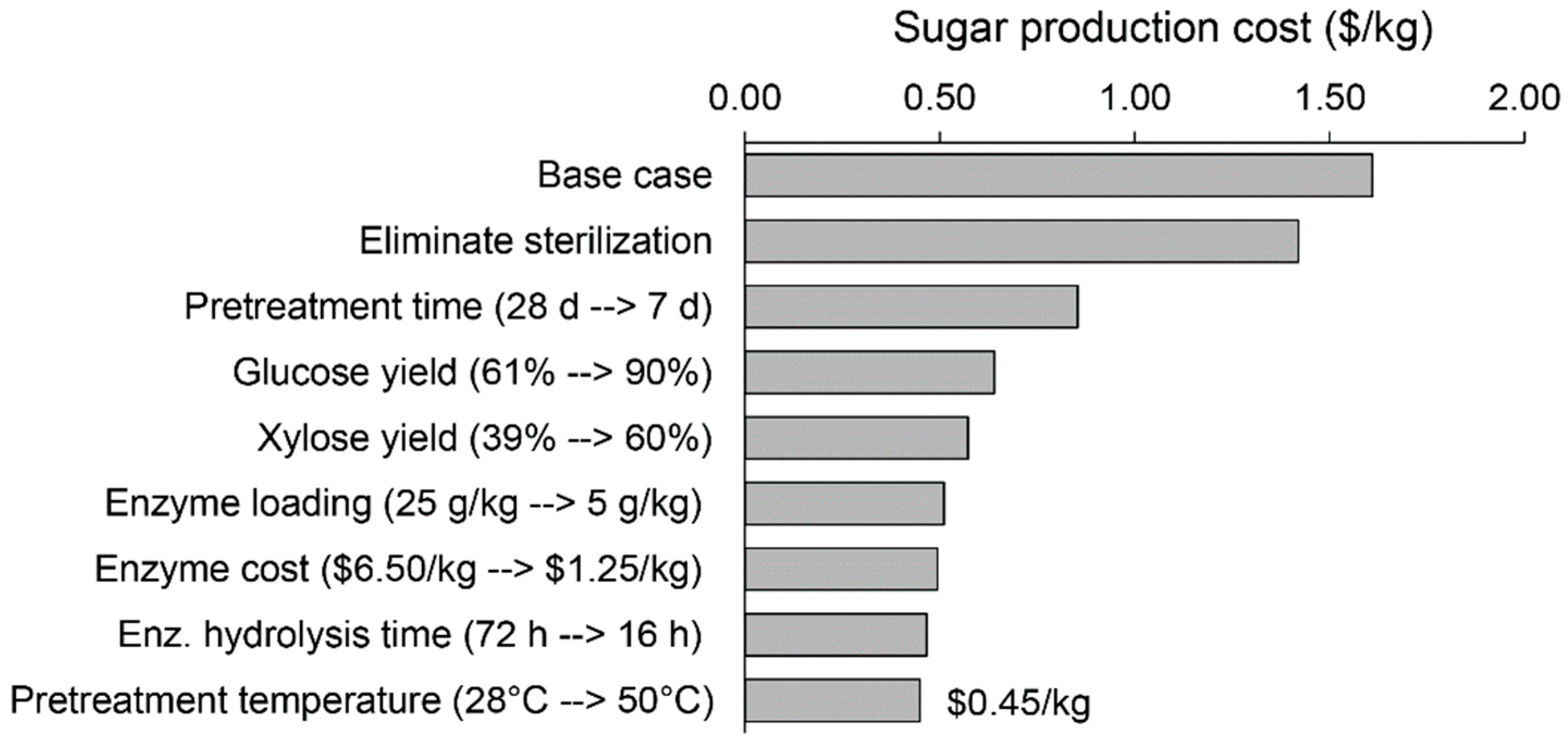
3.4. Potential Strategies to Reduce the Sugar Production Cost
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Time Parameters | Value | Financing Parameters | Value | Construction Plan | Value |
|---|---|---|---|---|---|
| Analysis year | 2017 | Equity (%) | 40 | 1st year (% of DFC) | 30 |
| Year construction starts | 2017 | Loan term (years) | 12 | 2nd year (% of DFC) | 40 |
| Construction period (months) | 18 | Loan interest (%) | 8 | 3rd year (% of DFC) | 30 |
| Startup period (months) | 12 | Depreciation method | Straight line | Plant direct cost parameters: | |
| Project life (years) | 30 | Depreciation period (years) | 15 | Process piping (% of PC) | 35 |
| Inflation rate (%) | 2 | Income tax rate (%) | 40 | Instrumentation (% of PC) | 40 |
| Fixed cost parameters: | Operating parameters: | Insulation (% of PC) | 5 | ||
| Auxiliary facilities (% of PC) | 40 | Annual operating time (h) | 7920 | Electrical (% of PC) | 10 |
| Engineering (% of TPDC) | 20 | Salvage factor (% of DFC) | 5 | Buildings (% of PC) | 45 |
| Construction (% of TPDC) | 20 | Startup cost (% of DFC) | 5 | Yard improvement (% of PC) | 15 |
| Contractor’s fee (% of TPC) | 5 | ||||
| Contingency (% of TPC) | 10 |
| Equipment | Characteristics | Feedstock | Reference/ Notes | |||
|---|---|---|---|---|---|---|
| Grasses | Corn Stover | Ag. Residues | Hardwood | |||
| Bale conveyor | Width (cm) | 100 | 100 | 100 | - | Set-up to fit bales |
| Length (m) | 100 | 100 | 100 | - | [80] | |
| Power (kW) | 15 | 15 | 15 | - | [109] | |
| Number | 1 | 1 | 1 | - | Calculated | |
| Unit cost ($) | 344,000 | 344,000 | 344,000 | - | [61] | |
| Bale grinder | Screen (in) | 2 | 2 | 2 | - | [17] |
| Throughput (kg/h) | 38,634 | 32,989 | 36,915 | - | Max. 40,000 kg/h [110] | |
| Specific power (kW/kg) | 0.025 | 0.03 | 0.025 | - | [97] | |
| Number | 2 | 2 | 2 | - | Calculated | |
| Unit cost ($) | 571,000 | 519,000 | 556,000 | - | [110] | |
| Bucket elevator | Power (kW) | 7.5 | 7.5 | 7.5 | 7.5 | [109] |
| Number | 1 | 1 | 1 | 1 | Calculated | |
| Unit cost ($) | 20,000 | 20,000 | 20,000 | 20,000 | [80] | |
| Belt conveyor | Width (cm) | 107 | 107 | 107 | 107 | [109] |
| Length (m) | 7.6 | 7.6 | 7.6 | 7.6 | [109] | |
| Power (kW) | 3.7 | 3.7 | 3.7 | 40 | [97,109] | |
| Number | 1 | 1 | 1 | 1 | Calculated | |
| Unit cost ($) | 70,000 | 70,000 | 70,000 | 70,000 | [80] | |
| Autoclave | Size (m3) | 94 | 92 | 96 | 96 | Max. 100 m3 [80] |
| Designed pressure (psi) | 100 | 100 | 100 | 100 | Set-up for sterilization | |
| Material | CS | CS | CS | CS | [110] | |
| Number | 11 | 12 | 14 | 17 | Calculated | |
| Unit cost ($) | 173,000 | 171,000 | 175,000 | 175,000 | [80] | |
| Screw conveyor 1 – feeding packed-bed bioreactor | Diameter (cm) | 86 | 90 | 91 | 98 | Max. 100 cm [80] |
| Length (m) | 10 | 10 | 10 | 10 | [80] | |
| Power (kW) | 94 | 101 | 130 | 150 | Calculated | |
| Material | CS | CS | CS | CS | [50] | |
| Number | 1 | 1 | 1 | 1 | Calculated | |
| Unit cost ($) | 140,000 | 118,000 | 136,000 | 229,000 | [50] | |
| Airlift fermenter (inoculum preparation bioreactor) | Size (m3) | 980 | 787 | 936 | 900 | Calculated |
| Material | SS316 | SS316 | SS316 | SS316 | [80] | |
| Number | 1 | 1 | 1 | 2 | Calculated | |
| Unit cost ($) | 1,208,000 | 1,054,000 | 1,174,000 | 1,146,000 | [80] | |
| Gas compressor for inoculum preparation | Pressure change (psi) | 16 | 16 | 16 | 16 | [50] |
| Efficiency (%) | 70 | 70 | 70 | 70 | [80] | |
| Power (kW) | 646 | 519 | 617 | 1188 | Calculated | |
| Number | 1 | 1 | 1 | 1 | Calculated | |
| Unit cost ($) | 257,000 | 225,000 | 250,000 | 370,000 | [50] | |
| Packed-bed bioreactor (fungal pretreatment) | Size (m3) | 3999 | 3972 | 3974 | 3976 | Max. 4000 m3 |
| Material | Concrete | Concrete | Concrete | Concrete | [50] | |
| Number | 85 | 92 | 120 | 142 | Calculated | |
| Unit cost ($) | 986,000 | 983,000 | 983,000 | 983,000 | [50] | |
| Gas compressor for fungal pretreatment | Pressure change (psi) | 16 | 16 | 16 | 16 | [50] |
| Efficiency (%) | 70 | 70 | 70 | 70 | [80] | |
| Power (kW) | 6000 | 6450 | 4208 | 4957 | Calculated | |
| Number | 1 | 1 | 2 | 2 | Calculated | |
| Unit cost ($) | 978,000 | 1,022,000 | 791,000 | 872,000 | [50] | |
| Screw conveyor 2 – feeding enzymatic hydrolysis reactor | Diameter (cm) | 78 | 80 | 91 | 92 | [80] |
| Length (m) | 15 | 15 | 15 | 15 | [80] | |
| Power (kW) | 115 | 119 | 156 | 197 | Calculated | |
| Material | CS | CS | CS | CS | [80] | |
| Number | 1 | 1 | 1 | 1 | Calculated | |
| Unit cost ($) | 165,000 | 138,000 | 157,000 | 270,000 | [50] | |
| Enzymatic hydrolysis reactor | Size (m3) | 2300 | 2321 | 2333 | 2482 | Max. 2,500 m3 [50] |
| Material | SS304 | SS304 | SS304 | SS304 | [50] | |
| Number | 11 | 8 | 10 | 22 | Calculated | |
| Unit cost ($) | 958,000 | 963,000 | 966,000 | 1,003,000 | [50] | |
| Cost Item | Grasses | Corn Stover | Ag. Residues | Hardwood |
|---|---|---|---|---|
| Feedstock | 50.5 | 40.7 | 48.4 | 72.9 |
| Enzymes | 22.9 | 16.8 | 15.2 | 40.7 |
| Other materials | 5.5 | 4.4 | 5.3 | 10.3 |
| Labor | 5.9 | 5.8 | 5.8 | 5.3 |
| Facility-dependent | 104.2 | 108.0 | 139.8 | 175.8 |
| Laboratory/QC/QA | 0.9 | 0.9 | 0.9 | 0.9 |
| Utilities | 36.7 | 41.1 | 58.9 | 68.0 |
| TOTAL | 226.6 | 217.6 | 274.2 | 373.8 |
| Grasses | Corn Stover | Ag. Residues | Hardwood | |||||
|---|---|---|---|---|---|---|---|---|
| Equipment | Quantity | Total Cost | Quantity | Total Cost | Quantity | Total Cost | Quantity | Total Cost |
| Packed-bed bioreactor | 85 | 83,810 | 92 | 90,436 | 120 | 117,960 | 142 | 139,586 |
| Enzymatic hydrolysis reactor | 11 | 10,538 | 8 | 7704 | 10 | 9660 | 22 | 22,066 |
| Autoclave | 11 | 1903 | 12 | 2052 | 14 | 2450 | 17 | 2975 |
| Airlift fermenter | 1 | 1208 | 1 | 1054 | 1 | 1174 | 2 | 2292 |
| Hammer mill | 2 | 1142 | 2 | 1036 | 2 | 1112 | - | - |
| Gas compressor for fungal pretreatment | 1 | 978 | 1 | 1022 | 2 | 1582 | 2 | 1744 |
| Bale conveyor | 1 | 344 | 1 | 344 | 1 | 344 | - | - |
| Gas compressor for inoculum | 1 | 257 | 1 | 225 | 1 | 250 | 1 | 370 |
| Screw conveyor 1 | 1 | 140 | 1 | 118 | 1 | 136 | 1 | 229 |
| Screw conveyor 2 | 1 | 165 | 1 | 138 | 1 | 157 | 1 | 270 |
| Belt conveyor | 1 | 70 | 1 | 70 | 1 | 70 | 1 | 70 |
| Bucket elevator | 1 | 20 | 1 | 20 | 1 | 20 | 1 | 20 |
| Other equipment | - | 25,181 | - | 26,080 | - | 33,762 | - | 42,469 |
| TOTAL | 125,756 | 130,299 | 168,677 | 212,091 | ||||
References
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- U.S. Department of Energy. 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstocks; U.S. Department of Energy: Oak Ridge, TN, USA, 2016.
- Williams, C.L.; Westover, T.L.; Emerson, R.M.; Tumuluru, J.S.; Li, C. Sources of biomass feedstock variability and the potential impact on biofuels production. BioEnergy Res. 2016, 9, 1–14. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefin. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Mosier, N.S.; Wyman, C.E.; Dale, B.E.; Elander, R.T.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M.R. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Baral, N.R.; Shah, A. Microbial inhibitors: Formation and effects on acetone-butanol-ethanol fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2014, 98, 9151–9172. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef]
- Li, M.-F.; Yang, S.; Sun, R.-C. Recent advances in alcohol and organic acid fractionation of lignocellulosic biomass. Bioresour. Technol. 2016, 200, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Travaini, R.; Martín-Juárez, J.; Lorenzo-Hernando, A.; Bolado-Rodríguez, S. Ozonolysis: An advantageous pretreatment for lignocellulosic biomass revisited. Bioresour. Technol. 2016, 199, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Grilc, M.; Likozar, B.; Levec, J. Kinetic model of homogeneous lignocellulosic biomass solvolysis in glycerol and imidazolium-based ionic liquids with subsequent heterogeneous hydrodeoxygenation over NiMo/Al2O3 catalyst. Catal. Today 2015, 256, 302–314. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.; Alam, M.Z.; Moniruzzaman, M.; Goto, M. Ionic liquid pretreatment as emerging approaches for enhanced enzymatic hydrolysis of lignocellulosic biomass. Biochem. Eng. J. 2016, 109, 252–267. [Google Scholar] [CrossRef]
- Mamilla, J.L.K.; Novak, U.; Grilc, M.; Likozar, B. Natural deep eutectic solvents (DES) for fractionation of waste lignocellulosic biomass and its cascade conversion to value-added bio-based chemicals. Biomass Bioenergy 2019, 120, 417–425. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef]
- van Osch, D.J.G.P.; Kollau, L.J.B.M.; van den Bruinhorst, A.; Asikainen, S.; Rocha, M.A.A.; Kroon, M.C. Ionic liquids and deep eutectic solvents for lignocellulosic biomass fractionation. Phys. Chem. Chem. Phys. 2017, 19, 2636–2665. [Google Scholar] [CrossRef]
- Baral, N.R.; Shah, A. Techno-economic analysis of cellulose dissolving ionic liquid pretreatment of lignocellulosic biomass for fermentable sugars production. Biofuels Bioprod. Biorefin. 2016, 10, 70–88. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Fungal pretreatment of lignocellulosic biomass. Biotechnol. Adv. 2012, 30, 1447–1457. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Ge, X.; Li, Y. Biological pretreatment of lignocellulosic biomass. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 561–586. ISBN 9780128023235. [Google Scholar]
- Singh, A.P.; Singh, T. Biotechnological applications of wood-rotting fungi: A review. Biomass Bioenergy 2014, 62, 198–206. [Google Scholar] [CrossRef]
- Saritha, M.; Arora, A.; Lata. Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J. Microbiol. 2012, 52, 122–130. [Google Scholar] [CrossRef]
- Nazarpour, F.; Abdullah, D.K.; Abdullah, N.; Zamiri, R. Evaluation of biological pretreatment of rubberwood with white rot fungi for enzymatic hydrolysis. Materials (Basel). 2013, 6, 2059–2073. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Effectiveness of microbial pretreatment by Ceriporiopsis subvermispora on different biomass feedstocks. Bioresour. Technol. 2011, 102, 7507–7512. [Google Scholar] [CrossRef]
- Ge, X.; Matsumoto, T.; Keith, L.; Li, Y. Fungal pretreatment of albizia chips for enhanced biogas production by solid-state anaerobic digestion. Energy Fuels 2015, 29, 200–204. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, T.; Cui, B. Biological pretreatment with white rot fungi and their co-culture to overcome lignocellulosic recalcitrance for improved enzymatic digestion. BioResources 2014, 9, 3968–3976. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, T.; Cui, B.; Dai, Y. Pretreatment of Populus tomentosa with Trametes velutina supplemented with inorganic salts enhances enzymatic hydrolysis for ethanol production. Biotechnol. Lett. 2012, 34, 2241–2246. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Guo, G.; Zhang, X.; Yan, K.; Xu, C. The effect of biological pretreatment with the selective white-rot fungus Echinodontium taxodii on enzymatic hydrolysis of softwoods and hardwoods. Bioresour. Technol. 2009, 100, 5170–5175. [Google Scholar] [CrossRef] [PubMed]
- López-Abelairas, M.; Álvarez Pallín, M.; Salvachúa, D.; Lú-Chau, T.; Martínez, M.J.; Lema, J.M. Optimisation of the biological pretreatment of wheat straw with white-rot fungi for ethanol production. Bioprocess. Biosyst. Eng. 2013, 36, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Zeng, J.; Laskar, D.D.; Deobald, L.; Hiscox, W.C.; Chen, S. Investigation of wheat straw biodegradation by Phanerochaete chrysosporium. Biomass Bioenergy 2011, 35, 1030–1040. [Google Scholar] [CrossRef]
- Zhi, Z.; Wang, H. White-rot fungal pretreatment of wheat straw with Phanerochaete chrysosporium for biohydrogen production: Simultaneous saccharification and fermentation. Bioprocess. Biosyst. Eng. 2014, 37, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Salvachúa, D.; Prieto, A.; López-Abelairas, M.; Lu-Chau, T.; Martínez, A.T.; Martínez, M.J. Fungal pretreatment: An alternative in second-generation ethanol from wheat straw. Bioresour. Technol. 2011, 102, 7500–7506. [Google Scholar] [CrossRef]
- Saritha, M.; Arora, A.; Nain, L. Pretreatment of paddy straw with Trametes hirsuta for improved enzymatic saccharification. Bioresour. Technol. 2012, 104, 459–465. [Google Scholar] [CrossRef]
- Canam, T.; Town, J.R.; Tsang, A.; McAllister, T.A.; Dumonceaux, T.J. Biological pretreatment with a cellobiose dehydrogenase-deficient strain of Trametes versicolor enhances the biofuel potential of canola straw. Bioresour. Technol. 2011, 102, 10020–10027. [Google Scholar] [CrossRef]
- García-Torreiro, M.; López-Abelairas, M.; Lu-Chau, T.A.; Lema, J.M. Fungal pretreatment of agricultural residues for bioethanol production. Ind. Crops Prod. 2016, 89, 486–492. [Google Scholar] [CrossRef]
- Taniguchi, M.; Suzuki, H.; Watanabe, D.; Sakai, K.; Hoshino, K.; Tanaka, T. Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. J. Biosci. Bioeng. 2005, 100, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Bak, J.S.; Kim, M.D.; Choi, I.G.; Kim, K.H. Biological pretreatment of rice straw by fermenting with Dichomitus squalens. N. Biotechnol. 2010, 27, 424–434. [Google Scholar] [CrossRef]
- Cianchetta, S.; Di Maggio, B.; Burzi, P.L.; Galletti, S. Evaluation of selected white-rot fungal isolates for improving the sugar yield from wheat straw. Appl. Biochem. Biotechnol. 2014, 173, 609–623. [Google Scholar] [CrossRef]
- Mishra, V.; Jana, A.K. Fungal pretreatment of sweet sorghum bagasse with combined CuSO4-gallic acid supplement for improvement in lignin degradation, selectivity, and enzymatic saccharification. Appl. Biochem. Biotechnol. 2017, 183, 200–217. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.D.S.; Ferraz, A. Biological pretreatment of sugarcane bagasse with basidiomycetes producing varied patterns of biodegradation. Bioresour. Technol. 2017, 225, 17–22. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Ge, X.; Li, Y. Fungal pretreatment of non-sterile miscanthus for enhanced enzymatic hydrolysis. Bioresour. Technol. 2016, 203, 118–123. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.L.; Tonnis, B.; Habteselassie, M.; Liao, X.; Huang, Q. Fungal pretreatment of switchgrass for improved saccharification and simultaneous enzyme production. Bioresour. Technol. 2013, 135, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, Y. Microbial delignification of corn stover by Ceriporiopsis subvermispora for improving cellulose digestibility. Enzyme Microb. Technol. 2010, 47, 31–36. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Microbial pretreatment of corn stover with Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol production. Bioresour. Technol. 2010, 101, 6398–6403. [Google Scholar] [CrossRef]
- Song, L.; Ma, F.; Zeng, Y.; Zhang, X.; Yu, H. The promoting effects of manganese on biological pretreatment with Irpex lacteus and enzymatic hydrolysis of corn stover. Bioresour. Technol. 2013, 135, 89–92. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, G.-L.; Wang, A.-J.; Ren, H.-Y.; Dong, D.; Liu, Z.-N.; Guan, X.-Y.; Xu, C.-J.; Ren, N.-Q. Fungal pretreatment of cornstalk with Phanerochaete chrysosporium for enhancing enzymatic saccharification and hydrogen production. Bioresour. Technol. 2012, 114, 365–369. [Google Scholar] [CrossRef]
- Sun, F.H.; Li, J.; Yuan, Y.X.; Yan, Z.Y.; Liu, X.F. Effect of biological pretreatment with Trametes hirsuta yj9 on enzymatic hydrolysis of corn stover. Int. Biodeterior. Biodegrad. 2011, 65, 931–938. [Google Scholar] [CrossRef]
- Saha, B.C.; Qureshi, N.; Kennedy, G.J.; Cotta, M.A. Biological pretreatment of corn stover with white-rot fungus for improved enzymatic hydrolysis. Int. Biodeterior. Biodegradation 2016, 109, 29–35. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chinn, M.S.; Sharma-Shivappa, R.R. Microbial pretreatment of cotton stalks by solid state cultivation of Phanerochaete chrysosporium. Bioresour. Technol. 2008, 99, 6556–6564. [Google Scholar] [CrossRef] [PubMed]
- Baral, N.R.; Shah, A. Comparative techno-economic analysis of steam explosion, dilute sulfuric acid, ammonia fiber explosion and biological pretreatments of corn stover. Bioresour. Technol. 2017, 232, 331–343. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process. Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol; National Renewable Energy Laboratory: Golden, CO, USA, May 2011.
- Martínez-Patiño, J.C.; Lu-Chau, T.A.; Gullón, B.; Ruiz, E.; Romero, I.; Castro, E.; Lema, J.M. Application of a combined fungal and diluted acid pretreatment on olive tree biomass. Ind. Crops Prod. 2018, 121, 10–17. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Li, Y. Solid-state anaerobic digestion of fungal pretreated Miscanthus sinensis harvested in two different seasons. Bioresour. Technol. 2015, 185, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Vasco-Correa, J. Investigation of solid-state fungal pretreatment of Miscanthus for biofuels production. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, July 2017. [Google Scholar]
- Sokhansanj, S.; Mani, S.; Turhollow, A.; Kumar, A.; Bransby, D.; Lynd, L.; Laser, M. Large-scale production, harvest and logistics of switchgrass (Panicum virgatum L.)—current technology and envisioning a mature technology. Biofuels Bioprod. Biorefin. 2009, 3, 124–141. [Google Scholar] [CrossRef]
- Edwards, W. Estimating a Value for Corn Stover; Ag Decision Maker; Iowa State University Extension: Ames, IA, USA, June 2014. [Google Scholar]
- Thoreson, C.P.; Webster, K.E.; Darr, M.J.; Kapler, E.J. Investigation of process variables in the densification of corn stover briquettes. Energies 2014, 7, 4019–4032. [Google Scholar] [CrossRef]
- Sultana, A.; Kumar, A. Optimal configuration and combination of multiple lignocellulosic biomass feedstocks delivery to a biorefinery. Bioresour. Technol. 2011, 102, 9947–9956. [Google Scholar] [CrossRef]
- Kargbo, F.; Xing, J.; Zhang, Y. Property analysis and pretreatment of rice straw for energy use in grain drying: A review. Agric. Biol. J. North. Am. 2010, 1, 195–200. [Google Scholar] [CrossRef]
- Lam, P.S.; Sokhansanj, S.; Bi, X.; Lim, C.J.; Naimi, L.J.; Hoque, M.; Mani, S.; Womac, A.R.; Ye, X.P.; Narayan, S. Bulk density of wet and dry wheat straw and switchgrass particles. Appl. Eng. Agric. 2008, 24, 351–358. [Google Scholar] [CrossRef]
- Miao, Z.; Phillips, J.W.; Grift, T.E.; Mathanker, S.K. Measurement of mechanical compressive properties and densification energy requirement of Miscanthus x giganteus and switchgrass. Bioenergy Res. 2015, 8, 152–164. [Google Scholar] [CrossRef]
- Swanson, R.M.; Satrio, J.a.; Brown, R.C.; Platon, A.; Hsu, D.D. NREL Report: Techno-Economic Analysis of Biofuels Production Based on Gasification; National Renewable Energy Laboratory: Golden, CO, USA, November 2010.
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Effects of compressive force, particle size and moisture content on mechanical properties of biomass pellets from grasses. Biomass Bioenergy 2006, 30, 648–654. [Google Scholar] [CrossRef]
- Searcy, E.; Hess, R. Uniform-Format Feedstock Supply System: A Commodity-Scale Design to Produce an Infrastructure-Compatible Biocrude from Lignocellulosic Biomass; Idaho National Laboratory: Idaho Falls, ID, USA, September 2010.
- Dupont, C.; Chiriac, R.; Gauthier, G.; Toche, F. Heat capacity measurements of various biomass types and pyrolysis residues. Fuel 2014, 115, 644–651. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol. Biofuels 2011, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Alibaba.com. Available online: www.alibaba.com (accessed on 14 October 2018).
- Davis, R.; Tao, L.; Tan, E.C.D.; Biddy, M.J.; Beckham, G.T.; Scarlata, C.; Jacobson, J.; Cafferty, K.; Ross, J.; Lukas, J.; et al. Process. Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Biological Conversion of Sugars to Hydrocarbons; National Renewable Energy Laboratory: Golden, CO, USA, October 2013.
- Saucedo-Castañeda, G.; Gutiérrez-Rojas, M.; Bacquet, G.; Raimbault, M.; Viniegra-González, G. Heat transfer simulation in solid substrate fermentation. Biotechnol. Bioeng. 1990, 35, 802–808. [Google Scholar] [CrossRef]
- Weber, F.J.; Oostra, J.; Tramper, J.; Rinzema, A. Validation of a model for process development and scale-up of packed-bed solid-state bioreactors. Biotechnol. Bioeng. 2002, 77, 381–393. [Google Scholar] [CrossRef]
- Shuler, M.L.; Kargi, F. Bioprocess. Engineering: Basic Concepts; Pearson: London, UK, 2014; ISBN 9781292025995. [Google Scholar]
- Doran, P.M. Bioprocess. Engineering Principles; Academic Press: London, UK, 2013; ISBN 9780122208515. [Google Scholar]
- Mitchell, D.A.; Berovič, M.; Krieger, N. (Eds.) Solid-State Fermentation Bioreactors – Fundamental of Desing and Operation; Springer: Heidelberg/Berlin, Germany, 2006; ISBN 978-3-540-31285-7. [Google Scholar]
- Liu, G.; Zhang, J.; Bao, J. Cost evaluation of cellulase enzyme for industrial-scale cellulosic ethanol production based on rigorous Aspen Plus modeling. Bioprocess. Biosyst. Eng. 2016, 39, 133–140. [Google Scholar] [CrossRef]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass: Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, March 2008.
- Hess, J.; Leitner, C.; Galhaup, C.; Kulbe, K.D.; Hinterstoisser, B.; Steinwender, M.; Haltrich, D. Enhanced formation of extracellular laccase activity by the white-rot fungus Trametes multicolor. In Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 2002; pp. 229–241. [Google Scholar]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Ulrich, G.D. A Guide to Chemical Engineering Process Design and Economics; Wiley: New York, NY, USA, 1984; ISBN 0471082767. [Google Scholar]
- Brown, R.C. Biorenewable Resources: Engineering New Products from Agriculture; Wiley: New York, NY, USA, 2003; ISBN 9780813822631. [Google Scholar]
- Aden, A.; Ruth, M.; Ibsen, K.; Jechura, J.; Neeves, K.; Sheehan, J.; Wallace, B.; Montague, L.; Slayton, A.; Lukas, J. Lignocellulosic Biomass to Ethanol Process. Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover; National Renewable Energy Laboratory: Golden, CO, USA, June 2002.
- Intelligen, Inc. SuperPro Designer-User’s Guide; Intelligen, Inc.: Scotch Plains, NJ, USA, 2014; Available online: http://www.intelligen.com/downloads/SuperPro_ManualForPrinting_v10.pdf (accessed on 24 July 2018).
- IndexMundi Sugar Monthly Price—US Dollars per Kilogram. Available online: www.indexmundi.com (accessed on 6 January 2019).
- Chevanan, N.; Womac, A.R.; Bitra, V.S.P.; Igathinathane, C.; Yang, Y.T.; Miu, P.I.; Sokhansanj, S. Bulk density and compaction behavior of knife mill chopped switchgrass, wheat straw, and corn stover. Bioresour. Technol. 2010, 101, 207–214. [Google Scholar] [CrossRef]
- Xu, C.; Ma, F.; Zhang, X.; Chen, S. Biological pretreatment of corn stover by Irpex lacteus for enzymatic hydrolysis. J. Agric. Food Chem. 2010, 58, 10893–10898. [Google Scholar] [CrossRef]
- Balan, V. Current challenges in commercially producing biofuels from lignocellulosic biomass. ISRN Biotechnol. 2014, 2014, 1–31. [Google Scholar] [CrossRef]
- Kazi, F.K.; Fortman, J.A.; Anex, R.; Hsu, D.D.; Aden, A.; Dutta, A.; Kothandaraman, G. Techno-economic comparison of process technologies for biochemical ethanol production from corn stover. Fuel 2010, 89, S20–S28. [Google Scholar] [CrossRef]
- Maheshwari, R.; Bharadwaj, G.; Bhat, M.K. Thermophilic fungi: Their physiology and enzymes. Microbiol. Mol. Biol. Rev. 2000, 64, 461–488. [Google Scholar] [CrossRef]
- Sokhansanj, S.; Kumar, A.; Turhollow, A.F. Development and implementation of integrated biomass supply analysis and logistics model (IBSAL). Biomass Bioenergy 2006, 30, 838–847. [Google Scholar] [CrossRef]
- Cui, Z.; Shi, J.; Wan, C.; Li, Y. Comparison of alkaline- and fungi-assisted wet-storage of corn stover. Bioresour. Technol. 2012, 109, 98–104. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Wu, S.; He, J.; Pang, C.; Deng, Y.; Dong, R. Fungal pretreatment by Phanerochaete chrysosporium for enhancement of biogas production from corn stover silage. Appl. Biochem. Biotechnol. 2014, 1907–1918. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V.; White, M.D.; Doering, A. Roll press briquetting and pelleting of corn stover and switchgrass. Trans. ASABE 2009, 52, 543–555. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V.; Tiffany, D.G. Economic and environmental analysis for corn stover and switchgrass supply logistics. BioEnergy Res. 2015, 8, 1433–1448. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V.; Schmidt, D.R. Roll press compaction of corn stover and perennial grasses to increase bulk density. Biomass Bioenergy 2013, 55, 322–330. [Google Scholar] [CrossRef]
- Wendt, L.M.; Smith, W.A.; Hartley, D.S.; Wendt, D.S.; Ross, J.A.; Sexton, D.M.; Lukas, J.C.; Nguyen, Q.A.; Murphy, J.A.; Kenney, K.L. Techno-economic assessment of a chopped feedstock logistics supply chain for corn stover. Front. Energy Res. 2018, 6, 90. [Google Scholar] [CrossRef]
- Agu, O.; Tabil, L.; Dumonceaux, T.; Agu, O.S.; Tabil, L.G.; Dumonceaux, T. Microwave-assisted alkali pre-treatment, densification and enzymatic saccharification of canola straw and oat hull. Bioengineering 2017, 4, 25. [Google Scholar] [CrossRef]
- Chico-Santamarta, L.; Godwin, R.J.; Chaney, K.; White, D.R.; Humphries, A.C. On-farm storage of baled and pelletized canola (Brassica napus L.) straw: Variations in the combustion related properties. Energy 2013, 50, 429–437. [Google Scholar] [CrossRef]
- Lu, D.; Tabil, L.G.; Wang, D.; Li, X.; Mupondwa, E. Comparison of pretreatment methods for wheat straw densification by life cycle assessment study. Trans. ASABE 2015, 58, 453–464. [Google Scholar] [CrossRef]
- Kenney, K.L.; Cafferty, K.G.; Jacobson, J.J.; Bonner, I.J.; Gresham, G.; Hess, J.R.; Ovard, L.P.; Smith, W.A.; Thompson, D.N.; Thompson, V.S.; et al. Feedstock Supply System Design and Economics for Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels; Idaho National Laboratory: Idaho Falls, ID, USA, September 2013.
- Lu, X.; Withers, M.R.; Seifkar, N.; Field, R.P.; Barrett, S.R.H.; Herzog, H.J. Biomass logistics analysis for large scale biofuel production: Case study of loblolly pine and switchgrass. Bioresour. Technol. 2015, 183, 1–9. [Google Scholar] [CrossRef]
- Martinkus, N.; Latta, G.; Morgan, T.; Wolcott, M. A comparison of methodologies for estimating delivered forest residue volume and cost to a wood-based biorefinery. Biomass Bioenergy 2017, 106, 83–94. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. Enzymatic hydrolysis of biomass at high-solids loadings—a review. Biomass Bioenergy 2013, 56, 526–544. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, Y.; Li, Y. Fungal pretreatment of yard trimmings for enhancement of methane yield from solid-state anaerobic digestion. Bioresour. Technol. 2014, 156, 176–181. [Google Scholar] [CrossRef]
- Zhao, J.; Ge, X.; Vasco-Correa, J.; Li, Y. Fungal pretreatment of unsterilized yard trimmings for enhanced methane production by solid-state anaerobic digestion. Bioresour. Technol. 2014, 158, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yu, H.; Ma, F.; Zhang, X. Biological pretreatment under non-sterile conditions for enzymatic hydrolysis of corn stover. Bioresources 2013, 8, 3802–3816. [Google Scholar] [CrossRef]
- Akin, D.E.; Rigsby, L.L.; Sethuraman, A.; Morrison, W.H.; Gamble, G.R.; Eriksson, K.-E.L. Alterations in structure, chemistry, and biodegradability of grass lignocellulose treated with the white rot fungi Ceriporiopsis subvermispora and Cyathus stercoreus. Appl. Envir. Microbiol. 1995, 61, 1591–1598. [Google Scholar]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment—a review. Renew. Sustain. Energy Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Jiao, X.; Cheng, X.; Abdullah, M.; Li, D.; Lin, Y.; Cai, Y.; Nie, F. Molecular characterization and overexpression of mnp6 and vp3 from Pleurotus ostreatus revealed their involvement in biodegradation of cotton stalk lignin. Biol. Open 2019, 8, bio036483. [Google Scholar] [CrossRef]
- Sharma, K.K.; Kuhad, R.C. Genetic transformation of lignin degrading fungi facilitated by Agrobacterium tumefaciens. BMC Biotechnol. 2010, 10, 67. [Google Scholar] [CrossRef]
- Coconi-Linares, N.; Ortiz-Vázquez, E.; Fernández, F.; Loske, A.M.; Gómez-Lim, M.A. Recombinant expression of four oxidoreductases in Phanerochaete chrysosporium improves degradation of phenolic and non-phenolic substrates. J. Biotechnol. 2015, 209, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Tao, L.; Scarlata, C.; Tan, E.C.D.; Ross, J.; Lukas, J.; Sexton, D. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Catalytic Conversion of Sugars to Hydrocarbons; National Renewable Energy Laboratory: Golden, CO, USA, March 2015.
- Kazi, F.K.; Fortman, J.; Anex, R.; Kothandaraman, G.; Hsu, D.; Aden, A.; Dutta, A. Techno-Economic Analysis of Biochemical Scenarios for Production of Cellulosic Ethanol; National Renewable Energy Laboratory: Golden, CO, USA, June 2010.
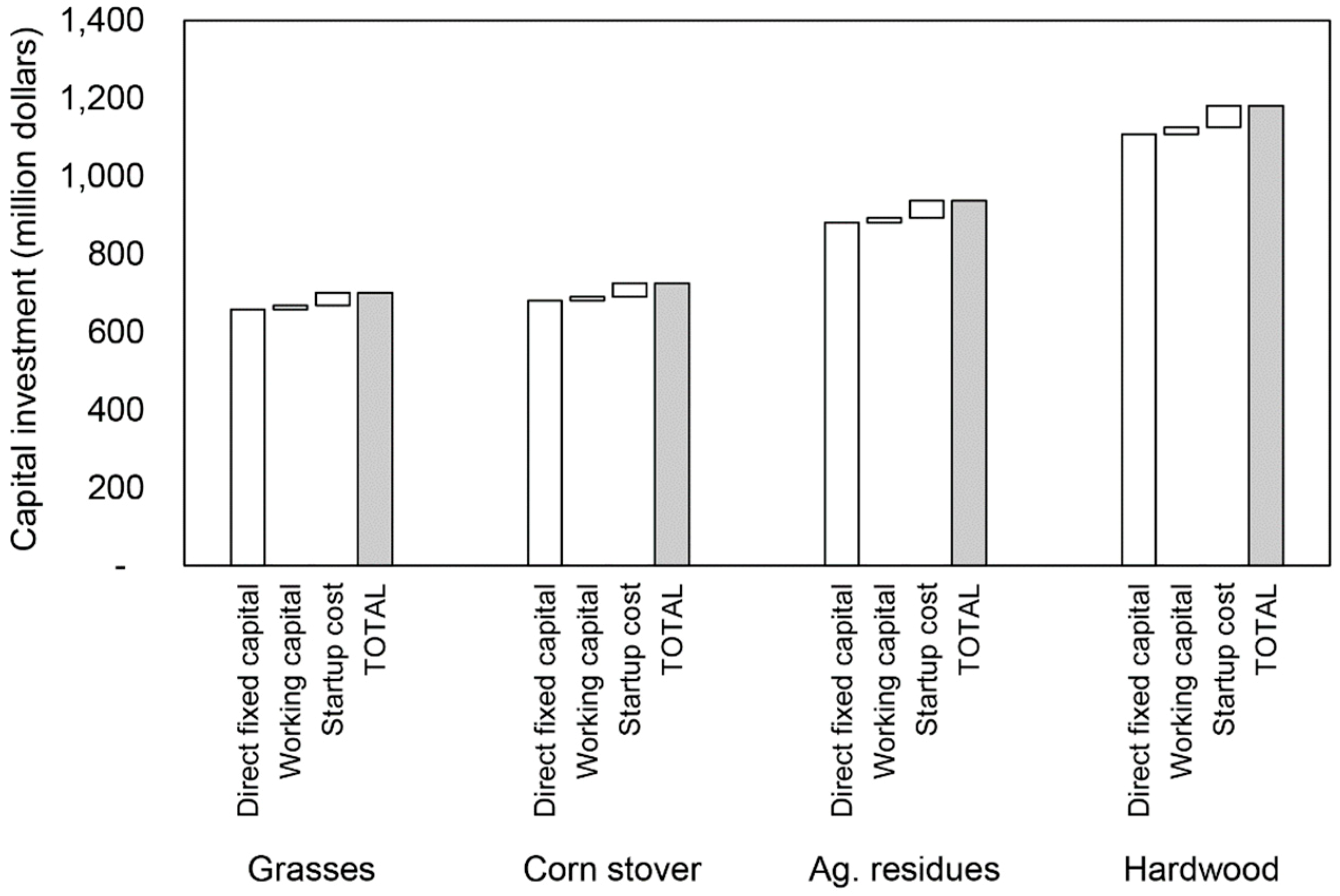
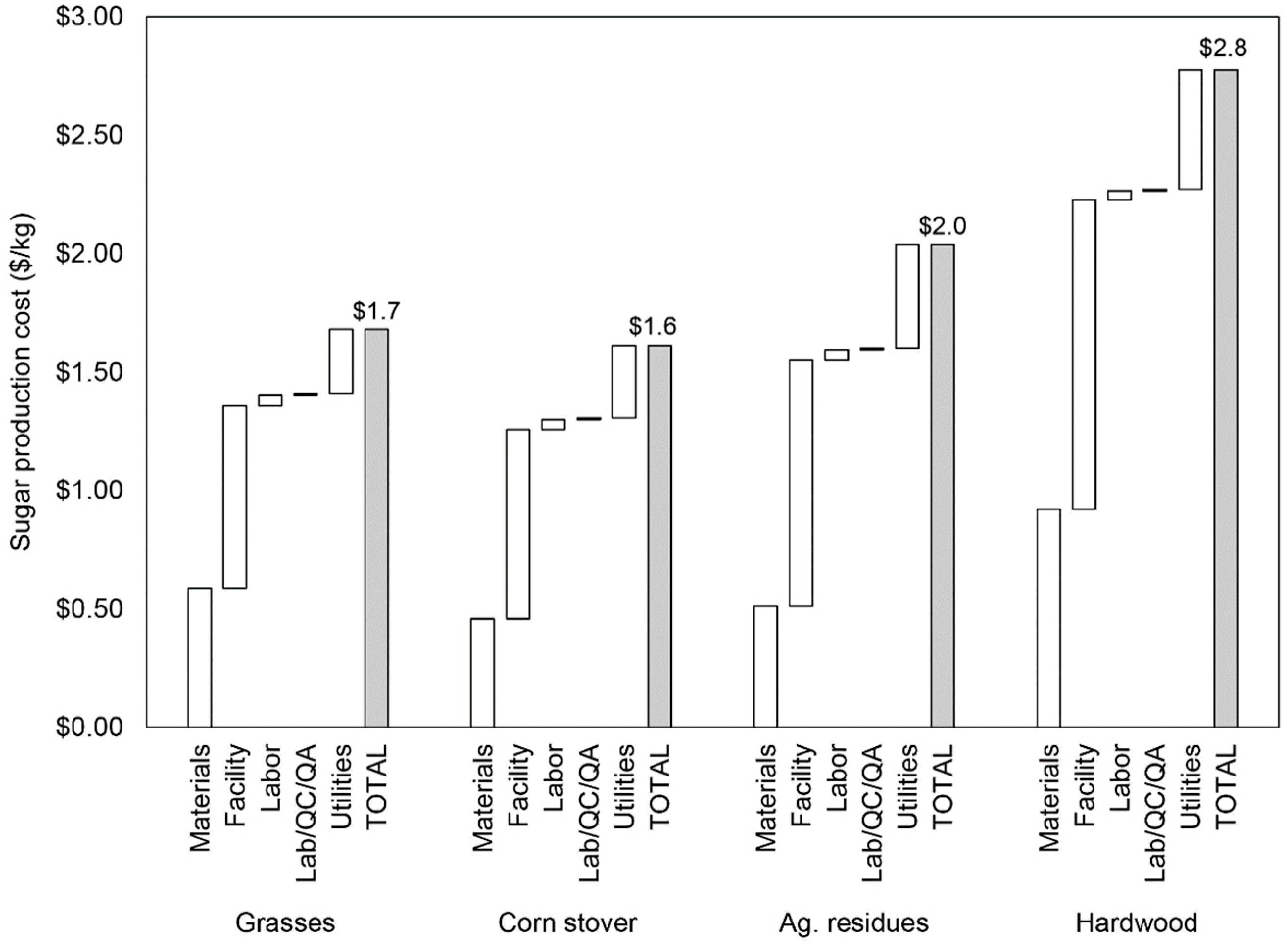
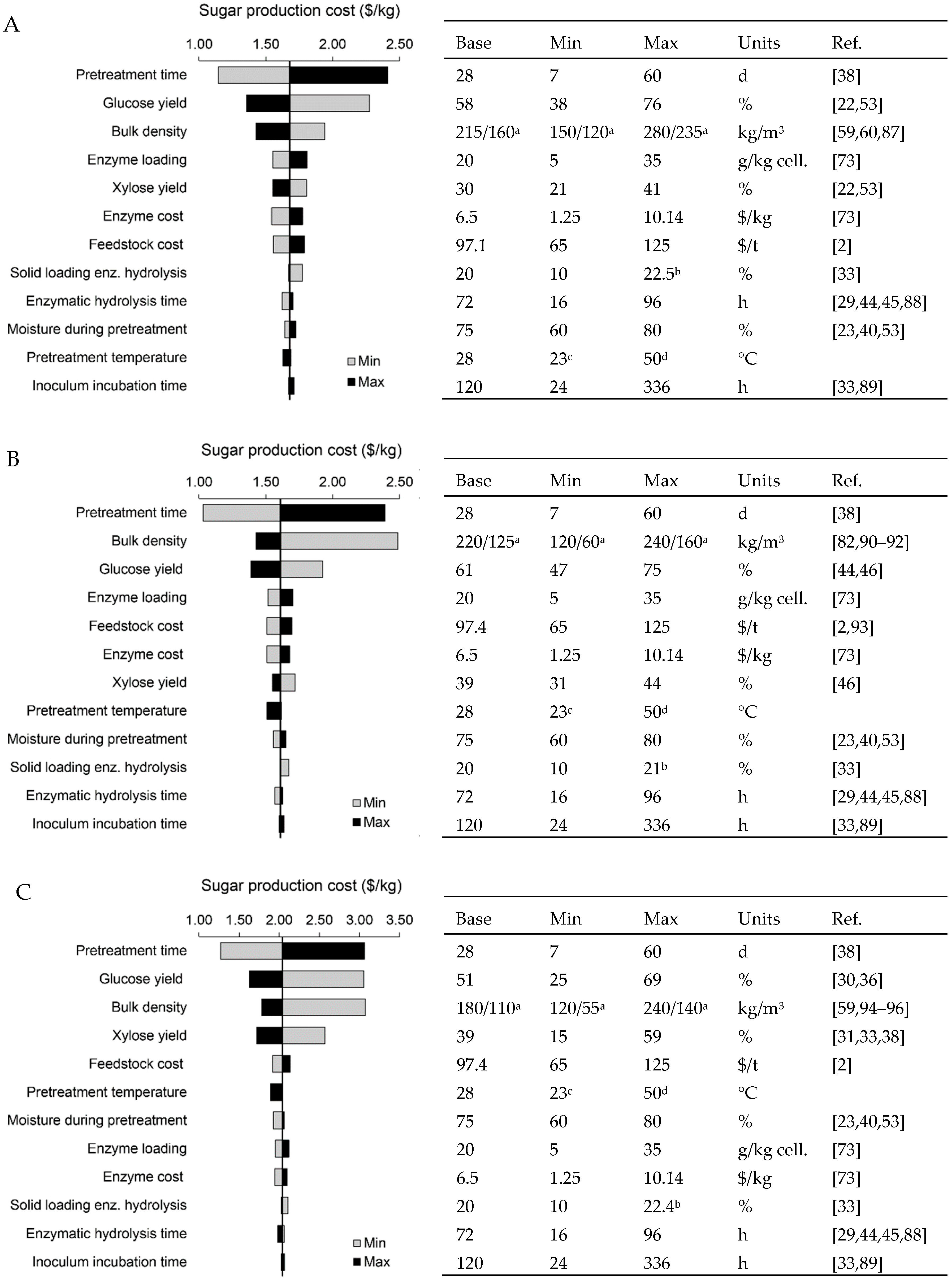
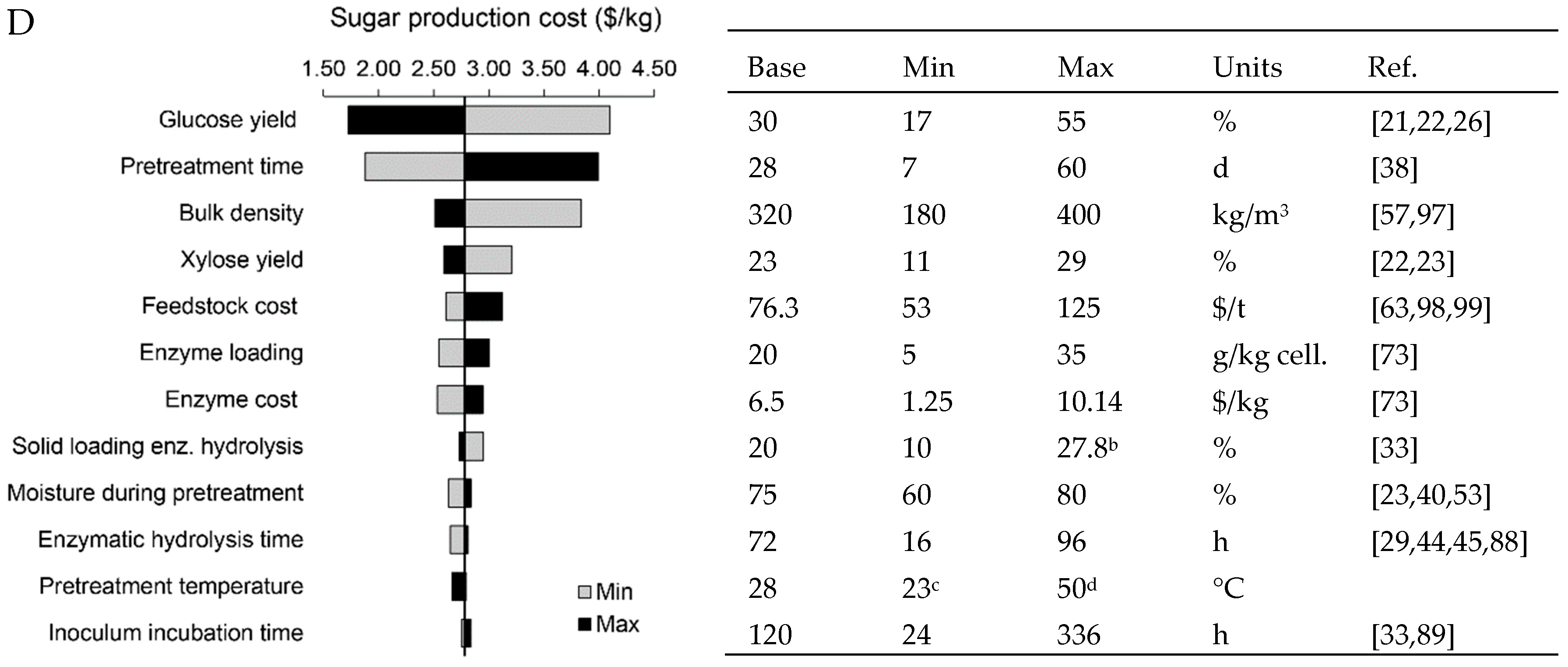
| Feedstock | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Property | Units | Grasses | Corn Stover | Ag. Residues | Hardwood | |||||
| Value | Ref. | Value | Ref. | Value | Ref. | Value | Ref. | |||
| Bulk density (baled) | kg/m3 | 215 | [54] | 220 | [55,56] | 180 | [57,58] | N/A | N/A | |
| Bulk density (grinded) | kg/m3 | 160 | [59,60] | 125 | [61] | 110 | [62] | 320 | [63] | |
| Composition | ||||||||||
| Cellulose * | 34.9 | [22,40,52,53] | 38.1 | [22,33,41,42,43,44,45,46] | 36.3 | [27,28,29,30,31,33,34,35,36,37,38] | 35.2 | [22,23,24,25,26,51,53] | ||
| Hemicellulose * | 20.8 | 25.0 | 24.5 | 16.6 | ||||||
| Lignin * | 21.5 | 19.0 | 20.5 | 23.9 | ||||||
| Ash * | 3.7 | 6.0 | 3.5 | 3.6 | ||||||
| Moisture | % | 15 | [2] | 20 | [2,50] | 15 | [2] | 50 | [57,63] | |
| Cost (facility gate) | $/dry t | 97.1 | [2] | 97.4 | [2] | 97.4 | [2] | 76.3 | [2] | |
| Specific heat | J/kg∙K | 1394 | [64] | 1395 | [64] | 1356 | [64] | 1330 | [64] | |
| Unit | Value | Reference | |
|---|---|---|---|
| Feedstock preparation | |||
| Storage residence time | days | 5 | [65] |
| Sterilization | |||
| Sterilization temperature | °C | 121 | |
| Sterilization time (time holding 121 °C) | min | 20 | |
| Total heating time | min | 40 | |
| Dry matter loss | % | 2 | [22] |
| Inoculum preparation | |||
| Medium cost | $/kg | 5 | [66] |
| Medium concentration | % | 2 | [22] |
| Aeration rate | vvm | 0.4 | [67] |
| Incubation time | h | 120 | [22,25,27] |
| Incubation temperature | °C | 28 | [14,15,16,18,19,21,25,27, 31,32,35,40] |
| Biomass yield (Yx/s) | kg fungal biomass/ kg glucose | 0.5 | [68] |
| Heat of reaction for fungal growth (ΔH°) | kJ/kg O2 | 1.4E4 | [69] |
| Fungal pretreatment | |||
| Biomass yield (Yx/s) | kg fungal biomass/ kg glucose | 0.5 | [70] |
| Residence time | days | 28 | [25,36,39,41,43] |
| Incubation temperature | °C | 28 | [14,15,16,18,19,21,25,27, 31,32,35,40] |
| Moisture | % | 75 | [21,22,27,29,33,36,37,40,41,45,51] |
| Inoculation rate | % (v/v) | 10 | [71] |
| Aeration rate | vvm | 0.01 | [72] |
| Heat of reaction for fungal growth (ΔH°) | kJ/kg O2 | 1.4E4 | [69] |
| Enzymatic hydrolysis | |||
| Reaction temperature | °C | 50 | [21,22,23,24,25,26,30,33,35,39,40,41,44,45,51] |
| Residence time | h | 72 | [22,23,26,31,35,36,37,38,39,40,41,43,46,51] |
| Solid loading | % | 20 | [50] |
| Enzyme cost | $/kg | 6.50 | [73] |
| Enzyme loading | g enzyme/ kg cellulose | 20 | [74] |
| Grasses | Corn Stover | Ag. Residues | Hardwood | |
|---|---|---|---|---|
| Fungal pretreatment | ||||
| Lignin degradation a | 20.8 | 41.3 | 33.1 | 20.2 |
| Cellulose degradation a | 1.0 | 17.3 | 33.9 | 5.2 |
| Hemicellulose degradation a | 12.8 | 40.3 | 38.3 | 18.3 |
| Total solids degradation b | 13.0 | 24.8 | 30.4 | 14.9 |
| Enzymatic hydrolysis | ||||
| Glucose yield c | 58 | 61 | 51 | 30 |
| Xylose yield c | 30 | 39 | 39 | 23 |
| Grasses | Corn Stover | Ag. Residues | Hardwood | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Annual Amount | Cost | Annual Amount | Cost | Annual Amount | Cost | Annual Amount | Cost |
| Labor (h) | 84,627 | 5.8 | 83,916 | 5.8 | 84,550 | 5.8 | 77,413 | 5.3 |
| Electricity (MWh) | 91,816 | 9.2 | 94,502 | 9.5 | 115,059 | 11.5 | 123,184 | 12.3 |
| Steam (million t) | 0.9 | 10.9 | 0.8 | 9.1 | 1.4 | 16.5 | 1.9 | 22.5 |
| Cooling water (million t) | 207.2 | 10.4 | 169.0 | 8.5 | 201.1 | 10.1 | 387.2 | 19.4 |
| Chilled water (million t) | 15.5 | 6.2 | 35.2 | 14.1 | 51.9 | 20.8 | 34.6 | 13.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasco-Correa, J.; Shah, A. Techno-Economic Bottlenecks of the Fungal Pretreatment of Lignocellulosic Biomass. Fermentation 2019, 5, 30. https://doi.org/10.3390/fermentation5020030
Vasco-Correa J, Shah A. Techno-Economic Bottlenecks of the Fungal Pretreatment of Lignocellulosic Biomass. Fermentation. 2019; 5(2):30. https://doi.org/10.3390/fermentation5020030
Chicago/Turabian StyleVasco-Correa, Juliana, and Ajay Shah. 2019. "Techno-Economic Bottlenecks of the Fungal Pretreatment of Lignocellulosic Biomass" Fermentation 5, no. 2: 30. https://doi.org/10.3390/fermentation5020030
APA StyleVasco-Correa, J., & Shah, A. (2019). Techno-Economic Bottlenecks of the Fungal Pretreatment of Lignocellulosic Biomass. Fermentation, 5(2), 30. https://doi.org/10.3390/fermentation5020030