1. Introduction
It is well established that the protein content and specific amino acid composition play a vital role in human and animal nutrition. Protein content and amino acid composition of animal feed diets also has a significant impact on growth rate, overall dietary protein need, and ultimately on environmental nitrogen release. Proper selection and formulation of the protein sources, in order to meet specific nutritional requirements, play a critical role in efficient and economic animal production.
Agricultural materials used in animal feed diets vary significantly in their nutritional content and value. Typically, feeds that are higher in protein concentration are more valuable; however, the amino acid profile also plays an important role in determining cost. Supplementation of animal diets with certain amino acids, such as
l-lysine or
l-threonine, has been shown to reduce the dietary crude protein requirements [
1]. A low lysine level in a certain feed could reduce its value further, due to the need for additional supplementation to balance the amino acid profile. Corn and corn-based feeds fall into this category, as they have a relatively low
l-lysine content and typically require blending or supplementation.
Ethanol production utilizing corn has greatly expanded the availability of distillers dried grains with solubles (DDGS). While the yeast component improves the nutritional value of the DDGS somewhat, the amino acid profile is still lacking in a number of essential amino acids including
l-lysine [
2].
l-lysine is a very important feed additive, as it is essential in animal diets. Chemical production of
d,
l-lysine has long been known; however,
l-lysine production is currently done using microbial fermentations. Industrial production of
l-lysine has been done using auxotrophic microbial mutants of
Corynebacterium glutamicum since 1958 [
3]. Currently,
l-lysine production exceeds 2 million metric tons per year and is expected to reach almost 3 million by 2020 [
4]. Other amino acids, such as
l-glutamate,
l-tryptophan and
l-threonine, can also be produced utilizing different variants of
Corynebacterium glutamicum [
5].
Media composition and feedstock selection for production of biobased products by fermentation can have a substantial impact on the overall economics. Soluble sugar feedstocks can have advantages relative to conversion of starchy feedstocks such as corn or grain sorghum. Sugarcane, sweet sorghum and tropical maize are all potential sources for soluble sugar feedstocks for biobased products.
The utilization of sweet sorghum juice (SSJ) for the small-scale production of lysine by fermentation could potentially be used to upgrade animal feeds that have low lysine contents. Mixing the lysine containing fermentation broth with a nearly dry product such as corn or sorghum DDGS from ethanol production could result in an improved lysine DDGS product having substantially higher value.
Many different production strains for lysine production have been developed with different levels of auxotrophy and conversion efficiencies.
Corynebacterium glutamicum ATCC 21513 is an auxotrophic mutant requiring
l-homoserine,
l-leucine, biotin, thiamin and pantothenic acid for growth [
6]. The research reported here investigates the production of
l-lysine using SSJ by fermentation using
Corynebacterium glutamicum ATCC 21513. Yields are compared with defined media having equivalent sugar and nitrogen content to investigate any potential inhibitory effects of the SSJ.
2. Materials and Methods
2.1. Materials
The SSJ was produced during the 2015-growing season by Delta Biorenewables and was frozen immediately after pressing and coarse filtering. The juice was later thawed and centrifuged, and the supernatant filtered through Whatman GF/F glass fiber filter prior to use in fermentation media. Corynebacterium glutamicum ATCC 21513 was obtained from the American Type Culture Collection. The culture was maintained by streaking on nutrient agar and storage at −80 °C in glycerol. Lysine (>98%), glucose, fructose, buffer salts and media components were obtained from Sigma-Aldrich (St. Louis, MO, USA). Amino acid reagents and standards were purchased from Agilent Technologies (Santa Clara, CA, USA). BD Bacto™ yeast extract and peptone were purchased from Fisher Scientific (Pittsburgh, PA, USA).
2.2. Shake Flask Fermentations
Shake flask fermentations were done in 125 mL Erlenmeyer flasks utilizing 25 mL of SSJ that had been either autoclaved or sterile filtered through 0.22 μ filters. Juice was inoculated with 0.5 mL of an overnight liquid culture grown in nutrient broth that had been inoculated from a single colony grown on nutrient agar. Flasks were incubated at 30 °C in a New Brunswick™ orbital shaking incubator at 250 rpm with foam stoppers.
2.3. Stirred Tank Bioreactor
Fermentations were done using utilizing an Applikon Biotechnology controller with a 2-liter capacity vessel filled with 1 liter of SSJ or defined media. Air was sparged at 1 liter per min into the vessel and the dissolved oxygen levels maintained at 40% by cascading to speed control. The pH was maintained at 7.0 with the addition of 5 M ammonium hydroxide.
2.4. Media
Media were prepared based on fermentation media described in [
7]. SSJ was supplemented with the following per liter: CaCl
2·2H
2O (1 g), (NH
4)
2SO
4 (30 g), MgSO
4·7H
2O (0.4 g), NaCl (0.05 g), MnSO
4·H
2O (0.0076 g), FeSO
4·7H
2O (0.001 g), KH
2PO
4 (1 g), K
2HPO
4 (1 g), urea (2 g), yeast extract (1.5 g), peptone (2 g), thiamine (0.2 mg),
d-biotin (0.5 mg), and
l-serine (0.1 mg). The defined media were prepared in deionized (DI) water instead of SSJ, and
d-glucose (70g) and
d-fructose (50g) were added, in addition to the supplements used with SSJ. The ammonium sulfate was prepared at 10× concentration in 100 mL of DI water and sterilized separately. It was then added to the bioreactor after autoclaving.
A richer growth medium with higher levels of yeast extract and peptone was also used with the SSJ as well as with the defined media. This medium utilized the same levels as above but the yeast extract and peptone contents were increased to 20 g/L and 4 g/L, respectively.
2.5. Inoculum Preparation
Inoculum for the bioreactors was prepared in two 250 mL Erlenmeyer flasks with 50 mL of BD Difco™ nutrient broth using a single colony from a petri plate culture. Flasks were incubated at 30 °C in a New Brunswick™ orbital shaking incubator at 250 rpm for 48 h.
2.6. HPLC Analysis for Lysine and Sugars
Sugars (glucose and fructose), organic acids (lactic, succinic and acetic acid) and amino acids were quantitated using HPLC analysis as previously described in Johnston and McAloon [
8]. Samples for lysine quantitation were diluted in DI water as necessary to be within the calibrated range (below 0.25 g/L). Duplicate injections were made for all samples.
2.7. Biomass Determination
Culture biomass contents were determined using 5 mL samples of culture media. The media were centrifuged, and the supernatant removed by aspiration. The pellet was washed one time with 5 mL of DI water and then transferred to a tared weigh boat and dried at 135 °C for 2 h to determine dry weight. Determinations were done in duplicate and results averaged.
2.8. Statistical Analysis
Fermentations in shake flasks and bioreactors were done in duplicate, and chemical analyses were performed at least in duplicate. A t-test was used to determine statistical significance between treatments at p ≤ 0.05. Mean and standard deviations are reported.
3. Results and Discussion
3.1. Shake Flask Cultivation for Lysine Biosynthesis
Initial experiments for lysine production were done in shake flasks.
Table 1 shows the cell mass, lysine produced, sugars and final pH of the flasks. Initial pH of the SSJ was adjusted to pH 6.5 and the final pH was measured to determine if there were changes. Using non-supplemented SSJ demonstrated that the juice alone was inadequate to support any significant growth of the organism.
Corynebacterium glutamicum ATCC 21513 is an auxotrophic mutant requiring
l-homoserine,
l-leucine, biotin, thiamin and pantothenic acid for growth [
6]. Supplementation with either urea or ammonium sulfate to alleviate the limited nitrogen content of the SSJ did allow growth and minimal lysine production. The growth and sugar utilization further indicated that additional supplementation was necessary.
Addition of yeast extract, which contains some quantity of the required nutrients, at 3 and 5 g/L, in addition to the nitrogen supplementation, improved growth and lysine production significantly. The level of production of organic acids, such as lactic acid, by the organism was so high that it was no longer possible to adequately maintain the pH within acceptable conditions for complete utilization of sugars in the SSJ (approximately 12% w/v).
The information developed from the shake flask experiments clearly demonstrated that it was necessary to better maintain the pH for optimal lysine production. Addition of a phosphate buffer helped but still did not allow complete sugar utilization either. Utilization of a pH-controlled bioreactor would therefore be necessary in order to better study the use of sweet sorghum juice for lysine production.
3.2. Lysine Biosynthesis in Stirred Tank Bioreactors (Using Low Supplementation Medium)
The defined media were made by utilizing the same components added to the SSJ, and additionally included glucose and fructose at concentrations similar to those in the SSJ. This was done in order to perform a comparison with the SSJ to determine if there were any inhibitory components present in the SSJ that may impact the growth or lysine production. SSJ is known to contain about 1% trans-aconitic acid [
9]. The cis- form of aconitic acid is an intermediate in the conversion of citrate to isocitrate; however, the trans- form commonly accumulates in range grasses, including sorghum [
10,
11]. Trans aconitic acid has been shown to have a range of inhibitory activities from antinutritional and antifeedant, nematicidal and antileishmanial properties [
10,
12,
13,
14,
15].
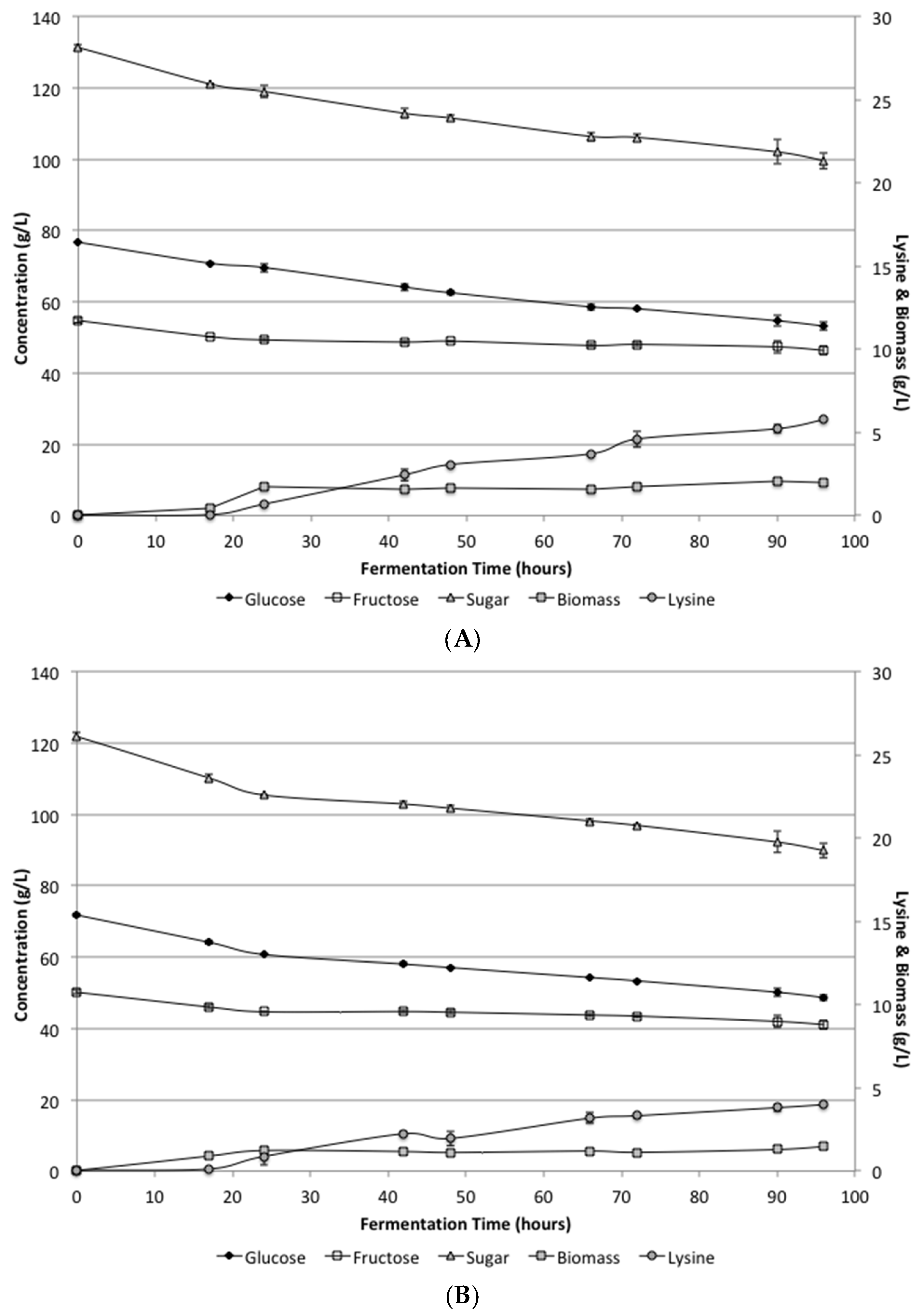
At the lower levels of yeast extract (1.5 g/L) and peptone (2.0 g/L) supplementation, the defined media and the supplemented SSJ both reached similar levels of lysine production.
Figure 1 shows the biomass, lysine, glucose and fructose levels during the fermentation period. While growth did occur, the lysine levels were much lower than expected. The average lysine levels for the SSJ and defined media after 96 h were 5.8 ± 0.06 and 4.0 ± 0.04 g/L, respectively. Additionally, there was still considerable unfermented sugar remaining in the bioreactor. Under these conditions, only about 25% of the total sugars available were utilized in the SSJ and in the defined media. These results were somewhat surprising, because shake flask experiments indicated that pH control was likely the problem with having incomplete sugar conversion, which should have resulted in increased lysine production.
Analysis of the biomass levels produced with the low supplementation indicated that there was still inadequate cell growth to complete the fermentation during the 96 h utilized. Additionally, a careful analysis of the rates further indicated that sugar was still being consumed and lysine was being produced at the same rates when the fermentation was stopped. The continued utilization was similar for both the defined media and the SSJ indicating that there was no apparent inhibition of the organism under these conditions.
3.3. Lysine Biosynthesis in Stirred Tank Bioreactors (Using High Supplementation Medium)
The results with the lower level of supplementation indicated that additional nutrients were necessary for adequate growth of the organism in SSJ. To address this issue, additional fermentations were done using higher levels of yeast extract and peptone, 20 g/L and 4.9 g/L respectively. It was likely that the levels of other added components were sufficient, and therefore, these were kept at the same levels as in the previous fermentation.
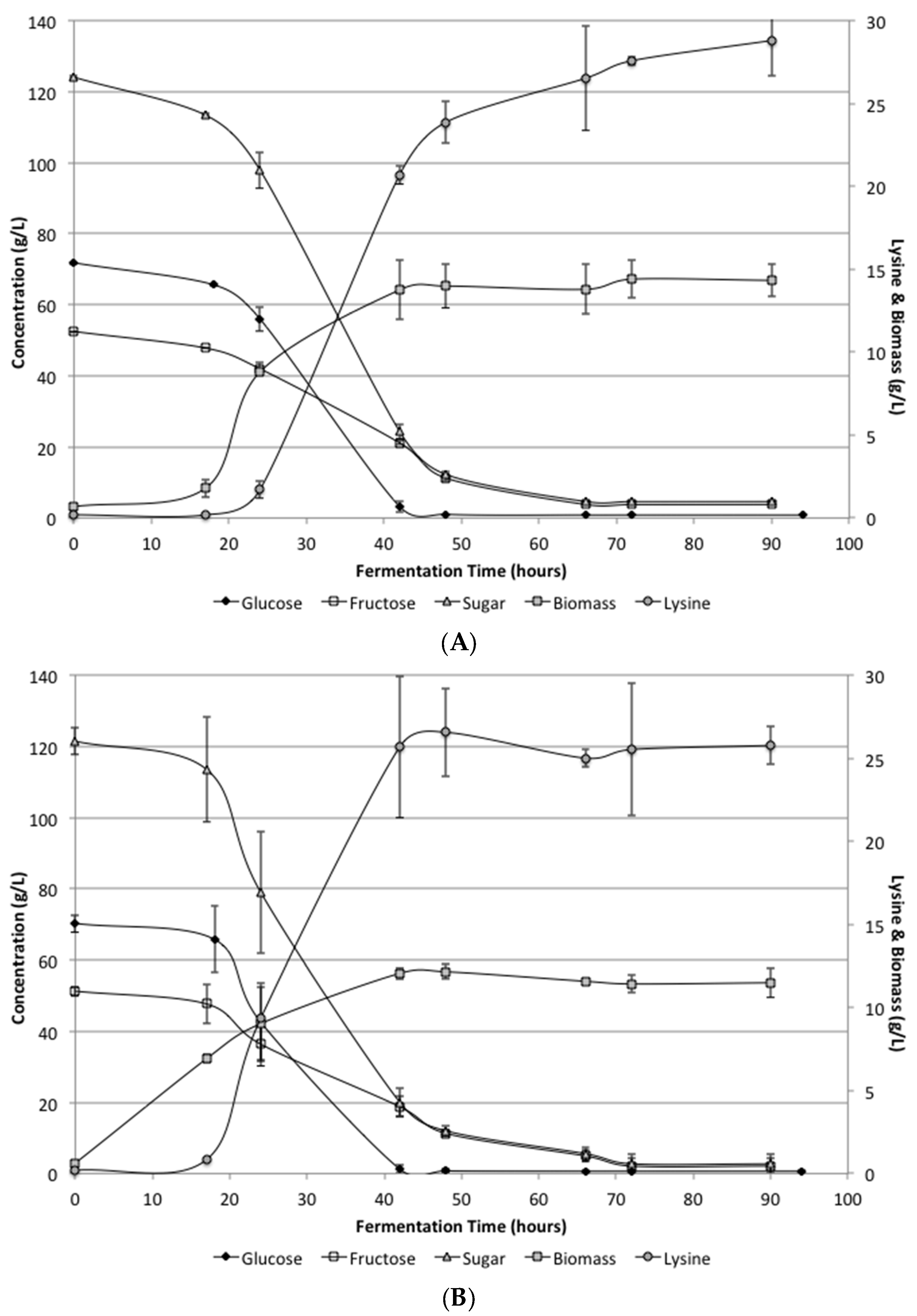
The defined media and SSJ fermentations again both showed nearly identical growth and conversion rates.
Figure 2 shows the biomass, lysine, glucose and fructose levels during the fermentation period. Unlike the low supplementation fermentation, these results showed complete utilization of the sugars. Glucose was completely consumed by 48 h. Fructose was utilized somewhat slower than glucose, but all sugars were consumed by 72 h. Biomass levels were determined during the fermentation and reach 12 g/L by 48 h in the defined media and 14 g/L in the SSJ fermentations.
Lysine levels were increased significantly over the low supplementation results and resulted in final concentrations in the defined media and SSJ of 25.7 ± 1.17 and 28.8 ± 2.14 g/L, respectively. There was a difference between the defined media and SSJ on average; however, it was not found to be statistically significant. The conversion efficiency for the defined media and SSJ were determined to be 0.21 and 0.23 g lysine/g sugar, respectively. Lysine concentrations and conversion efficiencies would likely be improved if a fed-batch system were to be utilized.