Optimized Co-Fermentation of Seed Melon and Z. bungeanum Seed Meal with Saccharomyces cerevisiae L23: Valorization into Functional Feed with Enhanced Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Chemicals
2.2. Pretreatment of Raw Materials
2.3. Optimization of SMCM
2.4. Preparation of SMCM
2.5. Preparation of SMFF
2.6. Standard Curve of S. cerevisiae L23
2.7. Optimization of SMFF Fermentation Processing
2.8. Sensory Evaluation of SMFF
2.9. Determination of Physiochemical Properties
2.10. Verification of Optimal Condition of SMFF
2.11. Determination of In Vitro Antioxidant Activity
2.11.1. DPPH Radical Scavenging Activity
2.11.2. ABTS Radical Scavenging Capacity
2.11.3. Hydroxyl Radical Scavenging Activity
2.11.4. Superoxide Anion Radical Scavenging Activity
2.12. Statistical Analysis
3. Results
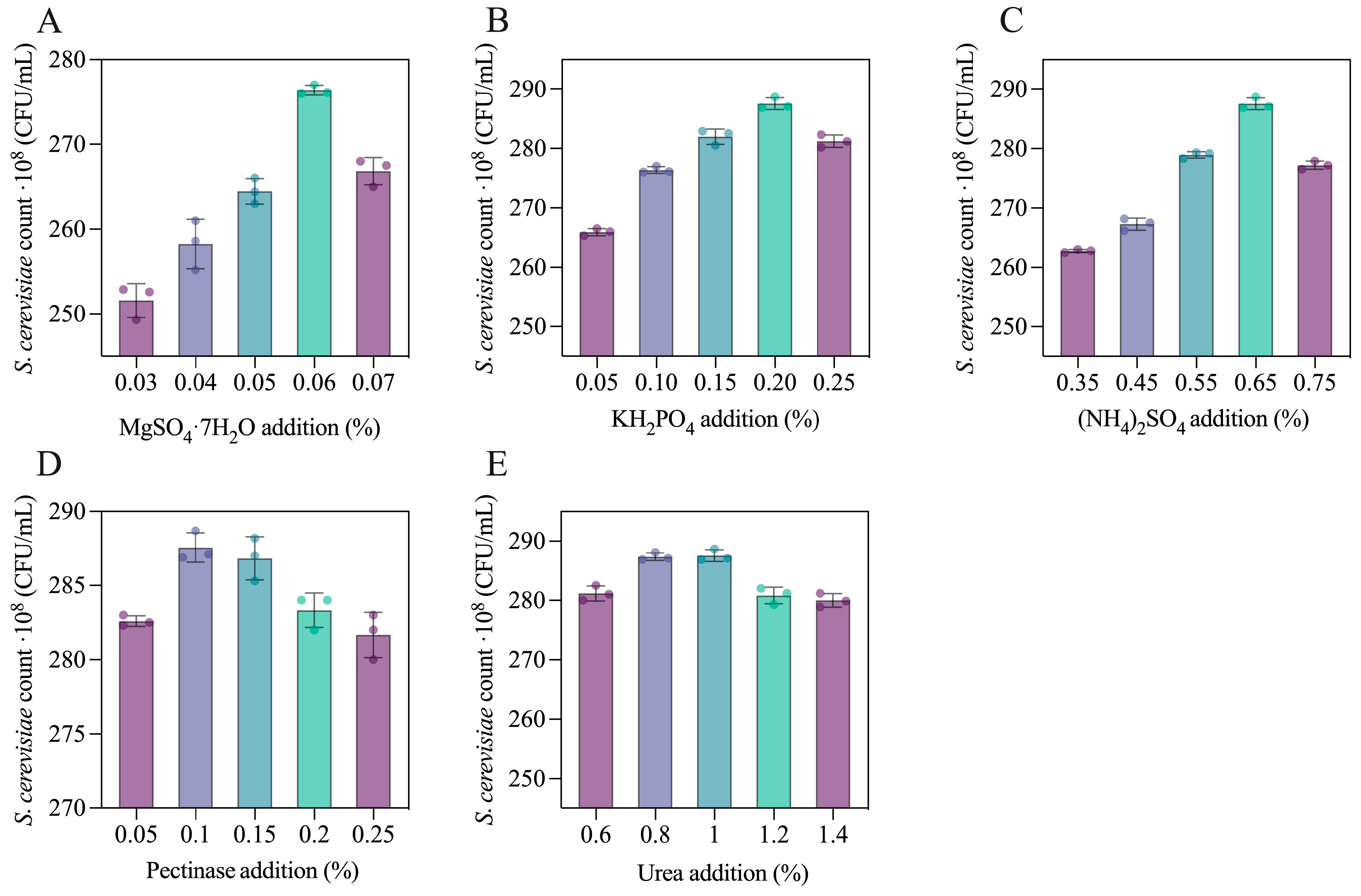
3.1. Optimization of the Culture Conditions of SMCM
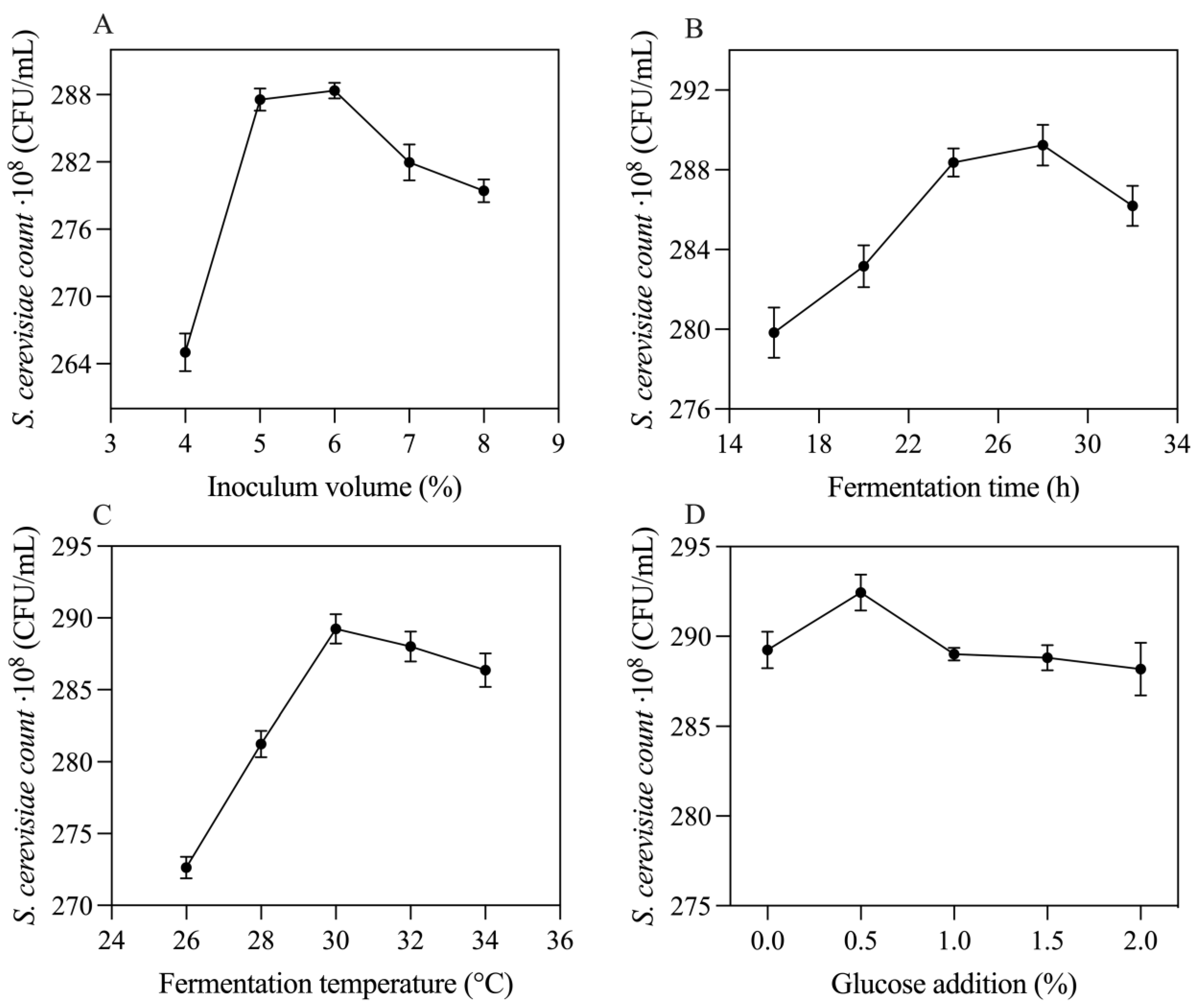
3.2. Optimization of SMCM Fermentation Process
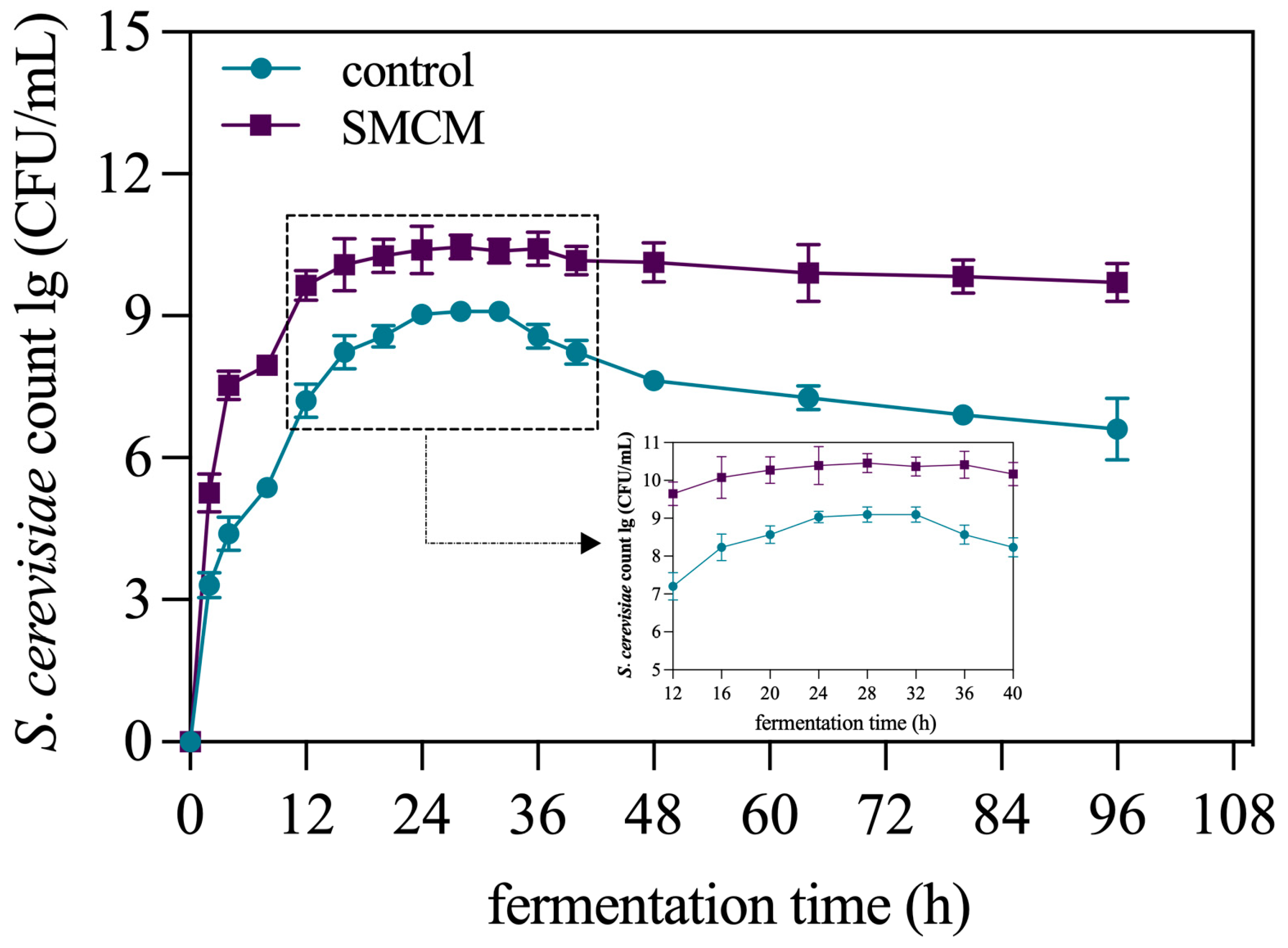
3.3. Growth Curve of S. cerevisiae L23
3.4. Optimization of SMFF Fermentation Processing
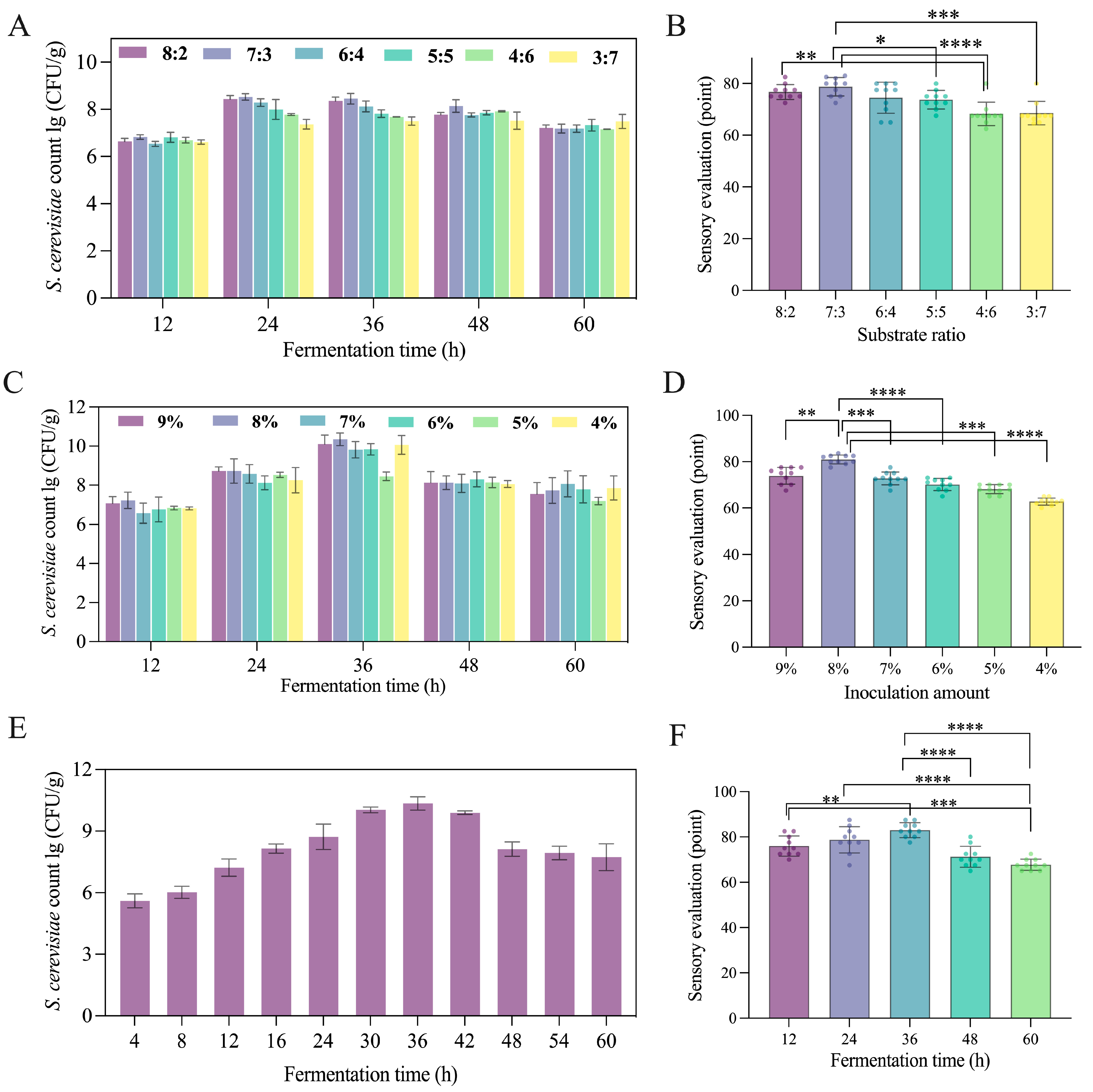
3.4.1. The Single-Factor Experimental of SMFF Fermentation Processing
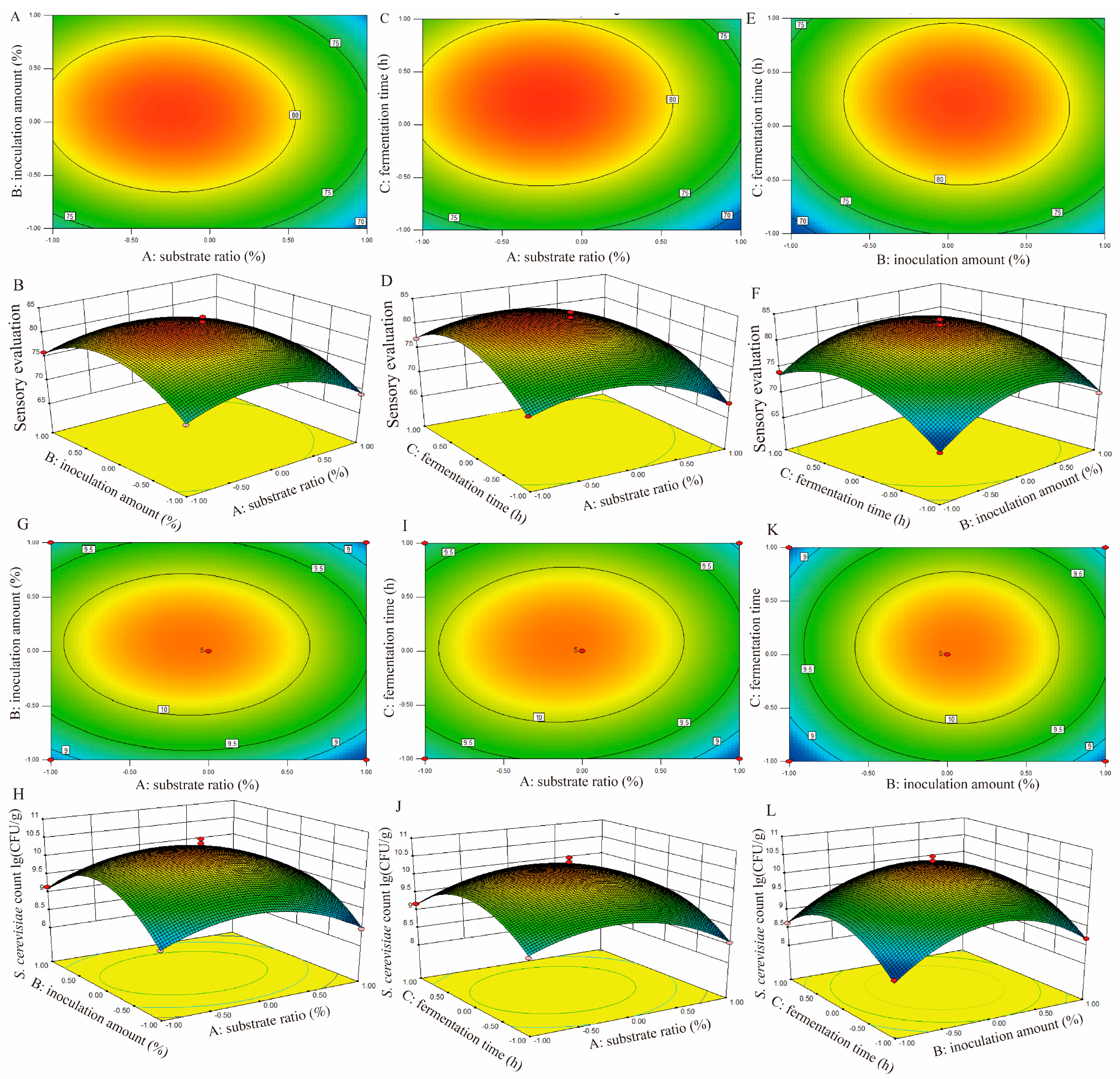
3.4.2. RSM Optimization of SMFF Fermentation Processing
3.5. Verification Experiment
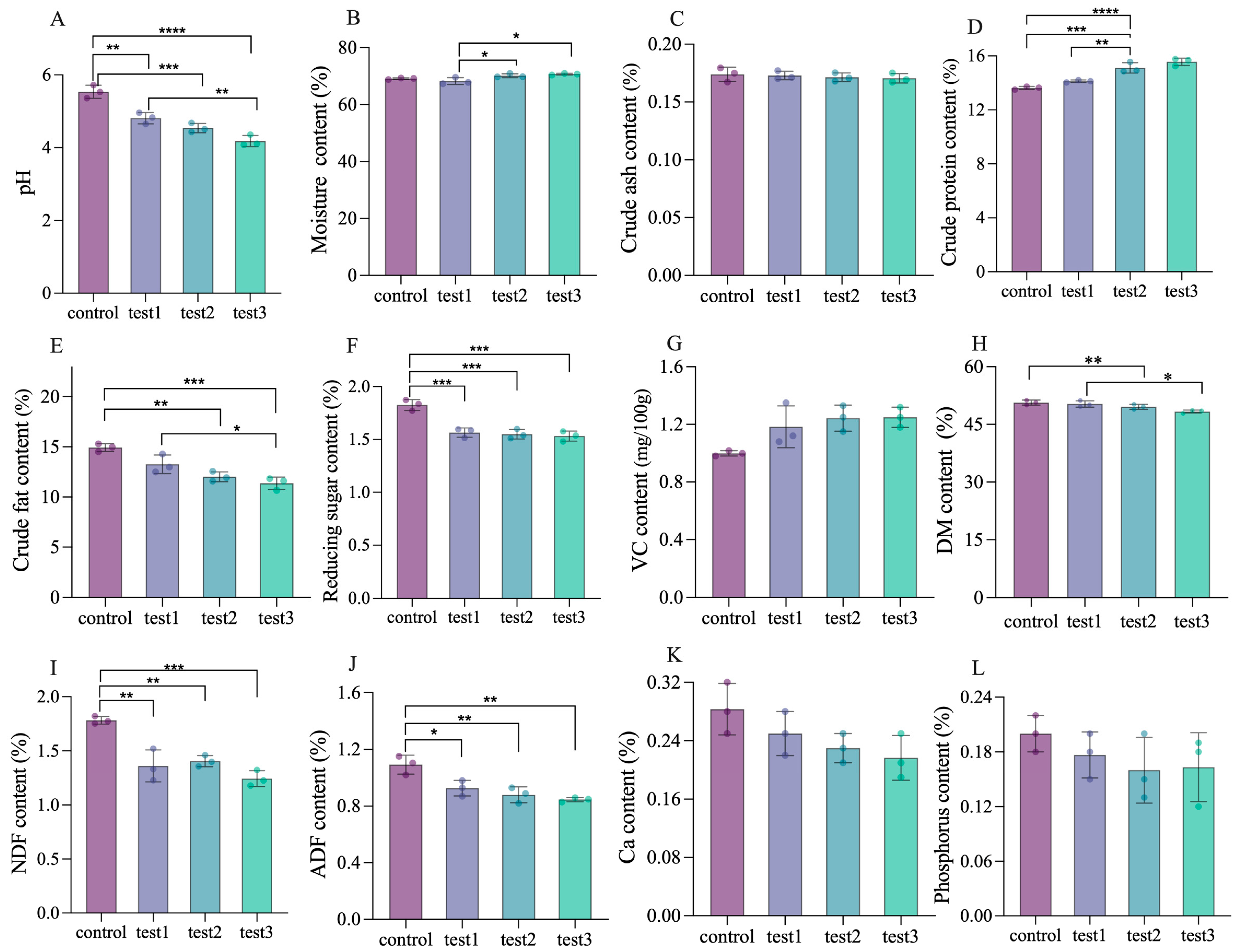
3.6. Determination of Fermentation Quality and Physiochemical Properties
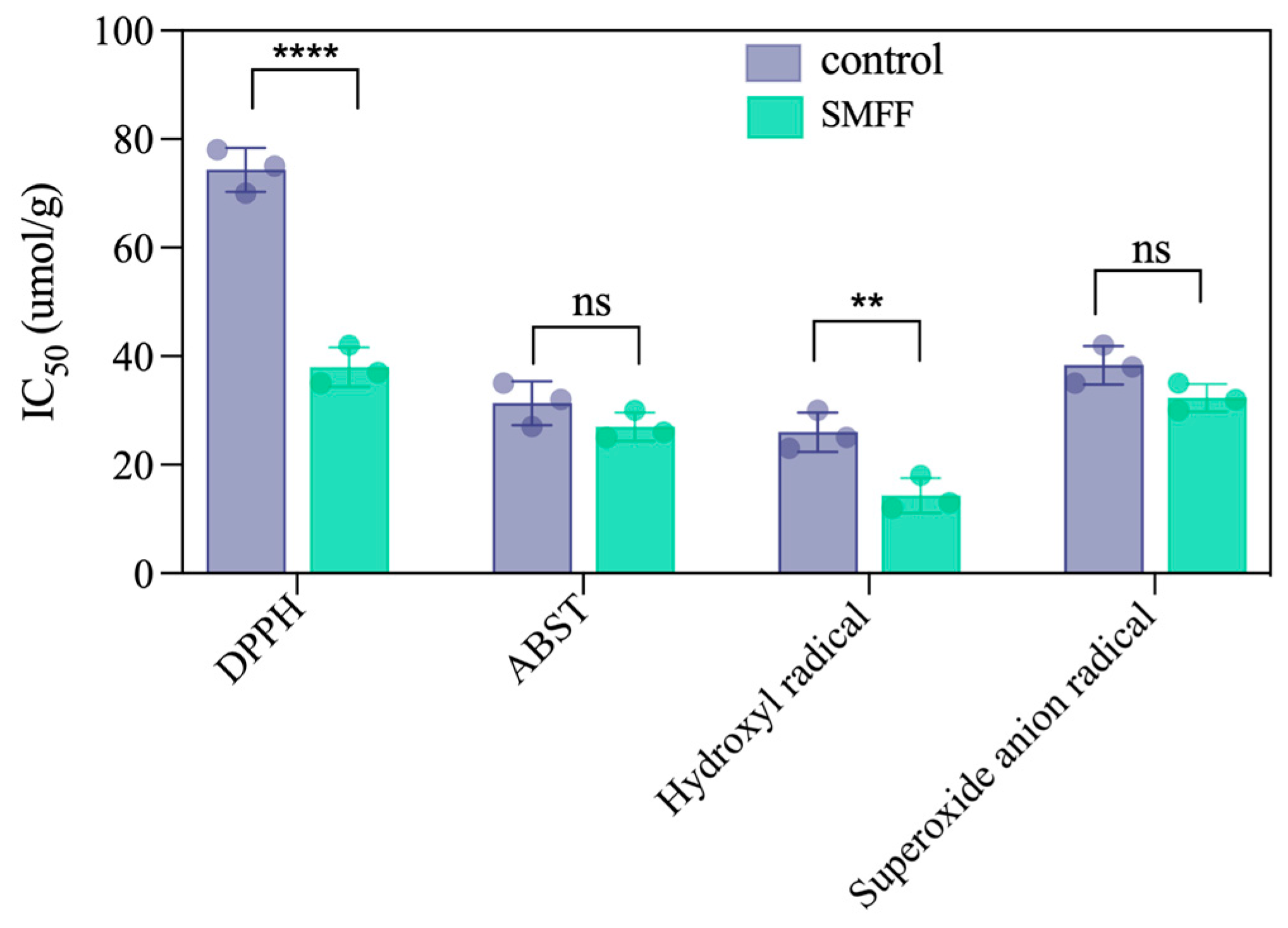
3.7. Determination of In Vitro Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMFF | Seed melon compound fermented feed |
| ZSM | Z. bungeanum Maxim seed meal |
| SMCM | Seed melon juice seed culture medium |
| DM | Dry matter |
| NDF | Neutral detergent fiber |
| ADF | Acid detergent fiber |
| LA | Lactic acid |
| AA | Acetic acid |
| PA | Propionic acid |
| BA | Butyric acid |
References
- Shi, L.; Wang, P.; Yang, J.; Wang, F.F.; Sun, X.H. Genetic Diversity Analysis and Construction of Seed Watermelon Core Germplasm Resources by SSR. Acta Bot. Boreal.-Occident. Sin. 2016, 36, 1125–1134. [Google Scholar]
- Thais, E.B.S.; Yasmim, P.O.; Leticya, B.A.C.; Jessica, A.B.D.S.; Marcos, D.S.L.; Rafael, F.; Cristiane, F.A.; Thais, S.P. Nanoparticles based on whey and soy proteins enhance the antioxidant activity of phenolic compound extract from Cantaloupe melon pulp flour (Cucumis melo L.). Food Chem. 2024, 464, 14173. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Li, Z.Q.; Dimitris, C. Upcycling of melon seed (Cucumis melo L.) oil processing by-product: Evaluation of functional properties and nutritional components as novel ingredient. Chem. Biol. Technol. Agric. 2024, 11, 101. [Google Scholar] [CrossRef]
- Ma, L.P.; Tian, L.P.; Zhang, Y.H.; Wang, J.H.; Xue, L. Study on Hypoglycemic and Hypolipidemic Activities of Seed Watermelon Juice Concentrate. Food Res. Dev. 2018, 39, 177–182. [Google Scholar] [CrossRef]
- Zhan, Y.Y.; Tian, L.P.; Liu, H.L.; Wang, J.H.; Xue, L. Preparation Process of the Seed Melon Peel Extract Capsule. Food Res. Dev. 2017, 38, 48–53. [Google Scholar] [CrossRef]
- Qi, L.; Huang, X.P.; Wu, J.F.; Mou, X.B.; Xu, Y.R.; Li, S.Y.; Ma, G.J.; Wang, F.X.; Peng, L.Z. Simulation Analysis and Parameter Optimization of Seed-Flesh Separation Process of Seed Melon Crushing and Seed Extraction Separator Based on DEM. Agriculture 2024, 14, 1008. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, B.W.; Lai, Q.H.; Gong, Y.; Yu, Q.X.; Chen, X. Determination of melon seed physical parameters and calibration of discrete element simulation parameters. PLoS ONE 2024, 19, e0300516. [Google Scholar] [CrossRef] [PubMed]
- Mai, E.A.H.; Yang, X.P.; Long, X.Q.; Yang, R.; Zhang, R.J. Research on the technological condition of citrullus lanatus fermenting feeds by response surface methodology. Feed Ind. 2020, 41, 23–28. [Google Scholar] [CrossRef]
- Lu, L.P.; Wang, M.Y.; Cui, R.J.; Zhu, Y.L.; Liu, S.S.; Yin, Z.Y.; Gao, D.D. Discrimination and Characterization Analysis of key flavor compounds from Tianshui Zanthoxylum bungeanum shells or seeds by GC×GC-TOFMS and chemometrics. Food Chem. X 2025, 102765. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Zhang, H.J.; Deng, X.X.; Wang, Y.B.; Ma, Y.; Zhao, X.Y.; Zhang, C. Characterization and Comparison of Unfermented and Fermented Seed-Watermelon Juice. J. Food Qual. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Uthpala, M.P.; Davis, D.A.; Daniel, E.W.; Harrison, C.E.; LaFrentz, B.R.; Beck, B.H.; Roy, L.A.; Mark, F.; Bruce, T.J. Influence of dietary fermented yeast products (Saccharomyces cerevisiae) on performance, health and microbiome of Nile tilapia (Oreochromis niloticus) and the influence of discharge water in the production of romaine lettuce (Lactuca sativa). Anim. Feed Sci. Technol. 2025, 325, 116348. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.Y.; Tian, J.J.; Yun, L.Y.; Zhang, M.; Tian, Y.J. Effects of Lactobacillus plantarum and Saccharomyces cerevisiae on rumen fermentation parameters, microbial diversity and metabolites of fermented feed in vitro. Anim. Feed Sci. Technol. 2025, 325, 116366. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, M.J.; Lee, B.; Jang, H.J.; Lee, S.W. Mixed Strains of Bacillus velezensis PBS-17, Lactiplantibacillus plantarum J-135, and Saccharomyces cerevisiae DAE-4 Increase the Storage Capacity of Fermented Feed and Silage. Fermentation 2024, 10, 621. [Google Scholar] [CrossRef]
- Huang, J.F.; Ou, Y.X.; Zhang, D.F.; Zhang, G.G.; Pan, Y.T. Optimization of the culture condition of Bacillus mucilaginous using Agaricus bisporus industrial wastewater by Plackett-Burman combined with box-Behnken response surface method. AMB Express 2018, 8, 141. [Google Scholar] [CrossRef]
- Wu, X.H.; Zhang, L.N.; Sui, A.L.; Qu, B.Q.; Wang, S.Y. Optimization of fermentation process parameters for ginsenoside re bioconversion by Plackett-Burman and Box-Benhnken Design. MATEC Web Conf. 2018, 238, 04001. [Google Scholar] [CrossRef][Green Version]
- ISO 13299: 2016(E); Sensory Analysis-Methodology-General Guidance for Establishing a Sensory Profile. International standard: Geneva, Switerland, 2016.
- Ma, Y.N.; Xu, X.; Chen, L.F.; Zhou, J.P.; Cao, Z.H.; Lin, Q.Y. Process optimization and evaluation of quality properties of natto with co-culture of Bacillus subtilis natto and Limosilactobacillus fermentum. Curr. Res. Microb. Sci. 2025, 8, 100347. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, Z.; Yu, H.C.; Luo, Z.Z.; Zhuang, Y.F. Effects of enzyme and fermented green juice on silage quality of Pennisetum sp. Pratacultural Sci. 2017, 34, 1755–1761. [Google Scholar] [CrossRef]
- Wang, H.J.; Chang, L.; Lin, Y.S. Changes in functionality of germinated and non-germinated brown rice fermented by Bacillus natto. Foods 2021, 10, 2779. [Google Scholar] [CrossRef]
- Wei, J.P.; Zhang, Y.X.; Qiu, Y.; Guo, H.; Ju, H.M.; Wang, Y.W.; Yuan, Y.H.; Yue, T.L. Chemical composition, sensorial properties, and aroma-active compounds of ciders fermented with Hanseniaspora osmophila and Torulaspora quercuum in co-and sequential fermentations. Food Chem. 2020, 306, 125623. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Huang, G.L. Extraction, purification and antioxidant activity of Juglans regia shell polysaccharide. Chem. Biol. Technol. Agric. 2023, 10, 75. [Google Scholar] [CrossRef]
- Zhou, D.; Hu, Y.F.; He, J.S.; Zhao, M.; Tang, Q.Y. Optimization of ultrasonic-assisted compound enzyme extraction process, structural characterization, and antioxidant activity of Gastrodia elata polysaccharides. J. Mol. Struct. 2025, 1327, 141214. [Google Scholar] [CrossRef]
- Shu, W.X.; Wu, Z.F.; Weng, P.F. Effects of Lactobacillus plantarum and Limosilactobacillus fermentum on fermentation quality and antioxidant activity of shaddock juice. Food Sci. 2019, 40, 152–158. [Google Scholar]
- Xu, S.Y.; Fu, B.; Zhang, L.J.; Liu, H. Bioconversion of H2/CO2 by acetogen enriched cultures for acetate and ethanol production: The impact of pH. World J. Microbiol. Biotechnol. 2015, 31, 41–50. [Google Scholar] [CrossRef]
- Liang, W.Y.; Yang, H.Y.; Lei, H.X.; Xiang, Z.B.; Duan, Y.Q.; Xin, H.L.; Han, T.; Su, J. Phytochemistry and health functions of Zanthoxylum bungeanum Maxim and Zanthoxylum schinifolium Sieb. et Zucc as pharma-foods: A systematic review. Trends Food Sci. Technol. 2024, 143, 104225. [Google Scholar] [CrossRef]
- Kumla, J.; Nakarin, S.; Kanaporn, S.; Watsana, P.; Pattana, K.; Kritsana, J.; Santhiti, V.; Saisamorn, L. Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production Through the Utilization of Agro-Industrial Waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef]
- Marlee, H.; Kelly, A.; Matthew, A.; Julia, B.; Summer, H.; Ilkyu, Y.; Jeffrey, W.; George, D.; Jodi, M.; Dörte, D. The Effect of Saccharomyces cerevisiae Fermentation Product Supplementation on Pro-Inflammatory Cytokines in Holstein Friesian Cattle Experimentally Inoculated with Digital Dermatitis. Animals 2024, 14, 3260. [Google Scholar] [CrossRef]
- Wang, F.Q.; Xie, H.; Chen, W.; Wang, E.T.; Du, F.G.; Song, A.D. Biological pretreatment of corn stover with ligninolytic enzyme for high efficient enzymatic hydrolysis. Bioresour. Technol. 2013, 144, 572–578. [Google Scholar] [CrossRef]
- Zheng, D.; Shimojo, M.; Shao, T. Effects of Wilting and Adding Green Juice Fermentation Liquid on Fermentation Quality of Hybrid Pennisetum Silage. Acta Agrestia Sin. 2011, 19, 273–276. [Google Scholar]
- Sharawy, Z.; Goda, A.M.S.; Hassaan, M.S. Partial or total replacement of fish meal by solid state fermented soybean meal with Saccharomyces cerevisiae in diets for Indian prawn shrimp, Fenneropenaeus indicus, Postlarvae. Anim. Feed Sci. Technol. 2016, 212, 90–99. [Google Scholar] [CrossRef]
- Dey, T.B.; Kuhad, R.C. Enhanced production and extraction of phenolic compounds from wheat by solid-state fermentation with Rhizopus oryzae RCK2012. Biotechnol. Rep. 2014, 4, 120–127. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.Z.; Zheng, A.J.; Wang, Z.D.; Chen, Z.M.; Liu, G.H. Effects of Zanthoxylum bungeanum Seed Meal Replacing Soybean Meal on the Growth Performance, Slaughter Performance, Serum Biochemical Indicators, Antioxidant Capacity, and Immune Function of Broilers. China Anim. Husb. Vet. Med. 2025, 52, 158–168. [Google Scholar]
| Level | Substrate Ratio (g/g): A | Inoculation Amount (%): B | Fermentation Time (h): C |
|---|---|---|---|
| −1 | 8:2 | 7% | 24 |
| 0 | 7:3 | 8% | 36 |
| 1 | 6:4 | 9% | 48 |
| Index | Evaluation Standard | ||
|---|---|---|---|
| Good | General | Worse | |
| Color | Chocolate-brown (21–25) | Yellowish-brown (11–20) | Brown-black (1–10) |
| Flavor | The unique fragrance of seed melon; pleasant and savory sour fragment (21–25) | Faint sour taste; delicate fragrance of seed melon; light pepper fragrance (11–20) | Heavier sour taste and numb-taste (1–10) |
| Texture | Well structured and loose (21–25) | Well structured with a few clumps (11–20) | Decay with large clumps (1–10) |
| Fermentation degree | Uniform, completely(21–25) | Uncompletely (11–20) | Decay and deterioration (1–10) |
| Runs | A Substrate Ratio (%) | B Inoculation Amount (%) | C Fermentation Time (h) | R1 Sensory Score | R2 S. cerevisiae L23 Count (lg(CFU/g)) |
|---|---|---|---|---|---|
| 1 | −1.00 | 1.00 | 0.00 | 76 | 9.15 |
| 2 | 1.00 | 0.00 | 1.00 | 72 | 8.98 |
| 3 | 1.00 | 0.00 | −1.00 | 68 | 8.56 |
| 4 | 0.00 | 1.00 | 1.00 | 74 | 8.79 |
| 5 | 0.00 | 1.00 | −1.00 | 70 | 8.68 |
| 6 | 0.00 | 0.00 | 0.00 | 83 | 10.38 |
| 7 | 0.00 | 0.00 | 0.00 | 80 | 10.02 |
| 8 | 0.00 | 0.00 | 0.00 | 84 | 10.65 |
| 9 | 1.00 | −1.00 | 0.00 | 69 | 8.46 |
| 10 | −1.00 | 0.00 | −1.00 | 73 | 9.02 |
| 11 | 1.00 | 1.00 | 0.00 | 70 | 8.67 |
| 12 | 0.00 | −1.00 | −1.00 | 68 | 8.49 |
| 13 | 0.00 | −1.00 | 1.00 | 74 | 8.63 |
| 14 | −1.00 | −1.00 | 0.00 | 73 | 8.78 |
| 15 | −1.00 | 0.00 | 1.00 | 77 | 9.18 |
| 16 | 0.00 | 0.00 | 0.00 | 83 | 10.38 |
| 17 | 0.00 | 0.00 | 0.00 | 84 | 10.53 |
| Variance Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Sensory score | ||||||
| Modle | 510.67 | 9 | 56.74 | 33.66 | <0.0001 | **** |
| A | 50.00 | 1 | 50.00 | 29.66 | 0.0010 | ** |
| B | 4.50 | 1 | 4.50 | 2.67 | 0.1463 | |
| C | 40.50 | 1 | 40.50 | 24.03 | 0.0017 | ** |
| AB | 1.00 | 1 | 1.00 | 0.59 | 0.4664 | |
| AC | −5.684 × 10−14 | 1 | −5.684 × 10−14 | −3.372 × 10−14 | 1.0000 | |
| BC | 1.00 | 1 | 1.00 | 0.59 | 0.4664 | |
| A2 | 101.09 | 1 | 101.09 | 59.97 | 0.0001 | *** |
| B2 | 146.57 | 1 | 146.57 | 86.95 | < 0.0001 | **** |
| C2 | 122.78 | 1 | 122.78 | 72.83 | < 0.0001 | **** |
| Residual | 11.80 | 7 | 1.69 | |||
| Lack of Fit | 1.00 | 3 | 0.33 | 0.12 | 0.9415 | not significant |
| Pure Error | 10.80 | 4 | 2.70 | |||
| Cor Total | 522.47 | 16 | ||||
| S. cerevisiae L23 count | ||||||
| Model | 9.79 | 9 | 1.09 | 30.85 | < 0.0001 | **** |
| A | 0.27 | 1 | 0.27 | 7.55 | 0.0286 | * |
| B | 0.11 | 1 | 0.11 | 3.06 | 0.1235 | |
| C | 0.086 | 1 | 0.086 | 2.44 | 0.1622 | |
| AB | 6.4 × 10−3 | 1 | 6.4 × 10−3 | 0.18 | 0.6830 | |
| AC | 0.017 | 1 | 0.017 | 0.48 | 0.5112 | |
| BC | 2.25 × 10−4 | 1 | 2.25 × 10−4 | 6.378 × 10−3 | 0.9386 | |
| A2 | 1.89 | 1 | 1.89 | 53.54 | 0.0002 | *** |
| B2 | 3.86 | 1 | 3.86 | 109.36 | < 0.0001 | **** |
| C2 | 2.61 | 1 | 2.61 | 73.97 | < 0.0001 | **** |
| Residual | 0.25 | 7 | 0.035 | |||
| Lack of Fit | 0.023 | 3 | 7.558 × 10−3 | 0.13 | 0.9343 | not significant |
| Pure Error | 0.22 | 4 | 0.056 | |||
| Cor Total | 10.04 | 16 | ||||
| Run | Variables | LA/% | AA/% | PA/% | BA/% | ||
|---|---|---|---|---|---|---|---|
| Substrate Ratio (g/g) | Inoculation Amount (%) | Fermentation Time (h) | |||||
| Control | 7:3 | - | 24 | 0.43 ± 0.05 c | 0.59 ± 0.04 d | 0.27 ± 0.08 c | 0.17 ± 0.04 a |
| Test 1 | 7:3 | 5 | 24 | 0.76 ± 0.18 b | 0.72 ± 0.15 b | 0.22 ± 0.07 d | 0.07 ± 0.06 b |
| Test 2 | 7:3 | 8 | 24 | 0.78 ± 0.14 b | 0.70 ± 0.16 c | 0.31 ± 0.05 b | 0.06 ± 0.07 b |
| Test 3 | 7:3 | 8 | 36 | 0.85 ± 0.12 a | 0.75 ± 0.13 a | 0.35 ± 0.09 a | 0.06 ± 0.08 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Zhang, X.; Yin, Z.; Zhou, R.; Zhu, Y.; Liu, S.; Gao, D. Optimized Co-Fermentation of Seed Melon and Z. bungeanum Seed Meal with Saccharomyces cerevisiae L23: Valorization into Functional Feed with Enhanced Antioxidant Activity. Fermentation 2025, 11, 533. https://doi.org/10.3390/fermentation11090533
Lu L, Zhang X, Yin Z, Zhou R, Zhu Y, Liu S, Gao D. Optimized Co-Fermentation of Seed Melon and Z. bungeanum Seed Meal with Saccharomyces cerevisiae L23: Valorization into Functional Feed with Enhanced Antioxidant Activity. Fermentation. 2025; 11(9):533. https://doi.org/10.3390/fermentation11090533
Chicago/Turabian StyleLu, Liping, Xue Zhang, Ziyi Yin, Rui Zhou, Yanli Zhu, Shanshan Liu, and Dandan Gao. 2025. "Optimized Co-Fermentation of Seed Melon and Z. bungeanum Seed Meal with Saccharomyces cerevisiae L23: Valorization into Functional Feed with Enhanced Antioxidant Activity" Fermentation 11, no. 9: 533. https://doi.org/10.3390/fermentation11090533
APA StyleLu, L., Zhang, X., Yin, Z., Zhou, R., Zhu, Y., Liu, S., & Gao, D. (2025). Optimized Co-Fermentation of Seed Melon and Z. bungeanum Seed Meal with Saccharomyces cerevisiae L23: Valorization into Functional Feed with Enhanced Antioxidant Activity. Fermentation, 11(9), 533. https://doi.org/10.3390/fermentation11090533






