1. Introduction
The global shift towards sustainable and healthier diets has driven a marked increase in demand for plant-based proteins driven by environmental concerns, ethical considerations, and growing consumer awareness of health benefits [
1]. Within this context, legumes represent a key protein source, and yellow pea (
Pisum sativum L.) flour (YPF) has emerged as a particularly promising ingredient. Its advantages include a high protein content (typically 20–25%), a rich starch fraction, an inherent gluten-free status suitable for the growing population with celiac disease or gluten sensitivity, and a relatively balanced amino acid profile, especially when complemented with cereals [
2]. Moreover, peas possess beneficial agronomic traits like nitrogen fixation, enhancing their sustainability credentials [
3].
However, the development of high-quality gluten-free (GF) bread remains a significant technological challenge. The absence of gluten—a unique protein network responsible for dough elasticity, gas retention, and structural integrity in wheat bread—typically results in GF products with poor texture (dense, crumbly, or gummy), low volume, rapid staling, and, often, compromised sensory acceptance [
4]. Current GF formulations often rely on complex blends of starches, hydrocolloids, and protein isolates to mimic gluten functionality, which can increase cost, reduce nutritional density, and involve ingredients with higher environmental footprints or less consumer appeal [
5]. Therefore, there is a pressing need to identify and optimize nutrient-dense naturally gluten-free flours such as YPF that can provide inherent structure-building potential within simpler formulations.
Despite its potential, the broader application of YPF in GF baked goods faces significant functional limitations. Key drawbacks include its relatively low water-holding capacity (WHC) and poor water binding, both of which negatively impact dough rheology, handling, and final product texture and moisture [
6]; undesirable beany or grassy off-flavors associated with lipid oxidation and specific volatile compounds [
7]; and suboptimal baking performance, often manifesting as low volume, excessively dense crumb structure, and rapid staling. These limitations stem largely from the native structure of its macromolecules (proteins and starch), the presence of antinutritional factors (e.g., phytic acid, tannins), and the aforementioned off-flavors, all of which are particularly detrimental in the context of GF systems lacking the cohesive properties of gluten.
Fermentation has become, in recent years, a natural and cost-effective label-friendly bioprocessing technique to counteract these effects. Fermentation can be performed either in a solid state or by the submerged method, and both have been extensively applied in this context. In general, solid-state fermentation is considered an economically favorable method due to its lower environmental impact, smaller fermenter size, and reduced requirements for downstream processing and stirring [
8]. Microbial activity during fermentation can improve the substrate by modifying its structural components through enzymatic hydrolysis (e.g., proteases, amylases) and metabolic transformations.
In this context, legume-based sourdough systems have shown promising effects on dough and bread quality. Previous studies have demonstrated improvements in protein solubility, dough rheology, loaf volume, and crumb structure, as well as enhanced sensory acceptance and extended bread shelf life. These benefits are often accompanied by improvements in nutritional properties and reductions in antinutritional factors and undesirable flavors [
9,
10,
11]. In addition, extracellular polysaccharides produced by fermentative microorganisms serve as natural alternatives to conventional thickeners, improving the stability, structure, and viscosity of gluten-free bakery products. Altogether, these findings provide a solid background, emphasizing the potential of fermentation strategies to enhance both the technological properties and nutritional performance of GF bread. While lactic acid bacteria (LAB) fermentation is common, exploring diverse microbial consortia could unlock further benefits for complex matrices like legumes [
12].
Kefir, a complex symbiotic culture of LAB, yeasts, and acetic acid bacteria, represents a particularly promising and underexplored fermentative agent for enhancing YPF functionality in GF bread. Beyond its well-documented probiotic benefits, kefir’s diverse enzymatic portfolio (proteases, amylases, lipases, phytases) and multifaceted metabolic activities (acidification, gas production—primarily CO
2 from yeasts—and exopolysaccharides synthesis) have shown significant potential to synergistically improve the functionality, structure-building capacity, and nutritional quality of plant matrices [
13]. While the liquid fermentation of legumes has been studied, the impact of solid-state fermentation using kefir directly on yellow pea flour remains notably underexplored, especially concerning its detailed structural modifications (protein conformation, starch crystallinity, surface properties), rheological behavior (pasting properties), and ultimate techno-functional performance in complex GF bread applications. Understanding these specific modifications induced by kefir solid-state fermentation is essential to unlock YPF’s full potential as a high-quality, nutritious, and palatable base for GF bread. Accordingly, this study aimed to investigate the fermentation of YPF with kefir microorganisms and to evaluate its effects on the structural, techno-functional, and sensory properties of GF bread.
2. Materials and Methods
2.1. Materials
Yellow pea flour (YPF) was provided by “BYO Alimentos S.A” (Chabás, Argentine). Milk kefir grains were purchased from “Salvia Morada Almacén Natural” (Lanús, Argentine). Whole milk powder for kefir propagation was purchased from “La Serenisima” (General Rodríguez, Argentine). Trinitro-benzene-sulfonic acid (TNBS) and 8-anilino-1-naphthalenesulfonic acid (ANS) were purchased from Merck (Darmstadt, Germany). All other chemical reagents employed were of analytical grade. For breadmaking, rice flour (Dimax, Cordoba, Argentine), maize starch (Dimax, Cordoba, Argentina), cassava starch (Dimax, Cordoba, Argentine), carboxymethylcellulose (CMC, Drogueria San Juan, Rosario, Argentine), sugar (Arcor, Arroyito, Argentine), sunflower oil (AGD, General Deheza, Argentine), dry yeast Levex (Lesaffre Argentina, Buenos Aires, Argentine), and tap water were used.
2.2. Yellow Pea Flour Fermentation
To prepare the kefir inoculum, reconstituted milk containing 10% total solids was prepared from whole milk powder. A volume of 450 mL of this milk was inoculated with 50 g of kefir grains and incubated at 25 °C for 24 h under temperature-controlled conditions (TDC-A-30, Tecnodalvo, Santa Fe de la Vera Cruz, Argentine) [
14]. After fermentation, the kefir grains were recovered by filtration and the fermented milk (hereafter referred to as kefir) was used as the inoculum for flour fermentation. For flour fermentation, 8 g of YPF was mixed with 12 g of kefir. Under these conditions, the moisture content (M
n) of the fermentation powder—calculated as detailed in equation 1—was fixed at 60%:
where M
n represents the moisture content of the material (expressed in %), W
w is the wet weight of the sample, and W
d is the dry weight of the sample.
Fermentation was performed by mixing YPF with kefir in glass Petri dishes (90 mm diameter × 15 mm height) and homogenized using a glass rod. The dishes were covered, allowing for air exchange during incubation for 0–30 h at 25 °C in an incubator (TDC-A-30, Tecnodalvo, Argentine). Fermentation was stopped by rapid cooling, and samples were stored at −18 °C before analysis.
Dispersions of fermented flours (5 g sample in 20 mL distilled water) were prepared to determine pH by direct measurement using a pH meter (HI 2550, HANNA Instruments). Titratable acidity (TTA) was assessed by titrating the dispersions with 0.0971 M NaOH up to pH 8.2, and the results were expressed as the volume (in mL) of NaOH required to reach this pH [
15]. Free amino acid content was assessed through the trinitro-benzene-sulfonic acid (TNBS) method [
16]. Briefly, 50 μL of a 1%
w/
v aqueous dispersion of each sample was mixed with 400 μL of phosphate buffer (0.2125 M, pH 8.2) and 400 μL of TNBS. The mixtures were incubated at 50 °C for 1 h. Then, 800 μL of 0.1 N HCl was added, vortexed for 30 min, and centrifuged at 600 g for 10 min. Finally, the absorbance of the supernatant was measured at 340 nm using a PWL 3100-V spectrophotometer (Paralwall, Rosario, Argentine). Free amino acid content was expressed as mmol glycine equivalents per gram of flour based on a calibration curve prepared with glycine as standard.
2.3. Fermented Flour: Techno-Functional and Structural Characterization
The structural and techno-functional properties of the fermented YPF were evaluated under the selected fermentation conditions. This sample was frozen and freeze-dried prior to analysis and was referred to as fermented flour (FF). Controls included untreated YPF (YPF) and a mixture of YPF with kefir milk at a 1:1.5 mass ratio, homogenized, frozen, and freeze-dried without fermentation (UF). All freeze-dried samples were ground using a mortar and passed through a 297 µm mesh. The commercial YPF was passed through the same 297 µm mesh.
2.3.1. Bulk and Tapped Density
Bulk and tapped densities were determined by weighing a 10 mL graduated cylinder filled with the sample before and after compaction with a glass rod and were calculated as the ratio of mass to volume [
17].
2.3.2. Oil and Water Absorption Capacity
Samples (0.5 g) were mixed with 5 g of water or oil, vortexed (1 min), rested (30 min), and centrifuged (25 min, 1600×
g) [
18]. The pellet was weighed, and the absorption capacities were expressed as grams of water or oil absorbed per gram of flour.
2.3.3. Color Parameters
Samples placed in Petri dishes were photographed inside a box under uniform white lighting. The color parameters L* (lightness), a* (red-to-green hue), and b* (yellow-to-blue hue) were determined through digital image analysis using ImageJ 1.47v software and the ‘Color Space Converter’ plugin. The yellowness index (YI) was calculated according to the following Equation (2) [
19,
20].
2.3.4. Surface Hydrophobicity
Surface hydrophobicity (S
0) was assessed by the binding of the fluorescent probe 8-anilino-1-naphthalenesulfonic acid (ANS) [
21]. A 10%
w/
v sample suspension was added in successive 25 μL aliquots to 2.5 mL of 40 μM ANS (medium: 10 mM Tris buffer, pH 7.0). Fluorescence intensity (FI) was measured at 25 °C using a Jasco FP-770 spectrofluorometer (Hachiōji-shi, Japan), with excitation and emission wavelengths set at 380 and 484 nm, respectively. S
0 was calculated from the initial slope of the FI versus sample concentration plot.
2.3.5. ATR-FTIR
Infrared spectra were obtained using a Shimadzu Prestige 21 spectrophotometer (Shimadzu Corp., Kyoto, Japan) equipped with a GladiATR diamond ATR accessory (Pike Technologies, Madison, WI, USA). Twenty scans per sample were acquired over a wavenumber range from 4000 to 600 cm−1 with a resolution of 4 cm−1.
2.3.6. X Ray Diffraction
Diffractograms were acquired using a D2 Phaser diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) operating at 30 kV and 10 mA, equipped with an SSD160-2 silicon strip detector, a 1 mm divergence slit, a 2 mm air scatter blade, and a 2.5 mm Soller slit. Samples were measured at room temperature using polymethylmethacrylate holders, with a rotation speed of 15 rpm. Measurements were performed in the 2θ range of 5–45° with a step size of 0.01° and a counting time of 1 s per step using Cu Kα radiation (1.54060 Å) and a 0.5 mm Ni filter.
2.3.7. Viscosity Profile
Pasting properties were determined using a Rapid Visco Analyzer (RVA-4, Newport Scientific Pty. Ltd., Warriewood, Australia), following the general method provided by the equipment. Three grams of sample (dry basis) were transferred to a canister and approximately 25 mL of distilled water was added. To ensure homogeneous dispersion of the sample, the suspension was stirred at 160 rpm while heating to 50 °C, held at 50 °C for 1 min, and subsequently heated to 95 °C at a rate of 9.4 °C/min with continuous stirring at 960 rpm. The sample was held at 95 °C for 2.5 min and then cooled to 50 °C at a rate of 11.8 °C/min. From the pasting curves, the pasting temperature, peak viscosity, final viscosity, breakdown, and setback were obtained.
2.4. Design and Production of Baked Products
2.4.1. Preparation of Baked Products
Three types of bread were prepared following a traditional recipe: a control with untreated YPF (named as YPF-B), a second control with UF (named as UF-B), and an experimental bread made with FF (named as FF-B). For YPF-B, the following dry ingredients were weighed and mixed: 19.5 g of rice flour, 19.50 g of corn starch, 14.50 g of cassava starch, 13.50 g of YPF, and 1.35 g of sugar. CMC (1.35 g) was hydrated in 43.50 g of tap water and dispersed using a paddle mixer, followed by the addition of 0.9 g of sunflower oil. An amount of 20.25 g of reconstituted whole milk (10% w/v) was prepared. The dry and liquid ingredients were then combined, and 1.35 g of yeast was added. The same procedure was applied to prepare UF-B, replacing the reconstituted milk with 20.25 g of kefir. For FF-B, 33.75 g of FF were used in place of YPF and kefir. Each dough (130 g) was placed in pre-oiled individual tins, fermented at 26 °C until doubled in volume, and baked at 180 °C for 30 min in a Dalton electric oven. Loaves were cooled for 1 h before characterization.
2.4.2. Determination of the Mass, Volume, and Specific Volume of the Loaves
Post-baking mass was determined using an analytical balance (Ohaus, Parsippany, NJ, USA), and the percentage weight loss was calculated according to Equation (3):
where %WL = percentage weight loss, WBL = weight of the baked loaf, and WD = weight of the dough.
Loaf volume was measured using the grain displacement method [
14]. Specific volume was calculated as the ratio of volume to mass.
2.4.3. pH Determination
For pH determination, ground samples (2.5 g) were mixed in Falcon tubes with 25 mL of distilled water, vortexed for 3 min, and allowed to rest for 1 h, and the pH of the supernatant was measured using a digital pH meter (HANNA Instruments HI 2550, Woonsocket, RI, USA).
2.4.4. Color Evaluation
Color parameters (L*, a*, b*) were assessed on raw doughs and on 2 cm thick crumb slices photographed under homogeneous lighting. Previously described image analysis procedures were applied. YI was calculated as previously described in Equation (2). The total color difference (∆E) was calculated to compare the UF-B and FF-B samples with the YPF-B control according to Equation (4):
where ∆a*, ∆b*, and ∆L* correspond to the difference in the values of a*, b*, and L* between FF-B (or UF-B) and the control YPF-B [
22].
2.4.5. Sensory Analysis
Quantitative descriptive analysis (QDA) of the bread samples was conducted by an eleven-member trained panel of nonsmoking individuals (five males and six females). The analysis was performed following the guidelines of the International Organization for Standardization (ISO 1132:2012 and 8589:2007) [
23,
24]. This research was conducted within the framework of the project “Sensory evaluation applied to the research and development of new foods. Formation of a trained expert sensory panel”. UNR. ACRE 2023. Project 80020220600045UR, Resolution C.S. No. 596/2022. 01/2023-12/2026 (Director: Dr. Emilce Llopart). The privacy of the subjects was respected during the experiment, and informed consent was obtained from all participants. Panelists were selected and trained for the evaluation of baked products and were recruited from the Food Research, Development, and Evaluation Laboratory at the Faculty of Biochemical and Pharmaceutical Sciences, National University of Rosario. The sensory evaluation was conducted on individual cabinets under controlled conditions. Bread samples were served as 2 cm thick slices and coded with randomly assigned three-digit numbers. Evaluations focused on texture, aroma and taste, using a 10 cm long continuous scale [
25].
2.5. Statistical Analysis
All analyses were conducted in at least triplicate. The significance of the evaluated factors was determined using analysis of variance (ANOVA), with differences considered significant at p < 0.05. When significant differences were found, Tukey’s post hoc test was applied. The normality of the data was assessed using the Shapiro–Wilk test. Total color differences (ΔE) were compared using a t-test, considering differences significant at p < 0.05.
3. Results
3.1. Selection of Fermentation Conditions
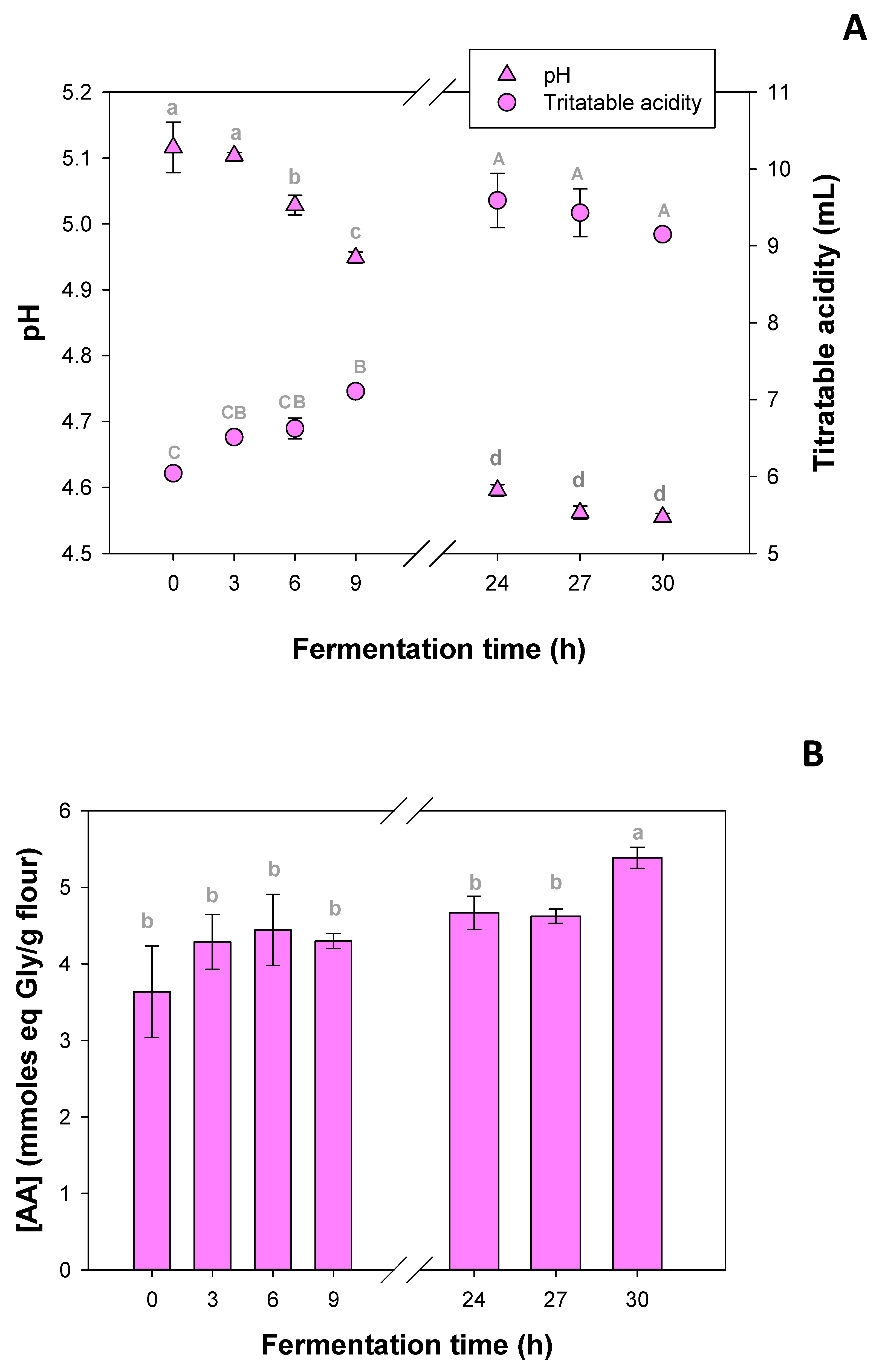
Figure 1A shows the changes in pH and titratable acidity during fermentation.
Significant differences in pH were observed after 6 h of fermentation compared to initial times. Additional differences were detected between 9 and 24 h, whereas no significant changes were found between 24, 27, and 30 h of incubation. The final pH value was 4.571 ± 0.008.
TTA significantly increased from 9 h onward, with a sharper rise at 24 h, remaining unchanged thereafter. After 30 h of fermentation, TTA reached 9.4 ± 0.2 mL of NaOH per 5 g of sample. The evolution of pH and TTA, which are key indicators of fermentation progress, reflects the activity of kefir microbiota and the resulting acidification.
As shown in
Figure 1B, a significant increase in the concentration of free amino acids was observed only after 30 h of fermentation. This rise may be attributed to the proteolytic activity associated with kefir microorganisms. Thus, this time was selected for further flour fermentation and characterization.
3.2. Characterization of the Fermented Flour
3.2.1. Bulk and Tapped Density
Bulk and tapped density are shown in
Table 1.
No significant differences were observed in the tapped density of FF compared to UF. However, both values were lower than that of the untreated YPF sample. These results suggest that the addition of kefir modified particle structures or their interactions, thereby influencing their behavior during compaction, while fermentation did not produce further relevant changes in packing capacity under pressure. By contrast, significant differences were observed in the bulk density among the three samples, with FF showing the lowest value, UF an intermediate value, and YPF the highest. These differences may be associated with the acidification of the medium caused by kefir and with the changes in macromolecular structure induced by fermentation.
3.2.2. Oil and Water Absorption Capacities
The water and oil absorption capacities of the samples were evaluated, and the results are presented in
Table 1.
Oil absorption capacity (OAC) did not differ significantly between FF and UF, although both exhibited significantly higher values than YPF. This behavior can be attributed to the higher protein content in FF and UF, derived from the addition of whole milk powder during kefir preparation. However, the similar OAC values of UF and FF suggest that the 30 h fermentation period did not induce further structural changes affecting lipid retention.
In terms of water-holding capacity (WHC), the highest value was observed in FF, followed by UF, while the lowest was recorded for YPF. The higher WHC observed in UF compared to YPF may be linked to its higher protein content, which increases the availability of polar groups capable of interacting with water molecules. As regards the enhanced WHC of FF, it can be attributed to the structural changes in macromolecules during fermentation.
3.2.3. Color Parameters
Color parameters are shown in
Table 1.
The YI of YPF increased with the addition of kefir milk, which could be attributed to interactions between the flour matrix and chromophoric compounds naturally present in the milk, such as riboflavin and carotenoids. These pigments, characterized by their yellow hues and present in whole milk powder used for the kefir inoculum, are prone to associating with protein- and starch-rich matrices like pea flour. On the other hand, fermentation led to a slight but significant decrease in the YI of FF compared to UF, although its value remained higher than that of YPF.
3.2.4. Surface Hydrophobicity
The S0 values obtained were 45 ± 1, 57.0 ± 0.9, and 59 ± 5 for YPF, UF, and FF, respectively. No significant differences were observed between FF and UF; however, both samples showed significantly higher values than YPF. The similarity between FF and UF suggests that the 30 h of fermentation did not markedly affect the exposure of hydrophobic residues on the protein surface. Therefore, the main effect on S0 could be attributed to the incorporation of kefir milk into the flour matrix.
3.2.5. X Ray Diffraction
Starch structural changes in YPF samples resulting from the fermentation process were assessed using X-ray diffraction analysis (
Figure 2).
As shown in
Figure 2, all samples exhibited diffraction patterns at 2θ angles around 15°, 17°, and 23°, which are characteristic of the C-type crystalline structure typically found in legume starches [
26]. Although the diffraction peak around 5.6° was not observed, the detected pattern was similar to that previously reported by Xu et al. [
27] for yellow pea starch.
After fermentation, no significant changes were observed in the position or intensity of the diffraction peaks. These results suggest that, under the experimental conditions employed, solid-state fermentation using kefir did not significantly affect the crystalline structure of starch in YPF samples.
3.2.6. ATR-FTIR
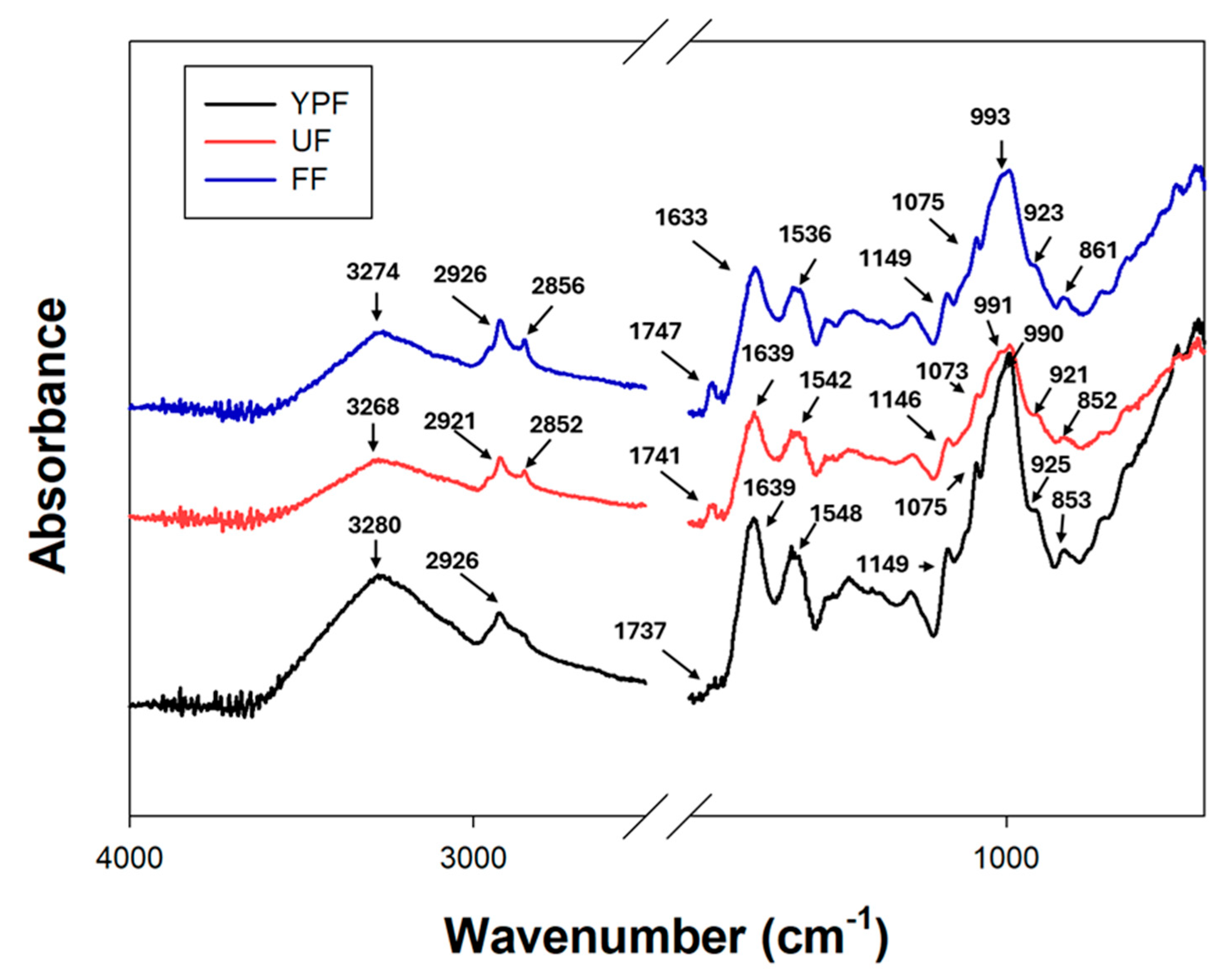
Figure 3 shows the ATR-FTIR spectra of YPF, UF, and FF samples.
The broad band observed in the 3000–3500 cm
−1 region, centered at 3280, 3274, and 3268 cm
−1 for YPF, FF, and UF, respectively, corresponds to –OH stretching vibrations, mainly associated with starch and intra-/intermolecular hydrogen bonding [
27,
28]. The band around 2930 cm
−1 (2926 cm
−1 for YPF and FF, 2921 cm
−1 for UF) is attributed to C–H stretching in glucopyranose rings [
28]. Bands near 2850 cm
−1, observed at 2856 cm
−1 (FF) and 2852 cm
−1 (UF), may result from the symmetric stretching of –CH
2– groups in lipid chains [
29]. The presence of these bands in the treated samples could be related to the addition of kefir milk, which contributes lipids to the matrix.
The absorption band near 1745 cm
−1 (1747, 1741, and 1737 cm
−1 for FF, UF, and YPF, respectively) corresponds to ester carbonyl stretching, characteristic of lipids, and the higher intensity observed in the treated samples may reflect the lipid contribution from kefir milk as well as effects of the fermentation process [
30].
Protein-related bands were also identified: amide I (1639 cm
−1 for YPF and UF, 1633 cm
−1 for FF), amide II (1548, 1542, and 1536 cm
−1 for YPF, UF, and FF, respectively), and amide III (1242 cm
−1 for YPF and UF, 1237 cm
−1 for FF) [
28,
31,
32]. In the fingerprint region (1200–900 cm
−1), bands around 1150, 1075, and 990 cm
−1 are primarily associated with C–O and C–O–C vibrations from starch glucose monomers. Additional peaks at approximately 920 and 850 cm
−1 are attributed to pyranose ring vibrations [
28].
The spectra did not reveal the presence of additional functional groups, suggesting that no major chemical modifications occurred during solid-state fermentation with kefir. Similar results have been reported in mung bean and rice flour fermented with some different
Lactobacillus strains [
28,
33].
3.2.7. Viscosity Profile
Table 1 summarizes the pasting parameters obtained from the RVA profiles of the studied flours.
During the initial heating phase, starch hydration was limited, and viscosity remained nearly constant until reaching the pasting temperature, after which it sharply increased. The pasting temperature did not differ significantly between YPF and FF, although both were higher than that of UF. As heating progressed, viscosity continued to increase, reaching a significantly higher peak value for FF compared to the control samples. Regarding breakdown viscosity, it significantly differed among the samples: it was lowest for YPF, intermediate for UF, and highest for FF. This parameter reflects paste stability and the results suggest greater mechanical susceptibility of UF and FF. Upon cooling, setback values were significantly lower in UF and FF compared to YPF, while no significant differences were observed in final viscosity between YPF and FF, although UF showed a lower value. These results suggest that fermentation did not impair the ability of YPF to form a viscous paste or gel after heating and cooling.
3.3. Bread Characterization
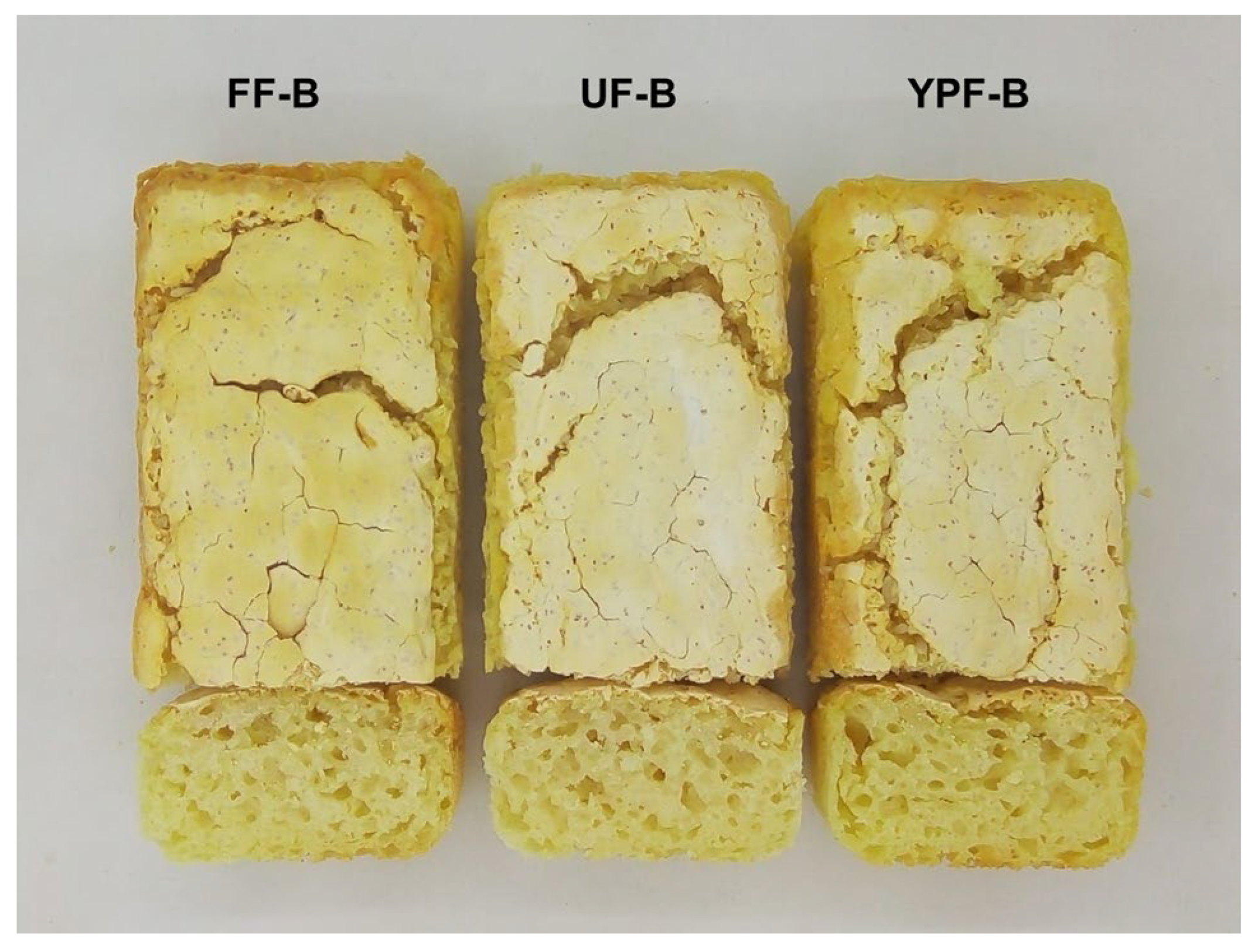
Figure 4 shows photographs of the formulated bread.
Table 2 shows the physicochemical and technological characteristics of the gluten-free breads.
Bread weight did not vary significantly among samples. YPF-B exhibited the highest baking loss, whereas UF-B and FF-B showed similar values. Regarding volume and specific volume, FF-B showed the highest values, followed by UF-B and YPF-B. In terms of pH, YPF-B presented values close to 6, while significantly lower pH values were observed in UF-B and FF-B. When analyzing the color parameters, no significant differences were observed among the raw doughs; therefore, a single mean is reported for each parameter, representing the average of the three samples: L*, a*, and b* values were 81.3 ± 0.3, 0.7 ± 0.3, and 6.6 ± 0.2, respectively. However, differences emerged after baking. FF-B showed a significantly lower L* value compared to UF-B and YPF-B, which did not differ significantly. All samples had small negative a* values, indicating a slight green hue. However, FF-B presented the highest value, followed by UF-B and YPF-B. For b*, all breads showed positive values, with FF-B being significantly higher than the others. The YI followed the same trend. The total color difference was calculated to compare the perceived visual differences between the experimental samples (UF-B and FF-B) and the control YPF-B. The ∆E for UF-B should be considered as ’different’ to the control, whereas a significantly higher ∆E value was observed for FF-B, which accounts for ‘significantly different’ samples.
3.4. Sensory Analysis
Figure 5 shows the spider plot corresponding to the QDA results for the attributes evaluated in the three bread samples.
For aroma, the pea-like odor was significantly lower in FF-B compared to UF-B and YPF-B, with no differences between the control breads. The acidic odor was significantly lower in YPF-B, while UF-B and FF-B exhibited similar values. For taste, FF-B showed a significantly lower pea-like taste than the control breads. As for acidic taste, a significantly higher value was observed in FF-B, with no differences between UF-B and YPF-B. Pea-like aftertaste did not differ between FF-B and UF-B, although both had lower values than YPF-B. When analyzing texture, no significant differences were found in crumb hardness. However, chewiness was significantly higher in FF-B.
4. Discussion
When analyzing the fermentation conditions, the observed pH reduction during fermentation was consistent with previous reports, in which pH values of 4.75 and 4.40 were obtained after 24 h of spontaneous fermentation of yellow pea flour at 30 and 37 °C, respectively [
34]. A significantly lower pH value was reported by Torino et al. [
8] after 96 h of liquid fermentation of lentils with
L. plantarum. Regarding TTA, values between 20.4 and 27.0 mL have been reported for 10 g of sourdoughs prepared with legume flours—including lentil, pea, bean, and chickpea—fermented with lactic acid bacteria at 30 °C for 24 h [
35]. When expressed as mL NaOH per gram of fermented product, these values are comparable to those obtained in the present study. In addition to reducing pH and markedly increasing TTA, fermentation for 30 h resulted in a higher content of free amino acids; therefore, this condition was selected for the subsequent characterization of the FF.
Both the presence of kefir and the fermentation process contributed to the significant differences observed among the samples, and the effects underlying both factors should be further analyzed. Milk kefir used in this study may have contributed to an increased protein content in UF and FF samples, thus providing additional hydrophilic and hydrophobic sites compared to YPF (also observed in terms of a greater surface hydrophobicity), and with kefiran, an exopolysaccharide secreted by kefir grains, with a high capacity to interact with water. Altogether, this led to an enhanced WHC and OAC in UF and FF when compared to YPF. Moreover, milk kefir also provides dairy-derived chromophoric compounds such as riboflavin and carotenoids, which enhance the YI of the untreated YPF.
When analyzing the fermentation process, no significant increase was observed in the exposure of hydrophobic protein groups, consistent with the similar OAC measured in UF and FF. However, other authors have reported different results. For instance, Çabuk et al. [
36] reported a significant increase in OAC and S
0 values in pea-protein-enriched flour following fermentation with
Lactobacillus plantarum for 9 h. Similarly, Emkani et al. [
37] suggested that fermentation of different cereals and legumes with lactic acid bacteria induces significant structural modifications in proteins, such as partial unfolding and exposure of hydrophobic residues, directly enhancing both S
0 and oil retention capacity. Interesting findings were reported by Batbayar et al. [
38] when studying the solid-state and submerged fermentations of pea-protein-enriched flour with some bacterial and fungal strains: S
0 decreased under all fermentation conditions; even both WHC and OAC increased after fermentation. These authors suggested that a higher ratio of hydrophilic protein moieties was liberated as a result of protein hydrolysis and that the exposure of previously buried hydrophobic sites can lead to aggregation and the formation of micro-capillaries that could physically entrap oil bodies and retain the oil through hydrophobic interactions. The highest increase was reported for OAC under submerged fermentation conditions. In the present work, fermentation did not additionally modify the binding of oil.
However, further modifications in the techno-functional and color properties of FF should be evaluated in light of the biochemical changes resulting from enzymatic activity, which is enhanced under acidic conditions. Regarding proteolytic enzymes, besides increasing the content of free amino acids, they likely disrupted protein–protein interactions within the native protein–starch matrix of YPF, weakening its dense and cohesive structure and exposing hydrophilic groups able to interact with water [
37,
39]. Since no significant changes were detected in either the short-range molecular order (FTIR) or the long-range crystalline structure (X-ray diffraction), starch is assumed to maintain its structural stability, even though some biochemical modifications could have been introduced by fermentation. For instance, microbial amylases may have partially hydrolyzed starch granules, while yeasts and heterofermentative bacteria present in kefir could have generated gas, forming voids within the matrix. These factors together may explain the lower bulk density of FF. Similar results have been reported for red bean and cowpea flours, attributed to comparable macromolecular modifications and increased matrix porosity. For instance, Xiao et al. reported a decrease in the bulk density of fermented red beans [
40]. Similarly, Chawla et al. observed a significant decrease in the bulk density of cowpea flour during fermentation with
Aspergillus oryzae at 30 °C over a period of 0 to 96 h [
41].
Moreover, these changes together with the possible contribution of exopolysaccharides production by LAB during fermentation may have enhanced WHC and the resulting pasting behavior of the FF: the greater WHC may have facilitated starch granule swelling, explaining the higher peak viscosity compared with YPF. In contrast, the marked reduction in setback (64% lower in FF than in YPF) and final viscosity indicates a reduced tendency toward retrogradation. These results are consistent with previous studies on lactic acid-treated legume flours. For instance, Sozer et al. [
42] described a substantial reduction in setback in faba bean flour, which was linked to pH-induced starch hydrolysis. These authors explained that acid-mediated cleavage of glycosidic bonds can compromise the molecular reassociation of starch chains during cooling, thereby attenuating retrogradation [
42]. Despite differences observed in FF compared to UF and YPF, the profile analysis indicated that its ability to form pastes or gels after thermal processing was preserved. This aspect is essential for its application in baked products.
Enzymatic activity also affected the color parameters of the flour. Microorganisms are known to produce lipases, peroxidases, and oxidoreductases, which have been linked to the degradation of phenolic compounds such as flavonoids, which are major contributors to the characteristic yellow coloration of legume-based matrices. Similar reductions in YI after fermentation have been reported in the literature [
15,
41], suggesting that microbial metabolism can modulate the pigment profile of plant-based substrates. Thus, the balance between pigment incorporation from milk and pigment degradation during fermentation likely defines the final color parameters of the FF.
To assess the technological functionality of the flours, GF breads were formulated and analyzed. The YPF-B formulation exhibited a pH close to 6, classifying it as a slightly acidic food product according to the pH range. In the UF-B, the presence of kefir led to a pH reduction, which may be mainly attributed to the metabolic activity of the microorganisms present in the kefir grains and their possible contribution to dough fermentation. In the FF-B, the production of acidic compounds is expected to be greater due to the flour fermentation pretreatment and the potential generation of low-molecular-weight fermentable compounds. As a result, a product with a pH value near 5 was obtained, slightly lower than those observed in other previously published works [
43,
44] but higher than other sourdough breads [
45].
The presence of kefir, through the contribution of kefiran, enhanced water retention in the breads and prevented the coalescence of cells in the dough, resulting in lower baking loss in UF-B and FF-B compared to YPF-B [
14,
46]. Additional effects were attributed to the flour fermentation process, which may have enhanced CO
2 production and promoted the formation of low-molecular-weight compounds, further contributing to leavening and resulting in an increased specific volume [
47,
48]. Although the specific volume remained lower than that of wheat-based breads, the values are consistent with those reported for gluten-free breads [
49,
50]. This increased volume may have influenced color parameters: the lower L* value likely reflects the greater incorporation of air bubbles and the higher specific volume observed in FF-B [
51]. In addition, the significant increase in b* indicates that fermentation intensified crumb yellowness, and the combined changes in both parameters account for the trend observed in YI measurements. When comparing raw dough and baked products, the observed color differences appear to result primarily from baking-induced reactions, such as Maillard reactions and sugar caramelization, which contribute to the sensory attributes of baked products [
52]. Moreover, the combination of a reduced pH, higher protein content, and a likely greater proportion of low-molecular-weight carbohydrates in FF-B may have further promoted Maillard reaction development, as previously reported [
53].
The breads developed in the present study were characterized by a more pronounced acidic taste and a reduced pea-like aftertaste compared to the YPF-B. Regarding texture, although fermentation increased the specific volume of the breads relative to both control samples, this did not translate into a perceptible reduction in crumb hardness, although higher chewiness was detected in FF-B. The fermentation of YPF significantly improved certain sensory attributes of the gluten-free breads. The reduction in pea-like odor and taste can be attributed to microbial fermentation, which may markedly modify the volatile profile of legumes by reducing compounds such as aldehydes, ketones, and furans [
54,
55]. This is generally considered beneficial, as it contributes to the improved sensory acceptability of legume-based baked products [
56]. Indeed, Jeradechachai and Hall [
57] previously emphasized the importance of effective de-flavoring treatments when analyzing the results they obtained.
5. Conclusions
The present study demonstrated that the fermentation of yellow pea flour with kefir for 30 h effectively modified its physicochemical and techno-functional properties and emerged as a promising strategy for improving the quality of gluten-free baked products.
Fermentation induced notable structural modifications at the macromolecular level evidenced by reduced bulk density and increased water and oil absorption capacities. These changes contributed to enhanced water-holding capacity and pasting behavior.
In GF baking applications, FF yielded breads with higher specific volume (vs. YPF control), lower baking loss, and intensified crumb yellowness (increasing b* and YI), potentiated by acid-mediated Maillard reactions. Sensorially, FF-B exhibited significantly reduced “pea-like” flavor/odor and aftertaste alongside balanced acidic notes, overcoming key organoleptic barriers in legume-based matrices. Although crumb hardness remained unchanged, increased chewiness and volume reflect textural improvements linked to synergistic kefir-driven mechanisms: yeast-generated CO2 for leavening, kefiran-mediated gas cell stabilization, and optimized flour rheology. These findings position kefir fermentation as a viable clean-label tool to enhance YPF’s technological functionality and sensory appeal in GF bread, reducing dependency on synthetic additives. The fermentation of yellow pea flour, a nutrient-dense, naturally gluten-free, and agronomically sustainable legume, reduces the need for complex and resource-intensive additives typically used in gluten-free baking. In addition to these techno-functional and sensory improvements, this approach contributes to broader sustainability goals by promoting plant-based proteins, improving resource efficiency, and supporting the development of sustainable bakery products. Future studies should further investigate the nutritional profile of fermented YPF, fine-tune sensory attributes, and evaluate shelf-life extension mediated by organic acids.