Characterization of Saccharomyces bayanus CN1 for Fermenting Partially Dehydrated Grapes Grown in Cool Climate Winemaking Regions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Grape Harvest, Desiccation and Processing
2.3. Winemaking
2.4. Grape, Must, and Fermentation Analysis
2.5. Color Evaluation
2.6. Sensory Evaluation
2.7. Statistical Analysis
2.8. Statement of Ethics
3. Results
3.1. Yeast Strain Identification
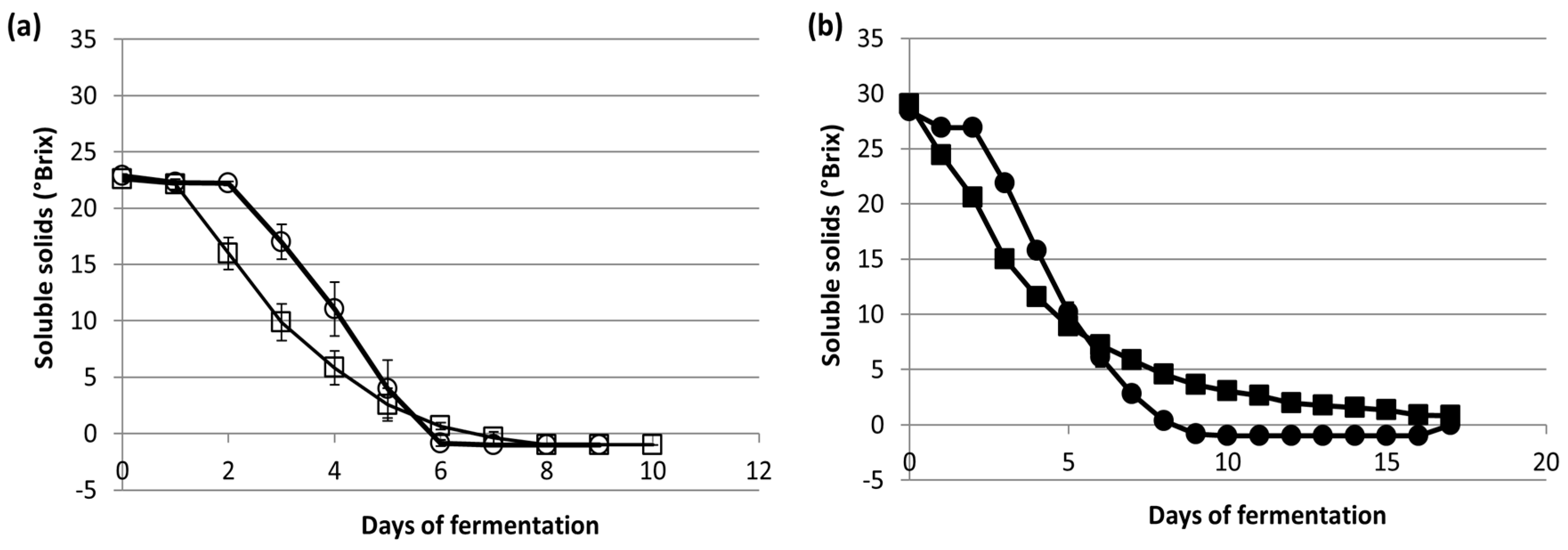
3.2. Fermentation Kinetics and Metabolites
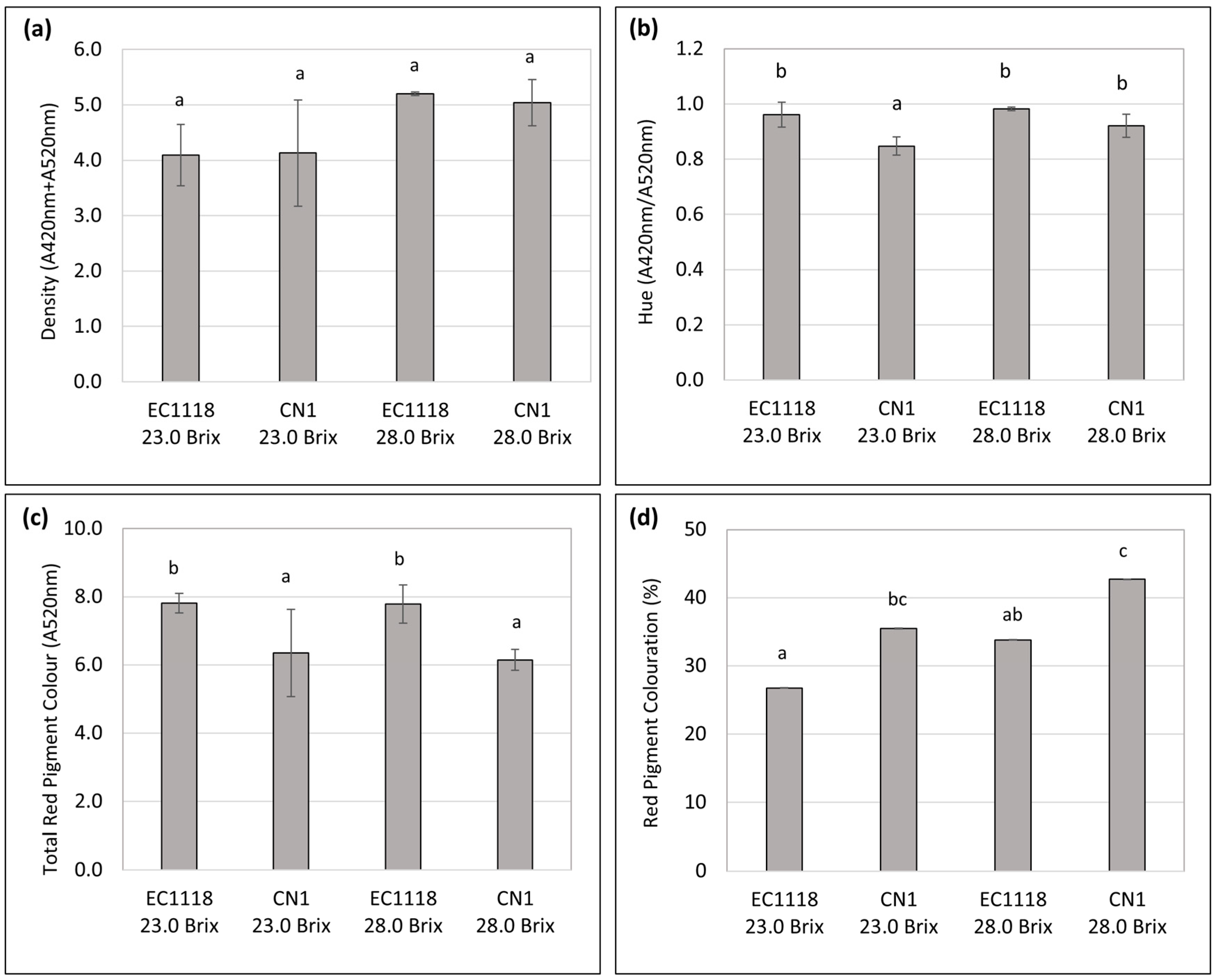
3.3. Color and Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aylward, D.K. A Documentary of Innovation Support among New World Wine Industries. J. Wine Res. 2003, 14, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Frank, A.; Eyler, R. The Economic Impact of the Wine and Grape Industry in Canada 2015. Available online: http://www.canadianvintners.com/wp-content/uploads/2017/06/Canada-Economic-Impact-Report-2015.pdf (accessed on 11 September 2018).
- Shaw, A.B. The Niagara Peninsula viticultural area: A climatic analysis of Canada’s largest wine region. J. Wine Res. 2005, 16, 85–103. [Google Scholar] [CrossRef]
- Shaw, T.B. Climate change and the evolution of the Ontario cool climate wine regions in Canada. J. Wine Res. 2017, 28, 13–45. [Google Scholar] [CrossRef]
- Cyr, D.; Kusy, M.; Shaw, A.B. Climate change and the potential use of weather derivatives to hedge vineyard harvest rainfall risk in the Niagara region. J. Wine Res. 2010, 21, 207–227. [Google Scholar] [CrossRef]
- Pagliarini, E.; Tomaselli, N.; Brenna, O.V. Study on sensory and composition changes in Italian Amarone Valpolicella red wine during aging. J. Sens. Stud. 2004, 19, 422–432. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Cancho-Grande, B.; Simal-Gándara, J. Effects on colour and phenolic composition of sugar concentration processes in dried-on- or dried-off-vine grapes and their aged or not natural sweet wines. Trends Food Sci. Technol. 2013, 31, 36–54. [Google Scholar] [CrossRef]
- Frangipane, M.T.; Torresi, S.; Santis, D.D.; Massantini, R. Effect of drying process in chamber at controlled temperature on the grape phenolic compounds. Ital. J. Food Sci. 2012, 24, 1–7. [Google Scholar]
- Bellincontro, A.; Matarese, F.; D’Onofrio, C.; Accordini, D.; Tosi, E.; Mencarelli, F. Management of postharvest grape withering to optimise the aroma of the final wine: A case study on Amarone. Food Chem. 2016, 213, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Bellincontro, A.; De Santis, D.; Botondi, R.; Villa, I.; Mencarelli, F. Different postharvest dehydration rates affect quality characteristics and volatile compounds of Malvasia, Trebbiano and Sangiovese grapes for wine production. J. Sci. Food Agric. 2004, 84, 1791–1800. [Google Scholar] [CrossRef]
- Ontario Regulation 406/00 Rules of Vintners Quality Alliance Ontario Relating to Terms for VQA Wine. Available online: https://www.ontario.ca/laws/regulation/000406/v27 (accessed on 7 September 2018).
- Heit, C. Acetic acid and ethyl acetate production during high Brix fermentations: Effect of yeast strain. Am. J. Enol. Vitic. 2013, 64, 416A. [Google Scholar] [CrossRef]
- Costantini, V.; Bellincontro, A.; De Santis, D.; Botondi, R.; Mencarelli, F. Metabolic changes of Malvasia grapes for wine production during postharvest drying. J. Agric. Food Chem. 2006, 54, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Cliff, M.A.; Pickering, G.J. Determination of odour detection thresholds for acetic acid and ethyl acetate in ice wine. J. Wine Res. 2006, 17, 45–52. [Google Scholar] [CrossRef]
- Nevoigt, E.; Stahl, U. Osmoregulation and glycerol metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 1997, 21, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, D.J.; Cliff, M.; van Vuuren, H.J.J. Impact of yeast strain on the production of acetic acid, glycerol, and the sensory attributes of Icewine. Am. J. Enol. Vitic. 2004, 55, 371–387. [Google Scholar]
- Yang, F.; Heit, C.; Inglis, D. Cytosolic redox status of wine yeast (Saccharomyces cerevisiae) under hyperosmotic stress during Icewine fermentation. Fermentation 2017, 3, 61. [Google Scholar] [CrossRef]
- Pigeau, G.M.; Inglis, D.L. Upregulation of ALD3 and GPD1 in Saccharomyces cerevisiae during Icewine fermentation. J. Appl. Microbiol. 2005, 99, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Pigeau, G.M.; Inglis, D.L. Response of wine yeast (Saccharomyces cerevisiae) aldehyde dehydrogenases to acetaldehyde stress during Icewine fermentation. J. Appl. Microbiol. 2007, 103, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Kontkanen, D.; Inglis, D.L.; Pickering, G.J.; Reynolds, A. Effect of yeast inoculation rate, acclimatization, and nutrient addition on Icewine fermentation. Am. J. Enol. Vitic. 2004, 55, 363–370. [Google Scholar]
- Garofalo, C.; Berbegal, C.; Grieco, F.; Tufariello, M.; Spano, G.; Capozzi, V. Selection of indigenous yeast strains for the production of sparkling wines from native Apulian grape varieties. Int. J. Food Microbiol. 2018, 285, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Garafalo, C.; Khoury, M.El.; Lucas, P.; Bely, M.; Russo, P.; Spano, G.; Capozzi, V. Autochthonous starter cultures and indigenous grape variety for regional wine production. J. Appl. Microbiol. 2015, 118, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Spano, G. Food microbial biodiversity and “microbes of protected origin”. Front. Microbiol. 2011, 2, 237. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Russo, P.; Spano, G. Microbial information regimen in EU geographical indications. World Pat. Inf. 2012, 34, 229–231. [Google Scholar] [CrossRef]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial terroir and food innovation: The case of yeast biodiversity in wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Rantsiou, S.; Campolongo, S.; Alessandria, V.; Rolle, L.; Torchio, F.; Cocolin, L. Yeast populations associated with grapes during withering and their fate during alcoholic fermentation of high-sugar must. Aust. J. Grape Wine Res. 2013, 19, 40–46. [Google Scholar] [CrossRef]
- Nurgel, C.; Inglis, D.L.; Pickering, G.J.; Reynolds, A.; Brindle, I. Dynamics of indigenous and inoculated yeast populations in Vidal and Riesling Icewine fermentations. Am. J. Enol. Vitic. 2004, 55, 435A. [Google Scholar]
- Eglinton, J.M.; McWilliam, S.J.; Fogarty, M.W.; Francis, I.L.; Kwiatkowski, M.J.; Hoj, P.B.; Henschke, P.A. The effect of Saccharomyces bayanus-mediated fermentation on the chemical composition and aroma profile of Chardonnary wine. Aust. J. Grape Wine Res. 2000, 6, 190–196. [Google Scholar] [CrossRef]
- Yang, F. Study of New Yeast Strains as Novel Starter Cultures for Riesling Icewine Production. Master’s Thesis, Brock University, St. Catharines, ON, Canada, November 2010. [Google Scholar]
- Nurgel, C.; Pickering, G.J.; Inglis, D.L. Sensory and chemical characteristics of Canadian ice wines. J. Sci. Food Agric. 2004, 84, 1675–1684. [Google Scholar] [CrossRef]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.; Nury, F.S. Wine Analysis and Production; Springer: New York, NY, USA, 1995; 621p. [Google Scholar]
- Iland, P.; Bruer, N.; Edwards, G.; Caloghiris, S.; Cargill, M.; Wilkes, E.; Iiland, J. Chemical Analysis of Grapes and Wine: Techniques and Concepts, 2nd ed.; Patrick Wine Promotions: Athelstone, Australia, 2013; pp. 88–89. [Google Scholar]
- Kemp, S.E.; Hollowood, T.; Hort, J. Sensory Evaluation a Practical Handbook, 1st ed.; Chichester Ames, Iowa; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Huang, C.H.; Lee, F.L.; Tai, C.J. The β-tubulin gene as a molecular phylogenetic marker for classification and discrimination of the Saccharomyces sensu stricto complex. Anton. Leeuw. 2009, 95, 135–142. [Google Scholar] [CrossRef] [PubMed]
- González, S.S.; Barrio, E.; Gafner, J.; Querol, A. Natural hybrids from Saccharomyces cerevisiae, Saccharomyces bayanus and Saccharomyces kudriavzevii in wine fermentations. FEMS Yeast Res. 2006, 6, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Través, L.; Lopes, C.A.; Querol, A.; Barrio, E. On the complexity of the Saccharomyces bayanus taxon: Hybridization and potential hybrid speciation. PLoS ONE 2014, 9, e93729. [Google Scholar] [CrossRef] [PubMed]
- Hittinger, C.T. Saccharomyces diversity and evolution: A budding model genus. Trends Genet. 2013, 29, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Sulo, P.; Szabóová, D.; Bielik, P.; Poláková, S.; Šoltys, K.; Jatzová, K.; Szemes, T. The evolutionary history of Saccharomyces species inferred from completed mitochondrial genomes and revision in the ‘yeast mitochondrial genetic code. DNA Res. 2017, 24, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, M.; Tosi, E.; Faccio, S.; Lorenzini, M.; Torriani, S.; Zapparoli, G. Selection of Botrytis cinerea and Saccharomyces cerevisiae strains for the improvement and valorization of Italian passito style wines. FEMS Yeast Res. 2013, 13, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Alessandria, V.; Giacosa, S.; Campolongo, S.; Rolle, L.; Rantsiou, K.; Cocolin, L. Yeast population diversity on grape during on-vine withering and their dynamics in natural and inoculated fermentations in the production of icewines. Food Res. Int. 2013, 54, 139–147. [Google Scholar] [CrossRef]
- Giordano, M.; Rolle, L.; Zeppa, G.; Gerbi, V. Chemical and volatile composition of three Italian sweet white passito wines. OENO ONE 2009, 43, 159–170. [Google Scholar] [CrossRef]
- Urso, R.; Rantsiou, K.; Dolci, P.; Rolle, L.; Comi, G.; Cocolin, L. Yeast biodiversity and dynamics during sweet wine production as determined by molecular methods. FEMS Yeast Res. 2008, 8, 1053–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papapetridis, I.; van Dijk, M.; van Maris, A.J.A.; Pronk, J.T. Metabolic engineering strategies for optimizing acetate reduction, ethanol yield and osmotolerance in Saccharomyces cerevisiae. Biotechnol. Biofuels 2017, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Heit, C.; Martin, S.J.; Yang, F.; Inglis, D.L. Osmoadaptation of wine yeast (Saccharomyces cerevisiae) during Icewine fermentation leads to high levels of acetic acid. J. Appl. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kilcast, D. Instrumental Assessment of Food Sensory Quality: A Practical Guide, 1st ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 1–658. [Google Scholar]
- Somers, T.C.; Evans, M.E. Wine quality: Correlations with colour density and anthocyanin equilibria in a group of young red wines. J Sci. Food Agric. 1974, 25, 1369–1379. [Google Scholar] [CrossRef]
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, phenolics, and color of Cabernet Franc, Merlot, and Pinot Noir Wines from British Columbia. J. Agric. Food Chem. 1999, 47, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Francis, L.; Noble, A.; Kwiatkowski, M.; Cheynier, V.; Waters, E. Taste and mouth-feel properties of different types of tannin-like polyphenolic compounds and anthocyanins in wine. Anal. Chim. Acta 2004, 513, 57–65. [Google Scholar] [CrossRef]
- Azzolini, M.; Fedrizzi, B.; Tosi, E.; Finato, F.; Vagnoli, P.; Scrinzi, C.; Zapparoli, G. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur. Food Res. Technol. 2012, 235, 303–313. [Google Scholar] [CrossRef]
| Gene Region | NCBI Database Strain for Sequence Comparison | GenBank Accession Number | Base Pairs * | Alignment Results | ||
|---|---|---|---|---|---|---|
| Max Score | Query Coverage | Sequence Identity | ||||
| β-tubulin | S. bayanus Strain BCRC 21818 | FJ238317.1 | 849/852 | 1555 | 100% | 99% |
| S. bayanus Strain BCRC 21964 | FJ238319.1 | 848/852 | 1550 | 100% | 99% | |
| S. bayanus Strain BCRC 21816 | FJ238316.1 | 847/852 | 1546 | 100% | 99% | |
| S. eubayanus Strain N/A | XM 018364800.1 | 815/852 | 1367 | 100% | 96% | |
| S. pastorianus Strain BCRC 21420 | FJ238324.1 | 813/852 | 1356 | 100% | 95% | |
| COXII | S. bayanus Strain CBS380T | KX657743.1 | 632/635 | 1157 | 99% | 99% |
| S. uvarum Strain CBS395T | KX657742.1 | 632/635 | 1157 | 99% | 99% | |
| S. bayanus Strain CBS380 | AP014933.1 | 632/635 | 1157 | 99% | 99% | |
| S. bayanus x S. uvarum Strain CECT1991 | JN676774.1 | 585/585 | 1081 | 91% | 100% | |
| S. eubayanus Strain CRUB1975 | KF530344.1 | 608/620 | 1079 | 97% | 98% | |
| Parameter | 23°Brix EC1118 | 23°Brix CN1 | 28°Brix EC1118 | 28°Brix CN1 |
|---|---|---|---|---|
| Reducing sugar (g L−1) | 218 ± 8 b | 198 ± 12 a | 300 ± 3 c | 301 ± 3 c |
| Glucose (g L−1) | 108 ± 4 b | 98 ± 6 a | 145 ± 2 c | 145 ± 1 c |
| Fructose (g L−1) | 111 ± 5 b | 100 ± 6 a | 155 ± 2 c | 156 ± 2 c |
| pH | 3.39 ± 0.05 a | 3.35 ± 0.01 a | 3.34 ± 0.03 a | 3.33 ± 0.03 a |
| Titratable acidity (g L−1 tartaric acid) | 5.8 ± 0.2 b | 6.1 ± 0.1 c | 4.8 ± 0.0 a | 4.9 ± 0.0 a |
| Ammonia nitrogen (mg N L−1) | 17 ± 9 b | 12 ± 2 a,b | 8 ± 1 a | 8 ± 2 a,b |
| Primary amino nitrogen (mg N L−1) | 62 ± 13 b | 47 ± 2 a | 61 ± 3 b | 63 ± 5 b |
| Ethanol (% v/v) | 0.009 ± 0.004 a | 0.005 ± 0.001 a | 0.030 ± 0.006 b | 0.031 ± 0.006 b |
| Glycerol (g L−1) | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.3 ± 0.1 b | 0.3 ± 0.0 b |
| Malic acid (g L−1) | 2.2 ± 0.3 a | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 2.0 ± 0.1 a |
| Lactic acid (g L−1) | 0.04 ± 0.00 a | 0.04 ± 0.11 a | 0.05 ± 0.00 b | 0.06 ± 0.00 b |
| Acetaldehyde (mg L−1) | <18 a | <18 a | <18 a | <18 a |
| Acetic acid (g L−1) | 0.01 ± 0.00 a | 0.00 ± 0.00 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b |
| Ethyl acetate (mg L−1) | n/d † | n/d † | n/d † | n/d † |
| Parameter | 23°Brix EC1118 | 23°Brix CN1 | 28°Brix EC1118 | 28°Brix CN1 |
|---|---|---|---|---|
| Reducing sugar (g L−1) | <0.07 a | 0.2 ± 0.0 a | <0.07 a | 15.8 ± 6.7 b |
| Glucose (g L−1) | <0.07 a | <0.07 a | <0.07 a | 1.1 ± 0.7 b |
| Fructose (g L−1) | <0.07 a | 0.1 ± 0.0 a | <0.07 a | 14.7 ± 6.0 b |
| pH | 3.78 ± 0.09 b | 3.54 ± 0.04 a | 3.74 ± 0.00 b | 3.59 ± 0.05 a |
| Titratable acidity (g L−1 tartaric acid) | 6.4 ± 0.3 a | 9.4 ± 0.3 c | 6.8 ± 0.2 a | 8.1 ± 0.3 b |
| Ammonia nitrogen (mg N L−1) | <6 a | <6 a | <6 a | <6 a |
| Primary amino nitrogen (mg N L−1) | 28 ± 3 a | 24 ± 3 a | 40 ± 2 b | 36 ± 4 b |
| Ethanol (% v/v) | 13.0 ± 0.3 a | 12.6 ± 0.4 a | 15.3 ± 0.7 b | 14.7 ± 0.2 b |
| Glycerol (g L−1) | 8.5 ± 0.4 a | 11.1 ± 0.6 b | 11.2 ± 0.1 b | 13.6 ± 0.2 c |
| Malic acid (g L−1) | 1.6 ± 0.4 a | 4.2 ± 0.2 c | 1.9 ± 0.1 a | 2.5 ± 0.1 b |
| Lactic acid (g L−1) | 0.45 ± 0.42 b | 0.04 ± 0.01 a | <0.03 a | <0.03 a |
| Acetaldehyde (mg L−1) | 56 ± 7 b | 38 ± 5 a | 88 ± 7 d | 70 ± 9 c |
| Acetic acid (g L−1) | 0.30 ± 0.02 c | 0.06 ± 0.01 a | 0.36 ± 0.02 d | 0.20 ± 0.02 b |
| Ethyl acetate (mg L−1) | 36 ± 3 b | 21 ± 3 a | 37 ± 13 b | 33 ± 2 a |
| Paired Treatments | Correct | Incorrect | Total | Significance |
|---|---|---|---|---|
| 23°Brix EC1118 vs. 23°Brix CN1 | 25 | 15 | 40 | p = 0.001 |
| 28°Brix EC1118 vs. 28°Brix CN1 | 34 | 6 | 40 | p = 0.001 |
| 23°Brix EC1118 vs. 28°Brix EC1118 | 25 | 15 | 40 | p = 0.001 |
| 23°Brix EC1118 vs. 28°Brix CN1 | 32 | 8 | 40 | p = 0.001 |
| 23°Brix CN1 vs. 28°Brix EC1118 | 26 | 14 | 40 | p = 0.001 |
| 23°Brix CN1 vs. 28°Brix CN1 | 37 | 3 | 40 | p = 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelly, J.; Yang, F.; Dowling, L.; Nurgel, C.; Beh, A.; Di Profio, F.; Pickering, G.; Inglis, D.L. Characterization of Saccharomyces bayanus CN1 for Fermenting Partially Dehydrated Grapes Grown in Cool Climate Winemaking Regions. Fermentation 2018, 4, 77. https://doi.org/10.3390/fermentation4030077
Kelly J, Yang F, Dowling L, Nurgel C, Beh A, Di Profio F, Pickering G, Inglis DL. Characterization of Saccharomyces bayanus CN1 for Fermenting Partially Dehydrated Grapes Grown in Cool Climate Winemaking Regions. Fermentation. 2018; 4(3):77. https://doi.org/10.3390/fermentation4030077
Chicago/Turabian StyleKelly, Jennifer, Fei Yang, Lisa Dowling, Canan Nurgel, Ailin Beh, Fred Di Profio, Gary Pickering, and Debra L. Inglis. 2018. "Characterization of Saccharomyces bayanus CN1 for Fermenting Partially Dehydrated Grapes Grown in Cool Climate Winemaking Regions" Fermentation 4, no. 3: 77. https://doi.org/10.3390/fermentation4030077
APA StyleKelly, J., Yang, F., Dowling, L., Nurgel, C., Beh, A., Di Profio, F., Pickering, G., & Inglis, D. L. (2018). Characterization of Saccharomyces bayanus CN1 for Fermenting Partially Dehydrated Grapes Grown in Cool Climate Winemaking Regions. Fermentation, 4(3), 77. https://doi.org/10.3390/fermentation4030077






