Transforming Tilapia into Indoleacetic Acid-Containing Biostimulants: Synergistic Effect of Enzymolysis and Multi-Strain Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment of Tilapia
2.3. Treatment of Fish Meat with Skin (FMS) with Bacteria Strains and Papain
2.4. Single or Mixed Microbial Fermentation of Fish Meat with Skin (FMS) in the Presence of Papain Synergism
2.5. Analytical Method
2.5.1. Determination of Soluble Protein and Trichloroacetic Acid (TCA)-Soluble Peptide
2.5.2. Analysis of Amino Acids and Hydrolysis Degree
2.5.3. Indoleacetic Acid Analysis
2.6. Protease Activity Analysis
2.7. Viable Bacteria Count Analysis
2.8. Determination of Growth Soybean Seedlings
2.9. Statistical Analysis
3. Results and Discussion
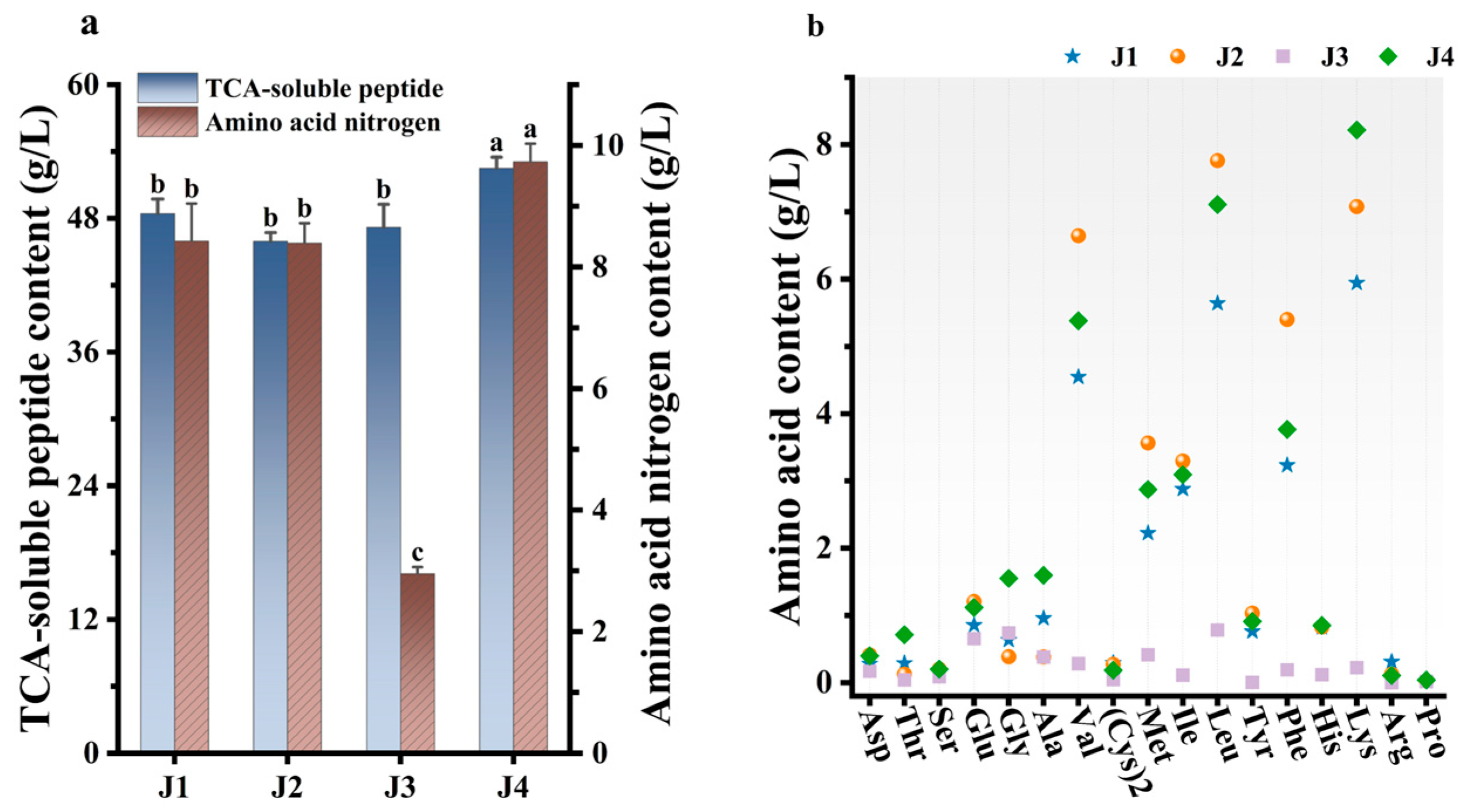
3.1. Synergistic Effect of Papain and Mixed Bacterial Strains on Fish Meat with Skin (FMS) Hydrolysis
3.2. Effects of Single and Mixed Strains on Fish Meat with Skin (FMS) Degradation
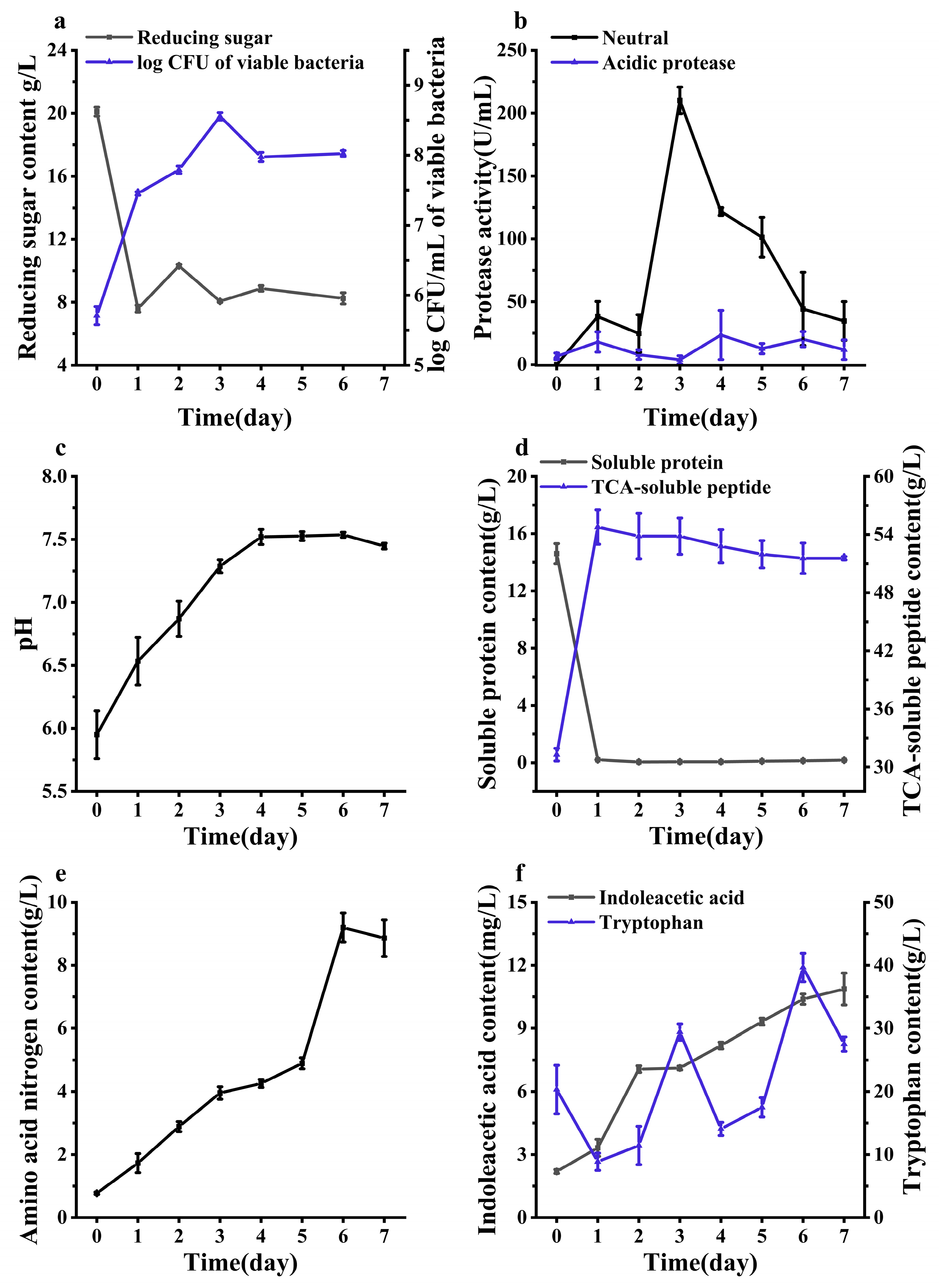
3.3. Indoleacetic Acid (IAA) Synthesis in Fish Meat with Skin (FMS) Fermentation with Single and Mixed Strains
3.4. Changes in Physicochemical Properties During the Transformation of Fish Meat with Skin (FMS)
3.5. Manurial Effect of Fish Meat with Skin (FMS) Hydrolysate on Soybean Seedlings Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lykogianni, M.; Bempelou, E.; Karamaouna, F.; Aliferis, K.A. Do pesticides promote or hinder sustainability in agriculture? The challenge of sustainable use of pesticides in modern agriculture. Sci. Total Environ. 2021, 795, 148625. [Google Scholar] [CrossRef]
- Elkhlifi, Z.; Iftikhar, J.; Sarraf, M.; Ali, B.; Saleem, M.H.; Ibranshahib, I.; Bispo, M.D.; Meili, L.; Ercisli, S.; Kayabasi, E.T.; et al. Potential role of biochar on capturing soil nutrients, carbon sequestration and managing environmental challenges: A review. Sustainability 2023, 15, 2527. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Burbano-Cuasapud, J.M.; Solarte-Toro, J.C.; Restrepo-Serna, D.L.; Alzate, C.A.C. Process Sustainability Analysis of Biorefineries to Produce Biofertilizers and Bioenergy from Biodegradable Residues. Fermentation 2023, 9, 788. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Nasri, R.; Abdelhedi, O.; Nasri, M.; Jridi, M. Fermented protein hydrolysates: Biological activities and applications. Curr. Opin. Food Sci. 2022, 43, 120–127. [Google Scholar] [CrossRef]
- Babu, N.S.; Suresh, P.V.; Kudre, T.G. Preparation and identification of a novel antioxidative peptide from fermented protein hydrolysate of chicken (Gallus gallus domesticus) meat. Process Biochem. 2025, 151, 167–176. [Google Scholar] [CrossRef]
- Zhou, J.; Li, D.; Duan, X.; Zhang, X.; Chen, C.; Chen, Y. Biological VFAs production from proteinaceous wastewater varied with protein type: The role of protein exposed enzyme cleavage sites and hydrolysates biotransformation capacity. Water Res. 2025, 275, 123201. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Gavlighi, H.A. Protein hydrolysates derived from aquaculture and marine byproducts through autolytic hydrolysis. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4872–4899. [Google Scholar] [CrossRef]
- Chiodza, K.; Goosen, N.J. Emulsion formation during enzymatic protein hydrolysis and its effect on protein recovery and molecular weight distribution of protein hydrolysates from sardine (Sardina pilchardus) by-products. Biomass Convers. Biorefin. 2024, 14, 24069–24080. [Google Scholar] [CrossRef]
- Sun, T.; Xiao, W.; Jiang, C.; Wang, J.; Liu, Z. Producing amino acid fertilizer by hydrolysis of the fermented mash of food waste with the synergy of three proteases expressed by engineered Candida utilis. Bioresour. Technol. Rep. 2019, 7, 100268. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Chen, Z.; Chen, Y.; Wang, C.; Yu, H.; Zuo, F.; Huang, W. Probiotic–enzyme synergy regulates fermentation of distiller’s grains by modifying microbiome structures and symbiotic relationships. J. Agric. Food Chem. 2025, 73, 5363–5375. [Google Scholar] [CrossRef]
- Zhong, B.; Xie, H.; Pan, T.; Su, B.; Xu, W.; Wu, Z. High acidity organic waste degradation and the potential to bioremediation of heavy metals in soil by an acid-tolerant Serratia sp. Environ. Geochem. Health 2024, 46, 321. [Google Scholar] [CrossRef]
- Neylon, E.; Nyhan, L.; Zannini, E.; Monin, T.; Münch, S.; Sahin, A.W.; Arendt, E.K. Food Ingredients for the Future: In-Depth Analysis of the Effects of Lactic Acid Bacteria Fermentation on Spent Barley Rootlets. Fermentation 2023, 9, 78. [Google Scholar] [CrossRef]
- Kononiuk, D.A.; Karwowska, M.; Bejarano, R.G.; Cacciola, F. Bioactive compounds in fermented sausages prepared from beef and fallow deer meat with acid whey addition. Molecules 2020, 25, 2429. [Google Scholar] [CrossRef] [PubMed]
- Yanohara, T.; Taoka, Y.; Yamamoto, M. Rapid Production of Fish Sauce from the Internal Organs of White Sturgeon, Acipenser transmontanus Richardson, 1836. Fermentation 2022, 8, 238. [Google Scholar] [CrossRef]
- Moropana, T.J.; Rensburg, E.L.J.V.; Makulana, L.; Phasha, N.N. Screening Aspergillus flavus, Talaromyces purpureogenus, and Trichoderma koningiopsis for plant-growth-promoting traits: A study on phosphate solubilization, IAA production, and siderophore synthesis. J. Fungi 2024, 10, 811. [Google Scholar] [CrossRef]
- Kumar, R.; Banerjee, T.K. Changes in the level of proteins, free amino acids and protease activities of clarias batrachus in response to sodium meta-arsenite intoxication. CLEAN—Soil Air Water 2013, 41, 1196–1200. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, P.; Xu, Y.; Xia, W.; Hua, Q.; Jiang, Q. Effect of Storage Conditions on Microbiological Characteristics, Biogenic Amines, and Physicochemical Quality of Low-Salt Fermented Fish. J. Food Prot. 2020, 83, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhao, X.; Hu, F.; Fu, J.; Zhang, Z.; Liu, Z.; Wang, B.; He, R.; Ma, H.; Ho, C.-T. The latest advances on soy sauce research in the past decade: Emphasis on the advances in China. Food Res. Int. 2023, 173, 113407. [Google Scholar] [CrossRef]
- Xu, J.; Cao, H.; Zhang, B.; Yao, H. The mechanistic effect of bromelain and papain on tenderization in jumbo squid (Dosidicus gigas) muscle. Food Res. Int. 2020, 131, 108991. [Google Scholar] [CrossRef]
- Li, N.; Shen, B.; Liu, Y.; Weng, P.; Wu, Z. Heterologous expression and characterization of Bacillus velezensis SW5 serine protease involved in the hydrolysis of anchovy protein. J. Sci. Food Agric. 2023, 103, 3468–3478. [Google Scholar] [CrossRef]
- Hathwar, S.C.; Bijinu, B.; Rai, A.K.; Narayan, B. Simultaneous recovery of lipids and proteins by enzymatic hydrolysis of fish industry waste using different commercial proteases. Appl. Biochem. Biotechnol. 2011, 164, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lambo, M.T.; Chang, X.; Liu, D. The recent trend in the use of multistrain probiotics in livestock production: An overview. Animals 2021, 11, 2805. [Google Scholar] [CrossRef]
- Smid, E.J.; Lacroix, C. Microbe–microbe interactions in mixed culture food fermentations. Curr. Opin. Biotechnol. 2013, 24, 148–154. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Wang, Y.; Wu, Y.; Chen, S. Improvement of the quality and safety of low-salt fish sauce by reconstruction of microbial community through cooperative fermentation of starters. Food Res. Int. 2025, 205, 115972. [Google Scholar] [CrossRef]
- Alkin, N.; Dunaevsky, Y.; Elpidina, E.; Beljakova, G.; Tereshchenkova, V.; Filippova, I.; Belozersky, M. Proline-specific fungal peptidases: Genomic analysis and identification of secreted DPP4 in alkaliphilic and alkalitolerant fungi. J. Fungi 2021, 7, 744. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, Y.; Sun, S.; Zhang, L.; Sun, L.; Weng, L.; Liu, G.; Cao, M. Aminopeptidase Play a Critical Role in the Accumulation of Free Amino Acids in Abalone (Haliotis discus hannai) During Cold Storage. J. Ocean Univ. China 2023, 22, 1049–1058. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Leon, M.J.; Millan-Linares, M.C.; la Paz, S.M. Antimicrobial plant-derived peptides obtained by enzymatic hydrolysis and fermentation as components to improve current food systems. Trends Food Sci. Technol. 2023, 135, 32–42. [Google Scholar] [CrossRef]
- Jeon, S.H.; Seong, H.-J.; Kim, H.; Kim, D.; Yang, K.-Y.; Nam, S.-H. Improvement of branched-chain amino acid production by isolated high-producing protease from Bacillus amyloliquefaciens NY130 on isolated soy/whey proteins and their muscle cell protection. Food Chem. 2024, 450, 139327. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhao, Y.; Hu, R.; Cheng, Y.; Qin, J.; Yang, J.; Fang, Y.; Lyu, M.; Wang, S. Screening and characteristics of marine Bacillus velezensis Z-1 protease and its application of enzymatic hydrolysis of mussels to prepare antioxidant active substances. Molecules 2022, 27, 6570. [Google Scholar] [CrossRef]
- Willett, J.L.E.; Robertson, E.B.; Dunny, G.M. The phosphatase Bph and peptidyl-prolyl psomerase PrsA are required for gelatinase expression and activity in Enterococcus faecalis. J. Bacteriol. 2022, 204, e00129-22. [Google Scholar] [CrossRef]
- Zeng, J.; Huang, W.; Tian, X.; Hu, X.; Wu, Z. Brewer’s spent grain fermentation improves its soluble sugar and protein as well as enzymatic activities using Bacillus velezensis. Process Biochem. 2021, 111, 12–20. [Google Scholar] [CrossRef]
- Yang, R.; Liu, P.; Ye, W.; Chen, Y.; Wei, D.; Qiao, C.; Zhou, B.; Xiao, J. Biological control of root rot of strawberry by Bacillus amyloliquefaciens strains CMS5 and CMR12. J. Fungi 2024, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Gao, M.; Zhou, S.; Liu, G.; Wang, P.; Liu, C.; Yang, F.; Fu, H. Antifungal activity of Bacillus velezensis X3-2 against plant pathogens and biocontrol effect on potato late blight. Agriculture 2024, 14, 2224. [Google Scholar] [CrossRef]
- Ma, M.-Y.; Hu, L.-L.; Xu, W.-Y.; Zhang, W. L—Tryptophan anaerobic fermentation for indole acetic acid production: Bacterial enrichment and effects of zero valent iron. Bioresour. Technol. 2024, 400, 130691. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rao, W.; Hu, S.; Zhu, S.; Ouyang, L.; Zhou, J. Study on the relationship between microbial community succession and physicochemical factors in the fermentation of rice-flavor Baijiu based on high-throughput and redundancy analysis techniques. LWT 2024, 213, 117031. [Google Scholar] [CrossRef]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wang, T.; Liu, R. Correlation analyses of amylase and protease activities and physicochemical properties of wheat bran during solid-state fermentation. Foods 2024, 13, 3998. [Google Scholar] [CrossRef]
- Wei, R.; Sun, X.; Chen, X.; Zhang, Y.; Li, Q.; Zhang, X.; Xu, N. Unraveling the microbial community and succession during the maturation of Chinese cereal vinegar Daqu and their relationships with flavor formation. Food Res. Int. 2025, 203, 115851. [Google Scholar] [CrossRef]
- Treece, T.R.; Tessman, M.; Pomeroy, R.S.; Mayfield, S.P.; Simkovsky, R.; Atsumi, S. Fluctuating pH for efficient photomixotrophic succinate production. Metab. Eng. 2023, 79, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jin, T.; Sahu, S.K.; Xu, J.; Shi, Q.; Liu, H.; Wang, Y. The distribution of tryptophan-dependent indole-3-acetic acid synthesis pathways in bacteria unraveled by large-scale genomic analysis. Molecules 2019, 24, 1411. [Google Scholar] [CrossRef]
- Liu, W.-H.; Chen, F.-F.; Wang, C.-E.; Fu, H.-H.; Fang, X.-Q.; Ye, J.-R.; Shi, J.-Y. Indole-3-acetic acid in Burkholderia pyrrocinia JK-SH007: Enzymatic identification of the indole-3-acetamide synthesis pathway. Front. Microbiol. 2019, 10, 2559. [Google Scholar] [CrossRef]
- Ambrosini, S.; Prinsi, B.; Zamboni, A.; Espen, L.; Zanzoni, S.; Santi, C.; Varanini, Z.; Pandolfini, T. Chemical characterization of a collagen-derived protein hydrolysate and biostimulant activity assessment of its peptidic components. J. Agric. Food Chem. 2022, 70, 11201–11211. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Kuang, Y.; Wang, N. Amino acids biostimulants and protein hydrolysates in agricultural sciences. Plants 2024, 13, 210. [Google Scholar] [CrossRef]
- Vaitkeviciene, R.; Burbulis, N.; Masiene, R.; Zvirdauskiene, R.; Jakstas, V.; Damasius, J.; Zadeike, D. Fermentation as a Promising Tool to Valorize Rice-Milling Waste into Bio-Products Active against Root-Rot-Associated Pathogens for Improved Horticultural Plant Growth. Fermentation 2022, 8, 716. [Google Scholar] [CrossRef]
- Ali, M.; Shi, L.; Khan, M.A.; Ali, A.; Hu, S.; Shen, J. Auxin biodynamics and its integral role in enhancing plant resilience to environmental cues. Physiol. Plant. 2025, 177, e70165. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, K.; Wang, G.; Tan, J.; Wei, F.; Ma, Y. Nitrogen addition regulates the growth of Pinus tabuliformis by changing distribution patterns of endogenous hormones in different organs. New For. 2023, 54, 853–865. [Google Scholar] [CrossRef]
- Pappalettere, L.; Bartolini, S.; Toffanin, A. Auxin-producing bacteria used as microbial biostimulants improve the growth of tomato (Solanum lycopersicum L.) seedlings in hydroponic systems. BioTech 2024, 13, 32. [Google Scholar] [CrossRef]
- Heidarzadeh, A. Role of amino acids in plant growth, development, and stress responses: A comprehensive review. Discov. Plants 2025, 2, 237. [Google Scholar] [CrossRef]
- Li, R.; Qin, M.; Yan, J.; Jia, T.; Sun, X.; Pan, J.; Li, W.; Liu, Z.; El-Sheikh, M.A.; Ahmad, P.; et al. Hormesis effect of cadmium on pakchoi growth: Unraveling the ROS-mediated IAA-sugar metabolism from multi-omics perspective. J. Hazard. Mater. 2025, 487, 137265. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Value |
|---|---|
| Moisture (%) | 77.99 ± 1.29 |
| Protein content (%) | 17.93 ± 0.13 |
| Ash content (%) | 0.88 ± 0.02 |
| Fat content (%) | 4.12 ± 0.27 |
| K2O (g/kg) | 18.27 ± 0.36 |
| P2O5 (g/kg) | 3.42 ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Zhong, B.; Zhang, Q.; Hu, X.; Xia, X.; Xie, H.; Wu, Z. Transforming Tilapia into Indoleacetic Acid-Containing Biostimulants: Synergistic Effect of Enzymolysis and Multi-Strain Fermentation. Fermentation 2025, 11, 525. https://doi.org/10.3390/fermentation11090525
Xie H, Zhong B, Zhang Q, Hu X, Xia X, Xie H, Wu Z. Transforming Tilapia into Indoleacetic Acid-Containing Biostimulants: Synergistic Effect of Enzymolysis and Multi-Strain Fermentation. Fermentation. 2025; 11(9):525. https://doi.org/10.3390/fermentation11090525
Chicago/Turabian StyleXie, Hanyi, Bin Zhong, Qimin Zhang, Xi Hu, Xuesen Xia, Hong Xie, and Zhenqiang Wu. 2025. "Transforming Tilapia into Indoleacetic Acid-Containing Biostimulants: Synergistic Effect of Enzymolysis and Multi-Strain Fermentation" Fermentation 11, no. 9: 525. https://doi.org/10.3390/fermentation11090525
APA StyleXie, H., Zhong, B., Zhang, Q., Hu, X., Xia, X., Xie, H., & Wu, Z. (2025). Transforming Tilapia into Indoleacetic Acid-Containing Biostimulants: Synergistic Effect of Enzymolysis and Multi-Strain Fermentation. Fermentation, 11(9), 525. https://doi.org/10.3390/fermentation11090525





