A Sequential Steam Explosion and Reactive Extrusion Pretreatment for Lignocellulosic Biomass Conversion within a Fermentation-Based Biorefinery Perspective
Abstract
:1. Introduction
2. Materials and Methods
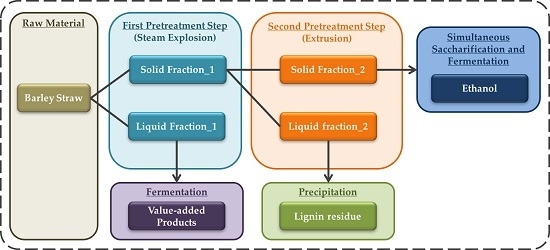
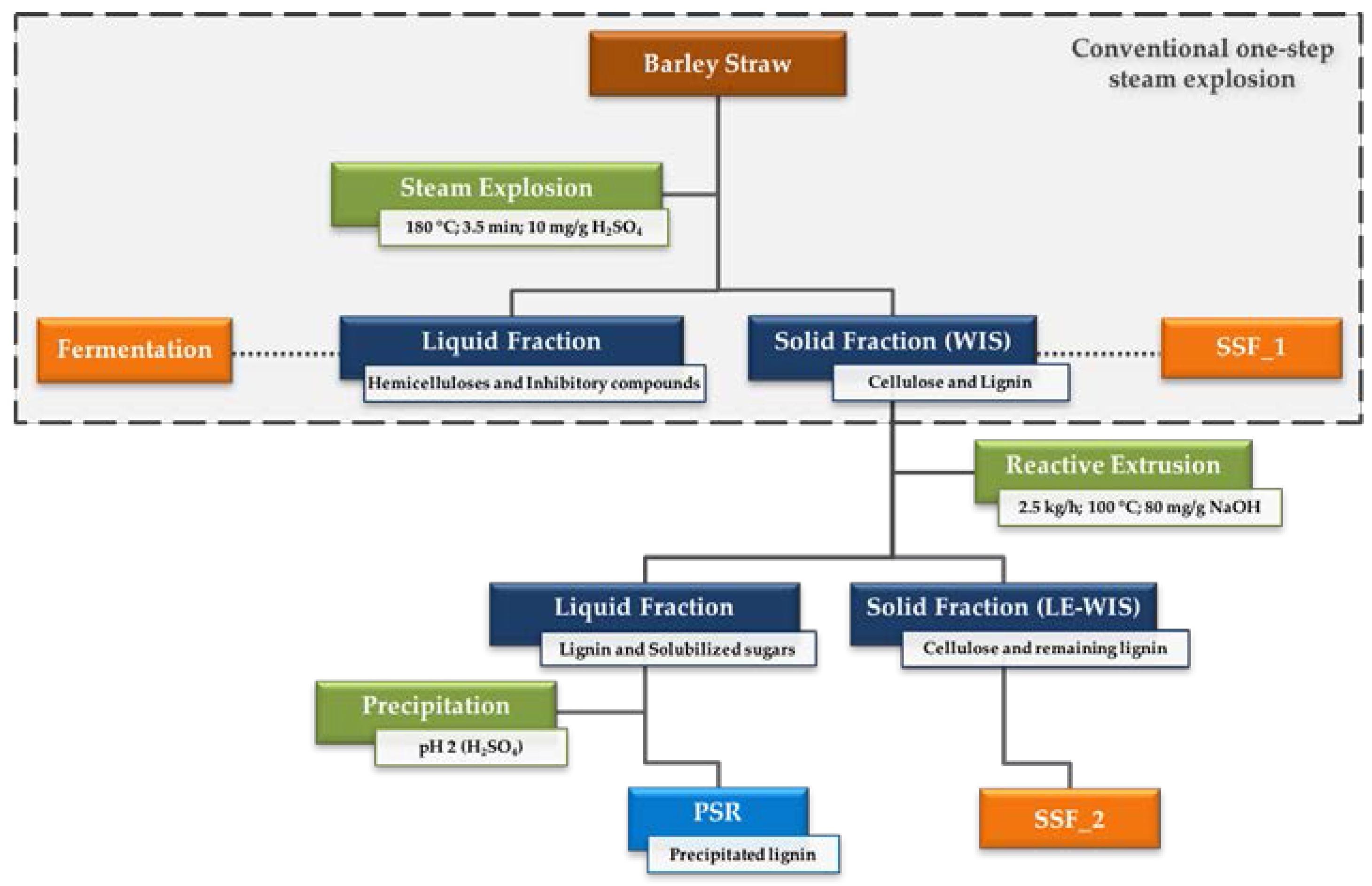
2.1. Raw Material and Pretreatment Process
2.2. Microorganisms and Growth Conditions
2.3. Enzymes
2.4. Fermentation of the Hemicellulosic-Rich Liquid Fraction
2.5. Hydrolysability and Fermentability Studies of the Pretreated Solid Fractions
2.5.1. Enzymatic Hydrolysis
2.5.2. Simultaneous Saccharification and Fermentation
2.6. Analytical Methods
2.6.1. Compositional Analysis of Biomass
2.6.2. ATR-FTIR Analysis of Solid Residues
2.6.3. Identification and Quantification of Metabolites
3. Results and Discussion
3.1. Pretreatment of Barley Straw
3.2. Characterization of Solid Residues by Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
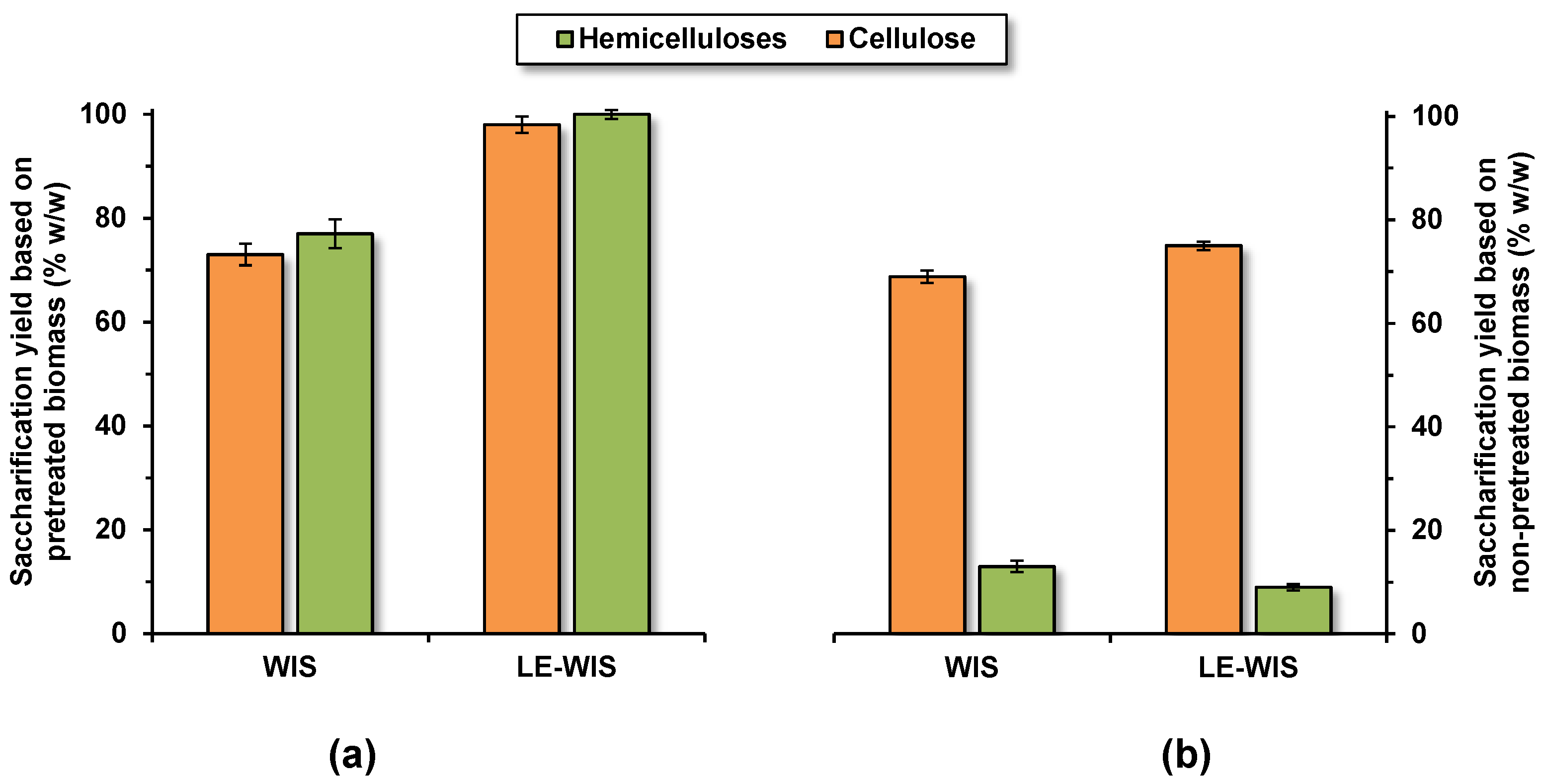
3.3. Saccharification of Pretreated Solid Residues
3.4. Conversion of Lignocellulosic Sugar by Microbial Fermentation Processes
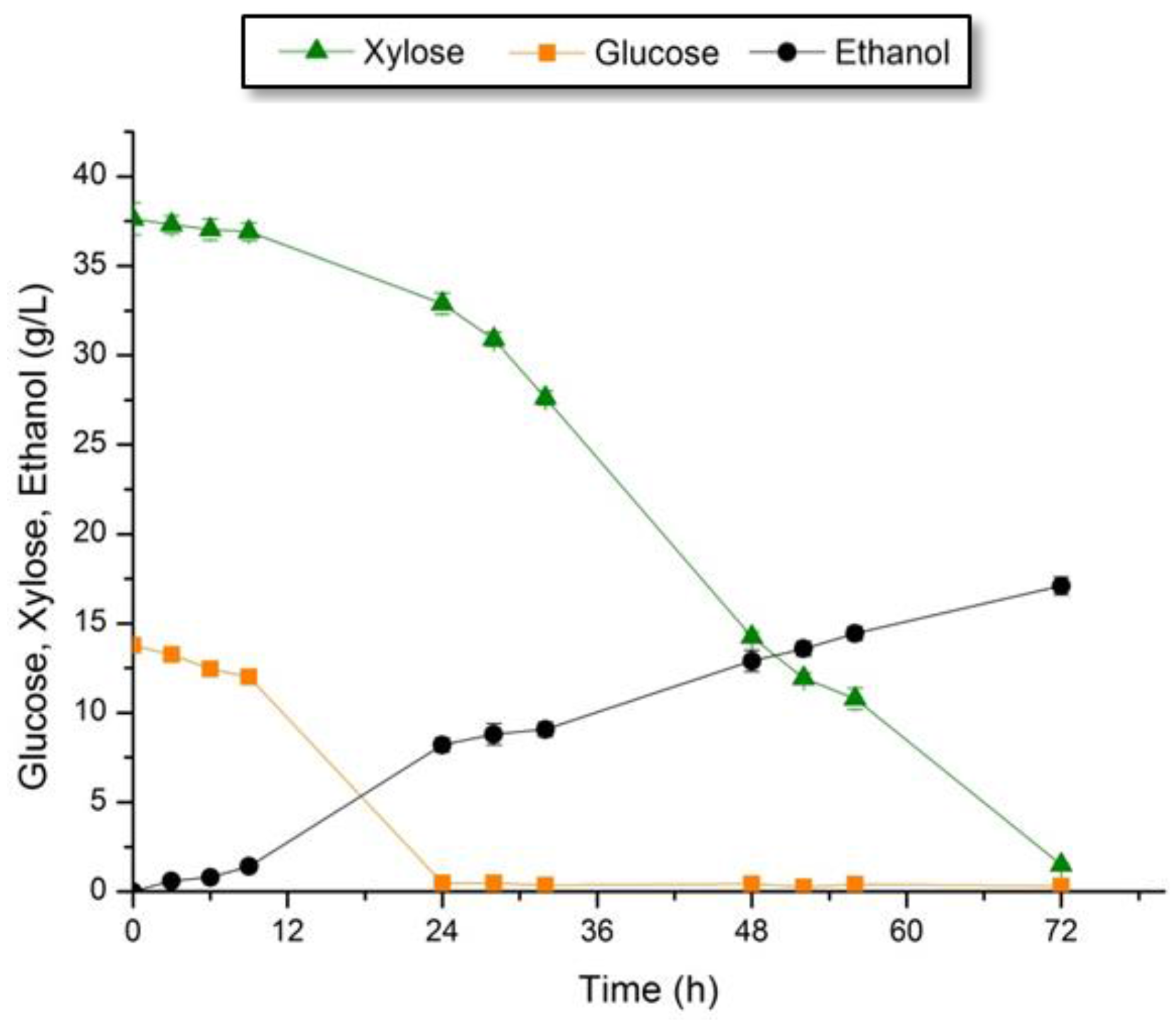
3.4.1. Fermentation of the Hemicellulose-Rich Liquid Fraction
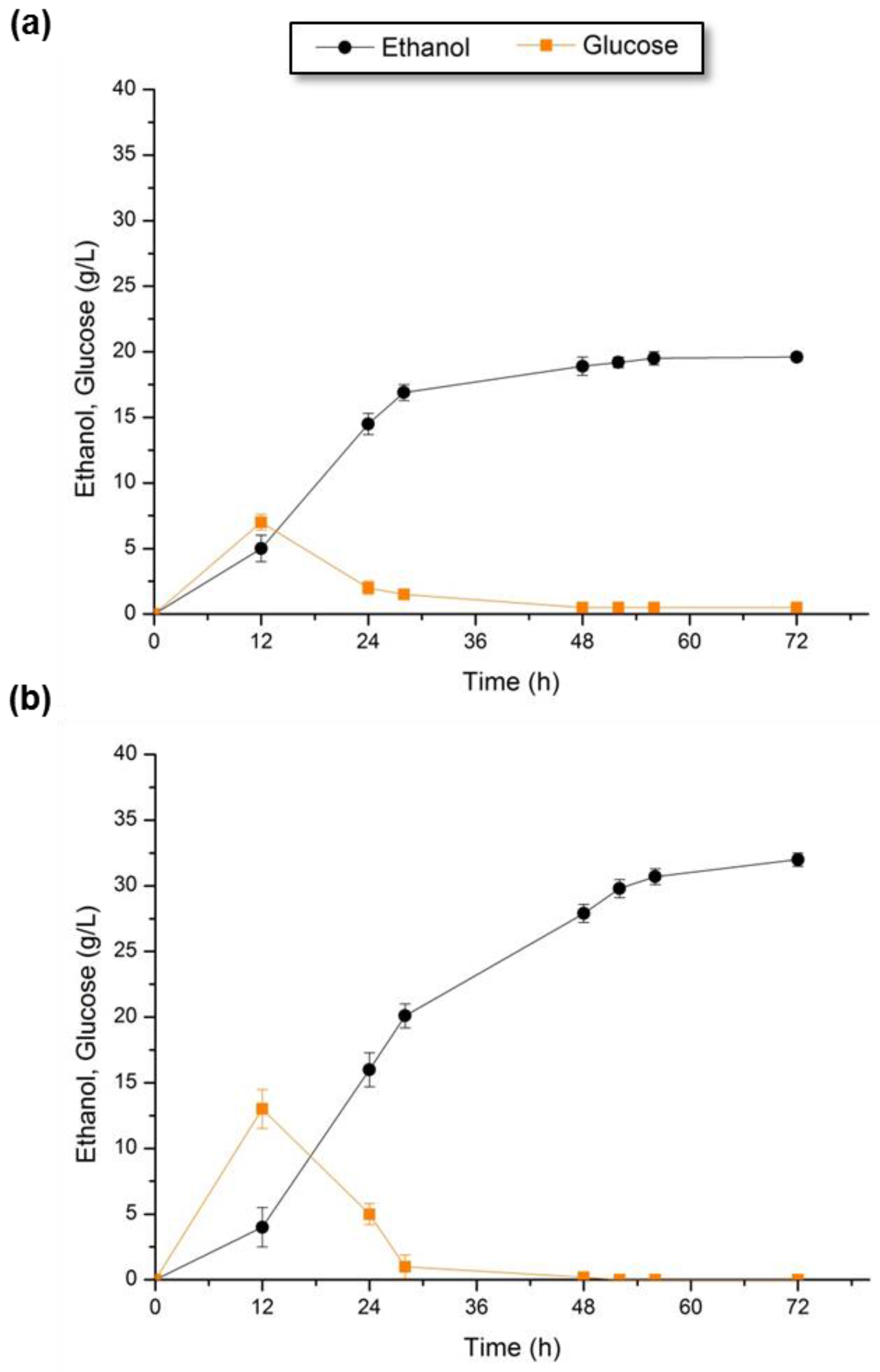
3.4.2. SSF of Pretreated Solid Fractions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 5-HMF | 5-hydroxymethylfurfural |
| ATR-FTIR | Attenuated Total Reflectance-Fourier Transform Infrared spectroscopy |
| CDW | Cell Dry Weight |
| DW | Dry Weight |
| EtOHmax | Maximum Ethanol concentration |
| FPU | Filter Paper Units |
| GC | Gas Chromatography |
| HPLC | High Performance Liquid Chromatography |
| LE-WIS | Lignin-Extracted Water Insoluble Solid fraction |
| NREL-LAP | National Renewable Energies Laboratory-Laboratory Analytical Procedures |
| PSR | Precipitated Solid Residue |
| QE | Ethanol Volumetric Productivity |
| WIS | Water Insoluble Solid fraction |
| YE/ET | Ethanol Yield based on the maximum theoretical ethanol |
| YE/S | Ethanol Yield based on potential sugars |
References
- Olsson, L.; Saddler, J. Biorefineries, using lignocellulosic feedstocks, will have a key role in the future bioeconomy. Biofuels Bioprod. Biorefin. 2013, 7, 475–477. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Dragone, G.M. Biomass pretreatment, biorefineries, and potential products for a bioeconomy development. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–22. [Google Scholar]
- Duque, A.; Manzanares, P.; Ballesteros, I.; Ballesteros, M. Steam explosion as lignocellulosic biomass pretreatment. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 349–368. [Google Scholar]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Inhibition of cellulases by phenols. Enzyme Microb. Technol. 2010, 46, 170–176. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.P.; Bura, R.; Mabee, W.E.; Berlin, A.; Pan, X.; Saddler, J.N. Substrate pretreatment: The key to effective enzymatic hydrolysis of lignocellulosics? Adv. Biochem. Eng. Biotechnol. 2007, 108, 67–93. [Google Scholar] [PubMed]
- Zheng, J.; Rehmann, L. Extrusion pretreatment of lignocellulosic biomass: A review. Int. J. Mol. Sci. 2014, 15, 18967–18984. [Google Scholar] [CrossRef] [PubMed]
- Vandenbossche, V.; Brault, J.; Vilarem, G.; Hernández-Meléndez, O.; Vivaldo-Lima, E.; Hernández-Luna, M.; Barzana, E.; Duque, A.; Manzanares, P.; Ballesteros, M.; et al. A new lignocellulosic biomass deconstruction process combining thermo-mechano chemical action and bio-catalytic enzymatic hydrolysis in a twin-screw extruder. Ind. Crops Prod. 2014, 55, 258–266. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, I.; Negro, M.J.; Oliva, J.M.; Sáez, F.; Ballesteros, M. Optimization of integrated alkaline–Extrusion pretreatment of barley straw for sugar production by enzymatic hydrolysis. Process Biochem. 2013, 48, 775–781. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- NREL. Chemical Analysis and Testing Laboratory Analytical Procedures. National Renewable Energy Laboratory: Golden, CO, USA, 2008. Available online: https://www.nrel.gov/bioenergy/biomass-compositional-analysis.html (accessed on 1 March 2017).
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Oliva, J.M.; Sáez, F.; Ballesteros, I.; González, A.; Negro, M.J.; Manzanares, P.; Ballesteros, M. Effect of lignocellulosic degradation compounds from steam explosion pretreatment on ethanol fermentation by thermotolerant yeast Kluyveromyces marxianus. Appl. Biochem. Biotechnol. 2003, 105–108, 141–153. [Google Scholar] [CrossRef]
- Norgren, M.; Edlund, H. Lignin: Recent advances and emerging applications. Curr. Opin. Colloid Interface Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- Sun, X.F.; Xu, F.; Sun, R.C.; Fowler, P.; Baird, M.S. Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr. Res. 2005, 340, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Gurram, R.N.; Al-Shannag, M.; Lecher, N.J.; Duncan, S.M.; Singsaas, E.L.; Alkasrawi, M. Bioconversion of paper mill sludge to bioethanol in the presence of accelerants or hydrogen peroxide pretreatment. Bioresour. Technol. 2015, 192, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Inoue, S.; Teramoto, Y.; Endo, T. Enzymatic saccharification of woody biomass micro/nanofibrillated by continuous extrusion process II: Effect of hot-compressed water treatment. Bioresour. Technol. 2010, 101, 9645–9649. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Xu, Y.Y.; Hwang, W.S.; Wang, J.B. Pretreatment of rice straw using an extrusion/extraction process at bench-scale for producing cellulosic ethanol. Bioresour. Technol. 2011, 102, 10451–10458. [Google Scholar] [CrossRef] [PubMed]
- E4tech; RE-CORD; WUR. From the Sugar Platform to Biofuels and Biochemicals. Final report for the European Commission Directorate-General Energy, contract No. ENER/C2/423-2012/SI2.673791: European Union. 2015. Available online: http://ibcarb.com/wp-content/uploads/EC-Sugar-Platform-final-report.pdf (accessed on 15 January 2017).
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Fermentation inhibitors in ethanol processes and different strategies to reduce their effects. In Biofuels Alternative Feedstocks and Conversion Processes; Pandey, A., Larroche, C., Ricke, S.C., Dussap, C.-G., Gnansounou, E., Eds.; Academic Press: Amsterdam, The Netherlands, 2011; pp. 287–311. [Google Scholar]
- Agbogbo, F.K.; Coward-Kelly, G. Cellulosic ethanol production using the naturally occurring xylose-fermenting yeast, Pichia stipitis. Biotechnol. Lett. 2008, 30, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. A review of biological delignification and detoxification methods for lignocellulosic bioethanol production. Crit. Rev. Biotechnol. 2015, 35, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Reimann, A.; Nilvebrant, N.-O.; Jönsson, L.J. Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl. Biochem. Biotechnol. 1999, 77, 91–103. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Fernández, J.L.; Ballesteros, M. Different laccase detoxification strategies for ethanol production from lignocellulosic biomass by the thermotolerant yeast Kluyveromyces marxianus CECT 10875. Bioresour. Technol. 2012, 106, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.M.; Ballesteros, I.; Negro, M.J.; Manzanares, P.; Cabañas, A.; Ballesteros, M. Effect of binary combinations of selected toxic compounds on growth and fermentation of Kluyveromyces marxianus. Biotechnol. Prog. 2004, 20, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Moreno, A.D.; Ibarra, D.; Sáez, F.; Ballesteros, M. Improving the fermentation performance of Saccharomyces cerevisiae by laccase during ethanol production from steam-exploded wheat straw at high-substrate loadings. Biotechnol. Prog. 2013, 29, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Zacchi, G.; Axelsson, A. Economic evaluation of preconcentration in production of ethanol from dilute sugar solutions. Biotechnol. Bioeng. 1989, 34, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Roberto, I.C. Xylitol production from high xylose concentration: Evaluation of the fermentation in bioreactor under different stirring rates. J. Appl. Microbiol. 2003, 95, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Ilmén, M.; Koivuranta, K.; Ruohonen, L.; Suominen, P.; Penttilä, M. Efficient production of L-lactic acid from xylose by Pichia stipitis. Appl. Environ. Microbiol. 2007, 73, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Fernández, D.; Lozano-Martínez, P.; Buey, R.M.; Revuelta, J.L.; Jiménez, A. Utilization of xylose by engineered strains of Ashbya gossypii for the production of microbial oils. Biotechnol. Biofuels 2017, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.B.; Felby, C.; Jørgensen, H. Yield-determining factors in high-solids enzymatic hydrolysis of lignocellulose. Biotechnol. Biofuels 2009, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kang, L.; Wei, H.; Arora, R.; Lee, Y.Y. Study on the decreased sugar yield in enzymatic hydrolysis of cellulosic substrate at high solid loading. Appl. Biochem. Biotechnol. 2011, 164, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Tomás-Pejó, E.; Ibarra, D.; Ballesteros, M.; Olsson, L. In Situ laccase treatment enhances the fermentability of steam-exploded wheat straw in SSCF processes at high dry matter consistencies. Bioresour Technol. 2013, 143, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Banat, B.M.; Hoshida, H.; Ano, A.; Nonklang, S.; Akada, R. High-temperature fermentation: How can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl. Microbiol. Biotechnol. 2010, 85, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Ballesteros, I.; Fernández, J.L.; Ballesteros, M. Ethanol from laccase-detoxified lignocellulose by the thermotolerant yeast Kluyveromyces marxianus-Effects of steam pretreatment conditions, process configurations and substrate loadings. Biochem. Eng. J. 2013, 79, 94–103. [Google Scholar] [CrossRef]
- Gurram, R.N.; Al-Shannag, M.; Knapp, S.; Das, T.; Singsaas, E.; Alkasrawi, M. Technical possibilities of bioethanol production from coffee pulp: A renewable feedstock. Clean Technol. Environ. 2016, 18, 269–278. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, J. A modified method for calculating practical ethanol yield at high lignocellulosic solids content and high ethanol titer. Bioresour. Technol. 2012, 116, 74–79. [Google Scholar] [CrossRef] [PubMed]

| WIS Fraction | ||||
| Component | % (w/w) | |||
| Cellulose | 55.1 ± 0.3 | |||
| Hemicellulose | 8.8 ± 0.2 | |||
| Lignin | 32.1 ± 1.9 | |||
| Ashes | 2.5 ± 0.3 | |||
| Others | ~1.5 | |||
| Liquid Fraction | ||||
| Sugar | Monomeric Form % (w/w) a | Oligomeric Form % (w/w) a | Inhibitor | % (w/w) a |
| Glucan | 0.7 ± 0.1 (1.7) | 2.8 ± 0.2 (7.6) | Acetic ac. | 0.23 ± 0.04 (0.6) |
| Xylan | 7.2 ± 0.4 (18.0) | 13.9 ± 1.2 (31.9) | Formic ac. | n.d. |
| Arabinan | 2.5 ± 0.3 (6.2) | 1.1 ± 0.2 (2.9) | Furfural | 0.17 ± 0.03 (0.4) |
| Galactan | 0.7 ± 0.2 (1.8) | 0.7 ± 0.1 (1.7) | 5-HMF | 0.04 ± 0.01 (0.1) |
| Vanillin | <0.01 (12 × 10−3) | |||
| Syringaldehyde | <0.01 (7 × 10−3) | |||
| p-courmaric ac. | 0.01 ± 0.00 (15 × 10−3) | |||
| Ferulic ac. | 0.01 ± 0.00 (21 × 10−3) | |||
| LE-WIS Fraction | |
| Component | % (w/w) |
| Cellulose | 64.2 ± 2.0 |
| Hemicellulose | 6.8 ± 0.1 |
| Lignin | 29.3 ± 0.6 |
| Ashes | 2.1 ± 0.0 |
| PSR Fraction | |
| Component | % (w/w) |
| Glucan | 0.9 ± 0.1 |
| Xylan | 2.5 ± 0.2 |
| Lignin | 85.1 ± 1.5 |
| Ashes | 8.6 ± 0.6 |
| Component | Steam Explosion | Extrusion | ||
|---|---|---|---|---|
| Solid a | Liquid | Solid b | Liquid | |
| Glucan | 90 | 9 | 75 | n.d. |
| Hemicellulose | 17 | 82 | 9 | n.d. |
| Lignin | 87 | n.d. | 55 | 32 c |
| Substrate (w/w) | Yeast | EtOHmax (g/L) | YE/S (g/g) | YE/ET (%) | QEmax (g/L·h) |
|---|---|---|---|---|---|
| Liquid fraction a | S. stipitis | 17.5 ± 0.2 | 0.34 ± 0.01 b | 66.7 | 0.46 ± 0.01 |
| 10% WIS | S. cerevisiae | 19.6 ± 0.1 | 0.29 ± 0.00 c | 56.9 | 0.83 ± 0.04 |
| 10% LE-WIS | S. cerevisiae | 31.7 ± 0.3 | 0.40 ± 0.01 c | 78.4 | 0.96 ± 0.09 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, I.; Chamorro, M.Á.; Sáez, F.; Ballesteros, M.; Moreno, A.D. A Sequential Steam Explosion and Reactive Extrusion Pretreatment for Lignocellulosic Biomass Conversion within a Fermentation-Based Biorefinery Perspective. Fermentation 2017, 3, 15. https://doi.org/10.3390/fermentation3020015
Oliva JM, Negro MJ, Manzanares P, Ballesteros I, Chamorro MÁ, Sáez F, Ballesteros M, Moreno AD. A Sequential Steam Explosion and Reactive Extrusion Pretreatment for Lignocellulosic Biomass Conversion within a Fermentation-Based Biorefinery Perspective. Fermentation. 2017; 3(2):15. https://doi.org/10.3390/fermentation3020015
Chicago/Turabian StyleOliva, José Miguel, María José Negro, Paloma Manzanares, Ignacio Ballesteros, Miguel Ángel Chamorro, Felicia Sáez, Mercedes Ballesteros, and Antonio D. Moreno. 2017. "A Sequential Steam Explosion and Reactive Extrusion Pretreatment for Lignocellulosic Biomass Conversion within a Fermentation-Based Biorefinery Perspective" Fermentation 3, no. 2: 15. https://doi.org/10.3390/fermentation3020015
APA StyleOliva, J. M., Negro, M. J., Manzanares, P., Ballesteros, I., Chamorro, M. Á., Sáez, F., Ballesteros, M., & Moreno, A. D. (2017). A Sequential Steam Explosion and Reactive Extrusion Pretreatment for Lignocellulosic Biomass Conversion within a Fermentation-Based Biorefinery Perspective. Fermentation, 3(2), 15. https://doi.org/10.3390/fermentation3020015