Abstract
Microbial enzymes, due to their efficiency, specificity, and sustainability, are central to innovative biotechnological strategies aimed at optimizing industrial processes such as winemaking. In this study, the potential of Aureobasidium pullulans m11-2, a native dimorphic fungus from the wine ecosystem, was evaluated as a source of hydrolytic enzymes capable of degrading grape cell wall polysaccharides. The strain was identified at the molecular level and characterised in terms of its morphology. To maximise enzyme production, various culture media were tested. Among the concentrations tested, the optimal levels of glucose and pectin were 1 g L−1 and 10 g L−1, respectively. The partially constitutive and inducible nature of the various polysaccharidase activities (pectinases, cellulases, and xylanases) was confirmed. The effect of grape skins (a winemaking by-product) on microbial growth and enzyme synthesis was evaluated, achieving a pectinase activity of 0.622 U mL−1 when combined with 1 g L−1 of glucose. Maximum enzyme yields were detected during the exponential growth phase in both citrus pectin and grape skin media, suggesting favorable conditions for continuous bioprocessing. These results confirm that A. pullulans m11-2 is an interesting microbial option for producing polysaccharidases that can be adapted to sustainable production systems.
1. Introduction
In recent decades, biotechnology has emerged as a strategic discipline for the economic development of many countries, driven by its applicability across various industries. Within this field, enzymes stand out as “eco-friendly” biocatalysts, widely used in industrial processes, including fruit juice production and winemaking [1]. In the latter, the biochemical conversion of grape must into wine occurs through the action of various enzymes, both intracellular and extracellular, which are present or produced by the yeast-like (dimorphic) microbiota.
The cell wall of the grape berry is composed of a complex matrix of cellulose, hemicelluloses, pectins, and proteins. Pectin acts as a cementing agent among the other structural polymers, and its complete degradation therefore requires the combined action of various enzymes. In this context, oenological preparations consist of mixtures of pectinases (polygalacturonase, pectinesterase, pectin lyase), complemented by other auxiliary activities such as cellulases, hemicellulases, and acid proteases [2,3].
Pectinases constitute a heterogeneous group of enzymes that catalyze the hydrolysis of pectin, a structural polysaccharide of the plant cell wall, primarily by cleaving the α-1,4-glycosidic bond of galacturonic acid [4]. Along with cellulases and xylanases, they enhance juice extraction and yield, as well as clarification and filterability. Moreover, they contribute to the release of color and aroma compounds trapped in the grape skin, thereby improving the wine’s bouquet and facilitating the liberation of phenolic compounds, ultimately enhancing both the sensory and technological properties of the final product [5,6,7,8,9,10].
The production of enzymes from microorganisms has become a research priority due to its rapid growth and broad diversification, in addition to its technical and commercial viability [11,12]. Currently, the food industry is primarily supplied with enzymes of fungal origin, mainly produced by species such as Aspergillus niger and Trichoderma sp., which are recognized as GRAS (Generally Recognized As Safe) and approved by the International Organisation of Vine and Wine (OIV) for enological applications [13]. While the filamentous fungi produce useful enzymes, they present challenges such as lower compatibility with the wine environment (pH, ethanol, SO2, anaerobiosis), and more complex processes for their control and elimination in the winery. Additionally, some filamentous fungi have risk of producing undesirable metabolites, such as mycotoxins, which can affect the quality or safety of wine.
In recent years, however, there has been growing interest in the use of non-Saccharomyces yeasts to improve the quality and biotechnological properties of wine, as they are increasingly considered adjunct starter cultures in mixed fermentations with Saccharomyces cerevisiae, where they can positively impact aroma, color, and overall sensory complexity [14]. These wine yeasts produce extracellular enzymes during the early stages of fermentation, contributing to color development and enhancing the aromatic and sensory complexity of the final product [6,8,15]. Unlike filamentous fungi, yeasts and dimorphic microorganisms generally exhibit greater technological versatility, produce enzymes more rapidly, and are less prone to secrete undesirable activities [16,17]. Thus, their enzymatic activities can be explored for the development of novel enzyme products or as starter cultures that produce exoenzymes for winemaking, offering a promising alternative to fungal pectinases [18].
In this context, Aureobasidium pullulans is a predominant species found on the surface of grapes, in fresh must, and during the very early stages of fermentation [19,20,21,22,23]. This dimorphic euascomycete fungus is notable for its ability to produce a wide range of enzymes, including pectinases, cellulases, proteases, lipases, xylanases, laccases, and amylases, among others, which significantly influence wine quality [6,24,25]. As has been described for other non-Saccharomyces yeasts, these extracellular enzymes can persist in the must even after the microorganisms themselves disappear, continuing to contribute to aroma, color, and clarity, which makes them a valuable biotechnological tool for the wine industry [14]. Although its enzymatic versatility has been the focus of recent studies, available information on the impact of this unconventional species on fermentation and wine quality remains limited. Nevertheless, previous studies from our research group have shown promising results when using this species in controlled vinifications [7,8,24,26].
The bioprospecting of native strains and the optimization of their cultivation conditions are essential to harness their biotechnological potential. Furthermore, using agro-industrial residues, such as grape pomace, is a cost-effective and sustainable way of inducing enzymatic synthesis, offering additional benefits in terms of waste reduction and the local valorization of resources [11,12]. The winemaking industry in regions such as Mendoza (Argentina) generates a substantial amount of grape skins as a primary by-product. These grape skins are particularly rich in valuable constituents, including structural polysaccharides (e.g., cellulose and pectins) and a diverse array of bioactive phenolic compounds. Although some of these compounds are extracted during winemaking, the grape skins retain considerable levels of these beneficial components, making them a promising and readily available substrate for various biotechnological applications [27,28].
This study aimed to characterise the A. pullulans m11-2 native strain at molecular and morphological levels. It also aimed to systematically evaluate the effect of various nutrient formulations—including combinations of grape skins with other carbon and nitrogen sources—on polysaccharidase enzyme production. The results are significant because they take an integrative approach, combining a native strain with targeted enzymatic profiling and the exploration of low-cost, enology-relevant media, in order to assess the strain’s potential as an innovative, sustainable source of polysaccharidase enzymes for use in winemaking.
2. Materials and Methods
2.1. Microorganisms and Culture Media
The A. pullulans m11-2 strain was previously isolated and selected by our research group from the surface of Malbec grape berries harvested in the San Rafael winegrowing region of Mendoza, Argentina [6], belonging to the “San Rafael Biodiversity” Culture Collection of the SCCM-AAM (Argentine Society for Microbiology, Buenos Aires, Argentina). The microorganism was cultivated in liquid YPD medium (g L−1: yeast extract, 10; peptone, 20; glucose, 20; pH 4.5) and on solid YPD medium when required (supplemented with 20 g L−1 agar-agar). Cultures were incubated in a thermostatic shaking bath (SHZ-88, Jiangsu Jinyi Instrument Technology Co. Ltd., Jintan, Changzhou, China) at 28 °C for 48–72 h at 100 rpm. Cells were harvested by centrifugation (3000× g, 20 min), washed, and resuspended in a 2.4% sodium glutamate protective solution. The cell suspensions were subsequently lyophilized using a Rificor S.H. freeze dryer (L-A-E50-CRT, Rificor, Buenos Aires, Argentina) for preservation. Lyophilized samples were stored at 4 °C until use.
2.2. Molecular Identification Through 26S rDNA Sequencing
2.2.1. Genomic DNA Extraction
The m11-2 strain was subjected to molecular identification using the protocol described by Querol et al. [29]. First, the strain was inoculated into YPD broth and incubated at 28 °C for 48–72 h. Cells were collected by centrifugation at 3500 rpm for 5 min, washed with 1 mL of sterile water, and resuspended in 500 µL of Solution 1 (0.9 mol L−1 sorbitol, 0.1 mol L−1 EDTA). Subsequently, 30 µL of a zymolyase solution (25 mg mL−1) and 30 µL of glucanase (25 mg mL−1) were added, the suspension was homogenized and incubated at 37 °C for 1 h. The mixture was then centrifuged at 7000 rpm for 10 min, and the supernatant was discarded. The pellet was resuspended in 500 µL of Solution 2 (50 mmol L−1 Tris-HCl, 20 mmol L−1 EDTA), followed by the addition of 13 µL of 10% SDS. The mixture was incubated at 65 °C for 5 min. Afterward, 200 µL of potassium acetate (5 mol L−1/3 mol L−1) was added, mixed by inversion, and placed on ice for 10 min. It was then centrifuged at 4 °C for 15 min, and the supernatants were transferred to clean microtubes. Genomic DNA was precipitated by adding isopropanol in a 1:1 ratio, mixed by inversion, incubated at −20 °C for 30–60 min, and centrifuged at 12,000 rpm for 10 min at 4 °C. The resulting DNA pellet was washed twice with 70% ethanol, air-dried at room temperature, and resuspended in sterile Milli-Q water. To eliminate RNA, 1 µL of RNase (2 mg mL−1) was added to each tube and incubated at 37 °C for 1 h.
2.2.2. PCR Amplification and Sequence Analysis
A fragment of approximately 500–600 nucleotides corresponding to the 5′ end of the gene encoding the 26S rRNA (large subunit), including the D1 and D2 domains, was amplified by polymerase chain reaction (PCR). The primers used were NL1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′), as previously described by Kurtzman and Robnett [30]. Amplification was performed in a TC-312 thermocycler (TECHNE). The reaction mixture was prepared in a final volume of 50 µL and contained: 1 µL of template DNA (12–60 ng µL−1), 5 µL of 10× PCR buffer, 3 µL of MgCl2 (50 mmol L−1), 4 µL of dNTPs (1 mmol L−1), 1 µL of each primer (10 µmol L−1), 0.25 µL of Taq DNA polymerase (5 U µL−1), and 34.75 µL of Milli-Q water. The thermal profile included an initial denaturation step at 95 °C for 5 min, followed by 35 cycles of 94 °C for 1 min, 52 °C for 1 min, and 72 °C for 2 min, and a final extension at 72 °C for 10 min.
The obtained sequence was edited using Chromas version 2.6.6, and sequence similarity was assessed by comparison with publicly available sequences using the Basic Local Alignment Search Tool (BLAST) provided by GenBank, available at the NCBI website (http://www.blast.ncbi.nlm.nih.gov/Blast.cgi, accesed on 15 May 2025). The 26S rRNA gene partial sequence was submitted to the GenBank database under the accession number: PV659872 (Aureobasidium pullulans). The identification was corroborated by high sequence similarity with reference strains of A. pullulans, including clone 71_JS_MRS0d1_11 (GenBank accession no. OQ067170), CBS 584.75 (NCBI accession no. NG_055734), isolate yuk02 (GenBank accession no. MW248427), strain G435 (GenBank accession no. OR334863), and strain GM-R-22 (GenBank accession no. JN572106), as well as with related species such as A. subglaciale culture CBS:123387 (GenBank accession no. MH874818) and A. melanogenum CBS 105.22 (NCBI accession no. NG_056960).
2.3. Morphological Characterization
Morphological characterization of the strain under study was performed using cultures grown on YPD agar medium at 28 °C after 3 to 7 days of incubation. For macroscopic analysis, colony characteristics such as shape, size, margin, texture, color, and pigment production were evaluated. Microscopic observation was conducted through simple wet mount preparations: a portion of the colony was collected with a sterile loop and placed on a microscope slide with a drop of sterile water, then covered with a coverslip. Observations were made using a light microscope at 40 ×and 100× magnifications to examine cell morphology and the formation of reproductive structures in both yeast-like (dimorphic) and mycelial phases.
2.4. Preliminary Test—Growth Kinetics and Enzyme Production
To investigate the growth kinetics and enzyme production of strain m11-2, the organism was inoculated into a medium based on citrus pectin, following the protocol proposed by Longhi et al. [6]. This medium consisted of a 50 mmol L−1 citrate-citric acid buffer (pH 3.8) with the following composition (g L−1): calcium chloride, 0.05; monobasic potassium phosphate, 0.2; manganese sulfate, 0.05; ammonium sulfate, 1; citrus pectin, 1; and glucose, 1. Incubation was carried out at 28 °C for 8 days under agitation (110 rpm). Samples were collected at regular time intervals. Cell-free supernatants (crude extracts) were obtained by centrifugation of the samples (10,000× g, 10 min, 4 °C) and were stored immediately in a freezer at −20 °C until enzymatic activity analysis.
Cell viability was assessed using the serial dilution method and colony-forming unit (CFU mL−1) counts on Petri dishes. Optical density (OD) was measured at 600 nm using a spectrophotometer (Metrolab 330, Metrolab S.A., Buenos Aires, Argentina). The maximum specific growth rate (µ) was estimated from the exponential phase of the growth curve by fitting an exponential model to OD600 data as a function of time.
2.5. Determination of Enzymatic Activities
2.5.1. Qualitative Detection of Pectinolytic Activity
The enzymatic ability to hydrolyze pectin was assessed using the halo clarification method, as described by Merín et al. [19]. A volume of 20 µL of enzyme extracts was inoculated into wells (5 mm in diameter) on pectin-agar medium (g L−1: calcium chloride, 0.05; potassium phosphate, 0.2; magnesium sulfate, 0.8; ammonium sulfate, 1; manganese sulfate, 0.05; yeast extract, 1; citrus pectin, 2; agar, 15; pH 4.5), and incubated at 28 °C for 48–72 h. Clarification halos were visualized by the addition of Lugol’s iodine solution to the plates, which allowed for the identification of zones of enzymatic degradation.
2.5.2. Quantitative Determination of Enzymatic Activities
Pectinolytic activity in the enzyme extracts was determined by measuring the amount of reducing sugars released from a 0.25% (w v−1) dispersion of citrus pectin in 50 mmol L−1 citrate-citric acid buffer (pH 3.8) at 28 °C, following the DNS method [31]. One unit of pectinolytic activity (U) was defined as the amount of enzyme that releases 1 µmol of reducing sugars per minute under the assay conditions.
The conditions for determining cellulase and xylanase activities were the same as those described for pectinolytic activity, but using 0.25% (w v−1) carboxymethyl cellulose and 0.25% (w v−1) birchwood xylan, respectively, dispersed in 50 mmol L−1 citrate-citric acid buffer (pH 3.8). One unit of cellulase or xylanase activity was defined as the amount of enzyme required to produce 1 µmol of reducing sugars (glucose or xylose) per minute under the assay conditions.
Additionally, total protein content was determined using the Bradford method [32], with the reagent prepared according to the original protocol. Bovine serum albumin was used to construct a standard curve, and a blank was included for each batch of samples. Specific activity was then calculated accordingly.
All enzymatic determinations were performed in triplicate (biological and analytical replicates).
2.6. Evaluation of the Culture Medium for Enzyme Production
2.6.1. First Phase
In light of the preliminary study, an initial experiment was conducted to evaluate the effect of different concentrations of carbon sources (1, 10, and 20 g L−1 of glucose and 1, 5, and 10 g L−1 of citrus pectin). A basal medium was used with the following composition (g L−1): calcium chloride, 0.05; monobasic potassium phosphate, 0.2; manganese sulfate, 0.05; ammonium sulfate, 1, according to Longhi et al. [6]. A full factorial design (3 × 3) was employed, with glucose concentration and pectin concentration as the factors and polysaccharidase activities as the responses. This design comprised nine different medium formulations, corresponding to all possible combinations of glucose (1, 10, and 20 g L−1) and pectin (1, 5, and 10 g L−1) levels. Each treatment was carried out in triplicate, resulting in a total of 27 experimental units (3 glucose concentrations × 3 pectin concentrations × 3 replicates).
Cultures were incubated under agitation at 110 rpm and 28 °C for 72 h. Cells were removed by centrifugation (10,000× g, 10 min, 4 °C), and the resulting cell-free supernatants were used as the enzyme extracts under study. Extracts derived from media with high glucose concentrations (initial formulations containing 10 or 20 g L−1) were subjected to dialysis using tubing with a molecular weight cut-off (MWCO) of 14 kDa. For this purpose, the cell-free supernatants were placed into dialysis tubing and immersed in 50 mmol L−1 citrate-citric acid buffer (pH 4.5) containing 0.1 mol L−1 NaCl. Samples were kept under constant agitation at 4 °C for 24 h, with buffer replacement every 12 h.
2.6.2. Second Phase
In view of the results obtained, a second experiment was conducted to determine whether grape skins and/or yeast extract significantly influenced enzyme synthesis. For this purpose, the concentrations of glucose and pectin were fixed at 1 and 10 g L−1, respectively. The basal medium composition was the same as described in the previous section. The new formulations are detailed in Table 1. This comparative design aimed to evaluate the effect of grape-derived substrates and nutrients on growth and enzyme production under controlled conditions.

Table 1.
Culture media composition for the second phase of the study. G: 1 g L−1 glucose; P: 10 g L−1 pectin; GS: 15 g L−1 grape skins; YE: 1 g L−1 yeast extract.
The grape skins used in this study were obtained from Malbec grapes harvested during the 2023 vintage in the wine-producing region of San Rafael, Mendoza, Argentina. The skins were manually separated from the berries, washed with water, lyophilized, and used directly without any additional chemical pretreatment.
Cultures were incubated under agitation at 110 rpm and 28 °C for 7 days. Samples were collected at regular intervals to monitor cell growth by measuring optical density at 600 nm. All measurements were corrected using a blank consisting of the same medium without inoculation. Cell-free supernatants were obtained by centrifugation at 12,000× g for 10 min at 4 °C and used as crude extracts for enzymatic activity quantification.
2.7. Statistical Analysis
All experimental formulations, as well as microbiological and analytical determinations, were performed in triplicate (biological and analytical replicates). Experimental data are presented as mean values ± standard deviation (SD). All data were checked for normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test). Since assumptions were met, no data transformation was required. To assess the significance of differences among treatments, a one-way analysis of variance (ANOVA) was applied, followed by Fisher’s Least Significant Difference (LSD) multiple comparison test, with a significance level of α = 0.05. Statistical procedures were performed using InfoStat software (version 2022) and R (v. x64 4.1.1). Additionally, R was used to estimate the curvature of the evaluated variables, and response surface and contour plots were generated using Python (version 3.12).
3. Results and Discussion
3.1. Characterization of the A. pullulans m11-2 Strain
3.1.1. Molecular Identification by 26S rRNA Gene Sequencing
To confirm the identity of the strain under study, sequencing of the conserved 26S rRNA gene region was performed. Molecular identification based on comparative homology analysis of the amplified sequences using the BLAST algorithm (Basic Local Alignment Search Tool) revealed that strain m11-2 was conspecific with Aureobasidium pullulans, exhibiting 97% similarity with reference strain sequences of the same region available in public databases. This analysis corroborated the identity previously assigned through the PCR-RFLP method, as reported by Longhi et al. [24].
3.1.2. Morphological Characterization
Aureobasidium pullulans is a dimorphic euascomycete fungus. Despite having yeast phases of growth, it is multicellular and an excellent producer of lytic enzymes. Given the trophic and ecological roles of these fungi in degrading organic matter, they release enzymes for primary degradation into the environment, and during later stages, they provide yeasts that complete the degradation process. Macroscopically, as shown in Figure 1, colonies grown on YPD medium at 28 °C for 7 days appeared circular in shape, reaching diameters of 2–5 mm. They exhibited irregular margins formed by superficial radial hyphae, lacked aerial mycelium, and presented a smooth, viscous surface due to abundant sporulation. Colonies displayed a pinkish-yellow to brown coloration, which intensified over time, with dark brown to black areas appearing during prolonged incubation or storage, as observed in colonies after 7, 30 and 60 days (Figure 1A–C). Microscopically, both yeast-like (dimorphic) and mycelial morphologies were observed. In the yeast phase (Figure 1D), pyriform conidia reproducing via budding were evident. In the filamentous phase (Figure 1E), reproductive hyphae bearing intercalary or terminal conidiophores with endoconidia were observed. The dimorphic nature of the strain, exhibiting both morphologies simultaneously, is shown in Figure 1F.
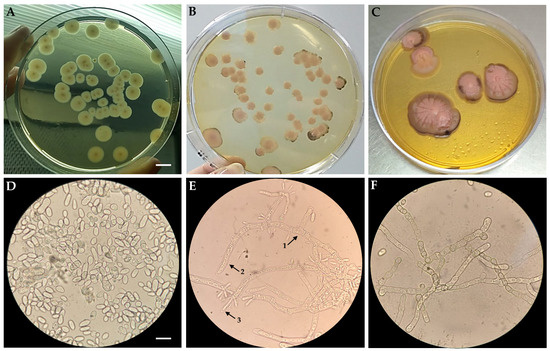
Figure 1.
Macromorphology of A. pullulans strain m11-2 grown on YPD medium at 28 °C after 7 days of incubation (A), and after storage at 4 °C for 30 (B) and 60 days (C). Microscopic observation of the yeast phase (D), showing budding vegetative cells (blastoconidia); filamentous phase (E), showing septate reproductive hyphae (1), intercalary conidiophores (2), and terminal conidiophores with endoconidia (3); and dimorphic phase with both yeast and filamentous structures (F). Scale bars: (A–C) = 5 mm; (D–F) = 20 µm.
Based on these morphological characteristics and in accordance with previous descriptions [33,34,35,36], strain m11-2 may be classified within A. pullulans var. pullulans. This variety is phenotypically characterised by rapidly expanding colonies that are cream to pinkish-yellow in color, often turning dark brown due to the development of thick-walled, melanized hyphae. Melanin, a key component that protects the organism under adverse environmental conditions, is secreted at the end of the exponential growth phase [17].
3.2. Preliminary Test—Growth Kinetics and Enzyme Production by Aureobasidium pullulans m11-2
The relationship between cell growth and extracellular pectinase production by A. pullulans m11-2 was preliminarily evaluated in citrus pectin medium at 28 °C for 8 days under shaking conditions (110 rpm). As shown in Figure 2A, enzymatic activity during the lag phase (0–12 h) remained basal, with values below 0.05 U mg−1. Exponential growth began after 12 h of incubation, accompanied by an increase in enzymatic activity. Both phases ended after 48 h, marking the start of the stationary phase, which extended up to 192 h. The maximum values were 2.43 × 107 CFU mL−1 of cellular concentration and 0.38 U mL−1 of total pectinolytic activity, corresponding to an optical density of 1.1 and a specific enzyme activity of 1.1 U mg−1. The specific growth rate (µ) was 0.033 h−1, as determined from the slope of the logarithmic growth curve. Pectinase production was further confirmed by qualitative assays on solid medium, showing clear halos in Petri dishes as evidence of pectin degradation (Figure 2B).
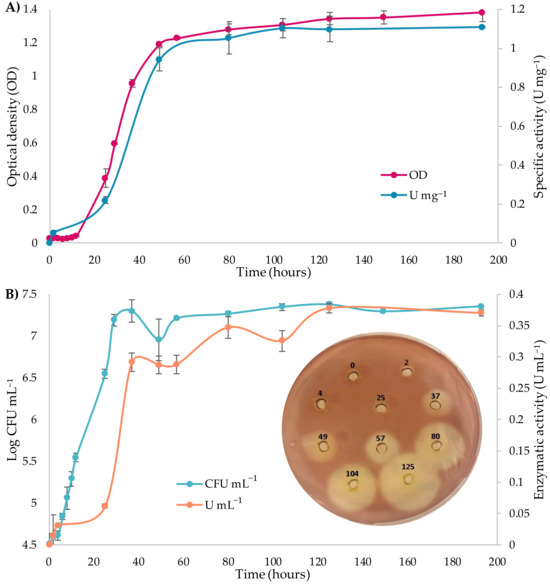
Figure 2.
Cell growth dynamics and pectinase production by A. pullulans m11-2 in citrus pectin medium over 8 days of incubation at 28 °C and 110 rpm. (A) Optical density curves (OD at 600 nm) and specific pectinase activity (U mg−1 of protein). (B) Cell growth curves (CFU mL−1) and total pectinase activity (U mL−1), along with the results of the qualitative assay on solid medium, evidenced by the formation of clarification halos in each well, corresponding to the time points indicated (in hours). Vertical bars represent standard deviations (n = 3).
The growth and enzymatic production dynamics of A. pullulans m11-2 observed in this study are consistent with those reported by Merín and Morata [7] for a different strain of the same species (GM R-22), in which a significant increase in pectinolytic activity was detected between the first and second day, coinciding with the end of the exponential growth phase. Enzymatic activity then remained stable for up to eight days post-inoculation. Similarly, Onetto et al. [37] reported that strains A. pullulans AWRI4229 and AWRI4231 reached peak activity between 48 and 96 h, followed by a decline, in contrast with the present study, where stable activity levels were sustained for a longer period. Furthermore, the specific growth rate observed here was lower than that reported by Xiao et al. [38] for A. pullulans NRRL 62031 (µ = 0.20 h−1), grown in a glucose-based medium.
The enzymatic activity values obtained in this study were also lower than those reported by Oskay [25] for A. pullulans P56, cultivated using a combination of glucose and pectin as carbon sources and yeast extract as the nitrogen source, under pH 6 and 28 °C, where activity reached 4.3 U mL−1. However, the present study was conducted under traditional winemaking conditions, based on previous studies with grape-surface yeast isolates [6,39], which employed a pH of 3.8 and a temperature of 28 °C to evaluate the potential application of these enzymes in winemaking processes.
3.3. Evaluation of the Culture Medium for Enzyme Production
3.3.1. First Phase
In this phase of the study, the influence of different carbon sources on pectinase (PA), cellulase (CA), and xylanase (XA) activities was evaluated. As shown in Table 2, the highest productions of pectinases, cellulases, and xylanases were achieved with 1 g L−1 of glucose and 10 g L−1 of pectin, yielding 0.140 ± 0.021, 0.194 ± 0.003, and 0.104 ± 0.013 U mL−1, respectively.

Table 2.
Cell growth based on optical density at 600 nm (OD600) and enzymatic activities (U mL−1) of the m11-2 strain after 72 h of incubation under different concentrations of glucose and pectin (g L−1) as carbon sources. Values are means ± SD (standard deviation) from three independent experiments.
Additionally, cell growth was highest at elevated glucose concentrations, which was expected since glucose is a readily assimilable carbon source that directly fuels biomass production. Although high concentrations of pectin have been reported to cause osmotic stress or viscosity effects that could restrict microbial growth [40], no inhibitory effect was observed in our study. However, enzymatic activity behaved differently: higher pectin concentrations promoted enzyme production, whereas high glucose partially suppressed enzyme production.
These trends were visualized through second-order fitted models and the corresponding response surface and contour plots (Figure 3). To this end, the model equations were obtained along with their coefficients of determination (R2), which were as follows:
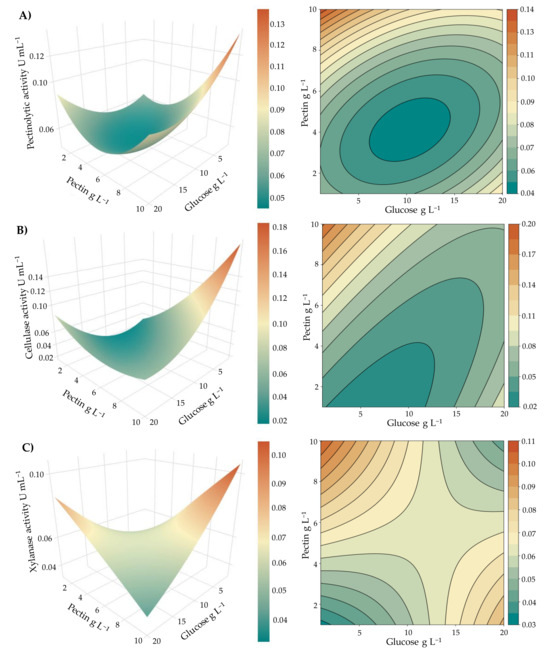
Figure 3.
Response surface plots and contour curves for the production of pectinases (A), cellulases (B), and xylanases (C) by A. pullulans m11-2 under different concentrations of glucose and pectin as carbon sources. Model statics: Pectinases, R2 = 0.7630, R2 (adj) = 0.7065, p < 0.001; Cellulases, R2 = 0.8651, R2 (adj) = 0.8329, p < 0.001; Xylanases, R2 = 0.8942, R2 (adj) = 0.8691, p < 0.001.
The fitted quadratic models adequately described the enzymatic activities of cellulases and xylanases, and showed an acceptable fit for pectinases. In all cases, the response surfaces revealed a common trend, with maximum enzymatic activities occurring under conditions of low glucose concentration (approximately 1 g L−1) and high pectin concentration (around 10 g L−1).
For pectinolytic activity (PA), the maximum value was observed a low glucose concentration (1 g L−1) and a high pectin concentration (10 g L−1). As shown in Figure 3A, enzymatic activity decreased markedly at intermediate and high glucose concentrations, even in the presence of excess pectin, indicating a potential inhibitory effect of glucose. Although some activity was detected at low pectin concentrations, the levels remained lower than those observed under high pectin availability.
Cellulolytic activity (CA) exhibited a similar behavior, with a maximum also observed at a minimal glucose concentration (1 g L−1) and a high pectin concentration (10 g L−1) (Figure 3B). A steep decline in activity was observed with increasing glucose concentration, particularly at intermediate levels of both variables. This suggests that glucose may exert a stronger inhibitory effect on cellulase production or secretion under these conditions.
Xylanase activity (XA) also reached its maximum at the same conditions (1 g L−1 glucose and 10 g L−1 pectin) but exhibited a more complex behavior (Figure 3C). High levels of activity were recorded both at low pectin and high glucose concentrations, and at high pectin and low glucose concentrations, with a slight predominance in the latter. In contrast, intermediate concentrations of both substrates resulted in the lowest activity values, suggesting that these conditions are not favorable for enzyme expression or activity. This saddle-shaped response indicates a more intricate regulation compared to the other polysaccharidases analyzed, possibly reflecting a bifasic regulation, partial catabolite repression, or shifts in carbon source preference. It should be noted that pectin, although varied here as a carbon source, is not directly hydrolyzed by xylanase; thus, the observed effects may be indirect. The increase in each substrate at low concentrations of the other stimulated activity, whereas their simultaneous increase or decrease resulted in lower levels. This regulatory pattern highlights the complexity of xylanase production and suggests that further specific studies will be required to elucidate its physiological meaning.
The results obtained in this study are consistent with those reported by other authors. Aureobasidium pullulans has demonstrated the ability to produce hydrolytic enzymes such as pectinases, cellulases, and xylanases, such as observed in strains NRRL 62031 [38] and NBB 7.2.1 [41], as well as specifically in strain m11-2 [6,24]. In this study, pectinase production was found to be partially constitutive. According to the present study, it can be hypothesized that the pectinase activity may be partially constitutive. While strain 11-2 had significant basal pectinolytic activity of 0.0162 ± 0.006 U mL−1, measured in the basal medium with glucose as the sole carbon source, it was increased in the presence of the specific substrate. However, constitutive production should be confirmed through enzymatic assays in a medium containing a carbon source that is neither an inducer nor a repressor, such as glycerol, for example. This behavior aligns with that reported by Biely et al. [42], Fontana et al. [43], and Merín and Morata [16], who documented both induction by pectin and repression by glucose in A. pullulans strains. Similar results have been observed in Aspergillus niger [44,45].
The values recorded in this study are comparable to those obtained by Longhi et al. and Merín et al. [6,39] under winemaking conditions (pH 3.8 and 28 °C), using citrus pectin and glucose as carbon sources and ammonium sulfate as the sole nitrogen source. However, the maximum pectinolytic activity recorded was lower than that reported by Oskay [25] for strain P56, which reached 8.2 U mL−1 under optimal conditions (30 °C, pH 5.5) in a medium supplemented with citrus pectin (10 g L−1) as the carbon source and an equimolar combination of ammonium sulfate and yeast extract as the nitrogen source.
In the case of cellulases, a similar behavior to that of pectinases was observed, with catabolite repression in the presence of glucose. This is consistent with the findings of Vieira et al. [46], who demonstrated that strain LB83 was capable of producing this enzyme using sugarcane bagasse as a carbon source. Finally, xylanase activity appeared to follow a less stringent regulatory pattern, with expression even in the absence of xylan, suggesting a partially constitutive nature. This may be related to the microorganism’s need to degrade structural polysaccharides from plant cell walls in coordination with other polysaccharidase activities, thereby accessing alternative carbon sources from within the plant cell.
Specific activities were also determined for each evaluated formulation (Figure 4). The results indicated that the most favorable condition corresponded to 1 g L−1 of glucose and 10 g L−1 of pectin, reaching maximum values of 0.318 ± 0.05 U mg−1, 0.443 ± 0.01 U mg−1, and 0.238 ± 0.03 U mg−1 for pectinase, cellulase, and xylanase activities, respectively.
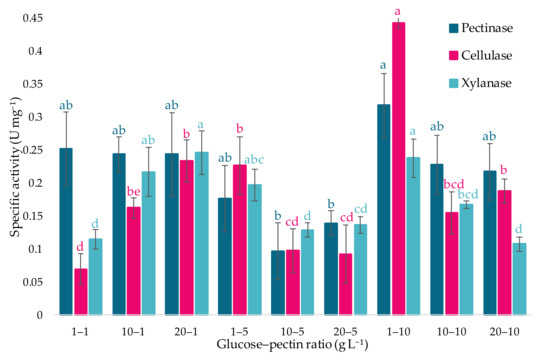
Figure 4.
Comparison of the specific activities (U mg−1 protein) of pectinases, cellulases, and xylanases from the A. pullulans strain m11-2 under different glucose and pectin concentrations as carbon sources. Values are means from three independent experiments and the vertical bars represent standard deviation (SD). Letters above the bars indicate significant differences among the same enzymes according to ANOVA (α = 0.05).
3.3.2. Second Phase
Based on the results obtained, a second experiment was conducted starting from the formulation that showed the highest enzymatic activity, to determine additional nutritional sources that could enhance the growth and enzymatic production of strain m11-2. In order to explore more sustainable and economically viable alternatives, grape skins were included as a nutrient-rich solid substrate, simulating an industrial residue with a high content of pectin and bioactive compounds [47,48]. This strategy is grounded in the growing interest in utilizing agro-industrial byproducts as sources for the production of value-added enzymes, due to their low cost and potential to reduce environmental impact [4,38,49]. The chemical composition of the grape skins from Malbec, a major variety in the Mendoza region, provides a strong basis for their use as a substrate. They are rich in structural polysaccharides such as cellulose and pectins, as well as phenolic compounds that include lignin [27,28].
Figure 5A shows the use of both ammonium sulfate as the sole nitrogen source and its combination with yeast extract. No statistically significant differences were observed in enzymatic production between the exclusive use of ammonium sulfate (0.602 ± 0.166 U mL−1) and its combination with yeast extract (0.449 ± 0.099 U mL−1), although the latter slightly increased optical density (3.35 ± 0.07 vs. 2.58 ± 0.71), indicating enhanced cell growth. These results suggest that ammonium sulfate was sufficient to meet the nitrogen requirements of the strain under the tested conditions. The findings differ from those reported by Oskay [25], who observed higher pectinolytic activity (8.2 U mL−1) and cell growth (4.0 mg mL−1) when using a 1:1 combination of ammonium sulfate and yeast extract, possibly due to strain-specific or cultivation condition differences.
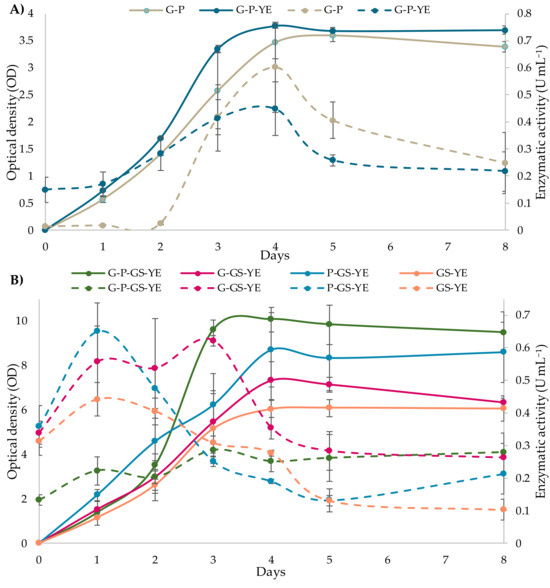
Figure 5.
Growth curves and pectinase production of Aureobasidium pullulans m11-2 under different culture medium conditions. Solid lines correspond to cell growth (OD), while dotted lines represent pectinase activity (U mL−1). (A) Influence of yeast extract (YE) as a complementary nitrogen source. (B) Influence of grape skins on microbial growth and enzymatic production. G: 1 g L−1 glucose; P: 10 g L−1 pectin; GS: 15 g L−1 grape skins; YE: 1 g L−1 yeast extract. Values are means from three independent experiments and the vertical bars represent standard deviation (SD).
Figure 5B displays the effect of grape skins as a supplementary nutrient and potential support for cell immobilization, with all formulations containing grape skins showing enhanced enzymatic activity and/or cell growth compared to the control medium without agro-residues. The highest cell growth was observed in the medium containing grape skins, pectin, and glucose, reaching a maximum OD of 10.08 ± 0.55, although the maximum enzymatic activity was 0.2858 ± 0.021 U mL−1 on the third day of incubation. In contrast, when using grape skins without the addition of other carbohydrates, a higher enzymatic activity was achieved (0.405 ± 0.042 U mL−1 at 48 h of incubation), despite a lower growth rate (OD of 6.10 ± 0.38). This behavior may be due to the low availability of simple sugars in the grape skin, which limits the growth rate but activates enzymatic degradation mechanisms to access nutrients. These findings are consistent with those reported by other authors [25], who also observed reduced growth when using grape residues as the sole carbon source, along with high enzymatic activity.
The most effective formulations in terms of enzymatic production were those combining grape skins with either glucose or pectin, reflecting the beneficial effect of this substrate relative to the control without agro-residues. This improvement can be attributed to the inherent composition of grape skins, particularly their complex and varied carbohydrates, which provide both a source of nutrients and a structural matrix for the microbial strain [27,28]. In the medium containing grape skins and glucose, cell growth reached an OD of 7.32 ± 0.86 on the fourth day, and significant enzymatic activity was detected within the first 24 h, peaking on the third day (0.622 ± 0.016 U mL−1), followed by a decline. By contrast, the combination of grape skins and pectin promoted greater growth, achieving an OD of 8.71 ± 1.67 on the fourth day of incubation. However, maximum enzyme activity was found to be 0.650 ± 0.088 U mL−1 at 24 h. The activity was maintained during the second day, but decreased markedly from the third day onwards. Thus, the data suggest that grape skins function as both structural support and a nutrient source. In combination with carbon sources such as pectin or glucose, they promote enzyme synthesis and biomass production, establishing them as a viable alternative.
In all formulations, enzymatic production was observed to be higher during the early stages of cell growth (beginning of the exponential phase), while enzymatic expression tended to decrease once sustained growth was achieved. This dynamic aligns with the findings of He et al. [50] in Aspergillus oryzae, where the expression of genes encoding hydrolytic enzymes increased significantly during the transition from the adaptation phase to the logarithmic phase, corresponding to a higher metabolic demand, and then stabilized in the stationary phase. In this context, it has been proposed that the use of continuous cultivation systems may represent an efficient strategy to maximize extracellular enzyme production by maintaining microorganisms in a constant state of active growth [17,51].
4. Conclusions
The results of this study confirm the biotechnological potential of the strain A. pullulans m11-2 as an innovative source of hydrolytic enzymes for use in the winemaking. The research involved evaluating different nutrient sources to determine the conditions that favour enzymatic production. It was demonstrated that these enzymes exhibit a partially constitutive and inducible profiles, with the highest activities were achieved under low glucose and high pectin concentrations. This enabled the establishment of optimal cultivation parameters. Furthermore, aiming to explore more sustainable and cost-effective alternatives, the use of grape skins as a model agro-industrial residue was evaluated, demonstrating that combining them with a low glucose concentration enabled optimal levels of growth and enzymatic production to be reached. The observation that maximum enzyme yields were recorded during the exponential growth phase, at a mid-to-late stage, reveals a kinetic profile that could potentially be exploited in future studies for continuous fermentation strategies. Maintaining cultures in this metabolically active phase remains a promising avenue for enhancing process productivity and will be addressed in subsequent research aimed at developing and optimising continuous bioprocessing systems for sustained, high-yield enzyme production.
Author Contributions
Conceptualization, V.I.M. and M.C.M.; methodology, M.E.S.; investigation, M.E.S., V.I.M. and M.C.M.; writing—original draft preparation, M.E.S., V.I.M. and M.C.M.; writing—review and editing, M.E.S., V.I.M. and M.C.M.; software, M.E.S.; visualization, M.E.S. and M.C.M.; supervision, V.I.M.; project administration, M.C.M. and V.I.M.; funding acquisition, M.C.M. and V.I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SIIP-UNCUYO (grant numbers 06/L004-T1, and 06/L013-T1), a PICT(MINCYT)-BID loan (grant number 2019-03446), and PIP-CONICET (grant number 2021-0074).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The partial sequence of the ribosomal RNA subunit gene acquired from the isolate investigated in this study has been submitted to the GenBank database (https://www.ncbi.nlm.nih.gov/nuccore/PV659872, accessed on 25 May 2025), for open access, reference code: PV659872. Also, the dataset is available on request from the authors.
Acknowledgments
The authors are grateful to Andrea Ridolfi, Nicolás Muzi, Francisca Julián and Camila Muñoz for their assistance with production the surface plots and statistical modelling.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANOVA | Analysis of Variance |
| BLAST | Basic Local Alignment Search Tool |
| CA | Cellulase activity |
| CFU | Colony-forming units |
| DNS | 3,5-Dinitrosalicylic acid |
| G | Glucose |
| GRAS | Generally Recognized As Safe |
| GS | Grape Skins |
| LSD | Least Significant Difference |
| OD | Optical Density |
| OIV | Organization of Vine and Wine |
| P | Pectin |
| PA | Pectinases activity |
| RSM | Response Surface Methodology |
| SD | Standard Deviation |
| U | One unit of enzymatic activity |
| XA | Xylanases activity |
| YE | Yeast Extract |
References
- Chen, J.; Liu, W.; Liu, C.M.; Li, T.; Liang, R.H.; Luo, S.J. Pectin Modifications: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698. [Google Scholar] [CrossRef]
- Dal Magro, L.; Hertz, P.F.; Fernandez-Lafuente, R.; Klein, M.P.; Rodrigues, R.C. Preparation and Characterization of a Combi-CLEAs from Pectinases and Cellulases: A Potential Biocatalyst for Grape Juice Clarification. RSC Adv. 2016, 6, 27242–27251. [Google Scholar] [CrossRef]
- Hüfner, E.; Haßelbeck, G. Application of Microbial Enzymes During Winemaking. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 365–658. [Google Scholar]
- Singh, B.; Soni, S.K.; Mathur, P.; Garg, N. Microbial Multienzyme Viz., Pectinase, Cellulase and Amylase Production Using Fruit and Vegetable Waste as Substrate—A Review. Appl. Microbiol. 2024, 4, 1232–1246. [Google Scholar] [CrossRef]
- Río Segade, S.; Pace, C.; Torchio, F.; Giacosa, S.; Gerbi, V.; Rolle, L. Impact of Maceration Enzymes on Skin Softening and Relationship with Anthocyanin Extraction in Wine Grapes with Different Anthocyanin Profiles. Food Res. Int. 2015, 71, 50–57. [Google Scholar] [CrossRef]
- Longhi, S.J.; Martín, M.C.; Fontana, A.; de Ambrosini, V.I.M. Different Approaches to Supplement Polysaccharide-Degrading Enzymes in Vinification: Effects on Color Extraction, Phenolic Composition, Antioxidant Activity and Sensory Profiles of Malbec Wines. Food Res. Int. 2022, 157, 111447. [Google Scholar] [CrossRef] [PubMed]
- Merín, M.G.; Morata de Ambrosini, V.I. Kinetic and Metabolic Behaviour of the Pectinolytic Strain Aureobasidium Pullulans GM-R-22 during Pre-Fermentative Cold Maceration and Its Effect on Red Wine Quality. Int. J. Food Microbiol. 2018, 285, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Merín, M.G.; Morata de Ambrosini, V.I. Application of a Grape Surface Majority Pectinolytic Species, Aureobasidium Pullulans, to Low-Temperature Red Winemaking: Development and Stability of Wine Colour. J. Wine Res. 2020, 31, 218–239. [Google Scholar] [CrossRef]
- Fratebianchi, D.; González, M.; Tenorio, C.; Cavalitto, S.F.; Ruiz-Larrea, F. Characterization and Winemaking Application of a Novel Pectin-Degrading Enzyme Complex from Aspergillus Sojae ATCC 20235. Vitis-J. Grapevine Res. 2017, 56, 85–93. [Google Scholar] [CrossRef]
- Claus, H.; Mojsov, K. Enzymes for Wine Fermentation: Current and Perspective Applications. Fermentation 2018, 4, 52. [Google Scholar] [CrossRef]
- Sharma, R.; Oberoi, H.S.; Dhillon, G.S. Fruit and Vegetable Processing Waste: Renewable Feed Stocks for Enzyme Production. In Agro-Industrial Wastes as Feedstock for Enzyme Production: Apply and Exploit the Emerging and Valuable Use Options of Waste Biomass; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 23–59. ISBN 9780128026120. [Google Scholar]
- Satapathy, S.; Rout, J.R.; Kerry, R.G.; Thatoi, H.; Sahoo, S.L. Biochemical Prospects of Various Microbial Pectinase and Pectin: An Approachable Concept in Pharmaceutical Bioprocessing. Front. Nutr. 2020, 7, 117. [Google Scholar] [CrossRef]
- Suhaimi, H.; Dailin, D.J.; Malek, R.A.; Hanapi, S.Z.; Ambehabati, K.K.; Keat, H.C.; Prakasham, S.; Elsayed, E.A.; Misson, M.; El Enshasy, H. Fungal Pectinases: Production and Applications in Food Industries. In Fungi in Sustainable Food Production; Springer: Cham, Switzerland, 2021; pp. 85–115. [Google Scholar]
- Escribano, R.; González-Arenzana, L.; Garijo, P.; Berlanas, C.; López-Alfaro, I.; López, R.; Gutiérrez, A.R.; Santamaría, P. Screening of Enzymatic Activities within Different Enological Non-Saccharomyces Yeasts. J. Food Sci. Technol. 2017, 54, 1555–1564. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces Pombe and Torulaspora Delbrueckii Strains in Mixed and Sequential Fermentations to Improve Red Wine Sensory Quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Merín, M.G.; de Ambrosini, V.I.M. Highly Cold-Active Pectinases under Wine-like Conditions from Non-Saccharomyces Yeasts for Enzymatic Production during Winemaking. Lett. Appl. Microbiol. 2015, 60, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Prasongsuk, S.; Lotrakul, P.; Ali, I.; Bankeeree, W.; Punnapayak, H. The Current Status of Aureobasidium Pullulans in Biotechnology. Folia Microbiol. 2018, 63, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.C.; Prendes, L.P.; Morata, V.I.; Merín, M.G. Biocontrol and Enzymatic Activity of Non-Saccharomyces Wine Yeasts: Improvements in Winemaking. Fermentation 2024, 10, 218. [Google Scholar] [CrossRef]
- Merín, M.G.; Martín, M.C.; Rantsiou, K.; Cocolin, L.; De Ambrosini, V.I.M. Characterization of Pectinase Activity for Enology from Yeasts Occurring in Argentine Bonarda Grape. Braz. J. Microbiol. 2015, 46, 815–823. [Google Scholar] [CrossRef]
- Pinto, C.; Pinho, D.; Cardoso, R.; Custódio, V.; Fernandes, J.; Sousa, S.; Pinheiro, M.; Egas, C.; Gomes, A.C. Wine Fermentation Microbiome: A Landscape from Different Portuguese Wine Appellations. Front. Microbiol. 2015, 6, 905. [Google Scholar] [CrossRef]
- Pertot, I.; Giovannini, O.; Benanchi, M.; Caffi, T.; Rossi, V.; Mugnai, L. Combining Biocontrol Agents with Different Mechanisms of Action in a Strategy to Control Botrytis Cinerea on Grapevine. Crop Prot. 2017, 97, 85–93. [Google Scholar] [CrossRef]
- Sternes, P.R.; Lee, D.; Kutyna, D.R.; Borneman, A.R. A Combined Meta-Barcoding and Shotgun Metagenomic Analysis of Spontaneous Wine Fermentation. Gigascience 2017, 6, gix040. [Google Scholar] [CrossRef]
- Bozoudi, D.; Tsaltas, D. The Multiple and Versatile Roles of Aureobasidium Pullulans in the Vitivinicultural Sector. Fermentation 2018, 4, 85. [Google Scholar] [CrossRef]
- Longhi, S.J.; Martín, M.C.; Merín, M.G.; Morata de Ambrosini, V.I. Yeast Multi-Enzymatic Systems for Improving Colour Extraction, Technological Parameters and Antioxidant Activity of Wine. Food Technol. Biotechnol. 2022, 60, 556–570. [Google Scholar] [CrossRef]
- Oskay, M. Production, Partial Purification, and Characterization of Polygalacturonase from Aureobasidium Pullulans P56 under Submerged Fermentation Using Agro-Industrial Wastes. Curr. Microbiol. 2022, 79, 296. [Google Scholar] [CrossRef]
- Merín, M.G.; Martín, M.C.; Carrión, R.O.; Morata, V.I. Co-Inoculation of a Pectinolytic Aureobasidium Pullulans Strain and Saccharomyces Cerevisiae for Low-Temperature Red Fermentation: A Strategy to Enhance the Colour and Sensory Properties of Malbec Wines. OENO One 2025, 59, 1. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of Polyphenols and Evaluation of Antioxidant Capacity in Grape Pomace of the Cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Querol, A.; Barrio, E.; Ramón, D. A Comparative Study of Different Methods of Yeast Strain Characterization. Syst. Appl. Microbiol. 1992, 15, 439–446. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Robnett, C.J. Identification of Clinically Important Ascomycetous Yeasts Based on Nucleotide Divergence in the 5 End of the Large-Subunit (26S) Ribosomal DNA Gene. J. Clin. Microbiol. 1998, 73, 331–371. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. ACS Publ. Most. Trusted. Most. Cited. Most. Read. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zalar, P.; Gostinčar, C.; de Hoog, G.S.; Uršič, V.; Sudhadham, M.; Gunde-Cimerman, N. Redefinition of Aureobasidium Pullulans and Its Varieties. Stud. Mycol. 2008, 61, 21–38. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, F.; Chi, Z.; Yue, L.; Liu, G.; Zhang, T. Bioproducts from Aureobasidium Pullulans, a Biotechnologically Important Yeast. Appl. Microbiol. Biotechnol. 2009, 82, 793–804. [Google Scholar] [CrossRef]
- Gostinčar, C.; Ohm, R.A.; Kogej, T.; Sonjak, S.; Turk, M.; Zajc, J.; Zalar, P.; Grube, M.; Sun, H.; Han, J.; et al. Genome Sequencing of Four Aureobasidium Pullulans Varieties: Biotechnological Potential, Stress Tolerance, and Description of New Species. BMC Genom. 2014, 15, 549. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, Y.; Liu, L.; Bai, R.; Zhang, S.; Hao, Y.; Xu, F.; Wei, B.; Zhao, H. Characteristic Analysis and Fermentation Optimization of a Novel Aureobasidium Pullulans RM1603 with High Pullulan Yield. J. Biosci. Bioeng. 2024, 137, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Onetto, C.A.; Borneman, A.R.; Schmidt, S.A. Investigating the Effects of Aureobasidium Pullulans on Grape Juice Composition and Fermentation. Food Microbiol. 2020, 90, 103451. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Driller, M.; Stein, K.; Blank, L.M.; Tiso, T. Genome Mining the Black-Yeast Aureobasidium Pullulans NRRL 62031 for Biotechnological Traits. BMC Genom. 2025, 26, 244. [Google Scholar] [CrossRef]
- Merín, M.G.; Mendoza, L.M.; Morata de Ambrosini, V.I. Pectinolytic Yeasts from Viticultural and Enological Environments: Novel Finding of Filobasidium Capsuligenum Producing Pectinases. J. Basic Microbiol. 2014, 54, 835–842. [Google Scholar] [CrossRef]
- Martins, L.C.; Monteiro, C.C.; Semedo, P.M.; Sá-Correia, I. Valorisation of Pectin-Rich Agro-Industrial Residues by Yeasts: Potential and Challenges. Appl. Microbiol. Biotechnol. 2020, 104, 6527–6547. [Google Scholar] [CrossRef]
- Rueda-Mejia, M.P.; Nägeli, L.; Lutz, S.; Hayes, R.D.; Varadarajan, A.R.; Grigoriev, I.V.; Ahrens, C.H.; Freimoser, F.M. Genome, Transcriptome and Secretome Analyses of the Antagonistic, Yeast-like Fungus Aureobasidium Pullulansto Identify Potential Biocontrol Genes. Microb. Cell 2021, 8, 184–202. [Google Scholar] [CrossRef]
- Biely, P.; Heinrichová, K.; Kruz, M. Induction and Inducers of the Pectolytic System in Aureobasidium Pullulans; Springer Inc.: New York, NY, USA, 1996; Volume 33. [Google Scholar]
- Fontana, R.C.; Salvador, S.; Da Silveira, M.M. Influence of Pectin and Glucose on Growth and Polygalacturonase Production by Aspergillus Niger in Solid-State Cultivation. J. Ind. Microbiol. Biotechnol. 2005, 32, 371–377. [Google Scholar] [CrossRef]
- Ahmed, I.; Zia, M.A.; Hussain, M.A.; Akram, Z.; Naveed, M.T.; Nowrouzi, A. Bioprocessing of Citrus Waste Peel for Induced Pectinase Production by Aspergillus Niger; Its Purification and Characterization. J. Radiat. Res. Appl. Sci. 2016, 9, 148–154. [Google Scholar] [CrossRef]
- Reginatto, C.; Rossi, C.; Miglioranza, B.G.; Santos, M.d.; Meneghel, L.; Silveira, M.M.d.; Malvessi, E. Pectinase Production by Aspergillus Niger LB-02-SF Is Influenced by the Culture Medium Composition and the Addition of the Enzyme Inducer after Biomass Growth. Process Biochem. 2017, 58, 1–8. [Google Scholar] [CrossRef]
- Vieira, M.M.; Kadoguchi, E.; Segato, F.; da Silva, S.S.; Chandel, A.K. Production of Cellulases by Aureobasidium Pullulans LB83: Optimization, Characterization, and Hydrolytic Potential for the Production of Cellulosic Sugars. Prep. Biochem. Biotechnol. 2021, 51, 153–163. [Google Scholar] [CrossRef]
- Ahmed, T.; Rana, M.R.; Zzaman, W.; Ara, R.; Aziz, M.G. Optimization of Substrate Composition for Pectinase Production from Satkara (Citrus Macroptera) Peel Using Aspergillus Niger-ATCC 1640 in Solid-State Fermentation. Heliyon 2021, 7, e08133. [Google Scholar] [CrossRef]
- Fasoli, M.; Dell’Anna, R.; Dal Santo, S.; Balestrini, R.; Sanson, A.; Pezzotti, M.; Monti, F.; Zenoni, S. Pectins, Hemicelluloses and Celluloses Show Specific Dynamics in the Internal and External Surfaces of Grape Berry Skin during Ripening. Plant Cell Physiol. 2016, 57, 1332–1349. [Google Scholar] [CrossRef]
- Govindaraji, P.K.; Vuppu, S. Characterisation of Pectin and Optimization of Pectinase Enzyme from Novel Streptomyces Fumigatiscleroticus VIT-SP4 for Drug Delivery and Concrete Crack-Healing Applications: An Eco-Friendly Approach. Saudi J. Biol. Sci. 2020, 27, 3529–3540. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Hu, Z.; Ma, L.; Li, H.; Ai, M.; Han, J.; Zeng, B. Transcriptome Analysis of Different Growth Stages of Aspergillus Oryzae Reveals Dynamic Changes of Distinct Classes of Genes during Growth. BMC Microbiol. 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Driller, M.; Dielentheis-Frenken, M.; Haala, F.; Kohl, P.; Stein, K.; Blank, L.M.; Tiso, T. Advances in Aureobasidium Research: Paving the Path to Industrial Utilization. Microb. Biotechnol. 2024, 17, e14535. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).