Abstract
Fumaric acid is a platform chemical with many applications in bio-based chemical and polymer production. Fungal cell morphology is an important factor that affects fumaric acid production via fermentation. In the present study, pellet and dispersed mycelia morphology of Rhizopus arrhizus NRRL 2582 was analysed using image analysis software and the impact on fumaric acid production was evaluated. Batch experiments were carried out in shake flasks using glucose as carbon source. The highest fumaric acid yield of 0.84 g/g total sugars was achieved in the case of dispersed mycelia with a final fumaric acid concentration of 19.7 g/L. The fumaric acid production was also evaluated using a nutrient rich feedstock obtained from soybean cake, as substitute of the commercial nitrogen sources. Solid state fermentation was performed in order to produce proteolytic enzymes, which were utilised for soybean cake hydrolysis. Batch fermentations were conducted using 50 g/L glucose and soybean cake hydrolysate achieving up to 33 g/L fumaric acid concentration. To the best of our knowledge the influence of R. arrhizus morphology on fumaric acid production has not been reported previously. The results indicated that dispersed clumps were more effective in fumaric acid production than pellets and renewable resources could be alternatively valorised for the biotechnological production of platform chemicals.
1. Introduction
Fumaric acid is considered a platform chemical with several applications in bio-based chemical and polymer production. It is used mainly as a food acidulant and as chemical feedstock for the production of paper resins, unsaturated polyester resins, alkyd resins, plasticizers, and miscellaneous industrial products []. The global fumaric acid market demand was estimated as 225.2 kt in 2012 and it is expected to be over 300 kt in 2020 []. The production of fumaric acid is based on the isomerization of maleic acid derived originally from n-butene []. The petrochemical method has the advantage of high production yields, compared with the biotechnological production through fermentation. The fermentation process yields around 85% w/w, using glucose as a carbon source, which is considered cheaper than the raw material of the chemical process []. Since there is an increasing concern over sustainability, the utilization of microbial strains for fumaric acid production has been appraised with the aim of its biotechnological production instead of the chemical route [].
Fermentative production of fumaric acid has been investigated using various fungal strains of Rhizopus arrhizus and Rhizopus oryzae. The oxidative TCA cycle is required for fungal growth, while fumaric acid production involves the reductive TCA cycle []. Many Rhizopus species have been reported to produce fumaric acid in greater or lesser extents. Some of them have shown a capability of producing significant amounts of fumaric acid (>90 g/L) []. The species R. oryzae and R. arrhizus are the two most studied species for fumaric acid production, however not all strains within these species can be used to produce fumaric acid. In particular, R. arrhizus NRRL 2582 and R. oryzae NRRL 1526 have presented the highest final concentrations and conversion yields [,,,].
Several studies have shown that fungal morphology is a significant factor affecting fumaric acid concentration and yield [,,,]. The fungal morphology of Rhizopus species is influenced by various factors such as inoculum size, agitation, working volume, pH, temperature, nutrients, and metal ions [,,,,,,]. Liao et al. reported that each factor affecting fungal morphology has different importance to the growth morphology of individual strains []. The formation of different fungal morphologies, such as clumps, pellets, and filaments, in submerged fermentations is affected by the growth conditions. The main disadvantage of filamentous and clump morphologies, during submerged fermentations, is the tendency to grow on bioreactor baffles, shaft and wall, which causes low oxygen transfer rates. The formation of fungal pellets promotes mass transfer due to lower medium viscosity [,]. However, the centre of large pellets could undergo autolysis due to nutrient limitation, which affects negatively fumaric acid production []. Other studies have reported that small pellets enhanced fumaric acid production in comparison to clump morphology [,].
The fungus R. arrhizus is considered as an important strain for the production of fumaric acid []. Hence, many studies have been carried out focusing on the morphology of several Rhizopus species [,,,,]. Still, studies dealing with the R. arrhizus NRRL 2582 strain are scarcely found in the literature. In this work, two different fungal morphologies of R. arrhizus, pellets and dispersed mycelia, were accessed in submerged fermentations using commercial nitrogen sources and glucose. The effect of ammonium sulphate concentrations on the production of fumaric acid was also evaluated. Subsequently, submerged fermentations were carried out using a nutrient-rich feedstock derived from soybean cake in order to replace the commercial nitrogen sources. Soybean cake feedstock was obtained through enzymatic hydrolysis. The soybean cake was initially utilised as a substrate for the production of a crude enzyme consortia by Aspergillus oryzae through solid state fermentation. The crude enzymes were then used for the hydrolysis process of soybean cake. Soybean cake is a renewable resource that could be efficiently utilised for the sustainable biotechnological production of platform chemicals. A previous study reported the utilization of a chemically hydrolysed soybean meal for fumaric acid production by R. oryzae ATCC 20344 [].
2. Materials and Methods
2.1. Microorganisms and Growth Media
Fumaric acid production was studied using the fungal strain Rhizopus arrhizus NRRL 2582, which was purchased from the ARS Culture Collection (NRRL) (Peoria, IL, USA). The fungus was cultivated in slopes containing 50 g/L soybean cake and 20 g/L agar, for six days at 30 °C and further propagated in Erlenmeyer flasks of 250 mL, which contained the same growth medium as in slopes, in order to form adequate quantities of spores. In particular, spores from slants were washed with 5 mL of sterilised water supplemented with 0.01% (v/v) of Tween 80 (Sigma-Aldrich, St. Louis, MO, USA) and then 2 mL were aseptically transferred to sporulation medium following incubation at 30 °C for three days. Fungal spore suspension was obtained by adding 50 mL sterilised water with 0.01% (v/v) Tween 80 and glass beads and the spores were liberated by vigorous shaking of the flasks []. The spore suspensions were collected and stored in cryovials, filled with pure glycerol, at −80 °C. The vials containing spore suspensions were used to inoculate pre-culture medium. All media were autoclaved at 121°C for 20 min.
The fungal strain Aspergillus oryzae was kindly provided by Prof. C. Webb (University of Manchester, UK) and was utilised for the production of crude proteolytic enzymes. The strain was originally isolated and purified by Wang et al. [], from a soy sauce industry (Amoy Food Ltd., Hong Kong, China). Storage, maintenance, sporulation, and inoculum preparations of the fungal strain A. oryzae have been described in previous studies [,].
2.2. Raw Materials Used as Fermentation Media
Soybean cake, a by-product of the biodiesel production process, was kindly provided by Petrobras (Rio de Janeiro, Brazil).
2.3. Crude Enzyme Production by Solid State Fermentation
Solid-state fermentations (SSF) were carried out in 250 mL Erlenmeyer flasks, containing 5 g (on dry basis, db) of soybean cake and sterilized at 121 °C for 20 min. Fungal spore suspension of Aspergillus oryzae was used to inoculate the SSF so as the inoculum spore concentration was approximately 2 × 106 spores/mL and the moisture content of the substrate was adjusted at 65% (w/w, db) [,]. All SSFs were incubated at 30 °C for up to 48 h. Then, the fermented solids were suspended in sterilised water, macerated using a kitchen blender and filtered in order to obtain the crude enzyme consortia suspension, which was subsequently used in soybean cake hydrolysis.
2.4. Enzymatic Hydrolysis of Soybean Cake
Enzymatic hydrolysis was performed in 1 L Duran bottles, containing a total concentration of 45 g/L of soybean cake using an initial proteolytic activity of 3.9 U/mL. Duran bottles containing soybean cake were sterilized and then the crude enzyme consortia suspension from SSF was added. The hydrolysis was carried out at different temperatures under agitation and uncontrolled pH. Samples were collected at regular intervals the first 10 h of hydrolysis and then every 24 h. The solids were separated via centrifugation (3000× g, 10 min) and the supernatant was used for the analysis of free amino nitrogen (FAN) and inorganic phosphorus (IP). Hydrolysis yield was expressed as the percentage of total Kjeldahl nitrogen (TKN) to FAN conversion. The experiments were carried out in duplicates and the results represent the mean values obtained.
After the hydrolysis, remaining solids were removed by centrifugation (3000× g, 5 °C, 15 min) following vacuum filtration. The pH of the hydrolysate was adjusted to 6 with 5 M KOH and then was filter-sterilized using a 0.2 μm filter unit (Polycap TM AS, Whatman Ltd., Maidstone, UK). The hydrolysate was further utilised as substitute of the commercial nitrogen sources for fumaric acid production.
2.5. Control of Fungal Morphology
The impact of two different cell morphologies (pellets and dispersed mycelium) of R. arrhizus on fumaric acid production was evaluated. The different media compositions and conditions were as follows.
2.5.1. Production of Pelletized Biomass
Pellets were formed in baffled flasks of 1 L filled with 50 mL of the pre-culture medium as depicted in Table 1. The pre-culture was incubated at 30 °C for 24 h in a rotary shaker with an agitation speed of 180 rpm. The inoculation of the fermentation medium was performed using a 10% (v/v) of the pre-culture.

Table 1.
Composition of different pre-culture media controlling the fungal morphology.
2.5.2. Production of Dispersed Mycelia
Uniformly dispersed mycelia were produced in Erlenmeyer flasks of 250 mL filled with 50 mL of the pre-culture medium, based on a modification of Rhodes et al. [] pre-culture medium synthesis (Table 1). The pre-culture was incubated at 30 °C for 24 h in a rotary shaker with an agitation speed of 180 rpm and dispersed mycelia was inoculated (10%, v/v) in the fermentation medium.
2.6. Fumaric Acid Production
Fumaric acid production by R. arrhizus was studied in 250 mL Erlenmeyer flasks through submerged fermentations. Shake flasks contained 50 mL of a medium, with the following composition (g/L): glucose at initial concentration of 25 or 50; CaCO3, 80% of the initial carbon source concentration used in each fermentation; KH2PO4, 0.6; MgSO4·7H2O, 0.4; ZnSO4·7H2O, 0.044; FeCl3·6H2O, 0.016; tartaric acid, 0.0075; corn steep liquor, 0.5 mL and methanol 15 mL [,]. The medium was autoclaved at 121 °C for 20 min. Corn steep liquor and ammonium sulphate were autoclaved separately. Methanol was filter sterilized and added to the sterilized medium under aseptic conditions. The pH was adjusted to 6 using 5 M KOH or 5 M HCl prior to sterilization. The effect of the initial ammonium sulphate concentration at 0.2 and 0.04 g/L on fumaric acid production was also evaluated. The fermentations were incubated at 30 °C with an agitation rate of 180 rpm. The experiments were carried out in duplicates and the results represent the mean values.
2.7. Fumaric Acid Production Using Soybean Cake Hydrolysate
The effect of soybean cake hydrolysate, as substitute of commercial nitrogen sources, was evaluated by adding different FAN concentrations in the pre-culture medium. The pre-culture medium without the nitrogen sources was autoclaved at 121 °C for 20 min and then the filter-sterilized hydrolysate was aseptically added. Incubation conditions were described in Section 2.2. Subsequently, batch fermentations were conducted in shake flasks as mentioned in Section 2.3.
2.8. Analytical Methods
The TKN (total Kjeldahl nitrogen) concentration was measured using a Kjeltek™ 8100 distillation Unit (Foss, Hillerød, Denmark). Ash, lipid, acid detergent fibre (ADF), acid detergent lignin (ADL), and neutral detergent fiber (NDF) content of soybean cake were also determined [,]. The results of ADL, ADF, and NDF were expressed as cellulose (ADF-ADL), hemicellulose (NDF-ADF), and lignin (ADL) content.
The enzyme activity of proteases during the SSF was determined according to the method reported by Kachrimanidou et al. []. Briefly, fermented solids obtained from SSF were mixed with citric acid-Na2HPO4 (0.2 M, pH 6) buffer solution for the extraction of proteases. After centrifugation (3000× g, 4 °C, 15 min) extracts were used for the determination of enzymatic activities. Protease activity was quantified by the FAN production during hydrolysis of 7.5 g/L of casein at 55 °C. One unit of protease activity (U) was defined as the protease required for the production of 1 μg FAN in one minute at 55 °C and pH 6.0. The results were expressed as U/g of fermented solids.
FAN and IP concentrations were determined according to the ninhydrin colorimetric method and the ammonium molybdate spectrophotometric method, respectively [,].
An inductively-coupled plasma mass spectrometer (ICP-MS) (Thermo X-Series II, Thermo Fischer Scientific, Schwerte, Germany) was used for elemental determination of the soybean cake hydrolysate. Calibration curves with at least eight points in the range of 0.5–100 μg/L were prepared for 33 trace elements: Ar Cl, As, Ba, Be, Br, Ca, Cd, Co, Cu, Cr, Fe, Ga, Hg, In, K, Kr, Li, Lu, Mo, Mn, Mg, Na, Ni, P, Pb, S, Sb, Se, Sr, Ti, Tl, V, and Zn. The calibration curve with the highest correlation coefficient was used for each element.
Fumaric acid determination in culture broths was carried out via dilution with deionized water and 3 M H2SO4 solution to dissolve the residual CaCO3 and reduce the pH to less than 1.0, following heating at 80 °C until the broth become clear []. Suspensions were filtered and the biomass was washed with deionized water. Samples from the clear filtrate were obtained and sugar consumption, fumaric acid and other by-products were monitored by HPLC equipped with a Bio-rad Aminex HPX-87H column with 300 mm length and 7.8 mm internal diameter. A 10 mM H2SO4 solution was used as mobile phase with 0.6 mL/min flow rate and 45 °C column temperature. The fumaric acid production was expressed as g/L. The yield was defined as grams of fumaric acid produced per gram of total glucose added to the fermentation medium.
The morphology of fungal biomass was monitored by image analysis software (Image-Pro Plus 7.0, Media Cybernetics, Warrendale, PA, USA) and the number of pellets or clumps as well as the mean diameter were determined. The mean diameter is defined as the average length of diameters measured at two degree intervals and passing through the centroid of the object. The data presented are the mean values of two replicates and error bars represent the standard deviation of those values (as calculated in Microsoft Excel).
3. Results and Discussion
The fungus R. arrhizus was studied in shake flask fermentations using two different pre-culture media in order to obtain two morphologies: pellets and uniformly-dispersed mycelia. The formation of pelletized biomass was initially carried out in flasks of 250 mL, but since the biomass was aggregated, baffled flasks of 1 L were employed in order to achieve pelletized biomass. Uniformly dispersed clumps were produced in a high-starch concentration medium, which led to a viscous liquid that inhibits the aggregation of the biomass of R. arrhizus.
The effect of the two types of morphologies on fumaric acid production was evaluated through submerged experiments using glucose as a carbon source. Moreover, the influence of ammonium sulphate was also assessed at 0.04 and 0.2 g/L initial concentrations. The formation of each morphology was studied by scanning the biomass at 48 h of each fermentation and the scanned images was processed by image analysis software.
3.1. Effect of Pelletized Mycelia on Fumaric Acid Production
The fumaric acid production using pelletized biomass of R. arrhizus is depicted in Figure 1. The highest fumaric acid production of 7 g/L with a yield of 0.29 g/g and a productivity of 0.10 g/L × h-1 was produced at 0.04 g/L initial ammonium sulphate concentration (Figure 1a). Fumaric acid production, yield, and productivity were increased two-fold when the ammonium sulphate concentration increased to 0.2 g/L (Figure 1b). Particularly, a final concentration of 14.7 g/L fumaric acid was observed with a yield of 0.60 g/g and a productivity of 0.20 g/L × h-1. The by-products succinic acid and ethanol were also produced up to 1.28 g/L and 0.2 g/L, respectively.
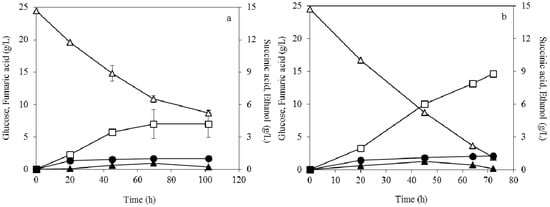
Figure 1.
Effect of pellet morphology on fumaric acid (□), ethanol (▲) and succinic acid (●) production during submerged fermentation of R. arrhizus NRRL 2582 at 25 g/L initial glucose concentration (△). The initial ammonium sulphate concentration was (a) 0.04 g/L and (b) 0.2 g/L.
The distribution of the pellets formed during fermentation containing 0.04 and 0.2 g/L of ammonium sulphate is shown in Figure 2. The pellet diameter was mainly ranged from 0.25–1 mm and 0.25–1.5 mm, while the average pellet diameter was 0.37 mm and 0.46 mm in the case of 0.04 and 0.2 g/L ammonium sulphate concentration, respectively. The results from image analysis showed that a total number of 168 ± 22 cells/mL and 268 ± 2 cells/mL were formed at 0.04 g/L and 0.2 g/L initial concentration of ammonium sulphate, respectively.
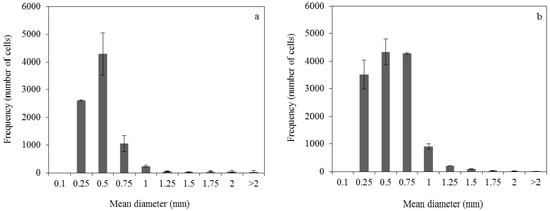
Figure 2.
Pellet diameter distribution at 48 h of submerged fermentation of R. arrhizus NRRL 2582 using 25 g/L initial glucose concentration. The initial ammonium sulphate concentration was (a) 0.04 g/L and (b) 0.2 g/L.
The initial concentration of ammonium sulphate seems to affect the metabolism of R. arrhizus, as the higher concentration enhanced fumaric acid production as well as the yield and the productivity. This could be related to the higher number of cells formed in the case of 0.2 g/L ammonium sulphate.
Pellet morphology has been utilised in several studies for fumaric acid production. Liao et al. [] and Zhou et al. [] have mentioned that fungal growth in pellet form enhanced fumaric acid production. Roa Engel et al. [] optimised the conditions in order to achieve small uniform pellets of R. oryzae ATCC 20344 with diameter less than 1 mm and achieved 30.2 g/L fumaric acid with a yield of 0.28 g/g. Zhou et al. [] found that pellet morphology of R. delemar NRRL 1526 produced higher concentration of fumaric acid (38.9 g/L) instead of suspended mycelia (31.8 g/L) using 100 g/L of glucose.
3.2. Effect of Dispersed Mycelia on Fumaric Acid Production
Figure 3 illustrates the kinetic profile of glucose consumption, fumaric acid and by-products formation during the fermentation using dispersed mycelia of R. arrhizus. A final concentration of 19.7 g/L fumaric acid was produced in the case of 0.04 g/L ammonium sulphate (Figure 3a). The corresponding yield and productivity were 0.84 g/g and 0.18 g/L × h-1, respectively. Similar results were observed during the fermentation at 0.2 g/L ammonium sulphate (Figure 3b). Particularly, a high fumaric acid production of 19.4 g/L was determined with a yield of 0.78 g/g and a productivity of 0.27 g/L × h-1. In the case of fermentation using dispersed mycelia, only the productivity of fumaric acid was affected by the different initial concentration of ammonium sulphate.
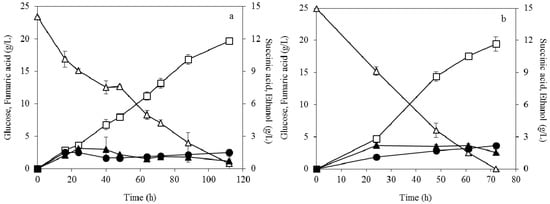
Figure 3.
Effect of dispersed mycelia morphology on fumaric acid (□), ethanol (▲) and succinic acid (●) production during submerged fermentation of R. arrhizus NRRL 2582 at 25 g/L initial glucose concentration (△). The initial ammonium sulphate concentration was (a) 0.04 g/L and (b) 0.2 g/L.
These results suggest that the utilisation of dispersed mycelia of R. arrhizus was more effective in fumaric acid production as the final concentration was significantly increased when compared with the results presented in Section 3.1. The fungus was able to consume glucose with higher efficiency and the yield was increased by 40%. The opposite effect has been reported in the case of R. delemar NRRL 1526 [].
The distribution of dispersed mycelia diameter (Figure 4) had wider range than the corresponding one of pellets. Specifically, in the fermentation with 0.04 g/L ammonium sulphate concentration, the cells diameter range was 0.25–2 mm with an average diameter of 0.86 mm (Figure 4a). When ammonium sulphate concentration of 0.2 g/L was utilised, the diameter of the dispersed mycelia was in the range of 0.25–1.5 mm and the average diameter was calculated at 0.43 mm (Figure 4b). The number of cells was determined as 58 ± 1 cells/mL at 0.04 g/L ammonium sulphate concentration and 332 ± 49 cells/mL at 0.2 g/L ammonium sulphate concentration.
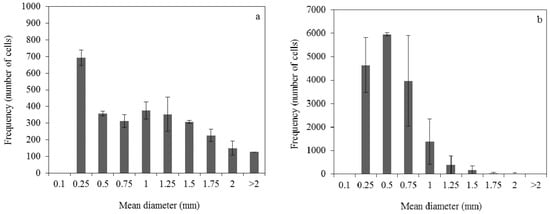
Figure 4.
Dispersed clumps diameter distribution at 48 h of submerged fermentation of R. arrhizus NRRL 2582 using 25 g/L initial glucose concentration. The initial ammonium sulphate concentration was (a) 0.04 g/L and (b) 0.2 g/L.
The dispersed mycelia formation of R. arrhizus has been efficiently utilised in fermentations for fumaric acid production by Rhodes et al. [,]. The dispersed mycelia of the fungus R. arrhizus has been previously cultivated in bioreactors, producing high concentrations (73–107 g/L) of fumaric acid with yields ranging from 0.72 g/g to 0.82 g/g using glucose as carbon source [,,]. The small and uniform mycelial clumps led to higher fumaric acid production than compact pellets maybe due to better oxygen transfer [].
The potential of the dispersed mycelia fungal morphology to produce high concentrations of fumaric acid was evaluated at fermentations containing higher glucose concentration. Figure 5 presents the results during fermentation of R. arrhizus at 45 g/L glucose. The highest fumaric acid production of 30.1 g/L with a yield of 0.66 g/g were observed at initial concentration of 0.04 g/L ammonium sulphate. The corresponding results were lower in the case of 0.2 g/L of ammonium sulphate (25.4 g/L fumaric acid with a yield of 0.53 g/g). The image analysis showed that the mean diameter and the number of the formed cells was almost the same as in fermentations with 25 g/L initial glucose concentration.
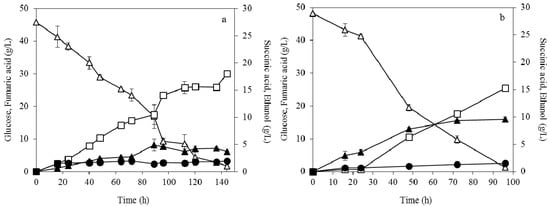
Figure 5.
Effect of dispersed mycelia morphology on fumaric acid (□), ethanol (▲) and succinic acid (●) production during submerged fermentation of R. arrhizus NRRL 2582 at 45 g/L initial glucose concentration (△). The initial ammonium sulphate concentration was (a) 0.04 g/L and (b) 0.2 g/L.
3.3. Enzymatic Hydrolysis of Soybean Cake
As depicted in Table 2, soybean cake is rich in protein thus it could be utilised as an alternative raw material for producing a nutrient-rich feedstock for microbial fermentations. The fungal strain A. oryzae was selected in the present study due to the efficient production of proteolytic enzymes on various substrates [,,]. The initial moisture content and pH value have been optimised in previous studies [,], thus the SSF on soybean cake was employed at an initial moisture content of 65% and uncontrolled pH. The kinetic profile of proteolytic activity during the SSF on soybean cake was evaluated and it is presented in Figure 6. The proteolytic activity increased during the fermentation reaching up to 205 U/g at 70 h. The SSF was utilised for hydrolysis process, when the proteolytic activity had reached the maximum value.

Table 2.
Composition of soybean cake.
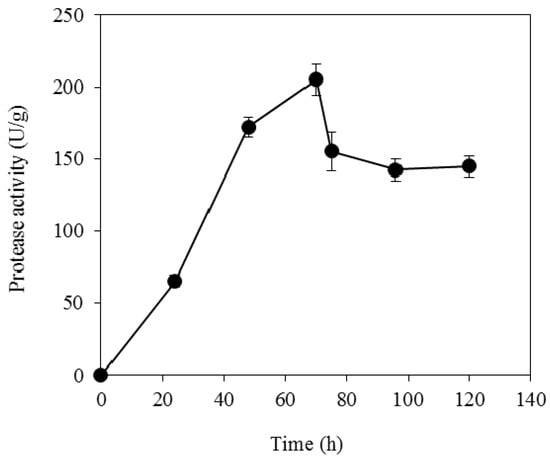
Figure 6.
Proteolytic activity (U/g of fermented solids) of A. oryzae during solid-state fermentation of soybean cake.
The hydrolysis of soybean cake was carried out at different temperatures in the range of 40 °C to 55 °C (Figure 7). The activity of proteases was enhanced at 45 °C during the hydrolysis of 50 g/L soybean cake, producing about 1.4 g/L FAN (free amino nitrogen) at 28 h of hydrolysis (Figure 7a). The IP production was similar at the different temperatures of hydrolysis reaching up to 113 mg/L at 45 °C (Figure 7b). The results showed that soybean cake was effectively hydrolysed by the proteases produced during SSF by A. oryzae. In particular, a TKN to FAN conversion yield of 43% was achieved.
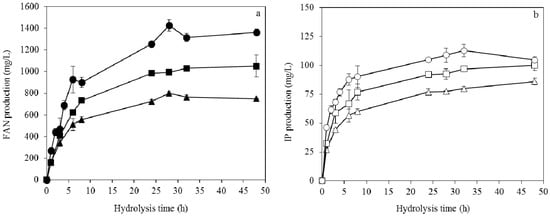
Figure 7.
FAN (a) and IP (b) production during hydrolysis of 45 g/L initial solids (soybean cake and fermented solids from SSF) at 45 °C (circles), 50 °C (squares) and 55 °C (triangles) using initial proteolytic activity of 3.9 U/mL.
Kachrimanidou et al. reported FAN production of around 0.6 g/L and 1.5 g/L at 45 °C when crude proteolytic enzymes of A. oryzae were employed for the hydrolysis of 45 g/L and 90 g/L of sunflower meal, respectively, using an initial proteolytic activity of 6.4 U/mL []. Higher FAN production of 2.3 g/L was achieved when the initial proteolytic activity increased to 16 U/mL []. Tsouko et al. achieved 451.6 mg/L FAN concentration and 161.3 mg/L IP concentration during hydrolysis of 98.7 g/L of palm kernel cake at 50 °C, using 6 U/mL initial proteolytic activity produced by A. oryzae []. The high FAN production obtained in this study was due to the high protein content of the soybean cake.
3.4. Fumaric Acid Production Using Soybean Cake Hydrolysate
The potential utilization of soybean cake hydrolysate as nutrient and nitrogen source for fumaric acid production by R. arrhizus strain was evaluated using dispersed mycelia morphology. The study focused on the replacement of the commercial nitrogen sources of the preculture by the soybean cake hydrolysate. Figure 8 presents the results during fermentation of R. arrhizus using 50 g/L initial glucose concentration and 0.2 g/L initial ammonium sulphate concentration. The preculture was conducted by substituting urea and peptone with soybean cake hydrolysate at initial FAN concentrations of 200 mg/L (Figure 8a) and 400 mg/L (Figure 8b). The results showed that the substitution of peptone and urea by the soybean cake hydrolysate influenced positively fumaric acid production. Particularly, the highest fumaric acid concentration of 33 g/L was achieved, when the initial FAN concentration of soybean cake was 200 mg/L. The corresponding yield was 0.69 g/g and the final productivity was 0.19 g/L × h-1.
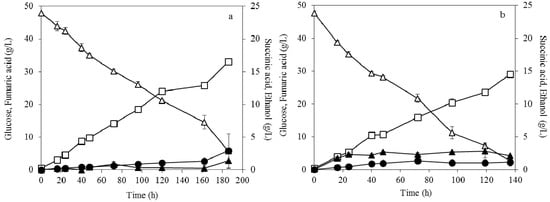
Figure 8.
Fumaric acid (□), ethanol (▲) and succinic acid (●) production during submerged fermentation of R. arrhizus NRRL 2582 at ~50 g/L of initial glucose (△) concentration using (a) 200 mg/L and (b) 400 mg/L FAN of soybean cake hydrolysate as nitrogen source in the preculture medium. The initial ammonium sulphate concentration was 0.2 g/L.
The fumaric acid production was improved when soybean cake hydrolysate was utilised as nitrogen source. The beneficial effect of commercial soybean peptone on the morphology of R. oryzae ATCC 20344 and R. oryzae NRRL 1526 has been reported in other studies [,,]. Zhang et al. [] showed that chemical hydrolysate derived from soybean meal can be utilised in the pre-culture medium of R. oryzae ATCC 20344 for fumaric acid production. Particularly, the utilisation of soybean meal hydrolysate led to 50 g/L fumaric acid production using 80 g/L glucose as carbon source, with a yield of 0.72 g/g and 0.36 g/L × h-1 productivity [].
The formed pelletized biomass and dispersed mycelia used in this study are presented in Figure 9. Although the significant differences found in morphology during image analysis, between the fermentations with commercial nitrogen sources (Figure 9a,b) and soybean cake hydrolysate (Figure 9c), the results indicated that fumaric acid production was also significantly affected by the nutritional parameters. Specifically, the dispersed mycelia was more uniform in fermentation with commercial nitrogen sources (Figure 9b) than soybean cake hydrolysate (Figure 9c), but the addition of soybean cake led to the highest fumaric acid production. ICP-MS analysis was performed in order to study the composition of soybean cake hydrolysate. The results showed that the hydrolysate was rich in microelements such as Mg (41.9 mg/L), Ca (11.5 mg/L), Fe (7.4 mg/L), and Zn (1.8 mg/L). Additionally, lower quantities (less than 0.2 mg/L) of Mn was detected.
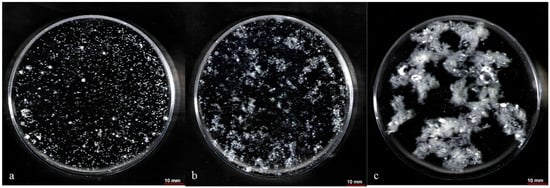
Figure 9.
Images of (a) pelletized biomass, (b) dispersed mycelia using commercial nitrogen sources, and (c) dispersed mycelia using 200 mg/L FAN of soybean cake hydrolysate as nitrogen sources. Images were taken at 48 h of submerged fermentation of R. arrhizus NRRL 2582 using 25 g/L of initial glucose and 0.2 g/L initial ammonium sulphate concentration.
4. Conclusions
The present study focused on the impact of the different morphologies on the fumaric acid production by the fungal strain R. arrhizus. The effect of fungus morphology on the fumaric acid production using different Rhizopus strains and on other microbial products such as citric acid [] has been studied in the past, but there is not any published research regarding the impact of R. arrhizus NRRL 2582 morphology on fumaric acid production. The biomass in the form of pellets and dispersed mycelia was produced and utilised in a synthetic medium for fumaric acid production. The results showed that uniformly dispersed mycelia led to higher fumaric acid concentrations and yields, compared with the results obtained using the pelletized biomass. Additionally, ammonium sulphate concentration in the fermentation medium had a key role in the metabolism of R. arrhizus, since the productivity was significantly improved at higher concentrations of ammonium sulphate. The potential substitution of commercial nitrogen sources by an enzymatic soybean cake hydrolysate was also evaluated. The soybean cake hydrolysate was effectively utilised by dispersed mycelia for high production of fumaric acid. This study demonstrated that agro-industrial biomass could be valorised for the sustainable biotechnological production of platform chemicals such as fumaric acid.
Acknowledgments
The authors would like to thank I. Mandala for providing access to Image Pro Plus Analysis Software. The work presented in this study has been funded by National Agency of Petroleum (ANP), Petrobras (Brazil) (project 00320-2/2012) and the National Council for Scientific and Technological Development of the Ministry of Science, Technology and Innovation (CNPq/MCTI) through the Special Visiting Researcher fellowship (process number: 313772/2013-4).
Author Contributions
Aikaterini Papadaki, Seraphim Papanikolaou, and Apostolis A. Koutinas conceived and designed the experiments; Nikolaos Androutsopoulos and Aikaterini Papadaki performed the experiments; Nikolaos Androutsopoulos carried out image analysis; Maria Patsalou and Michalis Koutinas contributed and performed the ICP-MS analysis; Aikaterini Papadaki, Nikolaos Kopsahelis, and Apostolis A. Koutinas were responsible for writing the paper, Aline Machado de Castro and Apostolis A. Koutinas revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Garcia, I.L.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef] [PubMed]
- Fumaric Acid Market Analysis by Application (Food & Beverages, Rosin Paper Sizes, UPR, Alkyd Resins) and Segment Forecasts to 2020. 2015. Available online: http://www.grandviewresearch.com/industry-analysis/fumaric-acid-market (accessed on 26 March 2017).
- Zhang, K.; Zhang, B.; Yang, S.-T. Production of citric, itaconic, fumaric and malic acids in filamentous fungal fermentation. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers, 1st ed.; Yang, S.-T., El-Enshasy, H.A., Thongchul, N., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Roa Engel, C.A.; Straathof, A.J.J.; Zijlmans, T.W.; van Gulk, W.M.; van der Wielen, L.A.M. Fumaric acid production by fermentation. Appl. Microbiol. Biotechnol. 2008, 78, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, J.K.; Lasure, L.L. Organic acid production by filamentous fungi. In Advances in Fungal Biotechnology for Industry, Agriculture and Medicine; Tracz, J.S., Lange, L., Eds.; Kluwer/Plenum: New York, NY, USA, 2004; pp. 307–340. [Google Scholar]
- Rhodes, R.A.; Lagoda, A.A.; Jackson, R.W.; Misenhei, T.J.; Smith, M.L.; Anderson, R.F. Production of fumaric acid in 20 liter fermentors. Appl. Microbiol. 1962, 10, 9–15. [Google Scholar] [PubMed]
- Kenealy, W.; Zaady, E.; Dupreez, J.C.; Stieglitz, B.; Goldberg, I. Biochemical aspects of fumaric acid accumulation by Rhizopus arrhizus. Appl. Environ. Microbiol. 1986, 52, 128–133. [Google Scholar] [PubMed]
- Gangl, I.C.; Weigand, W.A.; Keller, F.A. Economic comparison of calcium fumarate and sodium fumarate production by Rhizopus arrhizus. Appl. Biochem. Biotechnol. 1990, 24–25, 663–677. [Google Scholar] [CrossRef]
- Petruccioli, M.; Angiani, E.; Federici, F. Semi continuous fumaric acid production by Rhizopus arrhizus immobilized in polyurethane sponge. Process Biochem. 1996, 31, 463–469. [Google Scholar] [CrossRef]
- Liao, W.; Liu, Y.; Frear, C.; Chen, S. A new approach of pellet formation of a filamentous fungus–Rhizopus oryzae. Bioresour. Technol. 2007, 98, 3415–3423. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Du, G.; Hua, Z.; Zhou, J.; Chen, J. Optimisation of fumaric acid production by Rhizopus delemar based on the morphology formation. Bioresour. Technol. 2011, 102, 9345–9349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yu, C.; Yang, S.-T. Effects of soybean meal hydrolysate as the nitrogen source on seed culture morphology and fumaric acid production by Rhizopus oryzae. Process Biochem. 2015, 50, 173–179. [Google Scholar] [CrossRef]
- Roa Engel, C.A.; van Gulk, W.M.; Marang, L.; van der Wielen, L.A.M.; Straathof, A.J.J. Development of low pH fermentation strategy for fumaric acid production by Rhizopus oryzae. Enzyme Microb. Technol. 2011, 48, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Byrne, G.S.; Ward, O.P. Effect of nutrition on pellet formation by Rhizopus arrhizus. Biotechnol. Bioeng. 1989, 33, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Du, J.X.; Tsao, G.T. Mycelial pellet formation by Rhizopus oryzae ATCC 20344. Appl. Biochem. Biotechnol. 2000, 86, 779–789. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K. Enhanced fumaric acid production from brewery wastewater and insight into the morphology of Rhizopus oryzae 1526. Appl. Biochem. Biotechnol. 2014, 172, 2974–2988. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, R.A.; Moyer, A.J.; Smith, M.L.; Kelley, S.E. Production of fumaric acid by Rhizopus arrhizus. Appl. Microbiol. 1959, 7, 74–80. [Google Scholar] [PubMed]
- Kachrimanidou, V.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Yanniotis, S.; Kookos, I.; Koutinas, A.A. Utilisation of by-products from sunflower-based biodiesel production processes for the production of fermentation feedstock. Waste Biomass Valoriz. 2013, 4, 529–537. [Google Scholar] [CrossRef]
- Wang, R.; Law, R.C.S.; Webb, C. Protease production and conidiation by Aspergillus oryzae in flour fermentation. Proc. Biochem. 2005, 40, 217–227. [Google Scholar] [CrossRef]
- Dimou, C.; Kopsahelis, N.; Papadaki, A.; Papanikolaou, S.; Kookos, I.K.; Mandala, I.; Koutinas, A.A. Wine lees valorization: Biorefinery development including production of a generic fermentation feedstock employed for poly(3-hydroxybutyrate) synthesis. Food Res. Int. 2015, 73, 81–87. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists Inc. (AACC). Approved Methods of the American Association of Cereal Chemists, 8th ed.; American Association of Cereal Chemists Inc.: St. Paul, MN, USA, 1983. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Method 973.18, Fiber (Acid Detergent) and Lignin in Animal Feed, 15th ed.; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1990. [Google Scholar]
- Lie, S. The EBC-ninhydrin method for determination of free alpha amino nitrogen. J. Inst. Brew. 1973, 79, 37–41. [Google Scholar] [CrossRef]
- Harland, B.F.; Harland, J. Fermentative reduction of phytate in rye, white and whole wheat breads. Cereal Chem. 1980, 57, 226–229. [Google Scholar]
- Goldberg, I.; Lonberg-Holm, K.; Bagley, E.A.; Stieglitz, B. Improved conversion of fumarate to succinate Escherichia coli strains amplified for fumarate reductase. Appl. Environ. Microbiol. 1983, 1838–1847. [Google Scholar]
- Ng, T.K.; Hesser, R.J.; Stieglitz, B.; Griffiths, B.S.; Ling, L.B. Production of tetrahydrofuran/1,4-butanediol by a combined biological and chemical process. Biotechnol. Bioeng. Symp. 1986, 17, 344–363. [Google Scholar]
- Tsouko, E.; Kachrimanidou, V.; Dos Santos, A.F.; do Nascimento Vitorino Lima, M.E.; Papanikolaou, S.; de Castro, A.M.; Freire, D.M.; Koutinas, A.A. Valorization of By-Products from Palm Oil Mills for the Production of Generic Fermentation Media for Microbial Oil Synthesis. Appl. Biochem. Biotechnol. 2016, 181, 1241–1256. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Sikander, A.; Haq, I. Acidic pre-treatment of sugarcane molasses for molasses for citric acid production by Aspergillus niger NG-4. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 584–595. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).