Application of Hops (Humulus lupulus L.) and β-Acid Extract to Improve Aerobic Stability and In Vitro Ruminal Fermentation of Maralfalfa Grass Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Treatments, Microbial Growth
2.3. In Vitro Ruminal Fermentation
2.4. Statistical Analysis
2.4.1. Selection of Experimental Silage (Statistical Analysis)
2.4.2. Treatments, Microbial Growth, and Fermentation (Statistical Analysis)
2.4.3. In Vitro Ruminal Fermentation (Statistical Analysis)
3. Results
3.1. Preliminary Silages: Bromatological Analysis
3.2. In Vitro Fermentation and Microbial Growth: Characteristics in the Aerobic Phase
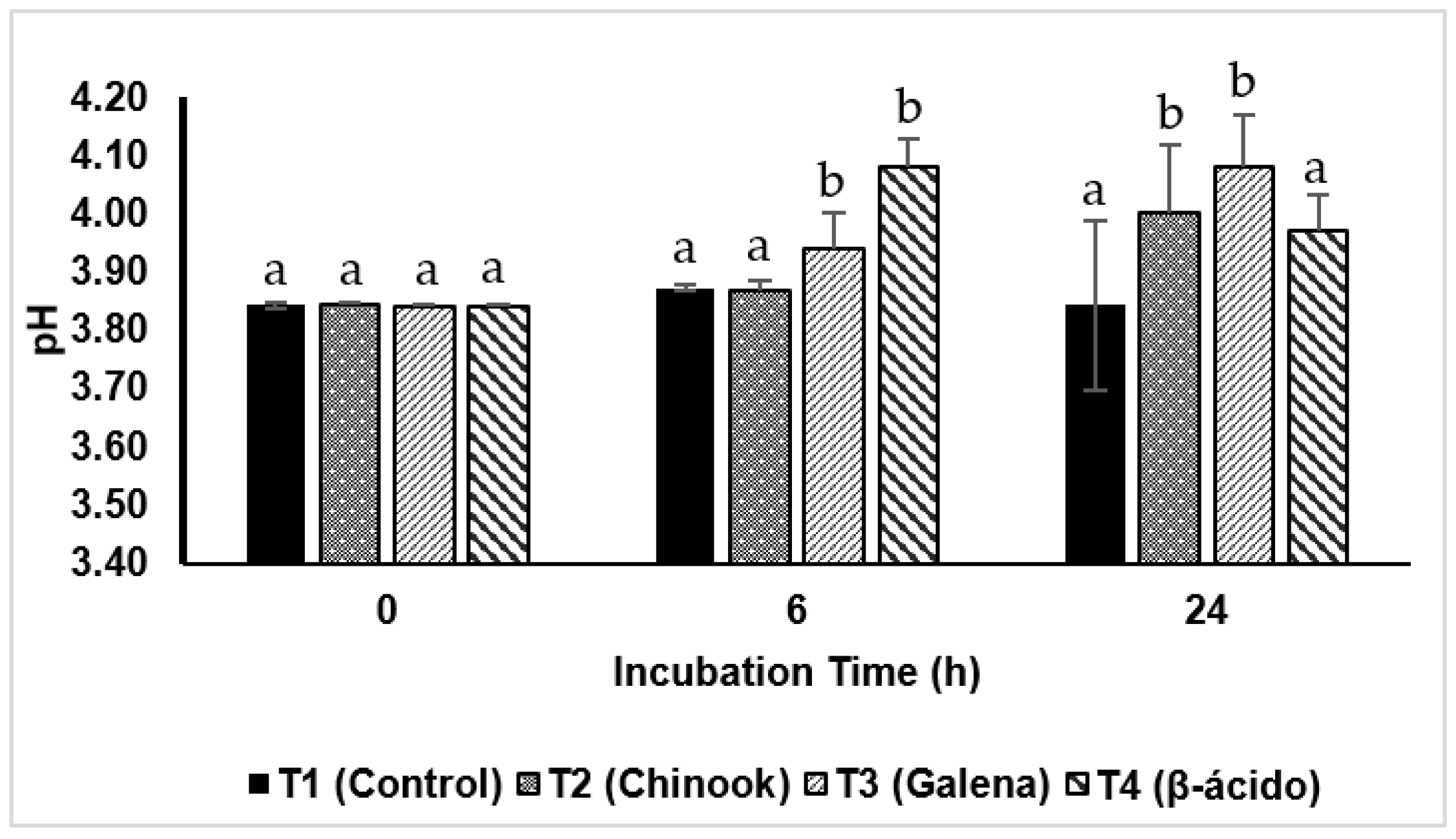
3.2.1. pH Values
3.2.2. Microbial Counts
3.2.3. Ammonia Concentrations
3.2.4. Acetic, Propionic, and Butyric Acid Concentrations
3.3. In Vitro Ruminal Fermentation Results
pH and Total Gas, Hydrogen, Methane, Ammonia Gas, Carbon Dioxide Production, and VFAs
4. Discussion
4.1. Preliminary Silages and Chemical Composition
4.2. Experimental Silages: Microbial Growth and Fermentation
4.3. In Vitro Ruminal Fermentation of Silage Treated with Hops and β-Acid Extract
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siefers, M.K. History of Silage, Sorghum Silage Production, and Nutritive Value of Pelleted Poultry by-Products and Restaurant Waste; Kansas State University: Manhattan, KS, USA, 2000; ISBN 049303742X. [Google Scholar]
- Ahmed, M.G.; Al-Sagheer, A.A.; El-Waziry, A.M.; El-Zarkouny, S.Z.; Elwakeel, E.A. Ensiling Characteristics, In Vitro Rumen Fermentation Patterns, Feed Degradability, and Methane and Ammonia Production of Berseem (Trifolium alexandrinum L.) Co-Ensiled with Artichoke Bracts (Cynara cardunculus L.). Animals 2023, 13, 1543. [Google Scholar] [CrossRef]
- Yıldız, S. Silage Quality, Nutrient Content and Relative Feed Value of Urea and Molasses Added Sweet Maize [Zea mays (L.) Saccharata Sturt.] Silage. Indian J. Anim. Res. 2024, 58, 1593–1598. [Google Scholar]
- Cao, X.; Cai, R.; Zuo, S.; Niu, D.; Yang, F.; Xu, C. Enhanced Lignin Degradation by Irpex Lacteus through Expanded Sterilization Further Improved the Fermentation Quality and Microbial Community during the Silage Preservation Process. Bioresour. Bioprocess 2024, 11, 14. [Google Scholar] [CrossRef]
- Mengistu, G.; Kebede, G.; Faji, M.; Feyissa, F.; Mohammed, K.; Kehaliew, A.; Geleti, D.; Minta, M.; Balehegn, M.; Rios, E.F. Morphological Characteristics, Dry Matter Yield, and Nutritive Value of Maralfalfa Grass (Pennisetum spp.) Grown under Different Planting Densities in the Central Highlands of Ethiopia. Front. Anim. Sci. 2024, 4, 1308911. [Google Scholar] [CrossRef]
- Henderson, N. Silage Additives. Anim. Feed Sci. Technol. 1993, 45, 35–56. [Google Scholar] [CrossRef]
- Kung, L. Silage Fermentation and Additives. Arch. Latinoam. Prod. Anim. 2018, 26. [Google Scholar]
- Santos da Silva, W.; Carvalho dos Santos, T.M.; Cavalcanti Neto, C.C.; Espíndola Filho, A.M.; Mesquita da Silva, S.G.; Neves Figueiredo, A.; Araújo de Melo, B. Características y Estabilidad Aeróbica de Ensilajes de Caña de Azúcar, Tratada Con Urea, NaOH y Maíz. Pastos Forrajes 2014, 37, 182–190. [Google Scholar]
- Ávila, C.L.S.; Carvalho, B.F. Silage Fermentation—Updates Focusing on the Performance of Micro-organisms. J. Appl. Microbiol. 2020, 128, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.O.; Wang, Y.; Gao, L.; Wu, Y.; Li, X.; Sun, J.; Jiang, J. The Performance of Lactic Acid Bacteria in Silage Production: A Review of Modern Biotechnology for Silage Improvement. Microbiol. Res. 2023, 266, 127212. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, F. Silage and the Safety and Quality of Dairy Foods: A Review. Agric. Food Sci. 2013, 22, 16–34. [Google Scholar] [CrossRef]
- López-Garrido, S.J.; Peralta, M.A.C.; Martinez, G.D.M.; Camacho-Escobar, M.A. The Effect of Commercial Additive (Toxic-Chec) and Propionic Acid on the Fermentation and Aerobic Stability of Silage with Pig Excreta. Am. J. Exp. Agric. 2014, 4, 1820. [Google Scholar] [CrossRef]
- Salfer, I.J.; Fessenden, S.W.; Stern, M.D. Evaluation of Iso-α-Acid and β-Acid Extracts from Hops (Humulus lupulus L.) on Fermentation by Rumen Microbes in Dual-Flow Continuous Culture Fermenters. Anim. Feed. Sci. Technol. 2020, 260, 114385. [Google Scholar] [CrossRef]
- Korpelainen, H.; Pietiläinen, M. Hop (Humulus lupulus L.): Traditional and Present Use, and Future Potential. Econ. Bot. 2021, 75, 302–322. [Google Scholar] [CrossRef]
- Castillo-Castillo, Y.; Solis, R.; Quintana, A.A.; Arzola, C.; Olivas-Palacios, A.L.; Salinas-Chavira, J.; Anderson, R. PSXI-17 Influence of Hops on in Vitro Ruminal Fermentation of Corn Grain. J. Anim. Sci. 2019, 97, 408. [Google Scholar] [CrossRef]
- Verzele, M.; De Keukeleire, D. Chemistry and Analysis of Hop and Beer Bitter Acids; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 1483290867. [Google Scholar]
- Szczepaniak, O.; Dziedzinski, M.; Kobus-Cisowska, J.; Szulc, P.; Szymanowska, D.; Sudyka, M.; Goryńska-Goldmann, E. Chmiel (Humulus lupulus L.) Jako Surowiec o Właściwościach Prozdrowotnych: Aktualny Stan Wiedzy. Tech. Rol. Ogrod. Leśna 2019, 3, 9–12. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Flythe, M.D. The Antimicrobial Effects of Hops (Humulus lupulus L.) on Ruminal Hyper Ammonia-producing Bacteria. Lett. Appl. Microbiol. 2009, 48, 712–717. [Google Scholar] [CrossRef]
- Flythe, M.D.; Harlow, B.E.; Aiken, G.E.; Gellin, G.L.; Kagan, I.A.; Pappas, J. Inhibition of Growth and Ammonia Production of Ruminal Hyper Ammonia-Producing Bacteria by Chinook or Galena Hops after Long-Term Storage. Fermentation 2017, 3, 68. [Google Scholar] [CrossRef]
- Sakamoto, K.; Konings, W.N. Beer Spoilage Bacteria and Hop Resistance. Int. J. Food Microbiol. 2003, 89, 105–124. [Google Scholar] [CrossRef]
- Chen, G.; Russell, J.B. More Monensin-Sensitive, Ammonia-Producing Bacteria from the Rumen. Appl. Environ. Microbiol. 1989, 55, 1052–1057. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and in Vitro Media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Chaparro-Escudero, A.; Domínguez-Viveros, J.; Castillo-Castillo, Y.; Román-Ponce, S.; Castillo-Rangel, F. Impacto de Diferentes Aditivos Sobre La Acidosis y Fermentación Ruminal En Corderos. Abanico Vet. 2023, 14, e2023-109. [Google Scholar]
- McDougall, E.I. Studies on Ruminant Saliva. 1. The Composition and Output of Sheep’s Saliva. Biochem. J. 1948, 43, 99. [Google Scholar] [CrossRef]
- Basurto, V.M. Cuál Es El Significado de Los Análisis de Los Ensilados. BMeditores 2020. Available online: https://bmeditores.mx/ganaderia/cual-es-el-significado-de-los-analisis-de-los-ensilados/ (accessed on 28 August 2025).
- González Moreno, E. Bromatología del Ensilado de Pasto Mar Alfalfa (Pennisetum sp.) Fertilizado Con ENTEC® e Inoculado Con Sil-All 4x4®; Universidad Autónoma Agraria Antonio Narro: Saltillo, México, 2015; Available online: http://repositorio.uaaan.mx:8080/xmlui/handle/123456789/6712 (accessed on 28 August 2025).
- Callejo Ramos, A. Conservación de Forrajes (XIV): Uso de Aditivos En El Ensilado. Frisona Española 2019, 92–100. Available online: https://oa.upm.es/56898/ (accessed on 28 August 2025).
- Núñez Hernández, G.; Payán García, J.A.; Pena Ramos, A.; González Castañeda, F.; Ruiz Barrera, O.; Arzola Alvarez, C. Caracterización Agronómica y Nutricional Del Forraje de Variedades de Especies Anuales En La Región Norte de México. Rev. Mex. Cienc. Pecu. 2010, 1, 85–98. [Google Scholar]
- Sánchez-Guerra, N.A.; Gonzalez-Ronquillo, M.; Anderson, R.C.; Hume, M.E.; Ruiz-Albarrán, M.; Bautista-Martínez, Y.; Zúñiga-Serrano, A.; Nájera-Pedraza, O.G.; Salinas-Chavira, J. Improvements in Fermentation and Nutritive Quality of Elephant Grass [Cenchrus purpureus (Schumach.) Morrone] Silages: A Review. Trop. Anim. Health Prod. 2024, 56, 171. [Google Scholar] [CrossRef]
- Morales, S.M.B.; Castelblanco, E.M.S.; Suárez, H.J.G. Aislamiento de Microorganismos Amilolíticos, Celulolíticos y Lignolíticos a Partir Del Suelo de Humedales de Bogotá. Rev. Sennova Rev. Sist. Cienc. Tecnol. E Innovación 2014, 1, 148–155. [Google Scholar]
- Castillo-Castillo, Y.; Arzola-Alvarez, C.; Fonseca, M.; Salinas-Chavira, J.; Ontiveros-Magadan, M.; Hume, M.E.; Anderson, R.C.; Flythe, M.D.; Byrd, J.A.; Ruiz-Barrera, O. Effects of Hops Treatment on Nitrogen Retention, Volatile Fatty Acid Accumulations, and Select Microbial Populations of Composting Poultry Litter Intended for Use as a Ruminant Feedstuff. Microorganisms 2023, 11, 839. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Rivière, C. An Overview of the Antimicrobial Properties of Hop. In Natural Antimicrobial Agents; Springer: Berlin/Heidelberg, Germany, 2018; pp. 31–54. [Google Scholar]
- Siragusa, G.R.; Haas, G.J.; Matthews, P.D.; Smith, R.J.; Buhr, R.J.; Dale, N.M.; Wise, M.G. Antimicrobial Activity of Lupulone against Clostridium Perfringens in the Chicken Intestinal Tract Jejunum and Caecum. J. Antimicrob. Chemother. 2008, 61, 853–858. [Google Scholar] [CrossRef]
- Bernal, L.; Ávila, P.; Ramírez, G.; Lascano, C.E.; Tiemann, T.; Hess, H. Degradación de Nutrientes y Emisión de Gases al Fermentar Ensilaje y Heno de Calliandra Calothyrsus y Vigna Unguiculata En El Sistema Rusitec. Asoc. Latinoam. De Prod. Anim. 2008, 16, 199–204. [Google Scholar]
- Rodríguez, R.; Sosa, A.; Rodríguez, Y. La Síntesis de Proteína Microbiana En El Rumen y Su Importancia Para Los Rumiantes. Rev. Cuba. De Cienc. Agrícola 2007, 41, 303–311. [Google Scholar]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Elferink, S.J.W.H.O.; Spoelstra, S.F. Microbiology of Ensiling. Silage Sci. Technol. 2003, 42, 31–93. [Google Scholar]
- Doyle, C.J.; Gleeson, D.; Jordan, K.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. Anaerobic Sporeformers and Their Significance with Respect to Milk and Dairy Products. Int. J. Food Microbiol. 2015, 197, 77–87. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage Review: Factors Affecting Dry Matter and Quality Losses in Silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef]
- Romero-Rodríguez, A.; Ruiz-Villafán, B.; Martínez-De la Peña, C.F.; Sánchez, S. Targeting the Impossible: A Review of New Strategies against Endospores. Antibiotics 2023, 12, 248. [Google Scholar] [CrossRef]
- Klein, G.; Rüben, C.; Upmann, M. Antimicrobial Activity of Essential Oil Components against Potential Food Spoilage Microorganisms. Curr. Microbiol. 2013, 67, 200–208. [Google Scholar] [CrossRef]
- Flythe, M.D.; Aiken, G.E. Effects of Hops (Humulus lupulus L.) Extract on Volatile Fatty Acid Production by Rumen Bacteria. J. Appl. Microbiol. 2010, 109, 1169–1176. [Google Scholar] [CrossRef]
- Flythe, M.D.; Harlow, B.E. Effects of Hops (Humulus lupulus L.) Beta-Acids on Short Chain Fatty Acid Production from Complex Carbohydrates by Rumen Microbiota. Adv. Microbiol. 2019, 9, 983–992. [Google Scholar] [CrossRef][Green Version]
- García-González, R.; López, S.; Fernández, M.; Bodas, R.; González, J.S. Screening the Activity of Plants and Spices for Decreasing Ruminal Methane Production in Vitro. Anim. Feed Sci. Technol. 2008, 147, 36–52. [Google Scholar] [CrossRef]
- Lavrenčič, A.; Levart, A.; Košir, I.J.; Čerenak, A. In Vitro Gas Production Kinetics and Short-Chain Fatty Acid Production from Rumen Incubation of Diets Supplemented with Hop Cones (Humulus lupulus L.). Animal 2015, 9, 576–581. [Google Scholar] [CrossRef]
- Staerfl, S.M.; Kreuzer, M.; Soliva, C.R. In Vitro Screening of Unconventional Feeds and Various Natural Supplements for Their Ruminal Methane Mitigation Potential When Included in a Maize-Silage Based Diet. J. Anim. Feed. Sci. 2010, 19, 651–664. [Google Scholar] [CrossRef]
- Flythe, M.D.; Kagan, I.A.; Wang, Y.; Narvaez, N. Hops (Humulus lupulus L.) Bitter Acids: Modulation of Rumen Fermentation and Potential as an Alternative Growth Promoter. Front. Vet. Sci. 2017, 4, 131. [Google Scholar] [CrossRef]
- Fahle, A.; Bereswill, S.; Heimesaat, M.M. Antibacterial Effects of Biologically Active Ingredients in Hop Provide Promising Options to Fight Infections by Pathogens Including Multi-Drug Resistant Bacteria. Eur. J. Microbiol. Immunol. (Bp) 2022, 12, 22–30. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Hession, A.O. Preventing in Vitro Lactate Accumulation in Ruminal Fermentations by Inoculation with Megasphaera elsdenii. J. Anim. Sci. 1995, 73, 250–256. [Google Scholar] [CrossRef]
- Driehuis, F.; Wilkinson, J.M.; Jiang, Y.; Ogunade, I.; Adesogan, A.T. Silage Review: Animal and Human Health Risks from Silage. J. Dairy Sci. 2018, 101, 4093–4110. [Google Scholar] [CrossRef]
| Silage | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Maralfalfa grass, % | 90.0 | 85.0 | 90.0 | 85.0 |
| Ground sorghum grain, % | 8.00 | 12.0 | 7.63 | 11.4 |
| Sorghum straw, % | 2.00 | 3.00 | 1.63 | 2.44 |
| Urea, % | - | - | 0.75 | 1.13 |
| Silages | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | SEM | p-Value | |
| pH | 6.08 b | 6.00 b | 8.19 a | 5.06 c | 0.13 | <0.01 |
| Ash % | 10.4 b | 10.2 b | 12.4 a | 11.8 a | 0.68 | <0.05 |
| Ether extract % | 2.66 | 2.65 | 3.65 | 2.68 | 0.22 | 0.07 |
| Crude protein % | 8.92 b | 5.71 c | 6.67 c | 11.9 a | 0.61 | <0.01 |
| NDF % | 61.8 | 56.7 | 63.9 | 61.7 | 14.0 | 0.09 |
| ADF % | 37.5 ab | 29.8 b | 42.4 a | 38.5 ab | 11.2 | <0.05 |
| Treatment | Time | SEM | p-Value | ||
|---|---|---|---|---|---|
| 0 | 6 | 24 | |||
| Control | 3.90 a | 4.82 ac | 5.40 b | 0.11 | 0.03 |
| Chinook | 3.90 a | 4.48 a | 5.05 bc | ||
| Galena | 3.90 a | 4.60 a | 4.78 a | ||
| β-acids | 3.90 a | 3.94 a | 3.74 a | ||
| Ammonia (µmol/g) | Acetic Acid (µmol/g) | Propionic Acid (µmol/g) | Butyric Acid (µmol/g) | |
|---|---|---|---|---|
| Control | 18.94 c | 8.58 | 1.54 | 0.56 |
| Chinook | 19.56 bc | 11.85 | 1.74 | 0.52 |
| Galena | 20.73 a | 8.56 | 1.63 | 0.54 |
| β-acids | 21.73 ab | 9.014 | 1.74 | 0.67 |
| SEM | 0.25 | 0.56 | 0.03 | 0.02 |
| Time | ||||
| 0 | 19.31 b | 20.03 a | 1.91 a | 0.94 a |
| 6 | 20.62 a | 6.25 b | 2.11 a | 0.44 b |
| 24 | 20.80 a | 2.22 b | 1.07 b | 0.33 b |
| SEM | 0.17 | 3.30 | 0.20 | 0.11 |
| Treatments | SEM | p-Value | ||||
|---|---|---|---|---|---|---|
| Control | Chinook Hops | Galena Hops | β-Acid Extract | |||
| Hydrogen 1, µmol/mL | ND | ND | 0.011 | ND | 0.009 | 0.44 |
| Methane 1, µmol/mL | 15.0 | 15.4 | 14.7 | 15.7 | 2.54 | 0.77 |
| Carbon dioxide 1, µmol/mL | 31.9 ab | 33.6 a | 28.4 b | 26.9 b | 3.26 | 0.05 |
| Ammonia, µmol/mL | 7.58 | 7.83 | 8.69 | 8.29 | 2.82 | 0.09 |
| pH | 7.39 | 7.35 | 7.44 | 7.45 | 0.07 | 0.36 |
| Total gas production, mL | 11.5 | 11.1 | 10.2 | 9.69 | 1.12 | 0.21 |
| Acetate, µmol/mL | 9.64 | 8.82 | 16.9 | 30.7 | 5.43 | 0.09 |
| Propionate, µmol/mL | 0.702 b | 1.04 b | 1.76 b | 6.02 a | 1.07 | 0.02 |
| Butyrate, µmol/mL | 0.261 | 0.485 | 0.791 | 1.88 | 0.19 | 0.07 |
| Acetate/Propionate ratio | 7.16 | 8.27 | 13.2 | 5.10 | 16.9 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Vilorio, L.; González-Mora, B.; Castillo-Castillo, Y.; Castillo-Rangel, F.; Vargas-Bello-Perez, E.; Dominguez-Viveros, J.; Felix-Portillo, M.; Anderson, R.C.; Hume, M.E.; Flythe, M.D.; et al. Application of Hops (Humulus lupulus L.) and β-Acid Extract to Improve Aerobic Stability and In Vitro Ruminal Fermentation of Maralfalfa Grass Silage. Fermentation 2025, 11, 529. https://doi.org/10.3390/fermentation11090529
Romero-Vilorio L, González-Mora B, Castillo-Castillo Y, Castillo-Rangel F, Vargas-Bello-Perez E, Dominguez-Viveros J, Felix-Portillo M, Anderson RC, Hume ME, Flythe MD, et al. Application of Hops (Humulus lupulus L.) and β-Acid Extract to Improve Aerobic Stability and In Vitro Ruminal Fermentation of Maralfalfa Grass Silage. Fermentation. 2025; 11(9):529. https://doi.org/10.3390/fermentation11090529
Chicago/Turabian StyleRomero-Vilorio, Lianne, Bexy González-Mora, Yamicela Castillo-Castillo, Francisco Castillo-Rangel, Einar Vargas-Bello-Perez, Joel Dominguez-Viveros, Monserrath Felix-Portillo, Robin C. Anderson, Michael E. Hume, Michael D. Flythe, and et al. 2025. "Application of Hops (Humulus lupulus L.) and β-Acid Extract to Improve Aerobic Stability and In Vitro Ruminal Fermentation of Maralfalfa Grass Silage" Fermentation 11, no. 9: 529. https://doi.org/10.3390/fermentation11090529
APA StyleRomero-Vilorio, L., González-Mora, B., Castillo-Castillo, Y., Castillo-Rangel, F., Vargas-Bello-Perez, E., Dominguez-Viveros, J., Felix-Portillo, M., Anderson, R. C., Hume, M. E., Flythe, M. D., Nájera-Pedraza, O. G., Salinas-Chavira, J., & Ruiz-Barrera, O. (2025). Application of Hops (Humulus lupulus L.) and β-Acid Extract to Improve Aerobic Stability and In Vitro Ruminal Fermentation of Maralfalfa Grass Silage. Fermentation, 11(9), 529. https://doi.org/10.3390/fermentation11090529









