2. Beer Flavour Production by Yeast
The aroma of beer (and spirits–details later) is the sum of several hundreds of flavour-active compounds produced during every stage of the brewing process [
3,
4]. The great majority (not all) of these substances are yeast secondary metabolites. They are produced during wort (malt extract) fermentation and consists of fermentation intermediates and by-products (metabolites). Higher alcohols (also called fusel oils), esters, vicinal diketones (VDKs) other carbonyls and sulphur compounds are the key (not all) flavour elements produced by yeast. These compounds (plus malt and hop constituents) determine a beer’s final quality, particularly when it is fresh [
6]. Higher alcohols and esters are desirable volatile beer constituents, with a few exceptions [
7]. Together with these compounds, yeast wort metabolism contributes to the biosynthesis of these other groups of beer flavour active compounds: organic acids, sulphur compounds (both organic and inorganic) and aldehydes [
8,
9]. This is an area of yeast activity that is currently termed metabolomics [
10]. Metabolomics is the “systematic study of the unique chemical fingerprints that specific cellular processes leave behind”. It is the study of relatively small-molecule metabolite profiles [
11]. Many of these small-molecules contribute to beer (and spirit) flavour profiles. Esters (and higher alcohols) are major contributors to beer (and spirit) flavour profile [
12] along with a plethora of other metabolites.
Obviously, flavour-active compounds must be maintained within certain limits. Otherwise, a single compound or group of compounds (for example, VDKs) may predominate and prejudice a beer’s flavour balance. Furthermore, flavour compounds such as esters often act in synergy with other compounds to affect beer flavour in concentrations well below their individual threshold values [
13,
14,
15].
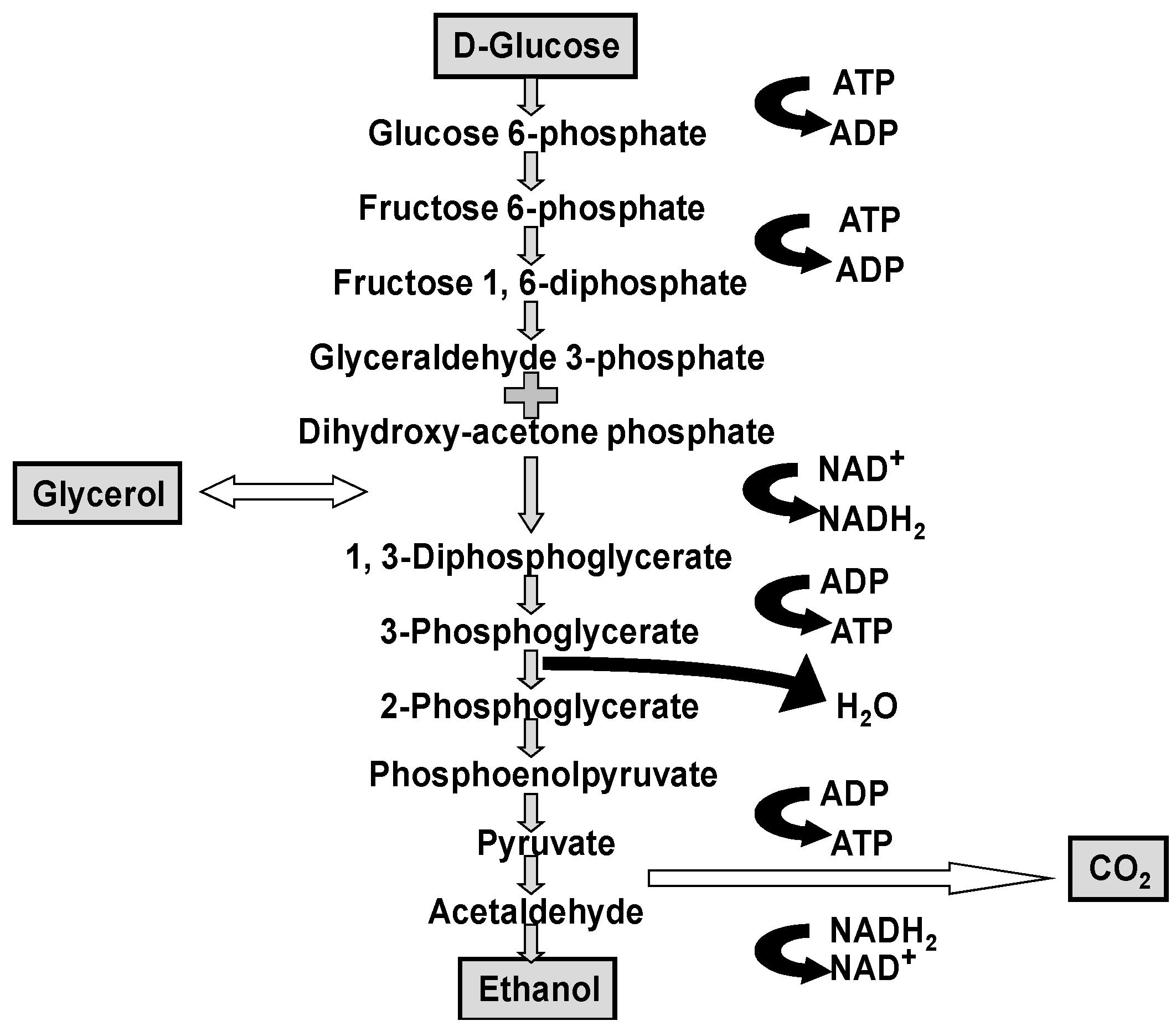
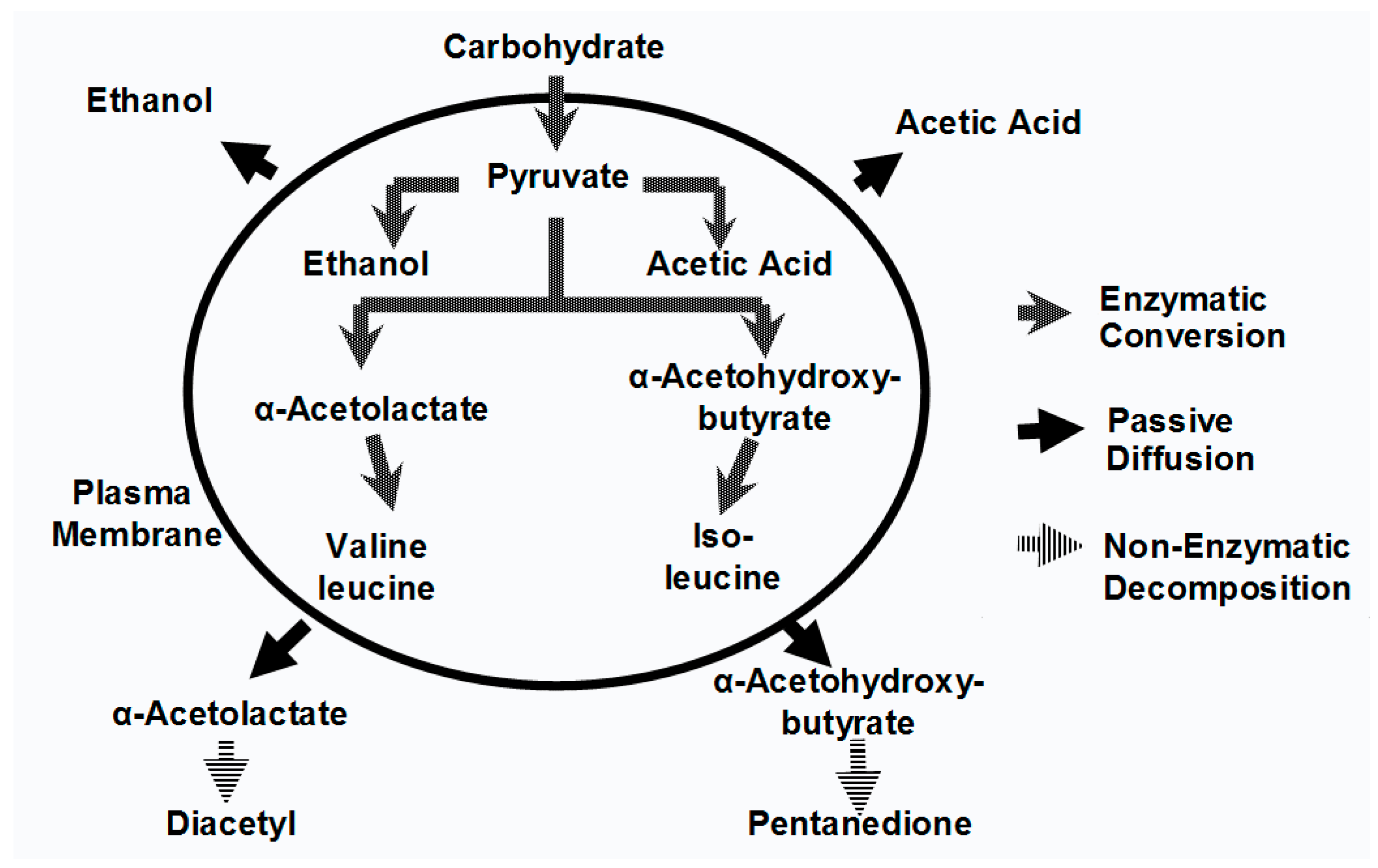
Although it is worth repeating that ethanol, carbon dioxide and glycerol are the major products produced by yeast during wort fermentation (
Figure 1), they have minimal impact on beer flavour [
1]. It is the type and concentration of other metabolites (excretion products), already discussed, which primarily determine beer flavour balance. Yeast strain, malt variety and type, fermentation temperature, adjunct (unmalted cereals and sugar) and percentage in the grist, fermenter design and geometry, wort pH, buffering capacity, wort concentration (gravity), etc., are all influencing factors particularly regarding VDK metabolism [
16]. In addition, hop variety and hopping procedures are important factors [
17].
Each type of beer has its own unique aroma triggered (but not exclusively) either by the yeast strain employed during the fermentation [
18,
19,
20] or by a plethora of process parameters used during fermentation [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30]. Only isoamyl acetate (banana-like aroma) concentration is usually above the threshold level in most lager beers, ales normally have ethyl acetate (solvent-like aroma) and ethyl hexanoate (apple-like aroma) as supplementary flavouring compounds with levels above their taste threshold [
29]. However, compounds such as diacetyl and other VDKs (with certain exceptions) should usually be below their flavour threshold values. Diacetyl contributes negatively to a buttery (stale milk) flavour in most beers.
Table 1 lists threshold values for the main esters and higher alcohols present in ale and lager beers [
4].
The biosynthesis and process variables of higher alcohols and esters during wort fermentation have been extensively reviewed by Pires and colleagues [
30] and the following discussion has been employed in this publication (with permission) (
Table 2). This also includes the effects of maltose concentration (compared to glucose) (
Table 3) in a synthetic medium on ester formation [
1,
30]. Reduced ester formation in wort has also been documented. In addition, material compiled in “An Introduction to Brewery Science and Technology” published by the Institute of Brewing and Distilling has been employed here [
1] together with “Whisky: Technology, Production and Marketing” [
2]—with permission.
3. Higher Alcohols
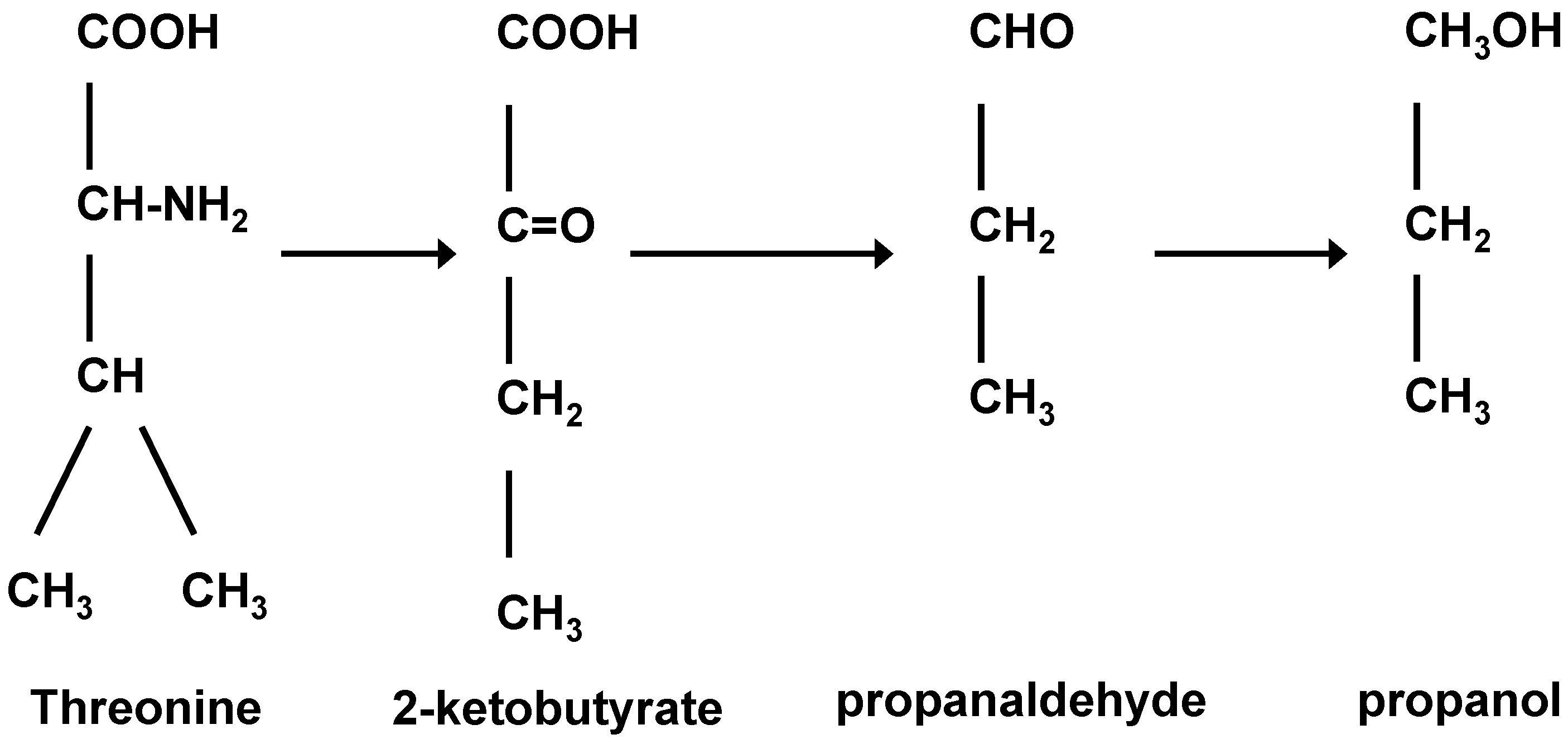
In flavour terms, the higher alcohols that occur in beer (and many spirits) are: n-propanol, isobutanol, 2-methyl-1-butanol, 3-methyl-1-butanol together with more than 40 further alcohols, have been identified. Regulation of higher alcohol biosynthesis is complex since they either have been produced as by-products of amino acid metabolism or via pyruvate and ethanol produced from carbohydrate metabolism (
Figure 1 and
Figure 2) [
39].
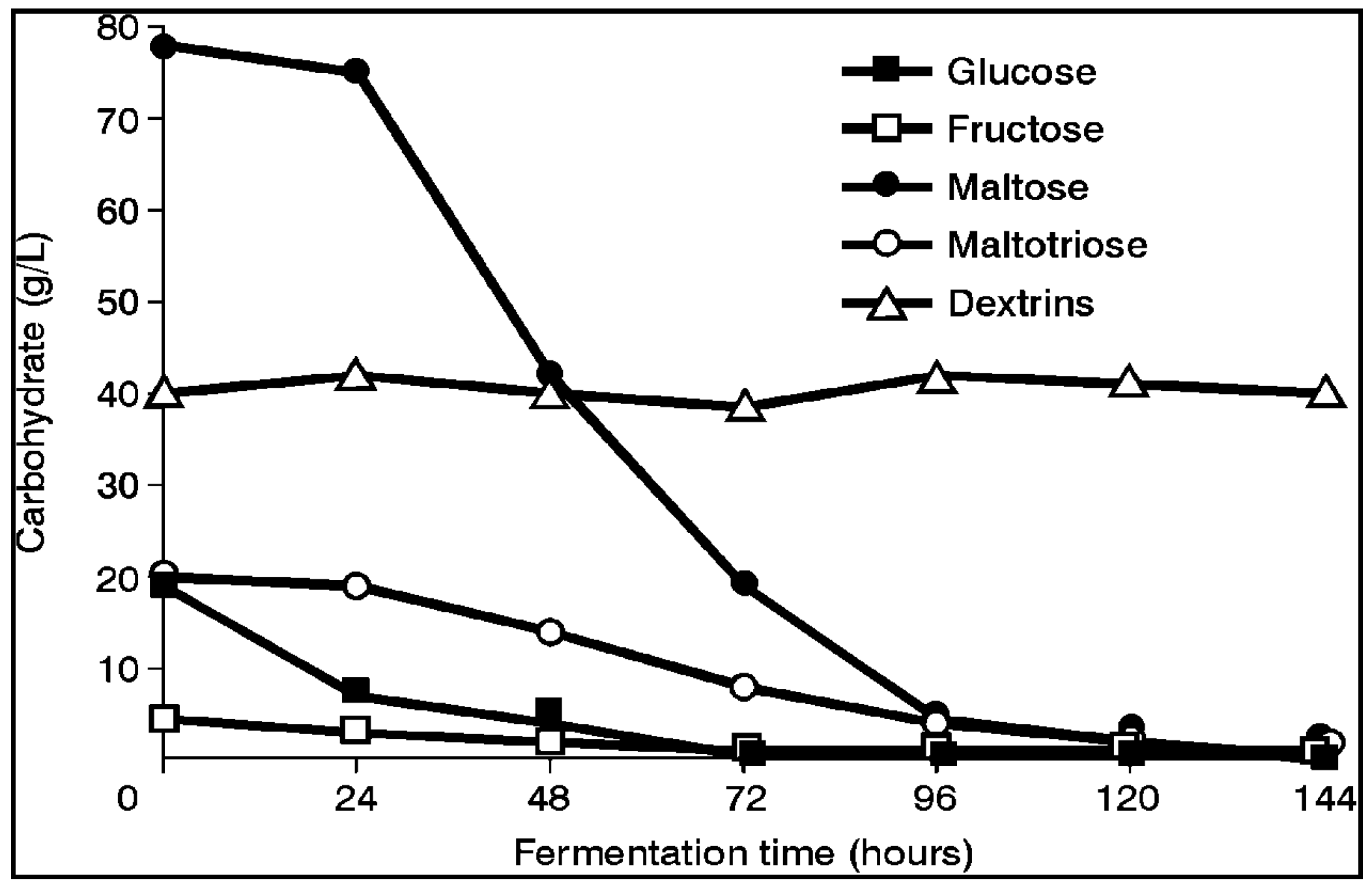
Both brewer’s and distiller’s yeast strains (similar to the five wort sugars (glucose, fructose, sucrose, maltose and maltotriose)—
Figure 3) [
1] absorb wort’s spectrum of amino acids and small peptides in a distinct order (
Figure 4 and
Table 4) [
40,
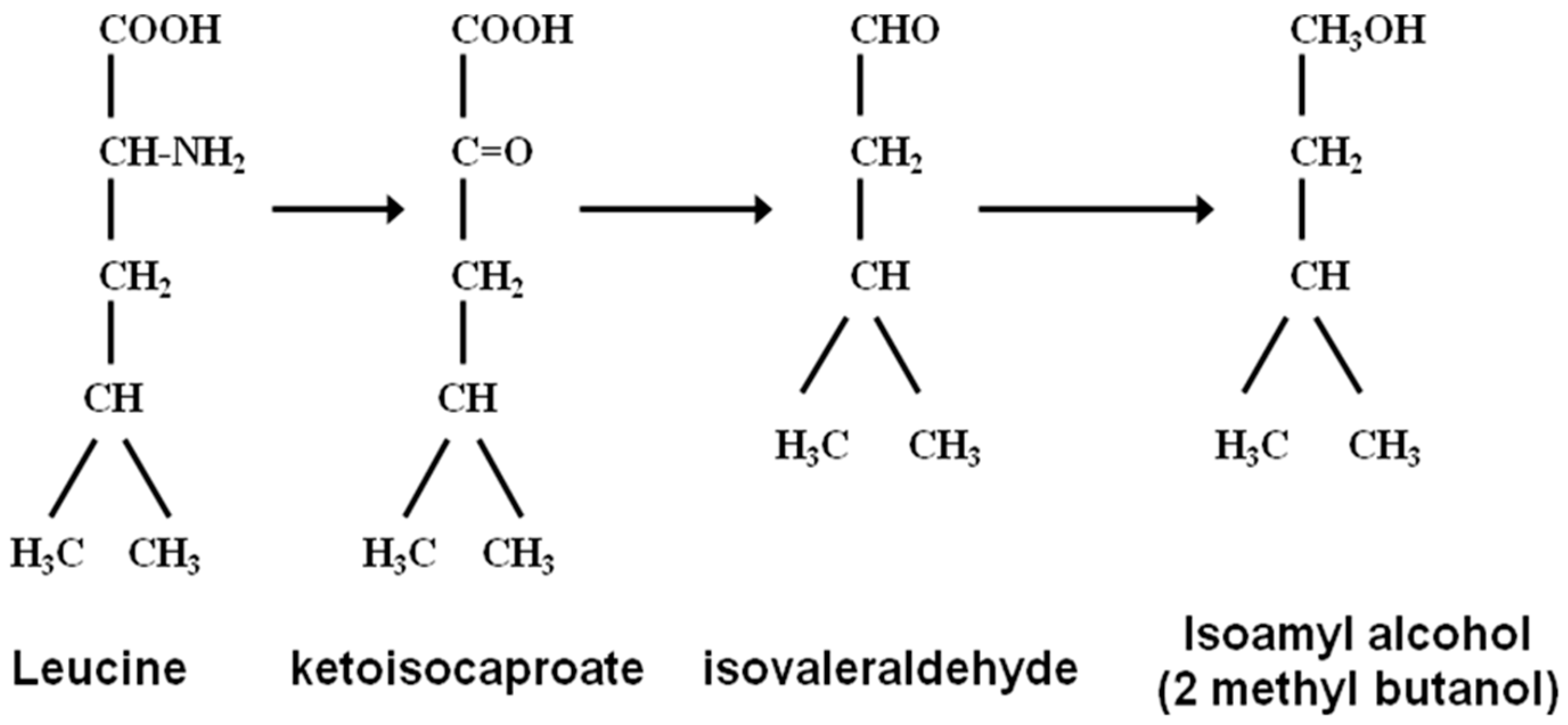
41] from which they take the amino group (Ehrlich Pathway) so that they can be incorporated into their own structures. What remains from the amino acids are α-keto acids which enter into an irreversible chain reaction that will ultimately form higher alcohols. This metabolic pathway was originally suggested by the German physician Paul Ehrlich (
Figure 5) who was intrigued with the structural molecular similarities between amyl alcohol and isoleucine and isoamyl alcohol with leucine (
Figure 6 and
Figure 7). Ehrlich was awarded the 1908 Nobel Prize in Physiology or Medicine for his contribution to immunology. His observations led to an investigation of the relationship between these amino acids and higher alcohol synthesis. When he supplemented a fermenting medium with these amino acids, increased higher alcohol formation was observed. Ehrlich also proposed that amino acids were enzymatically hydrolyzed to form the corresponding higher alcohol, along with ammonia and carbon dioxide. As ammonia was not detected in the medium, it was assumed that it was incorporated directly into yeast proteins. Subsequently, Neubauer and Fromherz [
42] proposed a few intermediate steps to the Ehrlich Pathway, completing a metabolic pathway that is currently accepted. However, a detailed enzymatic chain reaction was demonstrated. Several decades after Ehrlich’s original studies [
43] when the enzymatic sequence for the Ehrlich Pathway—transaminase, decarboxylase and alcohol dehydrogenase a detailed metabolic pathway for the Ehrlich Pathway has been elucidated. Although this pathway is the most studied in this context, higher alcohols are also formed during the upstream (anabolic pathway) biosynthesis of amino acids [
44,
45]. The most important specific pathway in the brewing context is the de novo synthesis of branched-chain amino acids (BCAAs) through the isoleucine-leucine (ILV) pathway (
Figure 7) [
39,
45].
4. Esters
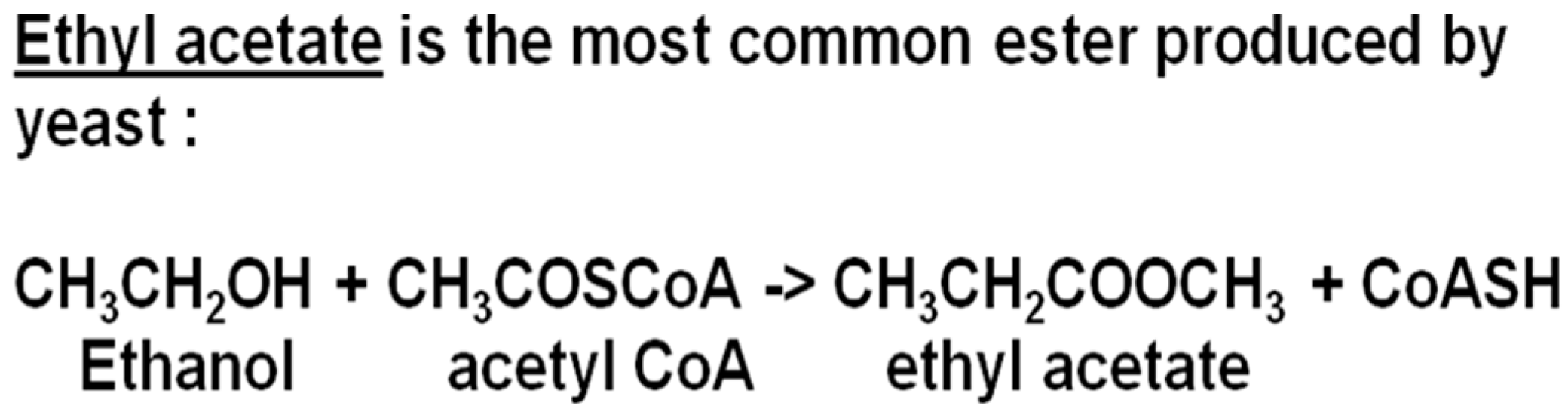
Esters are largely formed during the active phase of primary fermentation by the enzymatic condensation of organic acids with alcohols. Volatile esters in beer are divided into two major groups: the acetate esters and the medium-chain fatty acid (MCFA) ethyl esters. The acetate esters are synthesized from acetic acid (acetate) with ethanol or a higher alcohol (propanol, butanol, etc.) (
Figure 8). In the ethyl ester’s family, ethanol will form the alcohol radical and the acid side of the equation is an MCFA [
47]. Although dozens of different esters can be formed during the fermentation stage of any beer or spirit [
3,
48], of major importance as aroma constituents are: ethyl acetate (solvent-like aroma), isoamyl acetate (banana aroma), isobutyl acetate (fruity aroma), phenylethyl acetate (roses and honey aroma), ethyl hexanoate (sweet apple aroma) and ethyl octanoate (sour apple aroma) (further details on
Table 1). Esters are synthesized in the yeast cell’s cytoplasm, and they readily leave the cell because they are lipophilic. However, while small-chain acetate esters easily diffuse through the plasma membrane, MCFA ethyl esters may have their passage hindered [
49,
50,
51].
Ester synthesis involves organic acids linked to a coenzyme A module in order to form an acetyl-CoA molecule (
Figure 2). Acetyl-CoAs are highly energetic entities, which in the presence of oxygen can be β-oxidized (spliced) into smaller units (acetyl-CoA) in the yeast cell’s mitochondria. This will occur unless the organic acid involved is acetic acid, where it will be converted to acetyl CoA. The great majority of the acetyl-CoA produced by the yeast cell comes from the oxidative decarboxylation of pyruvate (
Figure 4). Aerobic conditions inside mitochondria will induce acetyl-CoA to enter the TCA Cycle (Krebs Cycle) to form ATP. In the absence of oxygen, acetyl-CoA will be enzymatically esterified with an alcohol to form acetate esters (
Figure 2). Also, MCFA ethyl esters are formed from longer chains of acyl-CoA with ethanol.
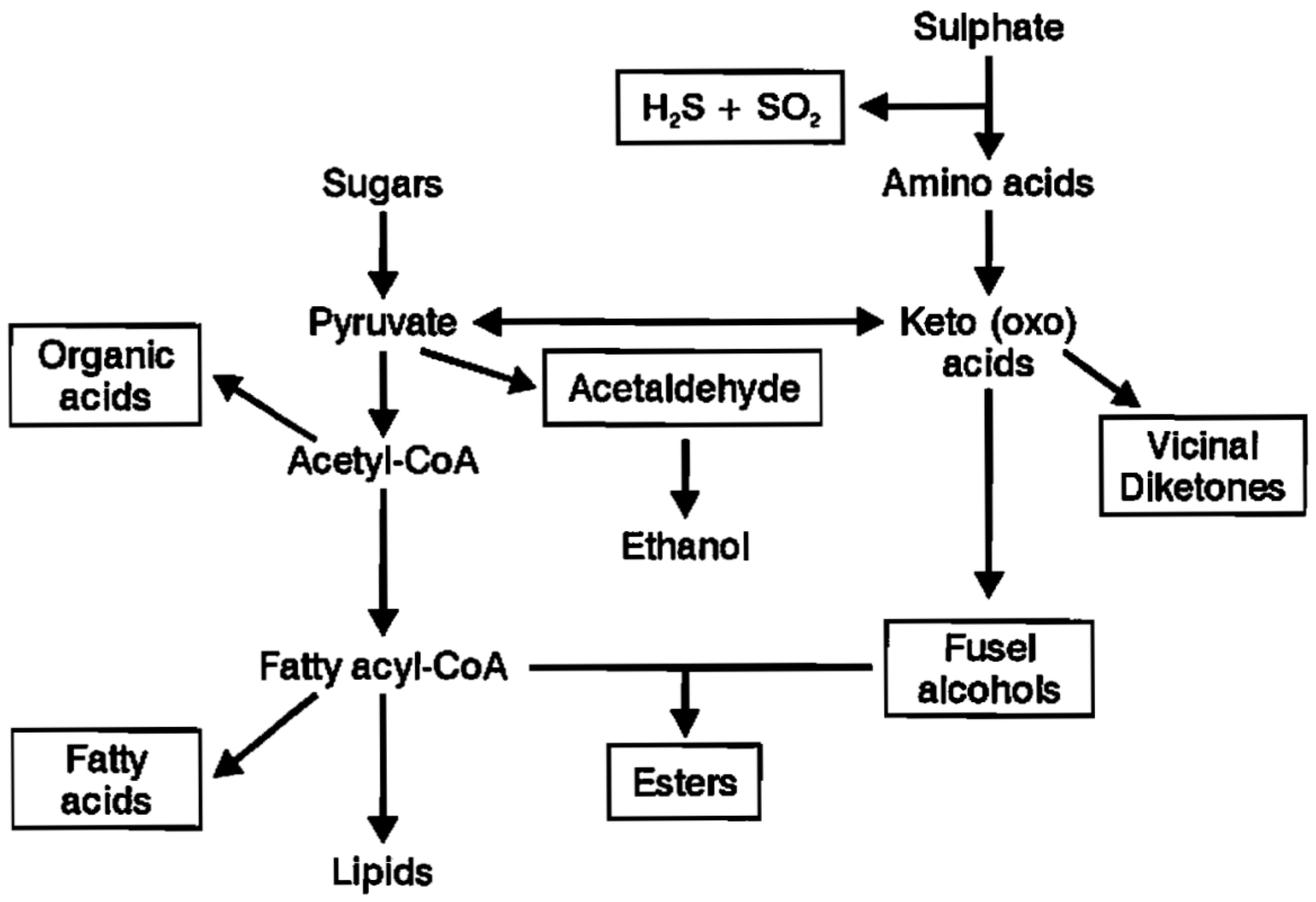
Figure 3 summarises the major metabolic routes by which brewer’s yeast strains synthesize both higher alcohols and esters.
4.1. Influence of Yeast Strains on Ester Formation
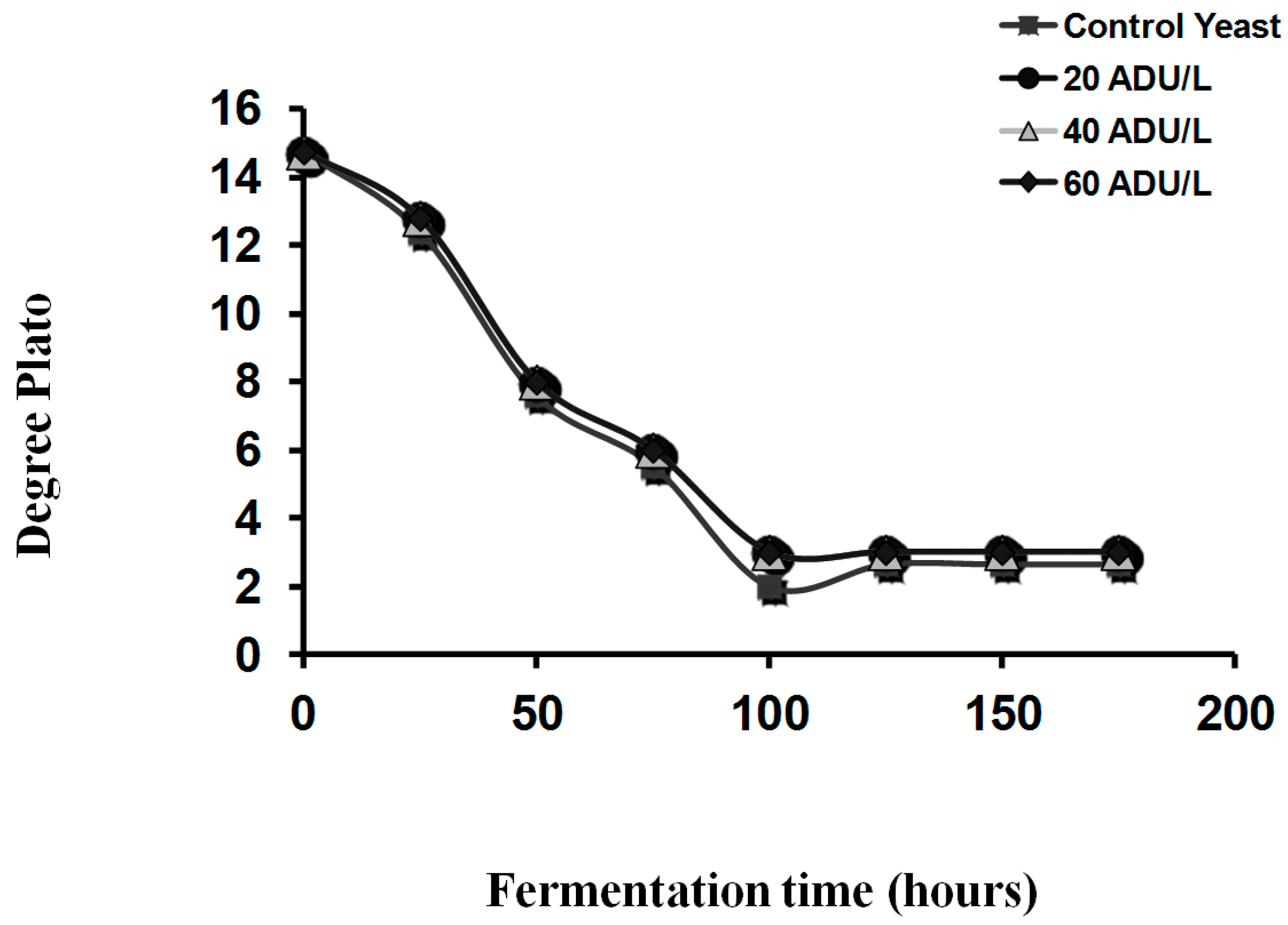
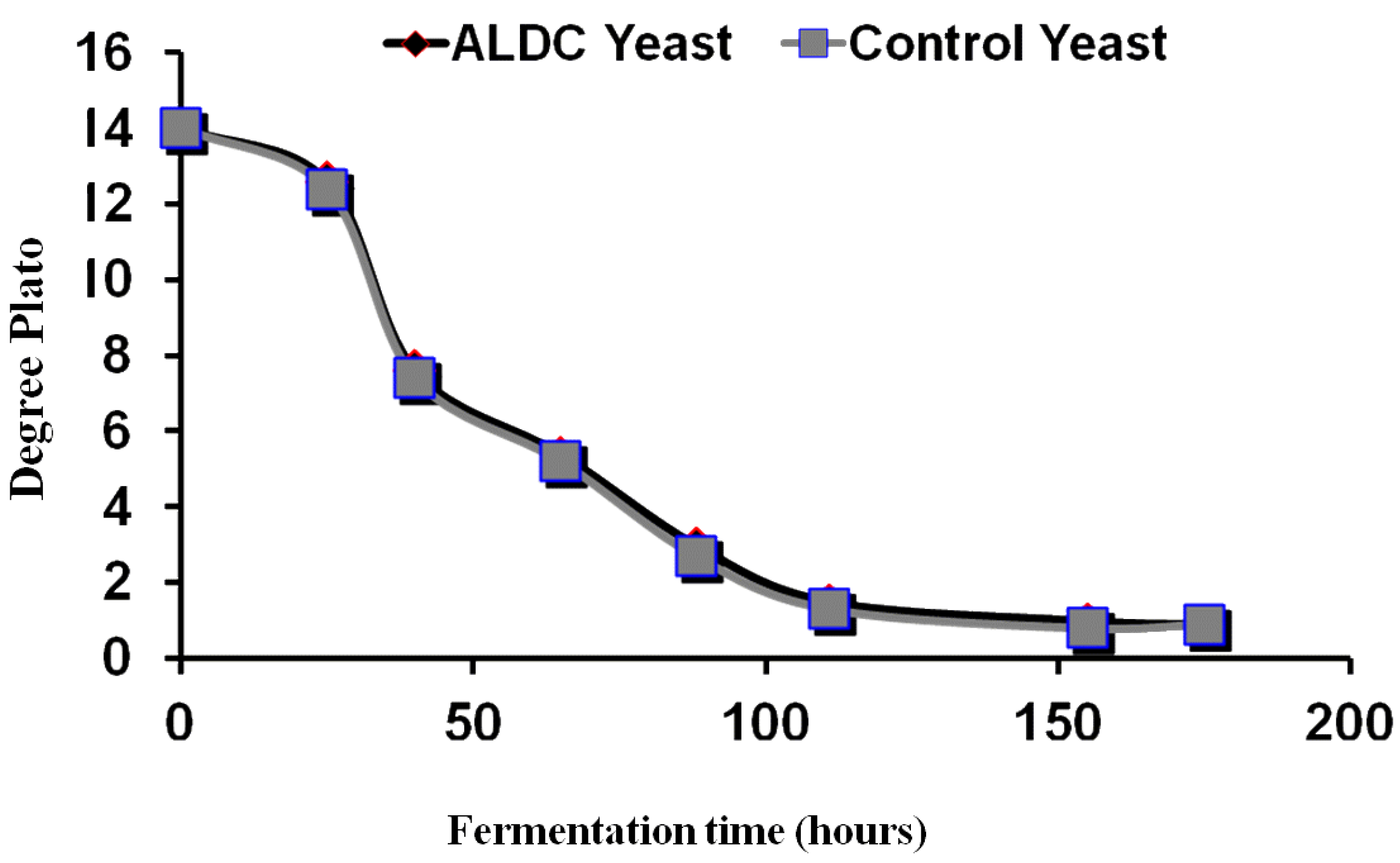
Brewers and distillers have at their disposal a large number of yeast strains from which to produce spirits, ales and lagers. The use of alternative yeast strains offers the prospect of more efficient fermentations, together with diverse characteristics and a variety of beer and spirit flavour characteristics. The characteristics of strain variation have been extensively reported but a basic understanding of the reasons for these differences is still necessary [
12]. To illustrate yeast strain diversity, ester production under standard conditions has been extensively studied. Fermentations were conducted in 1 L static fermenters with a 16°Plato all-malt wort at 20 °C (1°Plato ≡ 1004 SG) [
12]. When the fermentations were complete, ethyl acetate and isoamyl acetate were determined in the fermented wort samples (
Table 5). The results of differences regarding strain variations concerning the production of esters during wort fermentation illustrate strain differentiation between the two brewing and distilling yeast strains of
Saccharomyces cerevisiae (ale and distilling) and the two strains of
S. pastorianus (lager). Although both the
S. cerevisiae and the
S. pastorianus genomes have now been sequenced [
52,
53] and this has enabled an intense study of the genetic control of each species [
54,
55], an understanding of the reasons for ester production are still largely unclear.
4.2. Influence of Wort Clarity on Ester Formation
The level of solids carried over as a result of cereal mashing into wort, also solids resulting from grape crushing in the production of must, and their effect on yeast fermentation performance in the production of beer, whisky and wine has been the subject of considerable study [
31,
32,
35,
56,
57,
58].
Wort solids (also called trub) influence:
The removal of CO
2 from solution during fermentation by acting as nucleation sites Removal of CO
2 from fermenting wort can occur if no suspended particles are present but it contains a great deal of energy [
31].
Wort solids confer nutritive value to the fermentation medium. The rate of fermentation is more rapid in the presence of insoluble material. Wort solids are generally associated with higher levels of fatty acids [
51].
In some instances, yeast cells are able to attach themselves to wort solids and display enhanced growth because of the nutrient concentration that occurs at the particle’s surface [
32,
59].
In order to assess the influence of wort clarity on ester formation, a 15°Plato wort, produced in the Heriot Watt University 2hL pilot brewery (
Figure 9) and a number of other wort types were fermented in 1 L volume batches employing 2 L tall tubes at 27 °C with a
S. cerevisiae distiller’s yeast strain. Carbon dioxide evolution rates during fermentation together with ethyl acetate and isoamyl acetate concentrations at the completion of fermentation were determined. The following wort conditions were studied:
Cloudy wort
Clear wort
Clear wort plus 0.2 g/L diatomaceous earth (DE)
Clear wort plus DE and 5.5 mg/L C16:1 fatty acid
Clear wort plus 0.2 g/L bentonite.
Carbon dioxide concentrations during the fermentation of the wort types have been determined (
Table 6) [
32]. Cloudy wort, containing trub, and wort containing DE acted as nucleation sites and this increased CO
2 evolution out of the wort. Bentonite did not function as a nucleation site with clear wort. Consequently, the CO
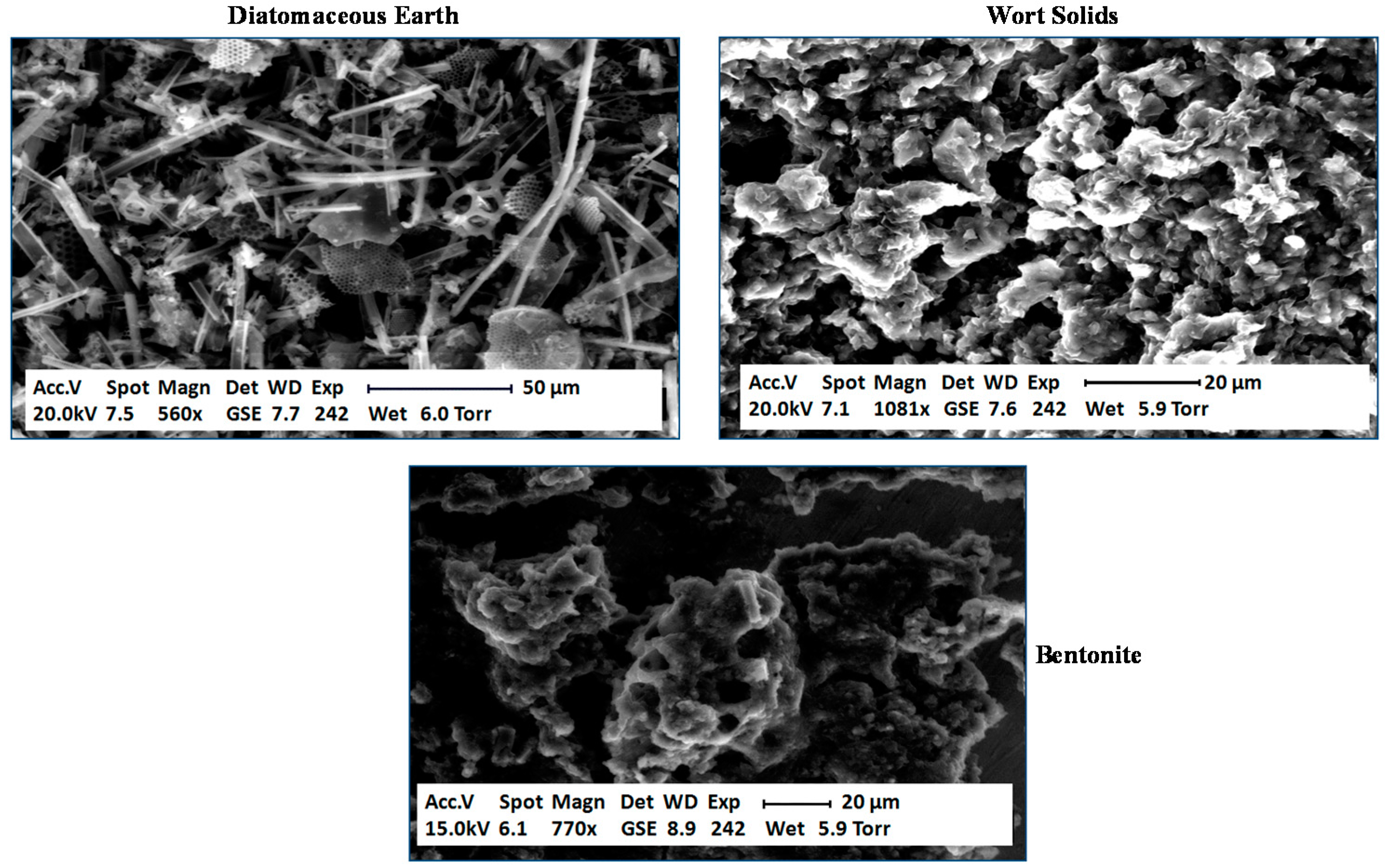
2 remained in the fermentation medium to a greater extent. Why was there a difference between trub, DE and bentonite? In order to examine each type of particle and obtain data on their surface characteristics, environmental confocal scanning electron microscopy (ESEM) was conducted (
Figure 10) [
55]. DE consisted of a heterogeneous mix of shapes and sizes of particles. The surface of most of the particles consisted of an extremely porous structure, as would be expected. The micrograph of the cloudy wort solids showed a mix of different structures, again porous in nature. Bentonite demonstrated a more homogenous structure. It did not appear to exhibit the same porous nature as DE or wort solids.
Ester levels are also influenced by the wort particle concentration and type. Ethyl acetate and isoamyl acetate concentrations are high in cloudy wort and DE that contained C16:1 fatty acid (
Table 7 and
Table 8). This reflects the fact that unsaturated fatty acids, that would be absorbed onto wort trub, induced the synthesis of esters [
32,
35,
57,
60,
61].
4.3. Biosynthesis of Acetate Esters
Research on enzymatic synthesis during ester production by yeast dates from the early 1960s [
62,
63] and the critical enzyme was purified and named alcohol acetyl-transferase (AATase). The most studied and comprehensively characterised enzymes responsible for ester synthesis are AATases I and II, which are encoded by the genes
ATFI and
ATF2 [
64,
65,
66]. It was also found that lager yeast strains have an additional
ATF1 homologous gene–
Lg-ATF1 [
8,
28,
47,
54,
56,
59,
60,
61,
65,
67] which encodes an AATase very similar to that encoded from the original
ATF1 gene. The additional gene expression on lager yeast strains compared to ale strains enhances acetate ester production and ultimately a beer’s aroma profile.
Earlier this decade, a brewer’s yeast strain was designed to increase the ester/higher alcohol ratio by overexpressing
ATF1 and decreasing the expression of a gene related to higher alcohol synthesis [
56]. Ester production by this genetically modified strain was considerably higher than the parental strain. It has also been shown that the level of
ATF genes has an impact on acetate ester production [
64,
65,
67,
68]. Overexpression of
ATF1 strains may have up to a 180-fold increased isoamyl acetate production and a 30-fold increased ethyl acetate production when compared to wild type yeast strains [
67]. Analysis has also revealed that
ATF1–encrypted ATTases appear to be responsible for the majority of acetate ester production. When
ATF1 and
ATF2 were deleted, no acetate esters were formed from alcohols with more than five carbon atoms, such as isoamyl acetate and phenyl ethyl acetate. This means that the formation of the required beer banana aroma (isoamyl acetate) depends on
ATF1- and
ATF2-encoded enzymes. Subsequently, Saerens and colleagues [
22] confirmed that the maximum expression levels of
ATF1 and
ATF2 are directly correlated to the final concentration of acetate esters. It is possible that there might be more ATTases involved in acetate ester production but currently published knowledge is unclear.
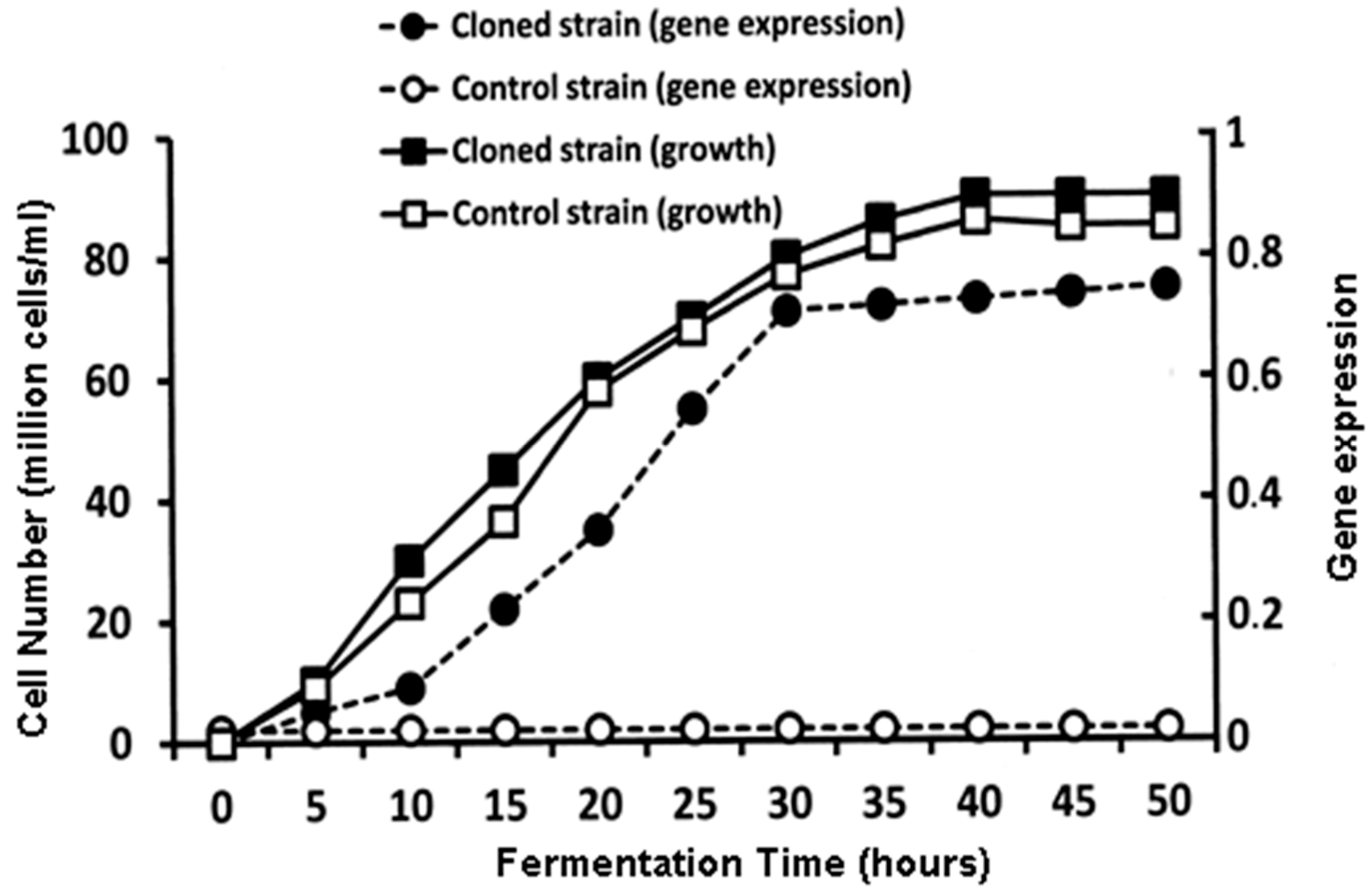
Figure 11 illustrates the time course during the production of the major acetate esters with the
ATF1 gene cloned into a yeast strain compared to the same yeast strain that does not contain the
ATF1 gene [
54].
Pires and colleagues [
30] have discussed the importance of acetate esters in alcohol-free beers (AFBs). AFBs can either be produced as a result of physical removal of ethanol from finished beer or control of the biological process involved in beer fermentation [
69]. Reduced ethanol affects the retention of volatile aroma-active compounds [
18]. A brewing yeast mutant capable of overproducing isoamyl acetate and isoamyl alcohol has been isolated [
68]. The sweet banana aroma of the resulting beer could be employed to overcome the undesirable worty off-flavour of AFB [
70]. As a consequence, sensory analyses showed that the enhanced level of isoamyl acetate ester had a positive effect on the fruity palate fullness and aroma intensity of the AFB.
4.4. Ester Metabolism during Beer Aging (Maturation)
During beer aging (maturation) following primary fermentation, a beer’s flavour will usually change. This change will usually occur as a result of the yeast culture’s metabolism in a secondary fermenter, cask, keg or bottle [
71] or by spontaneous chemical condensation of organic acids with ethanol during beer aging [
72,
73]. Some esters such as isoamyl acetate are known to be hydrolyzed during beer aging [
74]. Chemical hydrolysis and esterification are acid-catalysed [
72], but the remaining esterases from yeast autolysis can function in unpasteurized beers [
74]. Other ethyl esters such as ethyl nicotinate (medicinal, solvent), ethyl pyruvate (peas, freshly cut grass) and ethyl lactate (fruity, buttery) are also formed during beer aging [
73].
6. Sulphur Compounds
Sulphur compounds are secondary metabolic products of yeast strains produced during wort metabolism. Many such compounds make a significant contribution to beer and fresh spirit flavour prior to being matured in oak casks [
2]. Although small amounts of sulphur compounds can be acceptable, or even desirable in some beers, in excess they give rise to unpleasant off-flavours and special processes, such as purging with CO
2, use of a copper electrode [
114] and prolonged maturation times are necessary to remove them [
115]. Some of the sulphur compounds present in beer are not directly associated with fermentation and are derived from the brewing raw materials (malt, hops, etc.) employed. However, the concentrations of hydrogen sulphide (rotten egg aroma), sulphur dioxide (burnt match) and thiols (burnt rubber) are largely dependent on yeast metabolism. Failure to manage fermentation properly can result in unacceptably high levels of these compounds in finished beer. In addition, dimethyl sulphide (DMS) and their thioesters are flavour components in beer but they are largely derived from malt and hops not fermentation [
17,
107].
The concentration of hydrogen sulphide and sulphur dioxide [
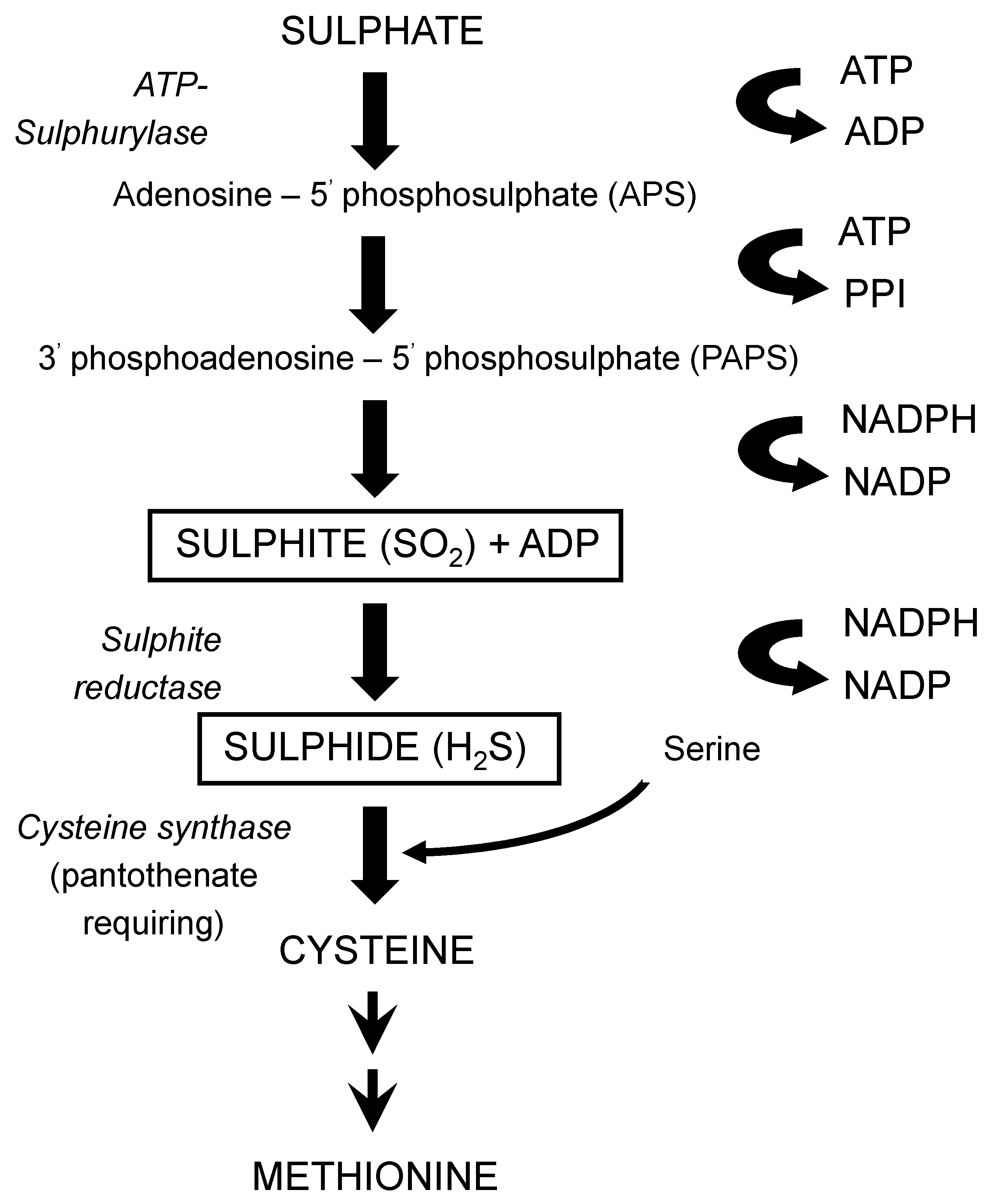
116], produced during fermentation, is primarily determined by the yeast strain employed, also wort composition and fermentation conditions are important factors, particularly where levels of these compounds are abnormally high. Both compounds arise as by-products from the synthesis of the sulphur-containing amino acids cysteine and methionine from sulphate (
Figure 20). These syntheses are influenced by wort composition in that the yeast will preferentially assimilate sulphur-containing amino acids such as methionine (
Table 4) compared to other amino acids and small peptides [
46]. It is only when wort is depleted of amino acids does the biosynthetic route(s) of amino acids come into operation [
101].
The peak of hydrogen sulphide and sulphur dioxide production occurs in the second to the fourth days of fermentation (depending on the yeast strain, the wort gravity and the incubation temperature) [
117]. Presumably, by this time, the sulphur-containing amino acids in wort will have been utilised. Yeast growth during wort fermentation is approximately synchronous with cell division occurring at the same time and hydrogen sulphide evolution seems to occur in a number of peaks which correspond to the growth phase of the yeast culture just prior to the onset of budding [
118].
The formation of excessive levels of hydrogen sulphide and sulphur dioxide during fermentation is associated with conditions that restrict yeast growth. In this regard, the provision of adequate oxygen to wort at the time of pitching is a critical factor. Since both hydrogen sulphide and sulphur dioxide are volatile metabolites, it follows that vigorous fermentation will enhance their removal as a result of carbon dioxide purging [
118]. The type and geometry of the fermentation vessel is also influential. Sulphur dioxide, as well as contributing to beer flavour (burnt match), has a number of other functions in beer (and other alcoholic beverages). It can act as an antimicrobial agent, an antioxidant [
6,
72,
115] and it retards the development of the beer staling characteristics. Bisulphite’s antimicrobial activity can only occur at concentrations in excess of 50 mg/L which is well above the permitted limit in beer by most countries (in the United States it is 10 mg/L, Canada less than 10 mg/L and the United Kingdom 25 mg/L). Due to the adverse effects of oxygen on the flavour stability of finished beer, some brewers (not all) add bisulphites or other antioxidants, such as ascorbic acid (vitamin C), to beer prior to packaging in order to provide protection against oxygen pickup. However, because of bisulphite’s allergenic properties its use is questionable and is decreasing.
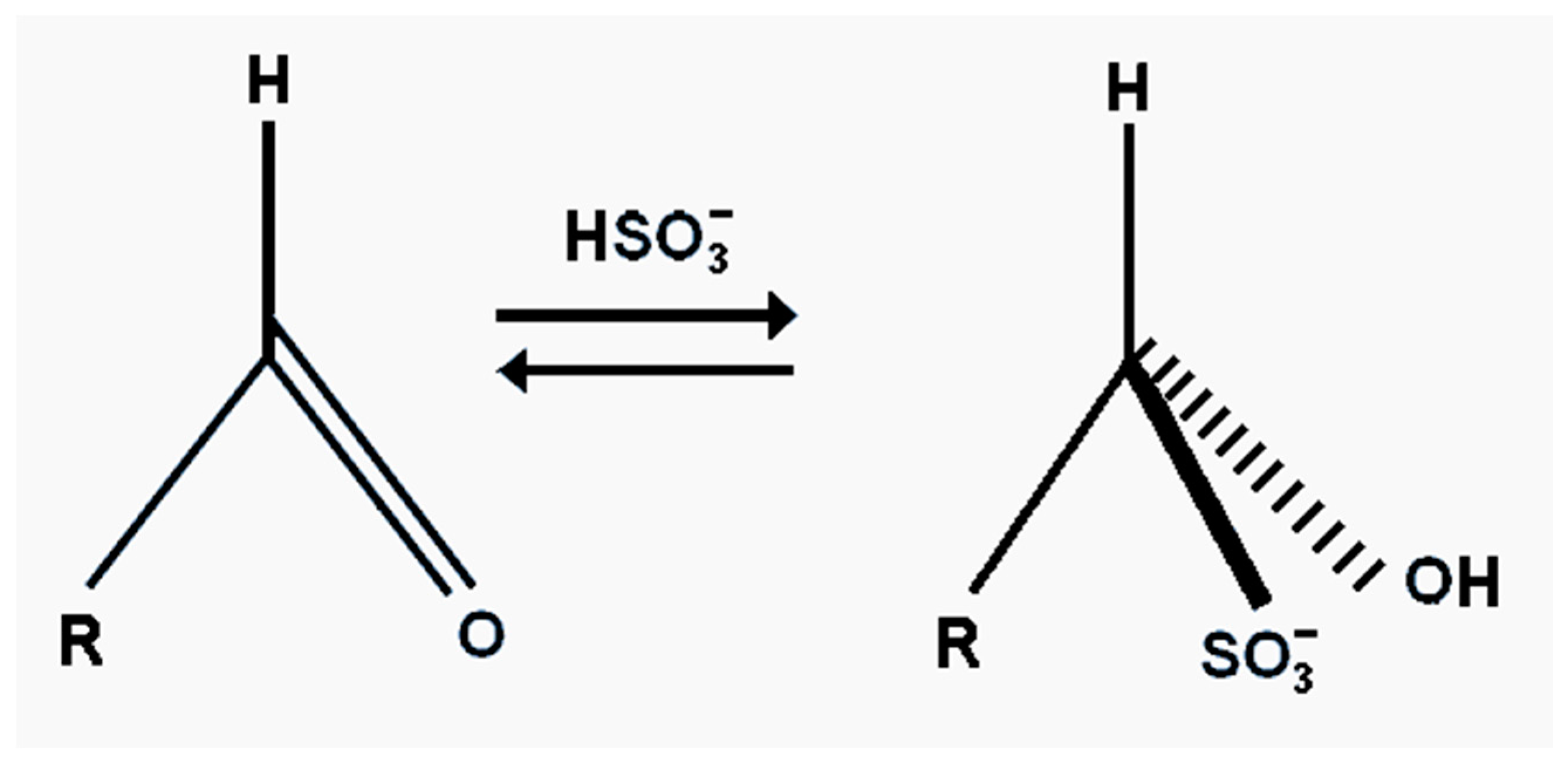
The effectiveness of bisulphite, besides its antioxidant properties, is also its ability to bind carbonyl compounds into flavour neutral complexes [
119]. This can improve beer flavour stability. The efficiency of bisulphite binding to carbonyl compounds does vary. The reaction is reversible, so that an excess of bisulphite will increase the yield of the flavour inactive adjuvant (
Figure 21). The addition of bisulphites to fresh beer minimizes increases in acetaldehyde concentrations during aging. In addition, when added to stale beer, bisulphite lowers the concentration of free aldehyde and affects the removal of the cardboard flavour [
119]. Over time, the bisulphite will be oxidized, by oxygen in the beer, to sulphate, thus increasing the concentration of free aldehydes [
120]. Studies at the Carlsberg Research Centre in Copenhagen have developed a genetically manipulated yeast strain that hyper-produces sulphur dioxide. Results would indicate that beer produced with this yeast strain has enhanced flavour stability [
121]. More recently, the development of a
S. cerevisiae mutant which produces higher levels of sulphur dioxide and glutathione but a lower level of hydrogen sulphide, with improved beer flavour stability, has been reported [
44]. This mutant has been developed by nongenetic engineering means employing ultra violet (UV) mutagenesis together with specific plate screening methods. The antioxidising ability of the mutant was significantly improved and will be safe for production use.
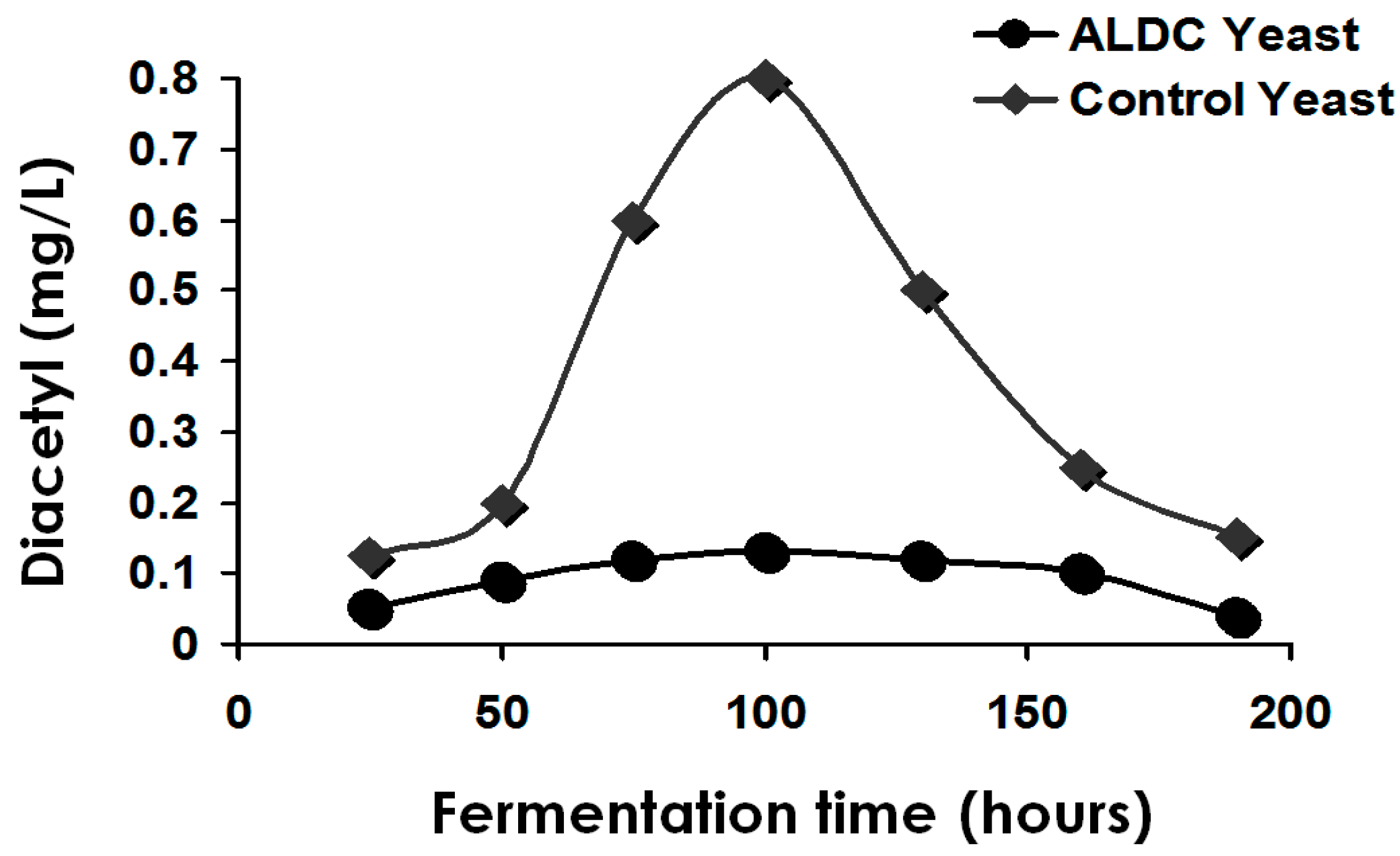
The process of maturation (aging or lagering) is complex (one aspect—the management of VDK—has already been discussed here). Maturation also manages the levels of unwanted sulphur compounds (for example, H
2S, SO
2 and thiols) during primary fermentation. The reduction of unwanted volatile sulphur compounds (inorganic and organic) occurs principally during secondary fermentation (lagering). For this purpose, some yeast must be present during secondary fermentation. The yeast employed for this role is usually (not always) the same yeast strain as that used for primary fermentation. Sometimes (rarely these days), a speciality lagering yeast strain is employed [
115] and the conditions that the yeast encounters during maturation are different to those during primary fermentation-lower pH, higher alcohol, carbon dioxide concentrations, CO
2 pressure, reduced osmotic pressure and lower temperature, etc. Copper ions in fermented wort have a positive effect on the reduction of these sulphur compounds [
114,
122]. The positive effects of copper in this regard can also be illustrated by its importance in the distilling industry [
2].
The use of copper in the construction of pot and continuous stills for malt and grain whisky distillation respectively (
Figure 22 and
Figure 23) [
2] is invaluable for the removal of unwanted sulphur compounds. The expanding application of stainless steel for the manufacture of brewing equipment, replacing copper, has reduced beer copper concentrations in many breweries since WWII. Copper ions can precipitate hydrogen sulphide (and other sulphur-containing compounds) as insoluble copper sulphide. The resultant CuS can subsequently be removed by filtration of the maturing beer or adsorbed by the filter bed. The traditional use of copper vessels, pipes, and plates during wort and beer production does not permit precise copper ion addition. Consequently, deliberate and careful treatment of beer with copper is advisable. Copper electrolysis of maturing beer is a potential alternative [
114]. It should be noted that Cu
2+ ions can have a negative effect on beer flavour stability. For example, Irwin and his colleagues reported [
123] that the rate of beer staling increased in the presence of small amounts of copper ions. As a transition metal ion, copper catalyzes the activation of molecular oxygen, which in turn can oxidize primary alcohols to beer-staling aldehydes. Nevertheless, the application of a copper electrode during maturation cannot be ignored. In a typical application [
114], a copper electrolysis system reduced H
2S levels in beer from 4 μg/L to undetectable levels and beer copper levels increased from 32 to 68 μg/L (
Table 9). Many years ago, in an attempt to reduce maturation times, copper sulphate was added to boiling wort or to the initial stages of fermentation to remove compounds such as copper sulphide [
122]. Although copper’s negative effect on beer flavour stability was unknown at the time, copper sulphate addition to wort has not been employed during the brewing process for many years!