Abstract
Microbially induced mineral precipitation is recognized as a widespread phenomenon in nature. A diverse range of minerals including carbonate, sulphides, silicates, and phosphates can be produced through biomineralization. Calcium carbonate (CaCO3) is one of the most common substances used in various industries and is mostly extracted by mining. In recent years, production of CaCO3 by bacteria has drawn much attention because it is an environmentally- and health-friendly pathway. Although CaCO3 can be produced by some genera of bacteria through autotrophic and heterotrophic pathways, the possibility of producing CaCO3 in different environmental conditions has remained a challenge to determine. In this study, calcium alginate was proposed as a protective carrier to increase the bacterial tolerance to extreme environmental conditions. The model showed that the highest concentration of CaCO3 is achieved when the bacterial cells are immobilized in the calcium alginate beads fabricated using 1.38% w/v Na-alginate and 0.13 M CaCl2.
1. Introduction
Calcium carbonate (CaCO3) comprises more than 4% of the earth’s crust. It can be found throughout the world in natural forms such as marble and limestone. CaCO3 is used in many industrial applications and mostly extracted by mining or quarrying. However, large scale industrial mining can be a serious risk to the environment. In recent years, microbially induced CaCO3 precipitation has emerged as an alternative approach to conventional CaCO3 extraction by mining. Bacterially induced CaCO3 precipitation has been successfully used for a wide range of applications including strengthening of sand and soil [1,2,3,4], removal of metal contaminants from the soil and groundwater [5], removal of calcium ions and polychlorinated biphenyls [6], remediation of monuments [7], CO2 sequestration [8], bio-deposition on porous materials such as limestone and brick [9,10], and, more recently, durability improvement of cementitious materials such as concrete [11,12,13].
Concrete is one of the most popular construction materials due to its availability, high compressive strength capacity, and relatively low cost. However, crack formation is the main issue associated with concrete structures. Low tensile strength coupled with internal and external stresses are recognized as the key causes of crack formation. Although the embedment of reinforcement bars limits the rate of crack growth, it cannot stop crack initiation in concrete. The initiated cracks accelerate structure degradation by allowing aggressive chemicals (fluids and gasses) to seep into the matrix [14]. This phenomenon brings about a reduction in concrete service life, increases maintenance costs, and, in severe cases, leads to structural failure. To replace concrete structures that failed due to cracking, more cement must be produced. Cement is the main ingredient of a concrete mixture and its production has a significant impact on the environment. Currently there are different types of techniques to seal the generated cracks. The application of chemical sealants is one of the most frequently used techniques for sealing the detected cracks. However, it has been reported that these materials are not environmentally friendly or permanent, and more importantly, they are applicable only for reachable cracks.
Recently, a biotechnological approach has been proposed as a green and viable approach to heal concrete cracks. In this approach, a bio agent containing bacteria and nutrient is incorporated in the concrete. When a crack occurs, CaCO3 precipitation is induced as a result of bacterial metabolic activity and the crack is bridged. Although the biotechnological approach seems to have potential for designing self-healing applications, there are a few challenges left to be addressed. One of the main issues with direct incorporation of bacterial cells into the concrete mixture is the high pH of concrete (~12). Our previous investigation showed that the bacterial viability decreases when they are exposed to a high pH environment (concrete pH) [15]. The same observation was noticed by Jonkers et al. [16] when free-floating bacteria were mixed with concrete ingredients. Shear forces on the bacterial cells during concrete mixing and casting, as well as shear and compressive stresses during gradual shrinkage of the concrete, could also damage the bacterial cells and negatively influence the performance of the self-healing mechanism. Water activity is another key stress parameter that may influence the bacterial metabolic activity in the concrete environment. It is known that different genera of bacteria have different water activity limits and solute tolerance [17]. Each bacterium has a very specific and narrow water activity for optimum metabolism and growth [18]. More recent studies show that Bacilli and closely related bacteria are moderately xerotolerant [19,20]. For near future applications of bio self-healing concrete, it is necessary to design a matrix capable of shielding the cells in a concrete environment.
Immobilization of bacterial cells into protective carriers can be a solution to increase the survival of cells in the concrete matrix. The encapsulation of bacterial cells into a polymeric matrix such as calcium alginate (Ca-alginate) is a promising example of this kind of solution. Furthermore, with encapsulation there is potential for the encapsulated bacterial cells to be separated and re-used. However, the concentrations of Na-alginate and CaCl2 used to form Ca-alginate significantly influence the permeability and the mass transfer capability of the beads and, consequently, the biomineralization of CaCO3 is affected.
Therefore, this investigation was performed to determine the optimum concentration of Na-alginate and CaCl2 for enhancing CaCO3 biosynthesis and its future applications in bio self-healing concrete.
2. Materials and Methods
2.1. Chemicals
In this study, a range of different chemicals were used for bacterial growth and fermentation process. Yeast extract, peptone, and calcium chloride were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium alginate (Na-alginate), urea, and glucose were purchased from a domestic supplier.
2.2. Microorganism and Growth Medium
Bacillus species (B. sphaericus NZRM 4381 and B. licheniformis ATCC 9789) were chosen from our previous studies since they showed the highest ability to produce CaCO3 [15,21]. To rehydrate the isolates, the sterilized growth medium, containing peptone (0.5% w/v), glucose (0.5% w/v), and yeast extract (0.05% w/v) was used. Thereafter, the bacterial strains were grown on nutrient agar plate and a sporulation process was performed to obtain a pure suspension of spores.
2.3. Synthesis of Calcium Alginate and Immobilization Process
The bacteria cells (4.5% v/v each strain) were dispersed in different concentrations of sterile Na-alginate solution (1–3% w/v). Different concentrations (0.1–0.3 M) of sterile calcium chloride solutions were also prepared. The Na-alginate solution was then polymerized to form a gel. As shown schematically in Figure 1, the cell suspension was extruded through a tube (diameter 0.25 mm) and injected through a nozzle into a sterile solution of calcium chloride at a constant rate, while the solution was continuously shaken. The beads were formed upon injection and the bacterial cells were entrapped accordingly. To increase the structural integrity of the fabricated beads, the mixture of calcium chloride was stirred for 20 min. Afterwards, a strainer was used to harvest the immobilized cells.
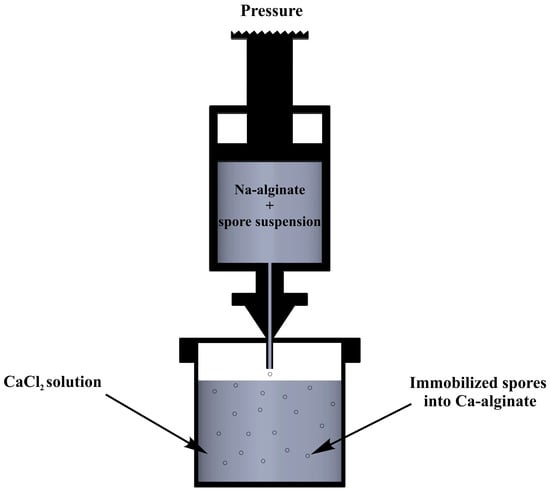
Figure 1.
Experimental set up for production of immobilized bacterial cells into Ca-alginate.
2.4. Experimental Design and Fermentation Procedure
Statistical software package (MODDE V11, Umetrics, Umeå, Sweden) was used for the optimization study. Response surface methodology (RSM), along with a central composite face-centered (CCF) design matrix, was used to determine the optimum concentrations of variables. In the optimization study, a total of 11 experiments were performed with three replications at the center point. The second-order polynomial regression model was employed to fit the experimental data for predicting the resulted biosynthesis product (CaCO3), according to Equation (1):
where Y is the concentration of CaCO3 in g/L, β0 is a constant coefficient, βi, βii, and βij represent the coefficients of the linear, quadratic, and synergic effects respectively, and Xi and Xj are the coded values of the significant factors.
In our previous study, the optimum fermentation media and operating conditions to induce the highest concentration of CaCO3 were determined [21]. In the present study, the fermentations were carried out in shaking flasks containing the optimum medium (40 g/L calcium chloride, 65 g/L urea, and 2 g/L yeast extract). As shown in Table 1, the flasks were then inoculated with the immobilized isolates, maintained at 35 °C and 100 rpm for 4.5 days. It is worth noting that the last three experimental runs are the replicates at the center point.

Table 1.
Level of variables examined in optimization using central composite face (CCF) design.
2.5. CaCO3 Extraction
The soluble calcium contained in the media was determined using a benchtop photometer. The precipitated CaCO3 was harvested by passing through filter paper (0.2 μm) and washed three times with distilled water. The precipitates were oven dried at 70 °C for 24 h.
2.6. Crystal Characterization and Morphological Observation
Scanning electron microscopy (SEM) was used for morphological characterization of precipitated CaCO3. The precipitated powder was placed in a carbon tape, and the sample was coated with platinum using a sputter coater (Hitachi E1030, Tokyo, Japan). The sample was mounted into the SEM instrument (Hitachi S-4700, Tokyo, Japan), and crystal observation was performed. The elemental analysis was also performed to analyze the compositions of the mineralization products using energy dispersive X-ray spectroscopy (EDS). SEM imaging and EDS analysis were performed at 5 KeV and 15 KeV, respectively.
The collected dry precipitates were well-crushed using a mortar and pestle for X-ray diffraction (XRD) analysis. The powder was then packed into sample holder and analysis was performed using a Panalytical Empyrean diffractometer (Almelo, The Netherlands) with CuKα radiation. The data were collected for an exploration range, step size, voltage, and current of 15–70° (2θ), 0.0530°, 45 KV, and 40 mA, respectively.
3. Results and Discussion
3.1. Immobilization of Bacterial Cell into Ca-Alginate
Immobilization of bacteria cells can be achieved through four main categories, namely: (1) attachment or adsorption onto solid carrier surfaces; (2) entrapment within a porous matrix; (3) self-aggregation by flocculation or with crosslinking agents; and (4) cell containment behind barriers [22]. Cell entrapment can be done into polysaccharide gel matrices, such as alginates, agar, and chitosan, or other polymeric matrixes including gelatin. In this study, polysaccharide gel entrapment (Ca-alginate) was used as an efficient immobilization approach to prevent the cells from diffusing into the surroundings. As shown in Figure 2a, the bacterial cells were successfully immobilized into uniformly sized Ca-alginate beads. An ideal immobilization approach must allow the mass transfer of nutrients to facilitate the bioprocess, while protecting the cells from the surrounding environment. The protective mechanism operates via triggering a stress response that produces a more robust cell. This phenomenon has previously been demonstrated at the level of the macromolecule [23]. Figure 2b illustrates the accumulation of bio-precipitates around the Ca-alginate beads in the fermentation media. This shows that that the fabricated Ca-alginate beads with the optimum concentrations of Na-alginate and CaCl2 were sufficiently permeable to promote the biosynthesis.
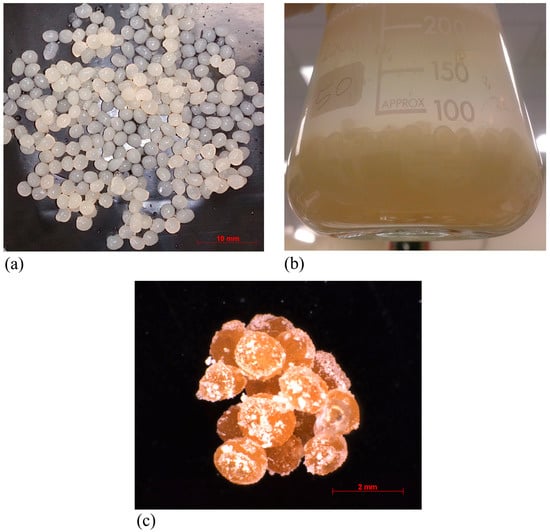
Figure 2.
(a) Immobilized bacterial cells into Ca-alginate beads; (b) fermentation medium inoculated with immobilized bacterial cells; and (c) precipitated CaCO3 crystals around Ca-alginate beads.
3.2. Bacterially Induced CaCO3 Precipitation
To maximize CaCO3 precipitation, the immobilization carriers should be permeable, while also being able to prevent cells from diffusing into the fermentation media. As given in Table 1, an optimization study was performed to identify the optimum concentrations of Na-alginate and CaCl2. Apart from the entrapment of the bacterial cells inside the beads, the immobilization into porous matrix such as polysaccharide gel can result in the bacterial cells moving to the outer surface of the beads. However, the effect the immobilization may influence the water activity, and as consequence metabolism will be affected [24].
In Figure 3, a 3D response surface plot was constructed to illustrate the synergistic effects of Na-alginate and CaCl2 concentrations on the response (CaCO3) and also provide a visual interpretation for the location of the optimal concentrations. The shape of the corresponding plots shows that the mutual interaction between variables is significant, and the response was considerably affected by the concentrations of Na-alginate and CaCl2.
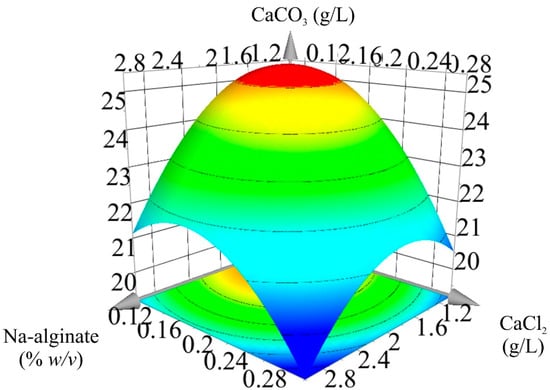
Figure 3.
3D response surface plot showing the interactive effects of Na-alginate and CaCl2 for bacterially induced CaCO3 precipitation.
To investigate the optimum levels of variables on the biosynthesis process, RSM, using a CCF design matrix, was used. The statistical analysis data containing the regression coefficients for the model are given in Table 2. As shown in Equation (2), a quadratic model was regressed for predicting the CaCO3 precipitation.
where Y, X1, and X2 represent the predicted CaCO3 concentration, Na-alginate concentration, and CaCl2 concentration, respectively. The statistical analysis of the quadratic regression model shows that the model is significant (p-value < 0.05), with R2 value of 0.977. Based on the results, all the single, linear, and quadratic terms are significant on CaCO3 precipitation. The statistical significance of the quadratic model was checked by F-test, and the results of analysis of variance (ANOVA) are listed in Table 3. The model was assessed for suitability by examining misfit, which was found insignificant for the model (p-value < 0.05). Lack of fit is used to evaluate the accuracy of the fitted model. When a mathematical model is well-fitted to the experimental results, the mean squared lack of fit reflects only the random errors inherent to the system [25]. According to the ANOVA results, the non-significant lack of fit (p-value < 0.367) and a significant regression (p-value < 0.000) suggest the high accuracy of the fitted model.

Table 2.
Statistical analysis from the central composite face-centered (CCF) design experiments for CaCO3 precipitation.

Table 3.
Analysis of variance for CaCO3 precipitation.
To determine the optimum levels of Na-alginate and CaCl2, the regression equations were solved within the experimental region. Independent fermentation runs were carried out at the conditions predicted by the model to verify the optimization results. The fermented medium was supplemented with the optimum concentrations of variables (1.38% w/v Na-alginate and 0.13 M CaCl2). The optimization shows that the highest concentration of CaCO3 (25.48 g/L) is achieved when the optimal concentrations of Na-alginate and CaCl2 are used.
3.3. Morphological Observation and Crystal Characterization
Calcite, aragonite, and vaterite are three crystalline polymorphs of CaCO3 found existing naturally. In general, physical and chemical characteristics of CaCO3 precipitates such as crystal size, specific surface area, morphology, purity, and brightness are the main criteria that are usually considered for industrial applications. Physical properties of CaCO3, including density, solubility, and hardness, largely depend on the fraction of each polymorph. Bioprecipitation of CaCO3 may result in the production of multiple different polymorphs. As vaterite has a lower density than calcite, a higher volume can be filled when vaterite particles are induced in a concrete crack, which contributes to enhancing the effectiveness of the self-healing mechanism.
SEM and XRD analysis were used to determine the morphology of precipitated CaCO3 crystals. Figure 4a presents an assemblage of vaterite particles produced through biomineralization via bacterial cells immobilized in Ca-alginate beads. The elemental compositions of the bioproducts were determined by EDS, and the spectra are given in Figure 4b. The results show that Ca, O, and C are the main elements found in the biominerals. The elemental ratios of the detected elements are very close to the pure CaCO3, and this confirms that the precipitated crystals are CaCO3. The XRD spectra for the CaCO3 crystals produced during fermentation are shown in Figure 5. The XRD spectra indicate that vaterite and calcite were the most predominant polymorphs produced when the media was inoculated with immobilized bacteria cells. The results also demonstrate that the immobilization had no significant effect on CaCO3 morphology. This means that this approach can be used as a promising tool for protecting bacterial cells in a harsh environment without influencing the metabolic activity.
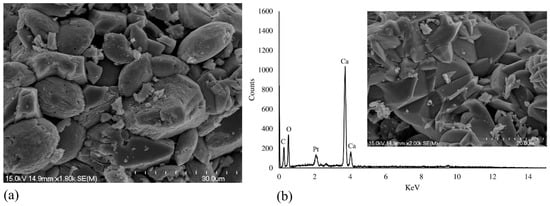
Figure 4.
(a) Scanning electron microscopy (SEM) micrograph of precipitated CaCO3 crystals using immobilized bacterial cells into Ca-alginate and (b) energy dispersive X-ray spectroscopy (EDS) spectra of the induced CaCO3 crystal.
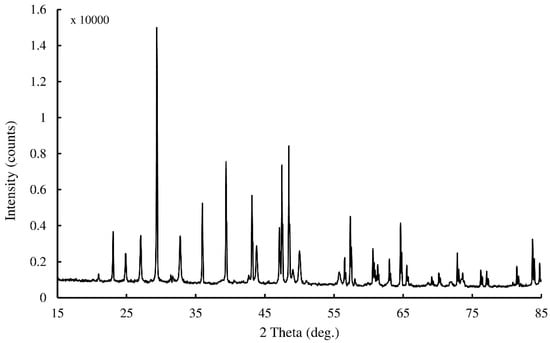
Figure 5.
X-ray diffraction (XRD) spectra for the precipitated CaCO3 precipitation via immobilized bacterial cells into Ca-alginate beads.
The reason for the production of different polymorphs during biosynthesis of CaCO3 is not well established. Different parameters such as media compositions, cell surface characteristics, bacteria metabolic activities, and extracellular polymeric substance (EPS) have been demonstrated to have an effect on the morphology of the precipitated particles [21]. The results obtained in this study show that the immobilization of bacteria in Ca-alginate beads is a promising approach to enhance the cell protection and recovery. This provides a new protocol to address the shortcomings associated with the future application of sustainable bio self-healing concrete.
4. Conclusions
The immobilization of Bacillus species in a polymeric matrix (Ca-alginate) proved a promising technique not only for protecting the bacterial cells from harsh environments but also for ease of separation and recovery. The model indicates that the implementation of the optimal concentrations of Na-alginate (1.38% w/v) and CaCl2 (0.13 M) results in the highest CaCO3 precipitation. Moreover, crystal characterization demonstrated that the entrapment of bacterial cells into Ca-alginate has no effect on the CaCO3 morphology formed. The results of this study can also be used for processing with recycled bacterial capsules by reducing the downstream processes involved.
Acknowledgments
This investigation was financially supported by The University of Waikato, New Zealand.
Author Contributions
Mostafa Seifan and Aydin Berenjian conceived the experiments and analyzed the data; Mostafa Seifan performed the experiments and wrote the manuscript; Aydin Berenjian and Shaun Hewitt revised the manuscript; Ali Khajeh Samani contributed analysis tools.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Paassen, L.A.; Daza, C.M.; Staal, M.; Sorokin, D.Y.; van der Zon, W.; van Loosdrecht, M.C.M. Potential soil reinforcement by biological denitrification. Ecol. Eng. 2010, 36, 168–175. [Google Scholar] [CrossRef]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Burbank, M.B.; Weaver, T.J.; Green, T.L.; Williams, B.; Crawford, R.L. Precipitation of calcite by indigenous microorganisms to strengthen liquefiable soils. Geomicrobiol. J. 2011, 28, 301–312. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. Upscaling effects of soil improvement by microbially induced calcite precipitation by surface percolation. Geomicrobiol. J. 2014, 31, 396–406. [Google Scholar] [CrossRef]
- Warren, L.A.; Maurice, P.A.; Parmar, N.; Ferris, G.F. Microbially mediated calcium carbonate precipitation: Implications for interpreting calcite precipitation and for solid-phase capture of inorganic contaminants. Geomicrobiol. J. 2001, 18, 93–115. [Google Scholar]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Tiano, P.; Biagiotti, L.; Mastromei, G. Bacterial bio-mediated calcite precipitation for monumental stones conservation: Methods of evaluation. J. Microbiol. Methods 1999, 36, 139–145. [Google Scholar] [CrossRef]
- Okyay, T.O.; Rodrigues, D.F. Biotic and abiotic effects on CO2 sequestration during microbially-induced calcium carbonate precipitation. FEMS Microbiol. Ecol. 2015, 91. [Google Scholar] [CrossRef] [PubMed]
- De Muynck, W.; Leuridan, S.; Van Loo, D.; Verbeken, K.; Cnudde, V.; De Belie, N.; Verstraete, W. Influence of pore structure on the effectiveness of a biogenic carbonate surface treatment for limestone conservation. Appl. Environ. Microbiol. 2011, 77, 6808–6820. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Improvement in strength properties of ash bricks by bacterial calcite. Ecol. Eng. 2012, 39, 31–35. [Google Scholar] [CrossRef]
- Sierra-Beltran, M.G.; Jonkers, H.M.; Schlangen, E. Characterization of sustainable bio-based mortar for concrete repair. Constr. Build. Mater. 2014, 67, 344–352. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; De Belie, N.; De Muynck, W.; Verstraete, W. Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 2010, 40, 157–166. [Google Scholar] [CrossRef]
- Seifan, M.; Samani, A.K.; Berenjian, A. Bioconcrete: Next generation of self-healing concrete. Appl. Microbiol. Biotechnol. 2016, 100, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Seifan, M.; Samani, A.K.; Burgess, J.J.; Berenjian, A. The effectiveness of microbial crack treatment in self healing concrete. In High Value Processing Technologies; Berenjian, A., Jafarizadeh-Malmiri, H., Song, Y., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2016; pp. 97–124. [Google Scholar]
- Seifan, M.; Samani, A.K.; Berenjian, A. New insights into the role of pH and aeration in the bacterial production of calcium carbonate (CaCO3). Appl. Microbiol. Biotechnol. 2017, 101, 3131–3142. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Stevenson, A.; Cray, J.A.; Williams, J.P.; Santos, R.; Sahay, R.; Neuenkirchen, N.; McClure, C.D.; Grant, I.R.; Houghton, J.D.; Quinn, J.P. Is there a common water-activity limit for the three domains of life? ISME J. 2015, 9, 1333–1351. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, A.; Burkhardt, J.; Cockell, C.S.; Cray, J.A.; Dijksterhuis, J.; Fox-Powell, M.; Kee, T.P.; Kminek, G.; McGenity, T.J.; Timmis, K.N. Multiplication of microbes below 0.690 water activity: Implications for terrestrial and extraterrestrial life. Environ. Microbiol. 2015, 17, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, A.; Hallsworth, J.E. Water and temperature relations of soil actinobacteria. Environ. Microbiol. Rep. 2014, 6, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Cray, J.A.; Connor, M.C.; Stevenson, A.; Houghton, J.D.; Rangel, D.E.; Cooke, L.R.; Hallsworth, J.E. Biocontrol agents promote growth of potato pathogens, depending on environmental conditions. Microb. Biotechnol. 2016, 9, 330–354. [Google Scholar] [CrossRef] [PubMed]
- Seifan, M.; Samani, A.K.; Berenjian, A. Induced calcium carbonate precipitation using Bacillus species. Appl. Microbiol. Biotechnol. 2016, 100, 9895–9906. [Google Scholar] [CrossRef] [PubMed]
- Kourkoutas, Y.; Bekatorou, A.; Banat, I.M.; Marchant, R.; Koutinas, A.A. Immobilization technologies and support materials suitable in alcohol beverages production: A review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
- Bell, A.N.; Magill, E.; Hallsworth, J.E.; Timson, D.J. Effects of alcohols and compatible solutes on the activity of β-galactosidase. Appl. Biochem. Biotechnol. 2013, 169, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Cray, J.A.; Stevenson, A.; Ball, P.; Bankar, S.B.; Eleutherio, E.C.; Ezeji, T.C.; Singhal, R.S.; Thevelein, J.M.; Timson, D.J.; Hallsworth, J.E. Chaotropicity: A key factor in product tolerance of biofuel-producing microorganisms. Curr. Opin. Biotechnol. 2015, 33, 228–259. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (rsm) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).