Aeration Rate in Tertiary Treatment of Anaerobic Effluent from Soft Drink Industry by Co-Cultivation Between Penicillium gravinicasei and Microalgae
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Collection, Storage and Characteristics
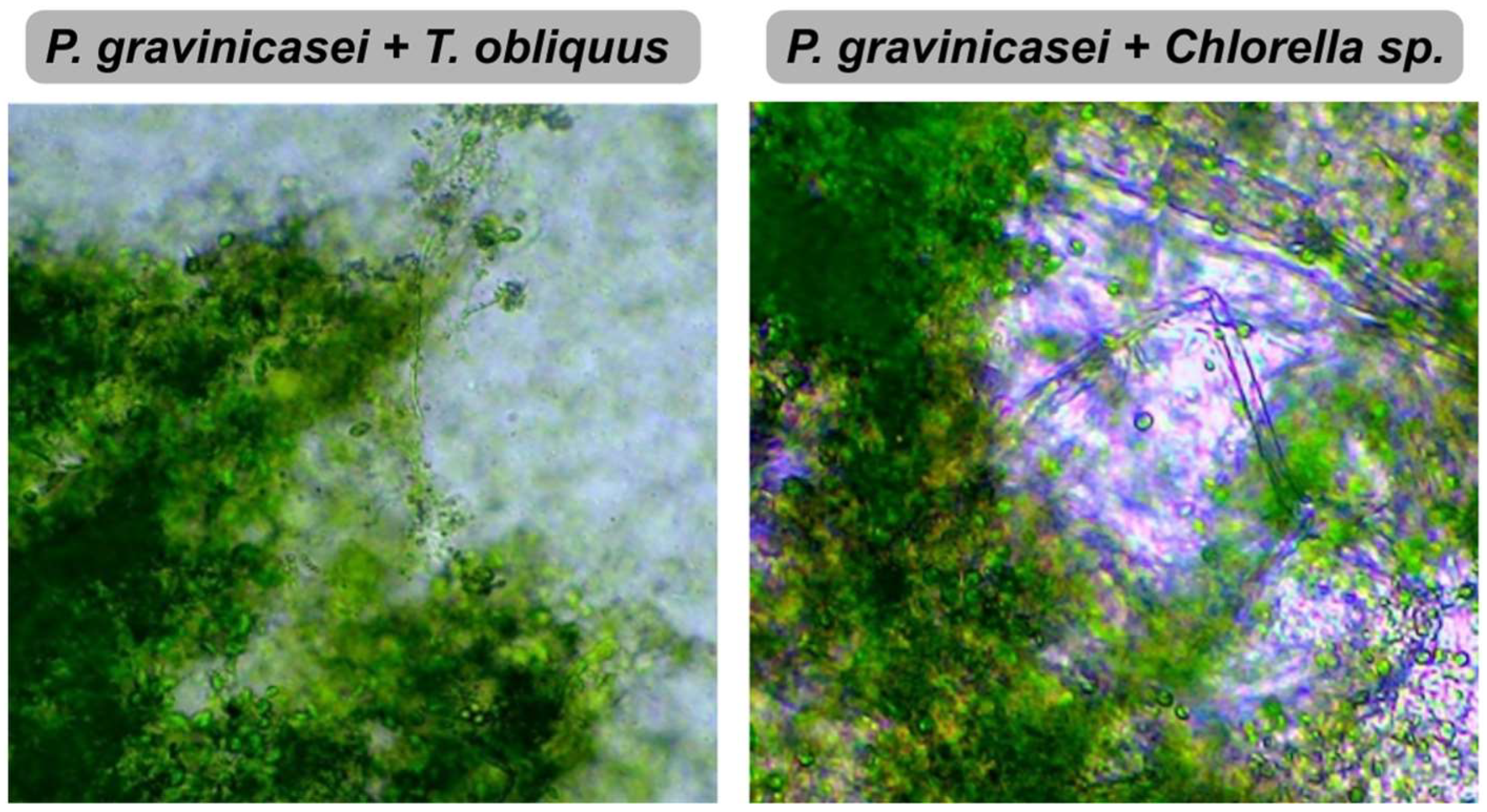
2.2. Species of Filamentous Fungus and Microalgae
2.3. Treatment System
2.4. Analyses
2.4.1. Microbial Sludge Produced and pH Determination
2.4.2. Chemical Oxygen Demand (COD) and Total Phosphorus (TP)
2.4.3. Total Nitrogen (TN or TKN)
2.4.4. Contaminant Removal Rate, Sludge Production Rate and Conversion Factor
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
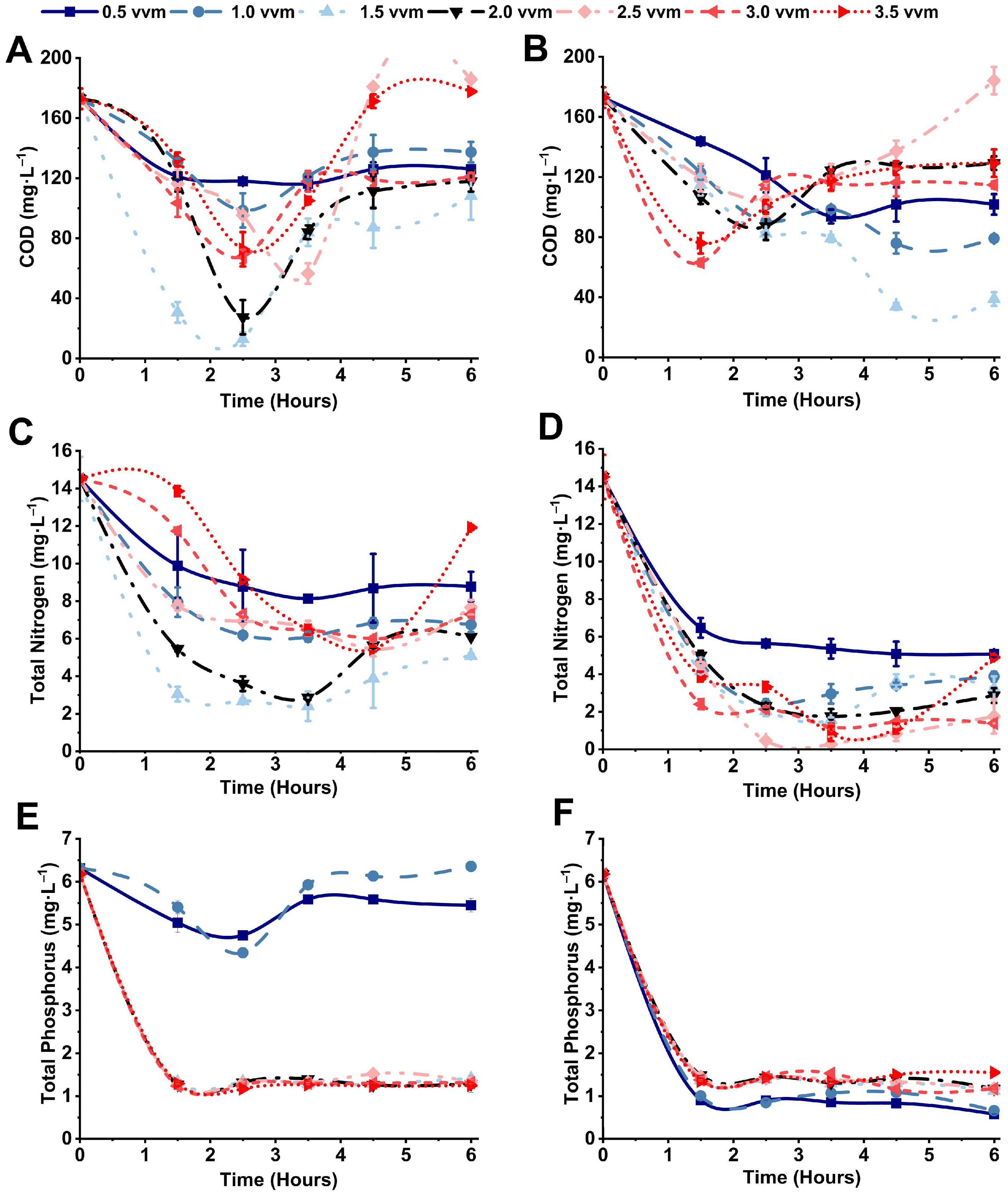
3.1. Effect on Contaminants Removal
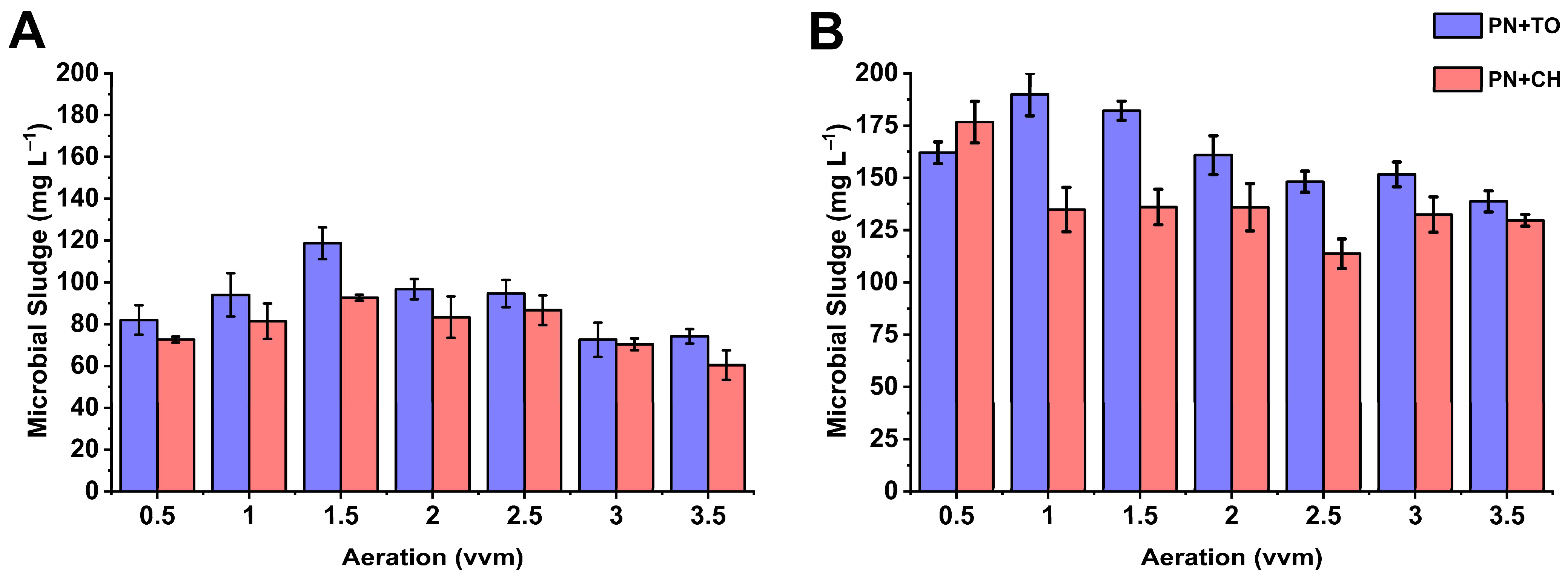
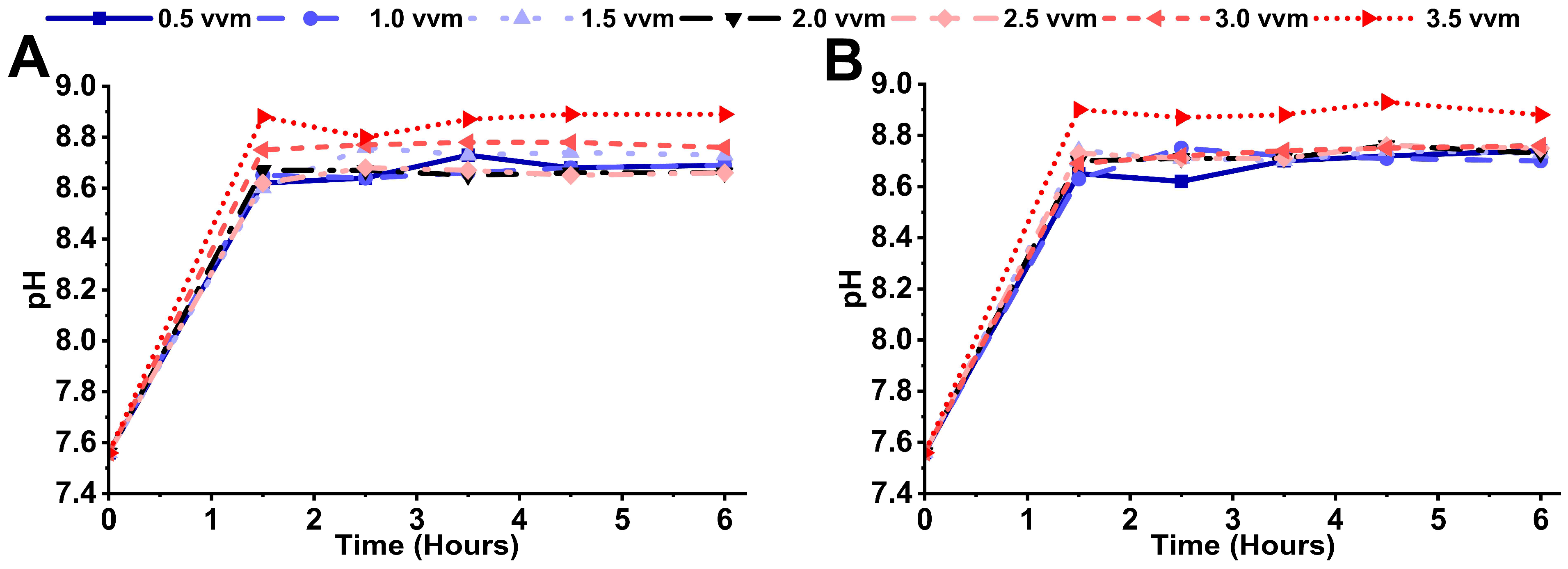
3.2. Microbial Sludge Production and pH Variation
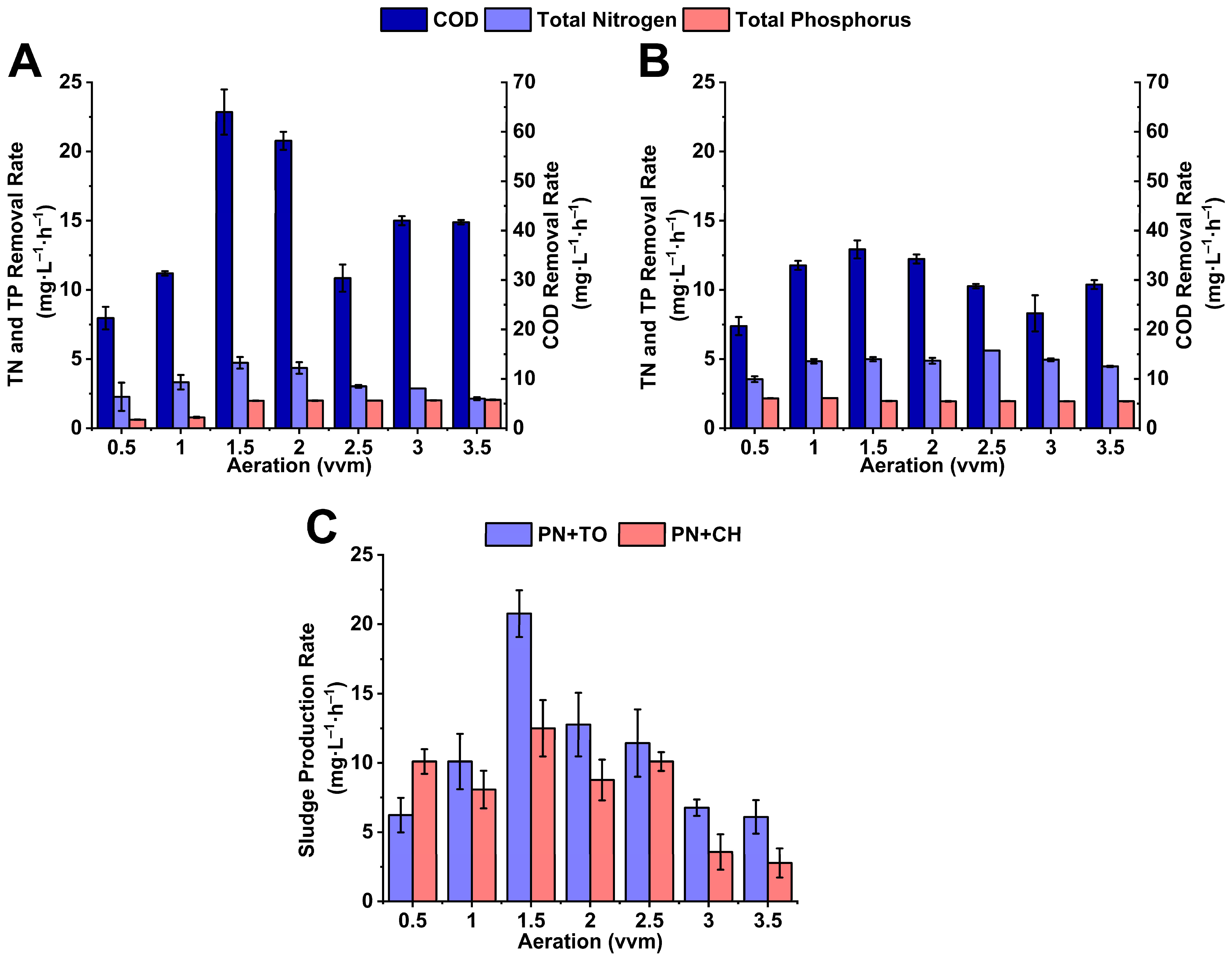
3.3. Contaminant Removal Rate and Sludge Production Rate
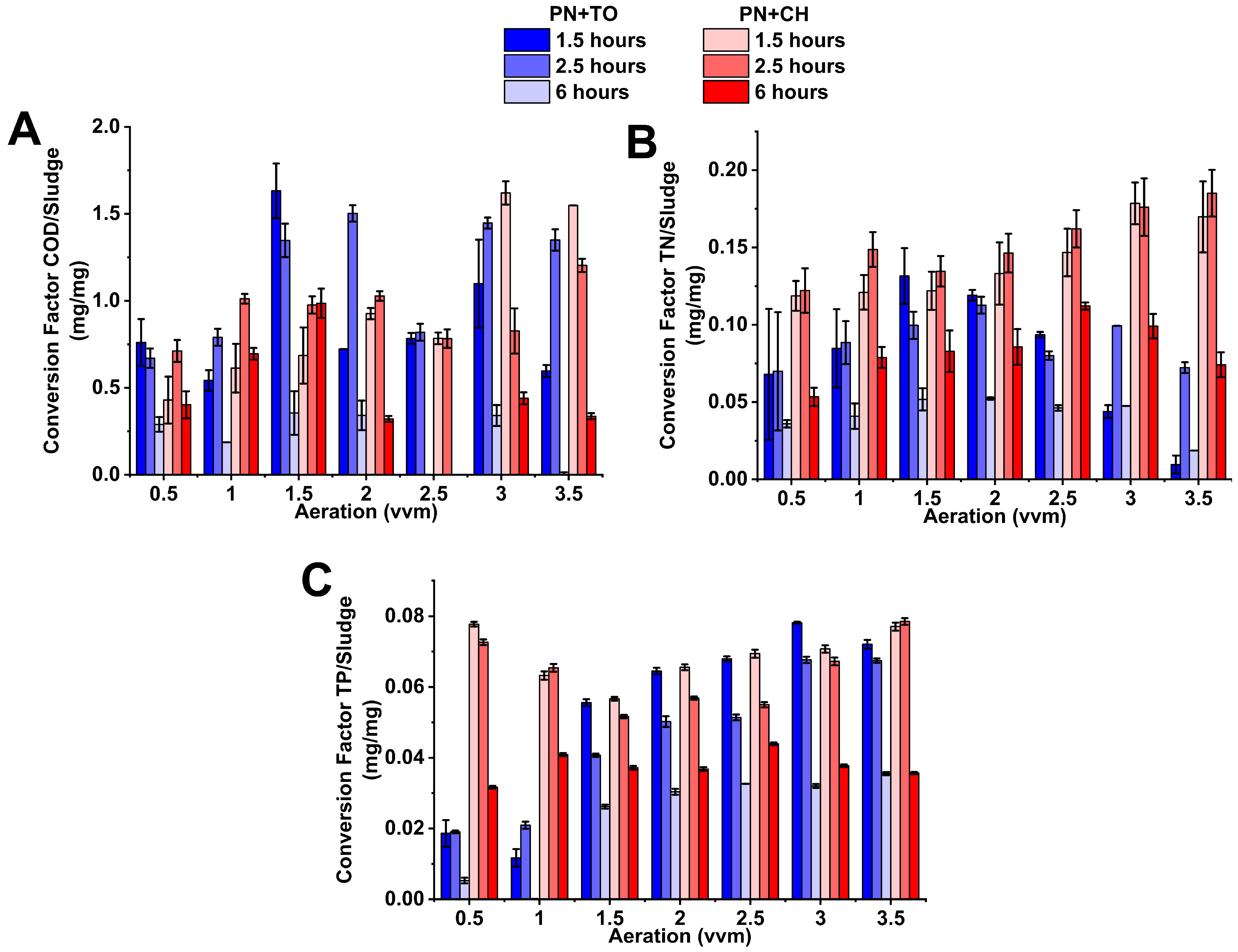
3.4. Conversion Factor of Contaminant Removed per Sludge Produced
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| vvm | Air volume (L) per medium volume (L) per minute |
| COD | Chemical oxygen demand |
| TOC | Total organic carbon |
| TN | Total nitrogen |
| TP | Total phosphorus |
References
- EMIS. Brazil Food and Beverage Sector 2022/2023 (2024). Available online: https://www.emis.com (accessed on 9 November 2024).
- Farhaoui, M.; Hasnaoui, L.; Derraz, M. Optimization of Drinking Water Treatment Process by Modeling the Aluminum Sulfate Dose. Curr. J. Appl. Sci. Technol. 2016, 17, 1–14. [Google Scholar] [CrossRef]
- Junior, D.A.A.; Oro, C.E.D.; Dos Santos, M.S.N.; Dallago, R.M.; Tres, M.V. Integration of Improved Methods for the Treatment of Wastewater from a Soft Drink Industry. Biointerface Res. Appl. Chem. 2021, 11, 12946–12957. [Google Scholar] [CrossRef]
- Szaja, A.; Montusiewicz, A.; Pasieczna-Patkowska, S.; Lebiocka, M. Technological and Energetic Aspects of Multi-Component Co-Digestion of the Beverage Industry Wastes and Municipal Sewage Sludge. Energies 2022, 15, 5395. [Google Scholar] [CrossRef]
- Weldehans, M.G. Optimization of distillery-sourced wastewater anaerobic digestion for biogas production. Clean. Waste Syst. 2023, 6, 100118. [Google Scholar] [CrossRef]
- Mahoney, K.; Lansing, S.; Amradi, K.N.; Sanders, D.; Loraine, G.; Hassanein, A. Waste-to-energy technologies: Integrating anaerobic digestion, microbial electrolysis cells, hydrodynamic cavitation, and electrocoagulation. J. Clean. Prod. 2024, 473, 143549. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Y.; Yuan, F.; Yang, Q. Optimization and control strategies of aeration in WWTPs: A review. J. Clean. Prod. 2023, 418, 138008. [Google Scholar] [CrossRef]
- Masłoń, A.; Czarnota, J.; Szaja, A.; Szulżyk-Cieplak, J.; Łagód, G. The Enhancement of Energy Efficiency in a Wastewater Treatment Plant through Sustainable Biogas Use: Case Study from Poland. Energies 2020, 13, 6056. [Google Scholar] [CrossRef]
- Myszograj, S.; Bocheński, D.; Mąkowski, M.; Płuciennik-Koropczuk, E. Biogas, Solar and Geothermal Energy—The Way to a Net-Zero Energy Wastewater Treatment Plant—A Case Study. Energies 2021, 14, 6898. [Google Scholar] [CrossRef]
- Abiusi, F.; Wijffels, R.H.; Janssen, M. Oxygen Balanced Mixotrophy under Day–Night Cycles. ACS Sustain. Chem. Eng. 2020, 8, 11682–11691. [Google Scholar] [CrossRef]
- Akkoyunlu, B.; Daly, S.; Cerrone, F.; Casey, E. Investigating Mass Transfer and Reaction Engineering Characteristics in a Membrane Biofilm Using Cupriavidus necator H16. Membranes 2023, 13, 908. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Li, Z.; Xie, J.; Tsybekmitova, G.T.; Wang, Y. Membrane aeration accelerated nitrifying biofilm formation and optimized spatial niche differentiation in aerobic biofilm-based reactors for enhanced nitrogen removals. Chem. Eng. J. 2025, 506, 160297. [Google Scholar] [CrossRef]
- Das, P.K.; Rani, J.; Rawat, S.; Kumar, S. Microalgal Co-cultivation for Biofuel Production and Bioremediation: Current Status and Benefits. BioEnergy Res. 2022, 15, 1–26. [Google Scholar]
- Rugnini, L.; Ellwool, N.T.W.; Costa, G.; Falsetti, A.; Congestri, R.; Bruno, L. Scaling-up of wastewater bioremediation by Tetradesmus obliquus, sequential bio-treatments of nutrients and metals. Ecotoxicol. Environ. Saf. 2019, 172, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.; Li, S.; Zhu, L.; Yin, Z.; Hu, D.; Liu, C.; Mo, F. A review on co-cultivation of microalgae with filamentous fungi: Efficient harvesting, wastewater treatment and biofuel production. Renew. Sustain. Energy Rev. 2021, 139, 110689. [Google Scholar] [CrossRef]
- Leng, L.; Li, W.; Chen, J.; Leng, S.; Chen, J.; Peng, J.L.; Zhou, W.; Huang, H. Co-culture of fungi-microalgae consortium for wastewater treatment: A review. Bioresour. Technol. 2021, 330, 110689. [Google Scholar]
- Medeiros, J.A.; de Farias Silva, C.E.; Santos, G.K.S.; do Nascimento, M.A.A.; de Andrade, F.P.; de Sá Filho, M.L.F.; da Gama, B.M.V.; da Silva, J.V.O.N.; Almeida, R.M.R.G. Tertiary treatment of dairy wastewater applying a microalga-fungus consortium. Environ. Technol. 2025, 46, 370–386. [Google Scholar]
- Yang, M.; Liu, Z.; Wang, A.; Nopens, I.; Hu, H.; Chen, H. High biomass yields of Chlorella protinosa with efficient nitrogen removal from secondary effluent in a membrane photobioreactor. J. Environ. Sci. 2024, 146, 272–282. [Google Scholar]
- De Andrade, F.P.; Da Silva Gonçalves, A.H.; De Farias Silva, C.E.; Macário, L.R.; Da Silva, J.V.O.N.; Da Gama, B.M.V.; Almeida, R.M.R.G.; Tonholo, J. Treatment of oil-produced water using a fungus–microalga consortium. Energy Ecol. Environ. 2024, 9, 144–158. [Google Scholar]
- Monteiro Dos Santos, L.; Barbosa Da Silva, J.C.; De Farias Silva, C.E.; Villar Da Gama, B.M.; Almeida Medeiros, J.; Markou, G.; Almeida, R.M.R.G.; de Souza Abud, A.K. Co-Cultivation between the Microalga Tetradesmus obliquus and Filamentous Fungus Cunninghamella echinulata Improves Tertiary Treatment of Cheese Whey Effluent in Semicontinuous Mode. Processes 2024, 12, 1573. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Directive 1998/15/EC. European Commission Directive of 27 February 1998 Amending Council Directive 91/271/EEC as Regards the Rights and Requirements Set out in Its Annex I, 1998/15/ECO, Official Journal of the European Union 1998 (L 67/29). Available online: http://data.europa.eu/eli/dir/1998/15/oj (accessed on 15 February 2024).
- Yang, B.; Yan, Y.; Jia, Y.; Chen, B.; Khanal, S.K.; Shu, W.-S.; Lu, H. Optimizing formation of microalgal-bacterial granular sludge for aquaculture wastewater treatment. Chem. Eng. J. 2025, 504, 158884. [Google Scholar]
- Huang, J.; Cheng, S.; Zhang, Y.; Teng, J.; Zhang, M.; Lin, H. Optimizing aeration intensity to enhance self-flocculation in algal-bacterial symbiosis systems. Chemosphere 2023, 341, 140064. [Google Scholar] [CrossRef]
- Radmehr, S.; Rissanen, T.; Kallioinen-Mänttäri, M.; Mänttäri, M. Reducing mechanical aeration in membrane bioreactors by inoculation of algal cells into activated sludge biomass. J. Water Process Eng. 2022, 49, 103047. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, W.; Zhou, X.; Jin, W.; Han, W.; Chi, K.; Chen, Y.; Zhao, Z.; He, Z.; Jiang, G. Sub-pilot scale cultivation of Tetradesmus dimorphus in wastewater for biomass production and nutrients removal: Effects of photoperiod, CO2 concentration and aeration intensity. J. Water Process Eng. 2022, 49, 103003. [Google Scholar] [CrossRef]
- Kumkaew, P.; Suaisom, P.; Mukkata, K.; Nitayavardhana, S. Decolorization of Biogas Power Plant Effluent by Phanerochaete chrysosporium TBRC 785. EnvironmentAsia 2023, 16, 76–86. [Google Scholar]
- Pérez-Cadena, R.; García-Esquivel, Y.; Castañeda-Cisneros, Y.E.; Serna-Díaz, M.G.; Ramírez-Vargas, M.R.; Muro-Urista, C.R.; Téllez-Jurado, A. Biological decolorization of Amaranth dye with Trametes polyzona in an airlift reactor under three airflow regimes. Heliyon 2020, 6, e05857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Xiong, Z.; Geng, Y.; Cui, D.; Pavlostathis, S.G.; Chen, H.; Luo, Q.; Qiu, G.; Dong, Q.; et al. Synergistic optimization of baffles and aeration to improve the Light/Dark cycle of microalgae photobioreactor for enhanced nitrogen removal performance: Computational fluid dynamics and experimental verification. Bioresour. Technol. 2024, 410, 131293. [Google Scholar] [CrossRef] [PubMed]
- Kusmayadi, A.; Leong, Y.K.; Lu, P.-H.; Huang, C.-Y.; Yen, H.-W.; Chang, J.-S. Simultaneous nutrients removal and bio-compounds production by cultivating Chlorella sorokiniana SU-1 with unsterilized anaerobic digestate of dairy wastewater. Algal Res. 2022, 68, 102896. [Google Scholar] [CrossRef]
- Molina, E.; Acién Fernández, F.G.; García Camacho, F.; Camacho Rubio, F.; Chisti, Y. Scale-up of tubular photobioreactors. J. Appl. Phycol. 2000, 12, 355–368. [Google Scholar] [CrossRef]
- Wang, C.; Lan, C.Q. Effects of shear stress on microalgae—A review. Biotechnol. Adv. 2018, 36, 986–1002. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Bashan, Y. Microalgal Heterotrophic and Mixotrophic Culturing for Bio-refining: From Metabolic Routes to Techno-economics. In Algal Biorefineries; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 61–131. [Google Scholar]
- Zhao, C.; Xie, S.; Pu, Y.; Zhang, R.; Huang, F.; Ragauskas, A.J.; Yuan, J.S. Synergistic enzymatic and microbial lignin conversion. Green Chem. 2016, 18, 1306–1312. [Google Scholar] [CrossRef]
- Satpati, G.G.; Dikshit, P.K.; Mal, N.; Pal, R.; Sherpa, K.C.; Rajak, R.C.; Rather, S.-U.; Raghunathan, S.; Davoodbasha, M. A state of the art review on the co-cultivation of microalgae-fungi in wastewater for biofuel production. Sci. Total Environ. 2023, 870, 161828. [Google Scholar] [CrossRef]
- Kondakindi, V.R.; Pabbati, R.; Erukulla, P.; Maddela, N.R.; Prasad, R. Bioremediation of heavy metals-contaminated sites by microbial extracellular polymeric substances—A critical view. Environ. Chem. Ecotoxicol. 2024, 6, 408–421. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Mohd Nasir, N.; Mohd Yunos, F.H.; Wan Jusoh, H.H.; Mohammad, A.; Lam, S.S.; Jusoh, A. Subtopic: Advances in water and wastewater treatment harvesting of Chlorella sp. microalgae using Aspergillus niger as bio-flocculant for aquaculture wastewater treatment. J. Environ. Manag. 2019, 249, 109373. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Guo, L.; Li, X.; Wang, Y. Effect of phosphorus concentration and light/dark condition on phosphorus uptake and distribution with microalgae. Bioresour. Technol. 2021, 340, 125745. [Google Scholar] [CrossRef] [PubMed]
- Kritmetapak, K.; Kumar, R. Phosphate as a Signaling Molecule. Calcif. Tissue Int. 2021, 108, 16–31. [Google Scholar]
- Luo, Y.; Lu, X.; Zhou, G.; Shen, H.; Li, H.; Li, S.; Pan, X.; Dao, G. Microalgae for phosphorus chemical wastewater treatment and recovery of phosphorus. Environ. Res. 2025, 276, 121511. [Google Scholar] [CrossRef]
- De Farias Silva, C.E.; Sforza, E. Carbohydrate productivity in continuous reactor under nitrogen limitation: Effect of light and residence time on nutrient uptake in Chlorella vulgaris. Process Biochem. 2016, 51, 2112–2118. [Google Scholar] [CrossRef]
- Geng, N.; Wang, S.; Hu, T.; Chen, Y.; Su, H. Fast granulation of flocculent activated sludge by mycelium pellet and wastewater biological treatment performance. J. Water Process Eng. 2022, 49, 103031. [Google Scholar]
- Ren, X.; Guo, L.; Chen, Y.; She, Z.; Gao, M.; Zhao, Y.; Shao, M. Effect of Magnet Powder (Fe3O4) on Aerobic Granular Sludge (AGS) Formation and Microbial Community Structure Characteristics. ACS Sustain. Chem. Eng. 2018, 6, 9707–9715. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Koutra, E.; Kornaros, M.; Farhadian, O.; Bhatnagar, A. Sequential cultivation of microalgae in raw and recycled dairy wastewater: Microalgal growth, wastewater treatment and biochemical composition. Bioresour. Technol. 2019, 273, 556–564. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Lu, Q.; Sun, X.; Wang, S.; Li, Q.; Wei, X.; Wang, Y. The influence of light intensity and organic content on cultivation of Chlorella vulgaris in sludge extracts diluted with BG11. Aquac. Int. 2021, 29, 2131–2144. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Zienkiewicz, K.; Vande, P.O.L.N.; Ostrom, N.E.; Benning, C.; Bonito, G.M. Algal-fungal symbiosis leads to photosynthetic mycelium. ELife 2019, 8, 47815. [Google Scholar]
- Chu, R.; Li, S.; Yin, Z.; Hu, D.; Zhang, L.; Xiang, M.; Zhu, L. A fungal immobilization technique for efficient harvesting of oleaginous microalgae: Key parameter optimization, mechanism exploration and spent medium recycling. Sci. Total Environ. 2021, 790, 148174. [Google Scholar]
- Jimenez, J.; Miller, M.; Bott, C.; Murthy, S.; De Clippeleir, H.; Wett, B. High-rate activated sludge system for carbon management—Evaluation of crucial process mechanisms and design parameters. Water Res. 2015, 87, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-X.; Yao, W.; Yang, C.-X.; He, W.-L.; Li, J.; Huang, B.-C.; Jin, R.-C. The nexus between aeration intensity and organic carbon capture in contact-stabilization process: Insights from molecular structure transition of dissolved organic matters. Water Res. 2025, 268 Pt B, 122769. [Google Scholar]
- Khalatbari, S.; Sotaniemi, V.-H.; Suokas, M.; Taipale, S.; Leiviskä, T. Microalgae technology for polishing chemically-treated fish processing wastewater. Groundw. Sustain. Dev. 2024, 24, 101074. [Google Scholar]
- Alava, D.D.; Mello, P.C.D.; Wagener, K. The relevance of the CO2 partial pressure of sodium bicarbonate solutions for the mass cultivation of the microalga Spirulina. J. Braz. Chem. Soc. 1997, 8, 447–450. [Google Scholar] [CrossRef]
- Mekpan, W.; Cheirsilp, B.; Maneechote, W.; Srinuanpan, S. Microalgae-fungal pellets as novel dual-bioadsorbents for dye and their practical applications in bioremediation of palm oil mill effluent. Bioresour. Technol. 2024, 413, 131519. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, Z.; Zhou, L.; Zhang, Z.; Wang, J.; Lan, C.Q. High cell density culture of Neochloris oleoabundans in novel horizontal thin-layer algal reactor: Effects of localized aeration, nitrate concentration and mixing frequency. Biochem. Eng. J. 2023, 192, 108839. [Google Scholar] [CrossRef]
- Yusuf, H.A.; Hossain, S.M.Z.; Khamis, A.A.; Radhi, H.T.; Jaafar, A.S. Optimization of CO2 biofixation rate by microalgae in a hybrid microfluidic differential carbonator using response surface methodology and desirability function. J. CO2 Util. 2020, 42, 101291. [Google Scholar] [CrossRef]
- Ran, C.; Zhou, X.; Yao, C.; Zhang, Y.; Kang, W.; Liu, X.; Herbert, C.; Xie, T. Swine digestate treatment by prior nitrogen-starved Chlorella vulgaris: The effect of over-compensation strategy on microalgal biomass production and nutrient removal. Sci. Total Environ. 2021, 768, 144462. [Google Scholar] [CrossRef]
- Dong, X.; Wei, J.; Huang, J.; Zhao, C.; Sun, S.; Zhao, Y.; Liu, J. Performance of different microalgae-fungi-bacteria co-culture technologies in photosynthetic and removal performance in response to various GR24 concentrations. Bioresour. Technol. 2022, 347, 126428. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, J.; Jin, Z.; Cheng, J.; Li, J.; Song, H.; Lu, Q.; Li, H.; Wan, T.; Fu, S.; et al. Enhancing Algal Yield and Nutrient Removal from Anaerobic Digestion Piggery Effluent by an Integrated Process-Optimization Strategy of Fungal Decolorization and Microalgae Cultivation. Appl. Sci. 2022, 12, 4741. [Google Scholar] [CrossRef]
- Taghavijeloudar, M.; Yaqoubnejad, P.; Amini-Rad, H.; Park, J. Optimization of cultivation condition of newly isolated strain Chlorella sorokiniana pa.91 for CO2 bio-fixation and nutrients removal from wastewater: Impact of temperature and light intensity. Clean Technol. Environ. Policy 2023, 25, 589–601. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Gao, F.; Liu, J.-Z.; Yang, J.-S.; Liu, M.; Ge, Y.-M.; Chen, D.-Z.; Chen, J.-M. Improving sedimentation and lipid production of microalgae in the photobioreactor using saline wastewater. Bioresour. Technol. 2022, 347, 126392. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, W.R.; Tagliaferro, G.V.; dos Santos, J.C.; Pereira, P.; Roma, C.; Silva, M.B.; Guimarães, D.H.P. Semi-continuous Cultivation of Chlorella minutissima in Landfill Leachate: Effect of Process Variables on Biomass Composition. Waste Biomass Valorization 2022, 13, 1627–1638. [Google Scholar] [CrossRef]
- de Sá Filho, M.L.F.; De Farias Silva, C.E.; de Oliveira, A.M.M.; de Andrade, F.P.; Medeiros, J.A.; Tonholo, J. Tetradesmus obliquus to treat groundwater contaminated with nitrate towards a semicontinuous process. Energy Ecol. Environ. 2023, 8, 262–272. [Google Scholar] [CrossRef]
- Qin, L.; Wang, Z.; Sun, Y.; Shu, Q.; Feng, P.; Zhu, L.; Xu, J.; Yuan, Z. Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Environ. Sci. Pollut. Res. 2016, 23, 8379–8387. [Google Scholar] [CrossRef]
- Kemel, S.; Marchal, L.; Habiba, W.N.; Marec, H.; Drouin, D.; Gonçalves, O.; Pruvost, J. Investigation of the colony size effect on light access and growth of the colonial microalga Botryococcus braunii in photobioreactor. Algal Res. 2025, 90, 104176. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Y.; Liao, Q.; Xia, A.; Fu, Q.; Zhu, X.; Fu, J. Boosting Nannochloropsis oculata growth and lipid accumulation in a lab-scale open raceway pond characterized by improved light distributions employing built-in planar waveguide modules. Bioresour. Technol. 2018, 249, 880–889. [Google Scholar] [CrossRef]
- Bullock, C.M.; Bicho, P.A.; Zhang, Y.; Saddler, J.N. A solid chemical oxygen demand (COD) method for determining biomass in waste waters. Water Res. 1996, 30, 1280–1284. [Google Scholar] [CrossRef]
- Sanctis, M.; Beccari, M.; Di Laconi, C.; Majone, M.; Rossetti, S.; Tandoi, V. Study of performances, stability and microbial characterization of a Sequencing Batch Biofilter Granular Reactor working at low recirculation flow. Bioresour Technol. 2013, 129, 624–628. [Google Scholar]
- Su, Y.; Mennerich, A.; Urban, B. A comparison of feasible methods for microalgal biomass determinations during tertiary wastewater treatment. Ecol. Eng. 2016, 94, 532–536. [Google Scholar]


| Aeration | P. gravinicasei + T. obliquus | P. gravinicasei + Chlorella sp. | |||||
|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | COD | TN | TP | COD | TN | TP |
| 0.5 | 1.0 | YES | YES | YES | YES | YES | NO |
| 0.5 | 1.5 | YES | YES | YES | YES | YES | YES |
| 0.5 | 2.0 | YES | YES | YES | YES | YES | YES |
| 0.5 | 2.5 | YES | YES | YES | NO | YES | YES |
| 0.5 | 3.0 | YES | YES | YES | NO | YES | YES |
| 0.5 | 3.5 | YES | NO | YES | YES | YES | YES |
| 1.0 | 1.5 | YES | YES | YES | NO | NO | YES |
| 1.0 | 2.0 | YES | YES | YES | NO | NO | YES |
| 1.0 | 2.5 | NO | YES | YES | NO | YES | YES |
| 1.0 | 3.0 | YES | YES | YES | YES | NO | YES |
| 1.0 | 3.5 | YES | YES | YES | NO | YES | YES |
| 1.5 | 2.0 | NO | YES | NO | NO | NO | NO |
| 1.5 | 2.5 | YES | YES | NO | YES | YES | NO |
| 1.5 | 3.0 | YES | YES | NO | YES | NO | NO |
| 1.5 | 3.5 | YES | YES | YES | YES | YES | NO |
| 2.0 | 2.5 | YES | YES | NO | YES | YES | NO |
| 2.0 | 3.0 | YES | YES | NO | YES | NO | NO |
| 2.0 | 3.5 | YES | YES | YES | NO | YES | NO |
| 2.5 | 3.0 | YES | NO | NO | NO | YES | NO |
| 2.5 | 3.5 | YES | YES | YES | NO | YES | NO |
| 3.0 | 3.5 | NO | YES | NO | NO | YES | NO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.V.O.N.d.; de Farias Silva, C.E.; Sampaio, J.N.; Santos, B.R.d.; Silva, T.S.d.; da Gama, B.M.V.; Silva, A.C.d.; Silva, A.E.d.; Almeida, R.M.R.G. Aeration Rate in Tertiary Treatment of Anaerobic Effluent from Soft Drink Industry by Co-Cultivation Between Penicillium gravinicasei and Microalgae. Fermentation 2025, 11, 539. https://doi.org/10.3390/fermentation11090539
Silva JVONd, de Farias Silva CE, Sampaio JN, Santos BRd, Silva TSd, da Gama BMV, Silva ACd, Silva AEd, Almeida RMRG. Aeration Rate in Tertiary Treatment of Anaerobic Effluent from Soft Drink Industry by Co-Cultivation Between Penicillium gravinicasei and Microalgae. Fermentation. 2025; 11(9):539. https://doi.org/10.3390/fermentation11090539
Chicago/Turabian StyleSilva, João Victor Oliveira Nascimento da, Carlos Eduardo de Farias Silva, Jânio Nunes Sampaio, Bruno Roberto dos Santos, Tácia Souza da Silva, Brígida Maria Villar da Gama, Anderson Correia da Silva, Albanise Enide da Silva, and Renata Maria Rosas Garcia Almeida. 2025. "Aeration Rate in Tertiary Treatment of Anaerobic Effluent from Soft Drink Industry by Co-Cultivation Between Penicillium gravinicasei and Microalgae" Fermentation 11, no. 9: 539. https://doi.org/10.3390/fermentation11090539
APA StyleSilva, J. V. O. N. d., de Farias Silva, C. E., Sampaio, J. N., Santos, B. R. d., Silva, T. S. d., da Gama, B. M. V., Silva, A. C. d., Silva, A. E. d., & Almeida, R. M. R. G. (2025). Aeration Rate in Tertiary Treatment of Anaerobic Effluent from Soft Drink Industry by Co-Cultivation Between Penicillium gravinicasei and Microalgae. Fermentation, 11(9), 539. https://doi.org/10.3390/fermentation11090539








