1. Introduction
Probiotics are live microorganisms which, when consumed in large quantities, confer health benefits [
1] to the consumer. The consumption of appreciable amounts of fermentative dairy products confers a health benefit by balancing intestinal microflora. The low viability of probiotic organisms is one of the most important problems in the processing and production of probiotic food supplements, because of their sensitivity to difficult conditions such as low pH in food and powerful enzymes of the stomach. A standardized probiotic food must contain a minimum of 10
6 CFU/g active and live organisms at the time of consumption [
2].
Research on the production of functional foods which promote consumers’ health has been on the increase in recent years. This development has brought a reduction in some of the diseases related to life style [
3]. “Functional foods” refers to foods with health-promoting ingredients enriched beyond the normal traditional nutrients [
4,
5]. These foods contain a reasonable quantity of some bioactive components, such as probiotic, prebiotic, and symbiotic components [
3].
For the most part, probiotics have been effectively incorporated in dairy products, but these products are known to contain a high amount of lactose, which places a limitation to lactose intolerant individuals, hence the need to find an alternative means of production that can make the product available for a larger population.
Sivudu et al. [
6] reported the probiotic action of mixed watermelon and tomato juice using
Lactobacillus strains.
The production of a probiotic mixture of black cherry and barberry juice by lactic acid bacteria (LAB) has been reported by Shahram et al. [
7]. He concluded that a mixture with 0.2% whey powder could be considered as a suitable matrix for the growth of probiotic bacteria and functional beverage production.
Pineapple (
Ananas comosus) is a tropical plant with edible multiple fruits; it is the most economically important plant in the
Bromeliaceae family [
8]. Pineapple can be consumed fresh, cooked, juiced, and preserved. The flesh and juice of the pineapple are used in cuisines around the world. It contains a high amount of vitamin C, magnesium, and calcium. The juice of the pineapple is served as a beverage, and it is also the main ingredient in cocktails [
9]. The presence of a high amount of vitamin C and sugar makes it an essential medium for the cultivation of probiotics.
There are many dairy and cereal probiotic foods, but the demand for vegetable probiotic products from fruits and vegetables has increased, due to an increase in the population of vegetarians and lactose intolerant individuals who are allergic to dairy products [
10,
11,
12,
13]. Vegetables and fruits are good sources of vitamins, minerals, dietary fibers, antioxidants, and bioactive compounds which have a positive effect on some vital organs in the body [
14]. Therefore, non-dairy items such as fruits and vegetables which lack some dairy allergens could be used [
5]. The ability of fresh-cut pineapple to serve as a new carrier of probiotic LAB and the use of probiotic lactic acid bacteria for the production of multifunctional fresh-cut cantaloupe has been reported [
15,
16].
Therefore, this study aimed at the production and storage of probiotic pineapple juice using probiotic LAB (single and mixed culture) as starter, and determination of the physicochemical parameters, vitamin content, viability, antagonistic activity, and sensory evaluation of the samples.
2. Materials and Methods
2.1. Sample Collection and Laboratory Preparation of Pineapple Juice (Ananas comosus)
Pineapple was purchased from Bodija market, Favours Farm, and one other market in Ibadan, Oyo State, Nigeria. The fruit was kept at 4 °C for further use. The fruits were washed thoroughly with running tap water, rinsed with distilled water, peeled, and rinsed again with sterile distilled water. The pineapple juice was then extracted using a juice extractor. The extracted juice was pasteurized using a pasteurizer at a temperature of 93 °C for 48 s. After cooling, samples were stored at 4 °C before they were used for further analysis.
2.2. Culture Collection and Inocula Preparation
Probiotic
Pediococcus pentosaceus LaG1,
Pediococcus pentosaceus LBF2, and
Lactobacillus rhamnosus GG were obtained from the culture collection of our previous work in the Department of Microbiology, University of Ibadan. The stock cultures were maintained on De Man Rogosa Sharpe (MRS) agar and stored at 4 °C. A 0.5 McFarland standard suspension with turbidity of 1.5 × 10
8 cfu/mL [
17] was used to standardize the inocula. The seed culture was grown in a 250 mL flask containing 50 mL of sterile MRS broth. Cells were harvested by centrifugation (10,000
g) for ten minutes and washed twice with sterile 0.1% peptone buffer [
18]. The cultures were diluted with sterile distilled water, and the optical density of the bacterial suspension was measured using a spectrophotometer at 625 nm. The optical density of the bacterial suspension was compared with the optical density of 0.5 McFarland containing 1.5 × 10
8 cfu/mL [
17]. Serial dilution was done to obtain a 10
7 cfu/mL dilution of the probiotic LAB.
2.3. Production of the Probioticated Juice Samples
Probiotication of pasteurized pineapple juice samples was done by inoculating 100 mL of the sample with 1% (v/v) equivalent to 10 mL of the probiotic LAB starter culture (0.5 McFarland standard containing 1.5 × 108 cfu/mL). The inoculated samples with the probiotic LAB (Pediococcus pentosaceus LaG1, Lactobacillus rhamnosus GG, Pediococcus pentosaceus LBF2, and a mixed culture of the three probiotic LAB strains) was labelled as: Propp1 (juice sample inoculated with Pediococcus pentosaceus LaG1), Propp2 (juice sample inoculated with Pediococcus pentosaceus LBF2), ProRhamno (juice sample inoculated with Lactobacillus rhamnosus GG), Proconsortium (juice sample inoculated with Pediococcus pentosaceus LaG1, Pediococcus pentosaceus LBF2, and Lactobacillus rhamnosus GG), and Pcontrol (un-inoculated juice samples). The inoculated samples were incubated at 37 °C for 72 h and stored at 4 °C for four weeks. Weekly analysis, including total soluble solids, colour, and pH was conducted.
2.4. Physicochemical Analysis
2.4.1. Quantitative Estimation of Lactic Acid and pH
The production of lactic acid was determined by titrating 10 mL of the homogenized sample against 0.25 mol·L
−1 NaOH using 1 mL of phenolphthalein indicator (0.5% in 50% alcohol). Acid equivalent is the amount of NaOH consumed in mL, while each mL of NaOH is equivalent to 90.08 mg of lactic acid [
19].
The pH of the probioticated juice samples stored at 4 °C for 1–4 weeks was determined using a pH meter (Titumum U9N model).
Colour assessment of the probioticated juice sample stored for different time intervals was done by using the colour meter. The colour parameters (Hunter L *, a *, and b * value) were determined for each sample using a spectrophotometer—colorimeter (CM-2500D, Minolta, Japan) per the method of Maskan [
20]. Total soluble solids (TSS) were determined using a hand refractometer (Erma, Tokyo, Japan) in terms of °Bx (°Brix) [
20].
2.4.2. Determination of Vitamin C (Ascorbic Acid)
Vitamin C (ascorbic acid) was determined by the titrimetric method as described by Mazumdar and Majumder [
21] and James [
22]. Ten millilitres of the probioticated juice samples was made up to 100 mL volume with 3% hydrogen phosphate (HPO
3) and filtered. Ten millilitres of the filtrate was titrated against standard redox dye (2,6-dichlorophenol indophenol, DCPIPH). Changes in colour to pink as a result of the unavailability of an electron to reduce the DCPIPH indicate the end point. The ascorbic acid was calculated in mg/100 mL.
2.5. Antagonistic Activity of Probioticated Substrate
The antagonistic activity of the probioticated substrate against some pathogenic bacteria (Escherichia coli, Salmonella typhimurium, Staphylococcus sp., L. monocytogenes, Klebsiella sp., B. subtilis, P. aeruginosa, E. faecalis, and Staphylococcus aureus) was investigated using agar well diffusion. The test pathogens containing 2.5 × 107 cfu/mL was seeded on a sterile molten nutrient agar. After solidification, wells were bore on seeded agar plates, and the probioticated juice sample was introduced into the wells. The plates were first incubated at 4 °C for 60 min to allow the test material to diffuse in the agar, and were then incubated at 37 °C for 18 h. After incubation, the diameter of the clear zone was measured in centimetres from the centre of the well.
2.6. Determination of the Survivability of the LAB Strain inside the Pineapple Juice
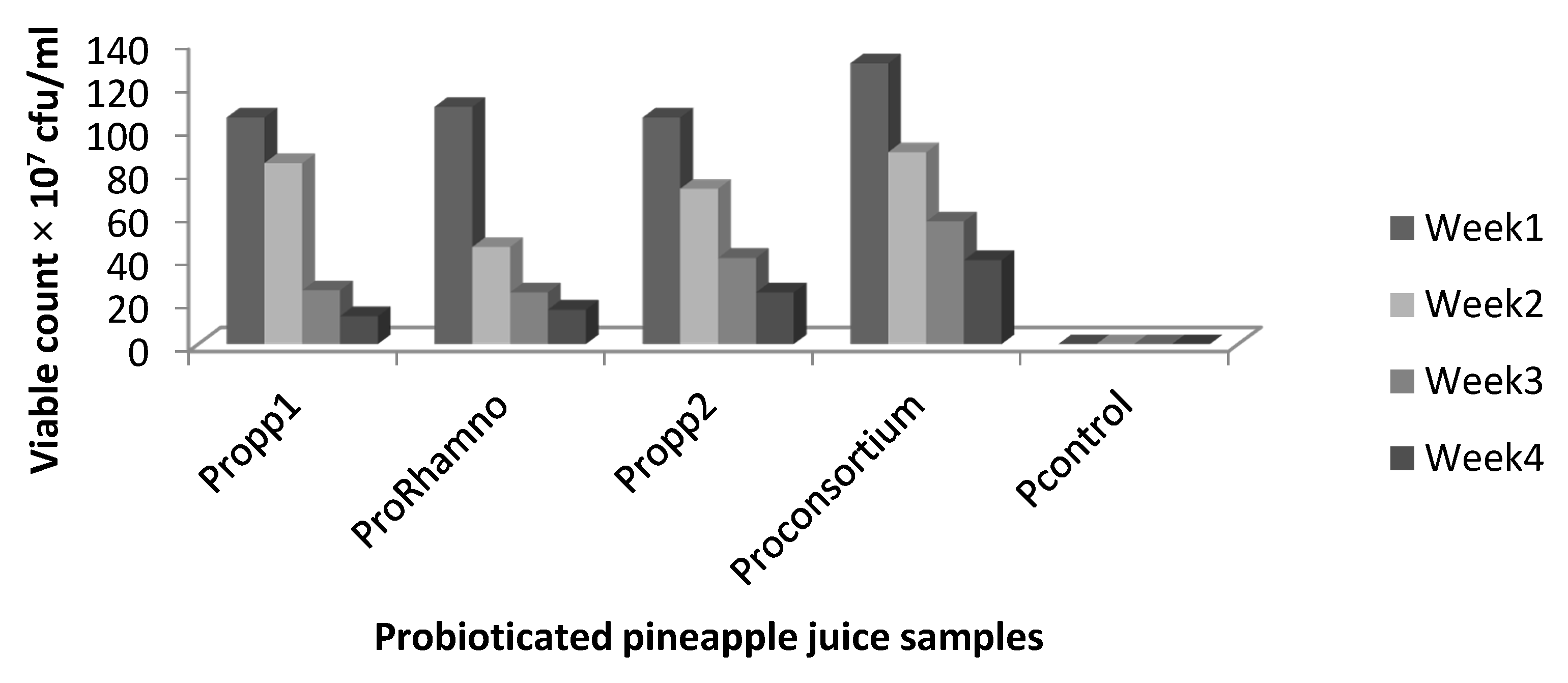
The survivability of the LAB strain in the probioticated juice samples was determined using a pour plate technique. The stored samples were pour plated at weekly intervals. Sample (1 mL) was inoculated on MRS agar plates, and the plates were incubated at 37 °C for 48 h. Viable counts were recorded by counting the visible colonies on the culture medium, and the number was multiplied by the reciprocal of the dilution factor and expressed as colony forming units (CFU).
2.7. Sensory Evaluation of Stored Probioticated Juice Samples
Coded samples of the probioticated juice samples were served to 10 trained panellists. The panellist was asked to rate the sample based on taste, aroma, colour, and appearance. Triplicate determinations were made per sample. The ratings were presented on a seven-point hedonic scale ranging from 1—extremely like, 7—extremely dislike. Obtained results were subjected to analysis of variance using one-way ANOVA, and the difference between means was separated using Duncan’s Multiple Ranged Test [
23].
4. Conclusions
In conclusion, the probiotic pineapple juice supported the growth and viability of probiotic LAB. During storage, the probiotic LAB candidate grew and had significant pH development and lactic acid production, increased vitamin C content, reduction in total soluble solids, and no significant changes in the sensory parameters. The stored juice had an antagonistic effect on some pathogens. The results obtained in this study will be useful for the development of probiotic fruit juice with health beneficial effects. This could serve as a nutraceutical with health benefits for vegetarians, lactose intolerant people, and those who are allergic to milk products. Further work is necessary to check the biological evaluation of the products and the production of probioticated juice using more LAB consortium and effect of immobilization on the viability and functionality.