Monensin Degradation and Methane Production from Sugarcane Vinasse in Two-Phase Thermophilic Anaerobic Fixed-Bed and Sludge Blanket Bioreactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Vinasse
2.2. Thermophilic Bioreactors
2.3. Two-Phase Reactor Operating Conditions
2.3.1. Acidogenic Phase Reactor
2.3.2. Methanogenic Phase Reactors
2.4. Experimental Monitoring
3. Results and Discussion
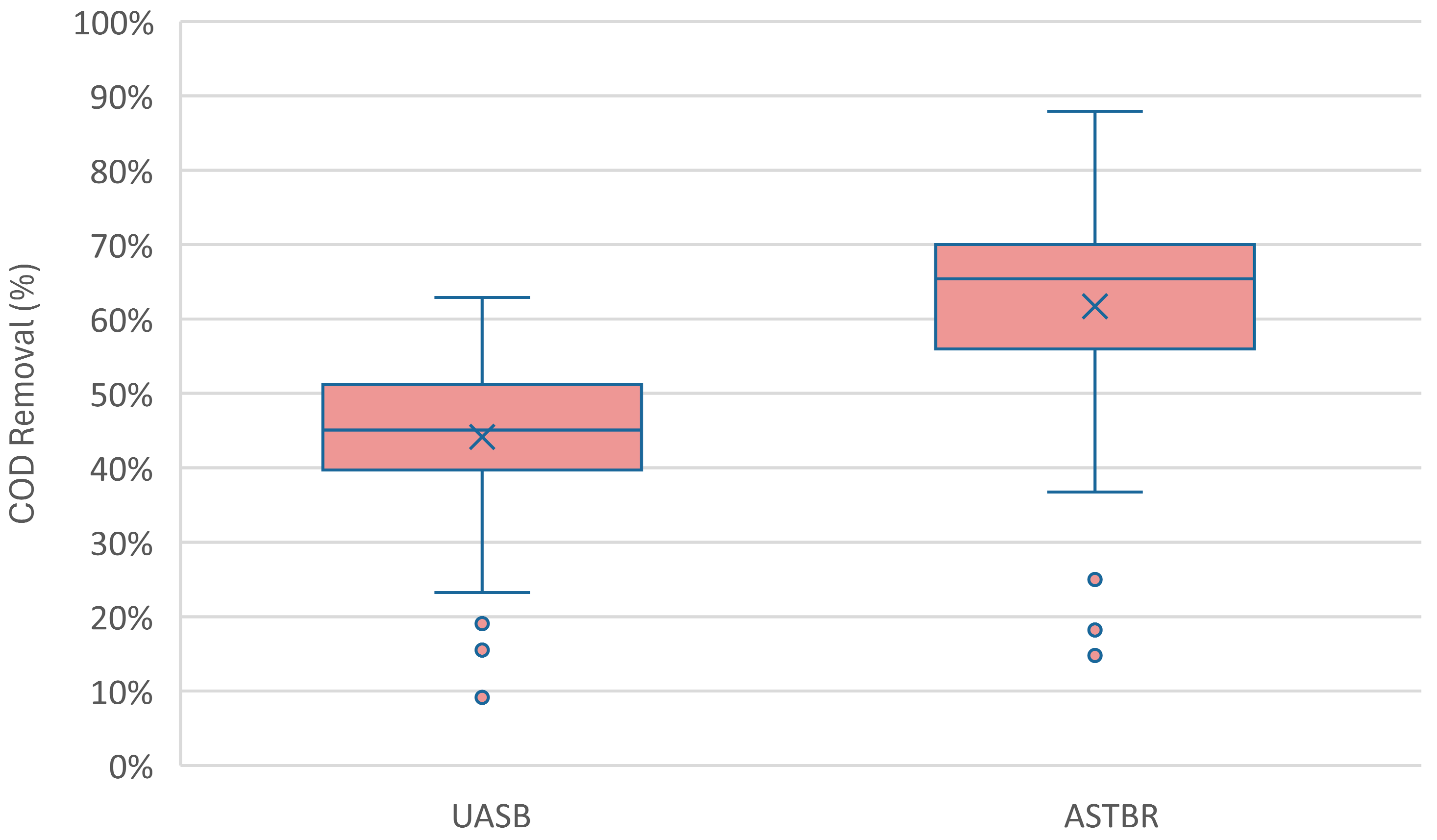
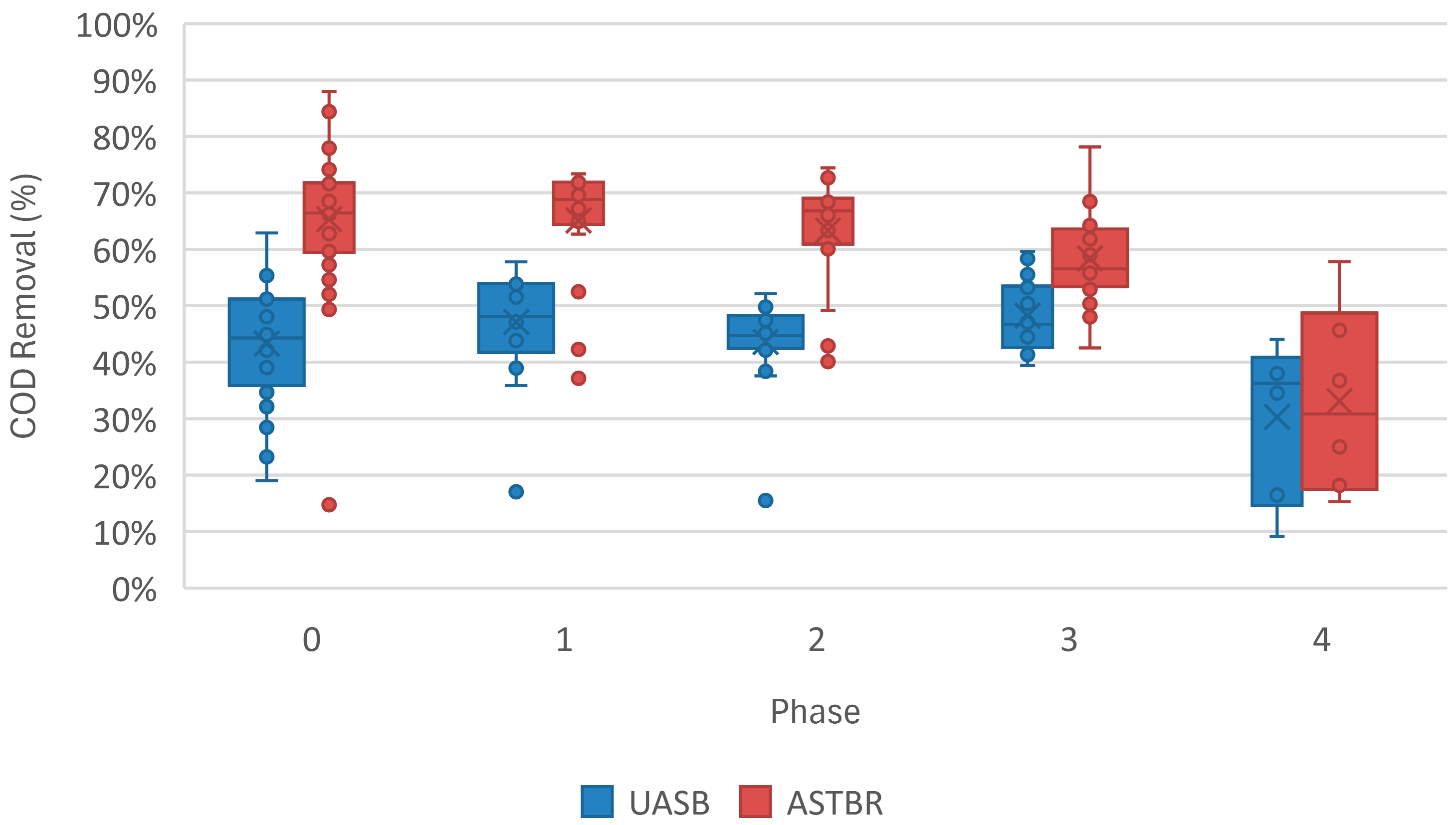
3.1. Reactor Performance
3.2. Acidogenic ASTBR
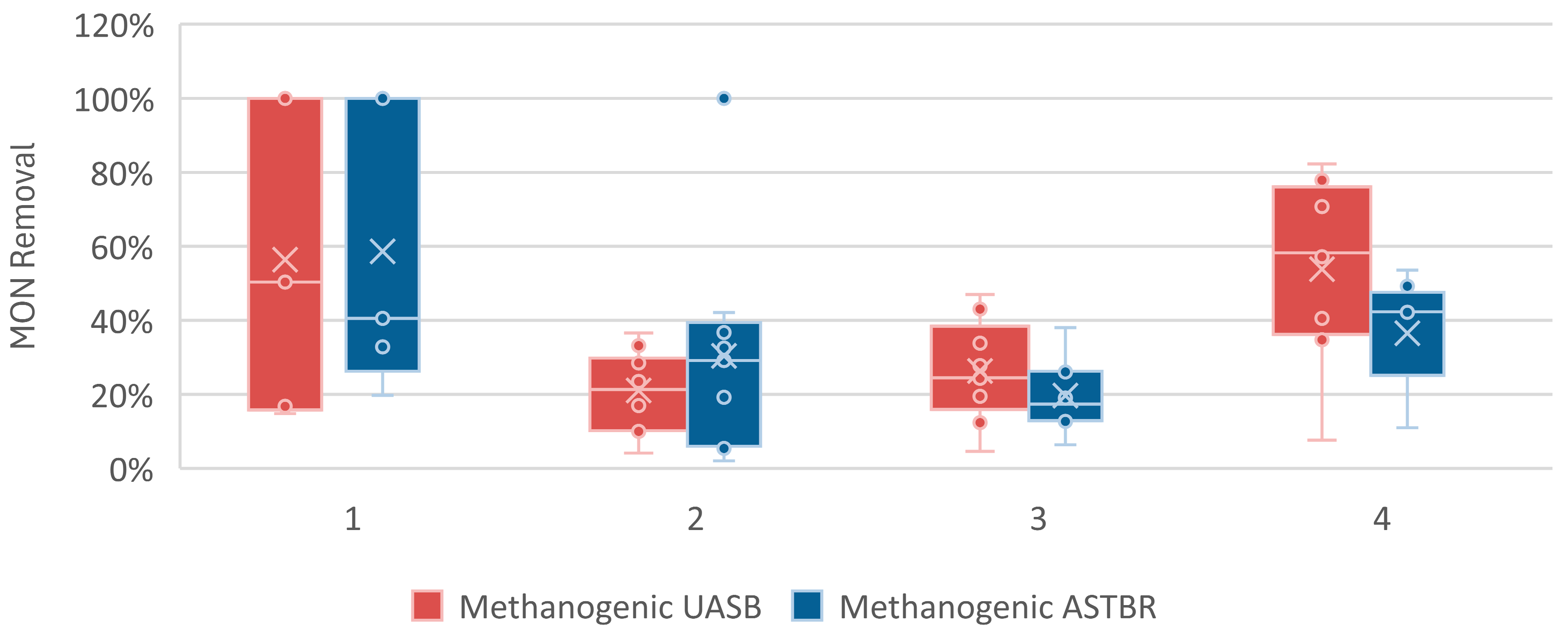
3.3. Methanogenic UASB and ASTBR
3.4. Two-Phase Reactor Global Monensin Removal
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASTBR | Anaerobic Structured-Bed Bioreactor |
| UASB | Upflow Anaerobic Sludge Blanket |
| COD | Chemical Oxygen Demand |
| OLR | Organic Loading Rate |
| HRT | Hydraulic Retention Time |
| VMP | Volumetric Methane Production |
| MY | Methane Yield |
| TSSs | Total Suspended Solids |
| VSSs | Volatile Suspended Solids |
| VOCs | Volatile Organic Compounds |
| MON | Monensin |
| VFAs | Volatile Fatty Acids |
| OASs | Organic Acids and Solvents |
| NTK | Total Kjeldahl Nitrogen (Nitrogênio Total Kjeldahl) |
| pH | Potential of Hydrogen (Acidity/Alkalinity Measure) |
References
- Fuess, L.T. Biodigestão Anaeróbia Termofílica de Vinhaça em Sistemas Combinados do Tipo Acidogênico-Metanogênico para Potencialização da Recuperação de Bioenergia em Biorrefinarias de Cana-de-Açúcar de Primeira Geração. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2017. [Google Scholar] [CrossRef]
- Poveda, M.M.R. Análise Econômica e Ambiental do Processamento da Vinhaça com Aproveitamento Energético. Master’s Thesis, Universidade de São Paulo, São Paulo, Brazil, 2015. [Google Scholar] [CrossRef]
- da Silva, G.A. Avaliação das Tecnologias de Disposição de Vinhaça de Cana de Açúcar Quanto ao Aspecto de Desenvolvimento Ambiental e Econômico. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2011. [Google Scholar] [CrossRef]
- Carpanez, T.G.; Moreira, V.R.; Assis, I.R.; Amaral, M.C.S. Sugarcane vinasse as organo-mineral fertilizers feedstock: Opportunities and environmental risks. Sci. Total Environ. 2022, 832, 154998. [Google Scholar] [CrossRef]
- Conab-Produção de Cana-de-Açúcar na Safra 2024/25 Sofre Redução Devido a Condições Climáticas Adversas. Available online: https://www.conab.gov.br/ultimas-noticias/5841-producao-de-cana-de-acucar-na-safra-2024-25-sofre-reducao-devido-a-condicoes-climaticas-adversas/ (accessed on 2 March 2025).
- Silva, D.F.D.S.E.; Bomtempo, J.V.; Alves, F.C. Innovation opportunities in the Brazilian sugar-energy sector. J. Clean. Prod. 2019, 218, 871–879. [Google Scholar] [CrossRef]
- Junior, A.D.N.F.; Etchebehere, C.; Perecin, D.; Teixeira, S.; Woods, J. Advancing anaerobic digestion of sugarcane vinasse: Current development, struggles and future trends on production and end-uses of biogas in Brazil. Renew. Sustain. Energy Rev. 2022, 157, 112045. [Google Scholar] [CrossRef]
- Ramos-Reyes, M.F.; González-López, M.E.; Barajas-Álvarez, P.; Garcia-Garcia, C.E.; Tuesta-Popolizio, D.A.; Mussatto, S.I.; Gradilla-Hernández, M.S. Exploring the potential of distillery vinasses through upcycling: Pathways to a circular economy. Environ. Technol. Innov. 2025, 38, 104072. [Google Scholar] [CrossRef]
- Sánchez, F.E.; Fuess, L.T.; Cavalcante, G.S.; Adorno, M.Â.T.; Zaiat, M. Value-added soluble metabolite production from sugarcane vinasse within the carboxylate platform: An application of the anaerobic biorefinery beyond biogas production. Fuel 2021, 286, 119378. [Google Scholar] [CrossRef]
- Fuess, L.T.; Kiyuna, L.S.M.; Ferraz, A.D.N.; Persinoti, G.F.; Squina, F.M.; Garcia, M.L.; Zaiat, M. Thermophilic two-phase anaerobic digestion using an innovative fixed-bed reactor for enhanced organic matter removal and bioenergy recovery from sugarcane vinasse. Appl. Energy 2017, 189, 480–491. [Google Scholar] [CrossRef]
- Borges, A.D.V.; Fuess, L.T.; Takeda, P.Y.; Rogeri, R.C.; Saia, F.T.; Gregoracci, G.B.; Damianovic, M.H.R.Z. Unleashing the full potential of vinasse fermentation in sugarcane biorefineries. Renew. Sustain. Energy Rev. 2025, 208, 115096. [Google Scholar] [CrossRef]
- Fuess, L.T.; Rogeri, R.C.; Eng, F.; Borges, A.D.V.; Bovio-Winkler, P.; Etchebehere, C.; Zaiat, M. Thermophilic fermentation of sugarcane vinasse: Process flexibility explained through characterizing microbial community and predicting metabolic functions. Int. J. Hydrogen Energy 2024, 77, 1339–1351. [Google Scholar] [CrossRef]
- Rogeri, R.C.; Fuess, L.T.; de Araujo, M.N.; Eng, F.; Borges, A.D.V.; Damianovic, M.H.R.Z.; da Silva, A.J. Methane production from sugarcane vinasse: The alkalinizing potential of fermentative-sulfidogenic processes in two-stage anaerobic digestion. Energy Nexus 2024, 14, 100303. [Google Scholar] [CrossRef]
- de Oliva-Neto, P.; Yokoya, F. Susceptibility of Saccharomyces cerevisiae and lactic acid bacteria from the alcohol industry to several antimicrobial compounds. Braz. J. Microbiol. 2001, 32, 10–14. [Google Scholar] [CrossRef][Green Version]
- Hynes, S.H.; Kjarsgaard, D.M.; Thomas, K.C.; Ingledew, W.M. Use of virginiamycin to control the growth of lactic acid bacteria during alcohol fermentation. J. Ind. Microbiol. Biotechnol. 1997, 18, 284–291. [Google Scholar] [CrossRef]
- De Miniac, M. US Patent for Use of Ionophoretic Polyether Antibiotics for Controlling Bacterial Growth in Alcoholic Fermentation Patent. U.S. Patent 5,888,788, 30 March 1999. Available online: https://patents.justia.com/patent/5888788 (accessed on 18 August 2021).
- Mollenhauer, H.H.; Morré, D.J.; Rowe, L.D. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim. Biophys. Acta (BBA)—Rev. Biomembr. 1990, 1031, 225–246. [Google Scholar] [CrossRef]
- Matsuoka, T.; Novilla, M.N.; Thomson, T.D.; Donoho, A.L. Review of monensin toxicosis in horses. J. Equine Vet. Sci. 1996, 16, 8–15. [Google Scholar] [CrossRef]
- Zidar, P.; Kos, M.; Vogel-Mikuš, K.; van Elteren, J.T.; Debeljak, M.; Žižek, S. Impact of ionophore monensin on performance and Cu uptake in earthworm Eisenia andrei exposed to copper-contaminated soil. Chemosphere 2016, 161, 119–126. [Google Scholar] [CrossRef]
- Watanabe, N.; Harter, T.H.; Bergamaschi, B.A. Environmental Occurrence and Shallow Ground Water Detection of the Antibiotic Monensin from Dairy Farms. J. Environ. Qual. 2008, 37, S-78–S-85. [Google Scholar] [CrossRef]
- Melchior, E.; Hales, K.; Lindholm-Perry, A.; Freetly, H.; Wells, J.; Hemphill, C.; Wickersham, T.; Sawyer, J.; Myer, P. The effects of feeding monensin on rumen microbial communities and methanogenesis in bred heifers fed in a drylot. Livest. Sci. 2018, 212, 131–136. [Google Scholar] [CrossRef]
- da Silva, J.J. Antibióticos na Produção de Etanol Combustível: Análise, Dispersão no Meio Ambiente e Efeitos na Biodigestão da Vinhaça. May 2021. Available online: http://hdl.handle.net/11449/204933 (accessed on 1 March 2025).
- da Silva, J.J.; da Silva, B.F.; Zanoni, M.V.B.; Stradiotto, N.R. Sample preparation and antibiotic quantification in vinasse generated from sugarcane ethanol fuel production. J. Chromatogr. A 2022, 1666, 462833. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Water Works Association: Washington, DC, USA, 2017. [Google Scholar]
- Taylor, K.A.C.C. A simple colorimetric assay for muramic acid and lactic acid. Appl. Biochem. Biotechnol. 1996, 56, 49–58. [Google Scholar] [CrossRef]
- Valdez, H.d.C.; Amado, R.S.; de Souza, F.C.; D’ELia, E.; Vieira, E.d.C. Free and total glycerol determination in biodiesel samples by enzymatic method with colorimetric detection. Química Nova 2012, 35, 601–607. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Leite, J.A.C.; Fernandes, B.S.; Pozzi, E.; Barboza, M.; Zaiat, M. Application of an anaerobic packed-bed bioreactor for the production of hydrogen and organic acids. Int. J. Hydrogen Energy 2008, 33, 579–586. [Google Scholar] [CrossRef]
- Fuess, L.T.; Kiyuna, L.S.M.; Garcia, M.L.; Zaiat, M. Operational strategies for long-term biohydrogen production from sugarcane stillage in a continuous acidogenic packed-bed reactor. Int. J. Hydrogen Energy 2016, 41, 8132–8145. [Google Scholar] [CrossRef]
- Perna, V.; Castelló, E.; Wenzel, J.; Zampol, C.; Lima, D.F.; Borzacconi, L.; Varesche, M.; Zaiat, M.; Etchebehere, C. Hydrogen production in an upflow anaerobic packed bed reactor used to treat cheese whey. Int. J. Hydrogen Energy 2013, 38, 54–62. [Google Scholar] [CrossRef]
- Adorno, M.A.T.; Hirasawa, J.S.; Varesche, M.B.A. Development and Validation of Two Methods to Quantify Volatile Acids (C2-C6) by GC/FID: Headspace (Automatic and Manual) and Liquid-Liquid Extraction (LLE). AJAC 2014, 5, 406–414. [Google Scholar] [CrossRef]
- Carneiro, R.B.; Gomes, G.M.; Camargo, F.P.; Zaiat, M.; Santos-Neto, Á.J. Anaerobic co-metabolic biodegradation of pharmaceuticals and personal care products driven by glycerol fermentation. Chemosphere 2024, 357, 142006. [Google Scholar] [CrossRef]
- Dias, M.E.S.; Lopes, J.C.; Carneiro, R.B.; Damianovic, M.H.R.Z.; Foresti, E. Using Compounds Derived from the Glycerol Fermentation as a Carbon Source for Denitrification and Biological Phosphorus Removal. Water Air Soil Pollut. 2021, 232, 339. [Google Scholar] [CrossRef]
- Lopes, J.C.; Silva, B.G.; Dias, M.E.S.; Carneiro, R.B.; Damianovic, M.H.R.Z.; Foresti, E. Enhanced biological nitrogen and phosphorus removal from sewage driven by fermented glycerol: Comparative assessment between sequencing batch- and continuously fed-structured fixed bed reactor. Environ. Sci. Pollut. Res. 2023, 30, 11755–11768. [Google Scholar] [CrossRef]
- Clomburg, J.M.; Gonzalez, R. Anaerobic fermentation of glycerol: A platform for renewable fuels and chemicals. Trends Biotechnol. 2013, 31, 20–28. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- García, O.D.V. Toxicidade Metanogênica da Monensina Sódica e Beta-Ácidos de Lúpulo e Seus Efeitos na Biodigestão Anaeróbia da Vinhaça no Contexto da Indústria Sucroalcooleira Brasileira. Ph.D. Thesis, Universidade Federal do ABC, Santo André, Brazil, 2021. [Google Scholar]
- Arikan, O.A.; Mulbry, W.; Rice, C.; Lansing, S. The fate and effect of monensin during anaerobic digestion of dairy manure under mesophilic conditions. PLoS ONE 2018, 13, e0192080. [Google Scholar] [CrossRef]
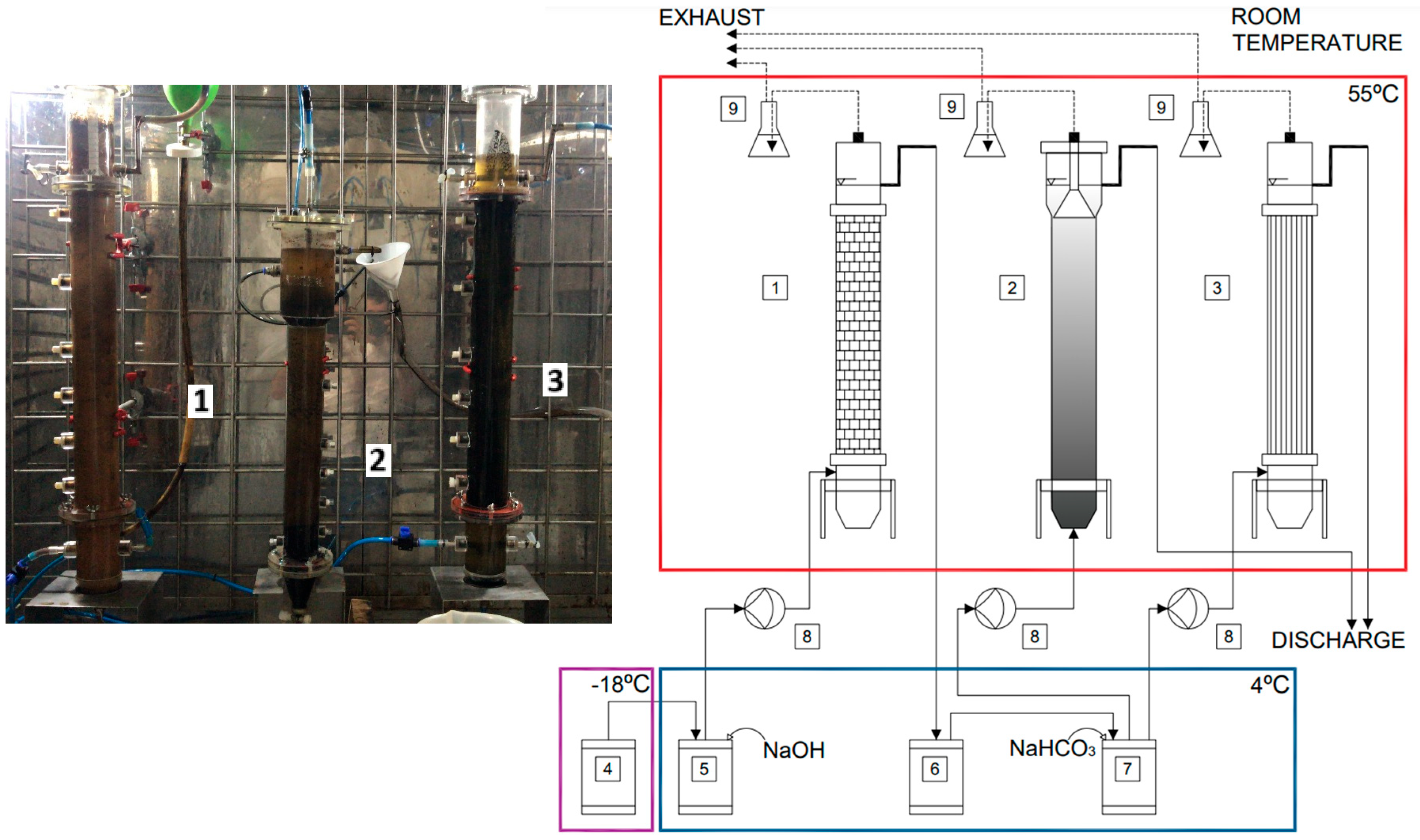
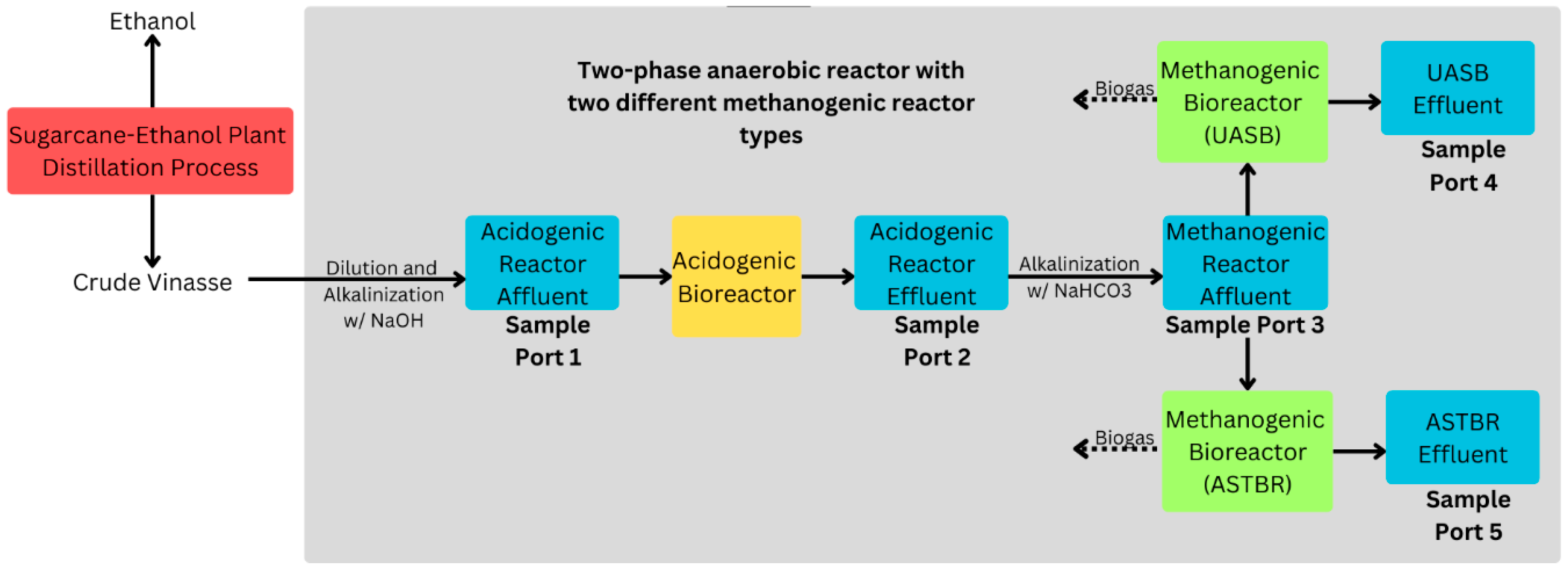
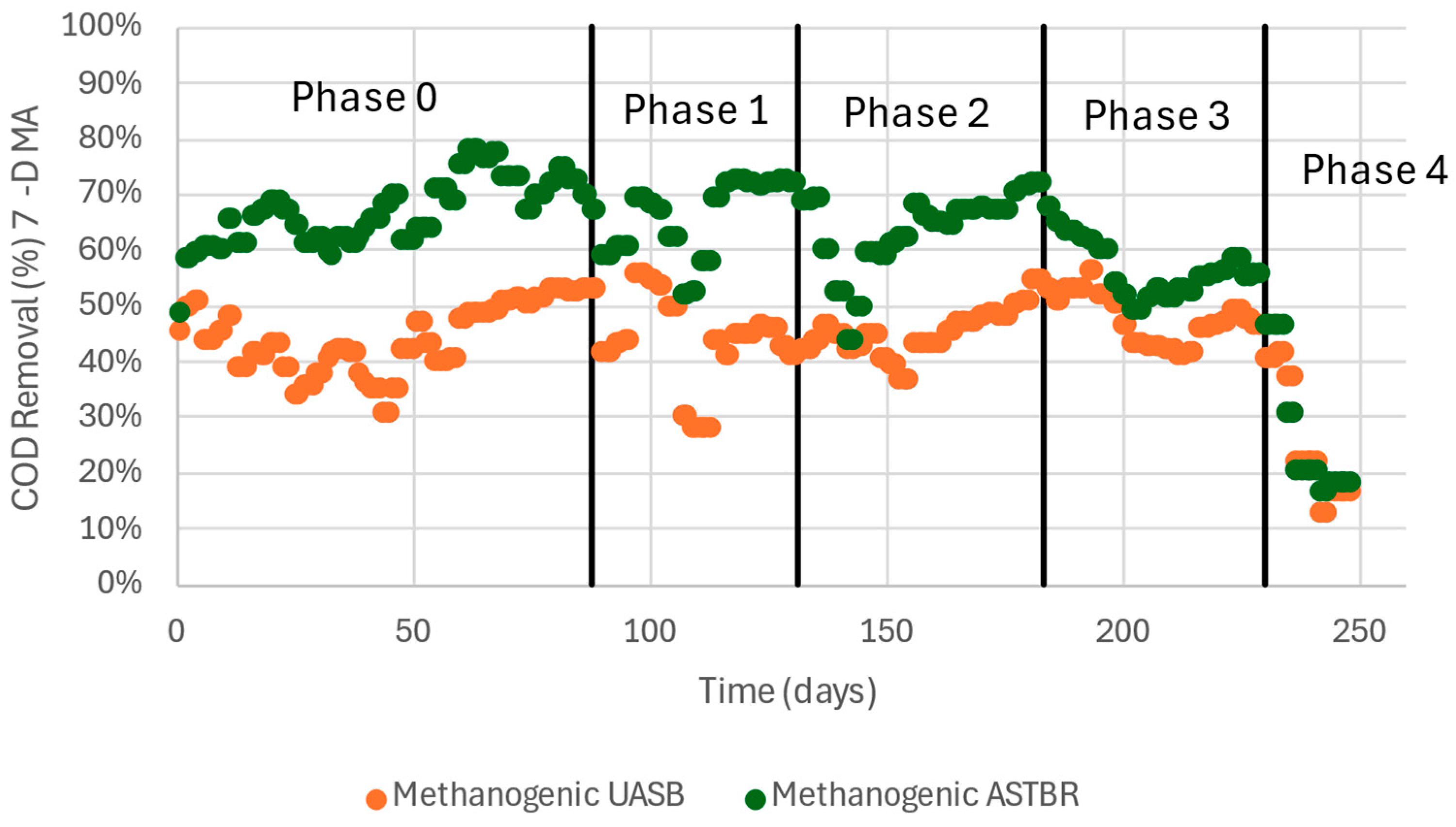


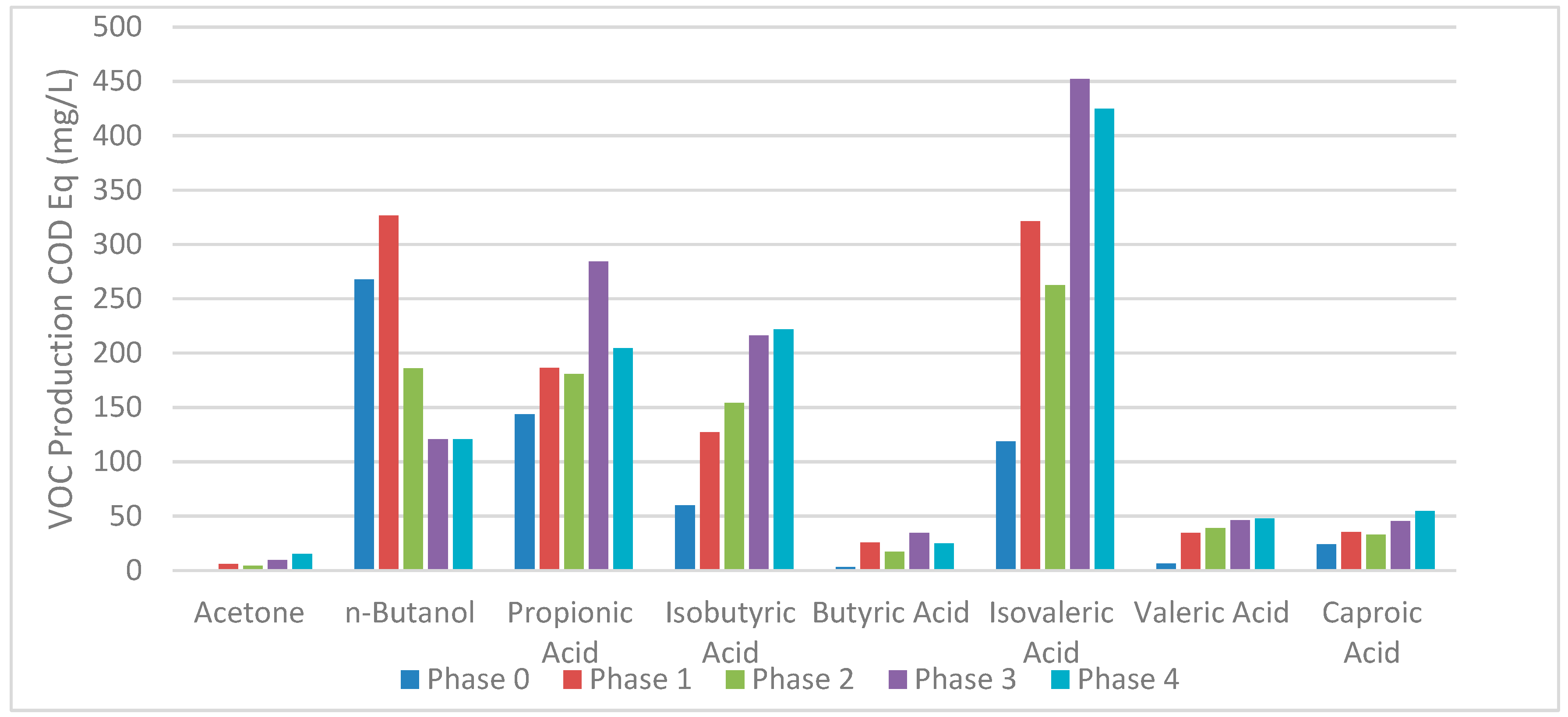
| Collection Lot | 21 July 2021 | 7 October 2021 | 24 June 2022 | Method |
|---|---|---|---|---|
| COD Total (mg·L−1) | 35,367 | 36,380 | 33,700 | APHA [24] |
| COD Soluble (mg·L−1) | 28,467 | 31,650 | 20,680 | APHA [24] |
| Lactic Acid (mg·L−1) | 2100 | 2120 | 720 | Taylor [25] |
| Glycerol (mg·L−1) | 3973 | 3956 | 2214 | Valdez [26] |
| Carbohydrates (mg·L−1) | 7760 | 7850 | 3750 | Dubois [27] |
| pH | 4.56 | 4.1 | 4.5 | APHA [24] |
| Sulfate (mg·L−1) | 2860 | 2000 | 1950 | APHA [24] |
| NTK (mg·L−1) | 1051.27 | 1205 | 1030 | APHA [24] |
| NH4+ (mg·L−1) | 154.09 | 147 | 92 | APHA [24] |
| Phenol (mg·L−1) | 1.29 | 3.49 | 2 | APHA [24] |
| Phosphorous (mg·L−1) | 47 | 53 | 60 | APHA [24] |
| Phase | Reactor | Configuration | Support Material | Total Height (mm) | Internal Diameter (mm) | Total Liquid Volume (L) |
|---|---|---|---|---|---|---|
| 1—Acidogenic | 1 | ASTBR | Structured Hollow Polyethylene Cylinders | 745 | 60 | 2.1 |
| 2—Methanogenic | 2 | UASB | None | 400 | 50 (main body) 80 (3-phase separator and headspace) | 1.6 |
| 3 | ASTBR | Vertically Arranged Polyurethane Foam Strips | 745 | 60 | 2.1 |
| Phase | Duration (Days) | Nominal Monensin Concentration (ng·mL−1) |
|---|---|---|
| 0 | 88 | 0 |
| 1 | 42 | 20 |
| 2 | 52 | 50 |
| 3 | 47 | 100 |
| 4 | 25 | 2000 |
| Monitored Parameter | Frequency | Method |
|---|---|---|
| pH | Daily | Electrometry [24] |
| Total Chemical Oxygen Demand (TCOD) (mg·L−1) | 3×/week | Closed Reflux Colorimetry [24] |
| Lactic Acid (HLa) (mg·L−1) | 3×/week | Calorimetry [25] |
| Glycerol (mg·L−1) | 3×/week | Enzymatic–Colorimetric [26] |
| Carbohydrates (mg·L−1) | 3×/week | Colorimetry [27] |
| Total Suspended Solids (TSSs) (mg/mL) | 1×/week | Gravimetry [24] |
| Volatile Suspended Solids (VSSs) (mg/mL) | 1×/week | Gravimetry [24] |
| Biogas Volumetric Production (L/d) | 5×/week | Mariotte’s Bottle |
| Biogas Composition | 5×/week | Gas Chromatography [30] |
| Volatile Organic Compounds (VOCs) (mg·L−1) | 3×/week | Gas Chromatography [31] |
| Monensin (MON) (ng·mL−1) | 3×/week | HPLC-MS/MS |
| Phase 0 | Phase 1 | Phase 2 | Phase 3 | Phase 4 | |
|---|---|---|---|---|---|
| Average MON Affluent Concentration (ng·mL−1) | - | 18.24 (5.79) | 49.76 (16.25) | 98.67 (29.68) | 1763.04 (472.05) |
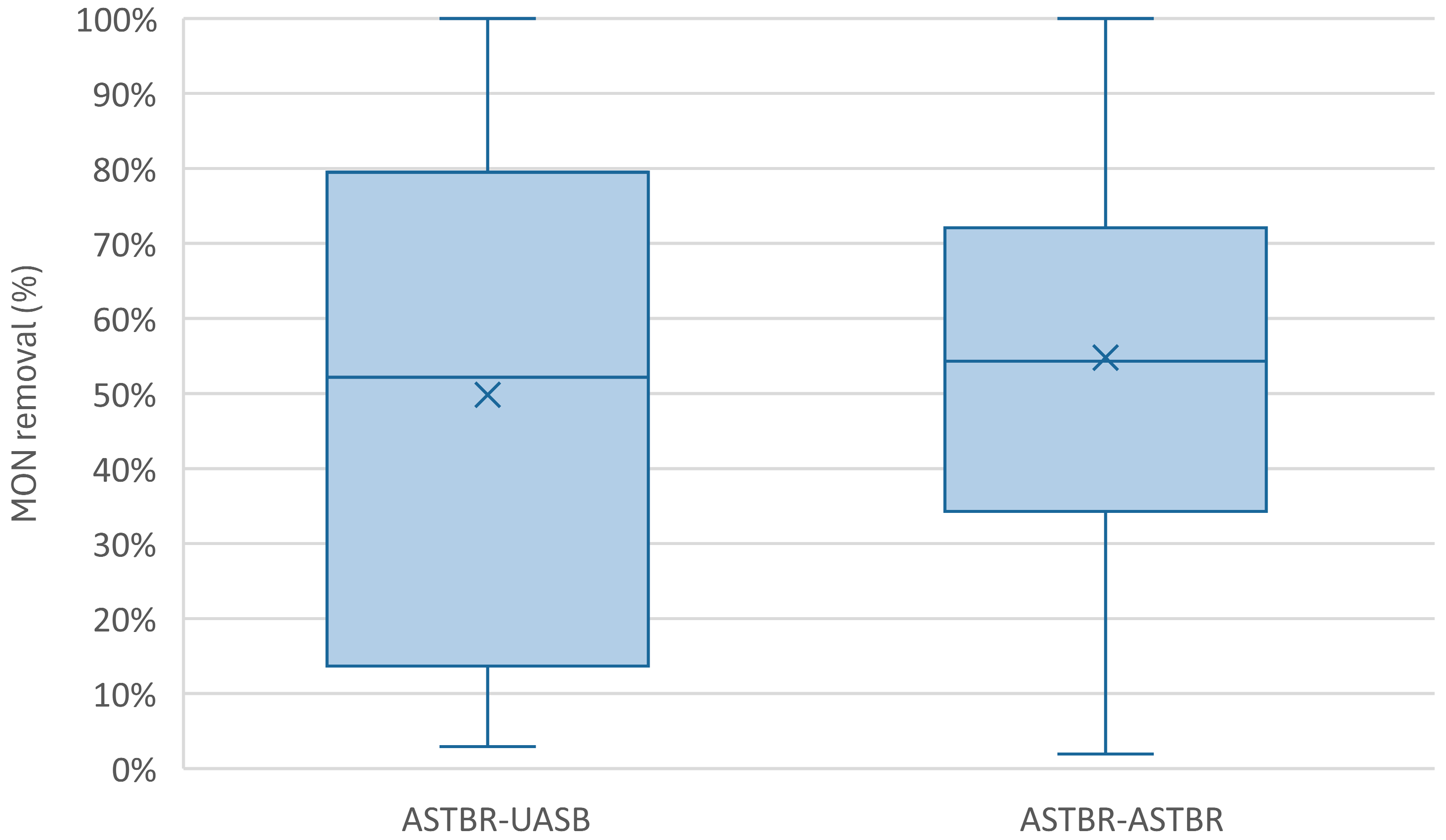
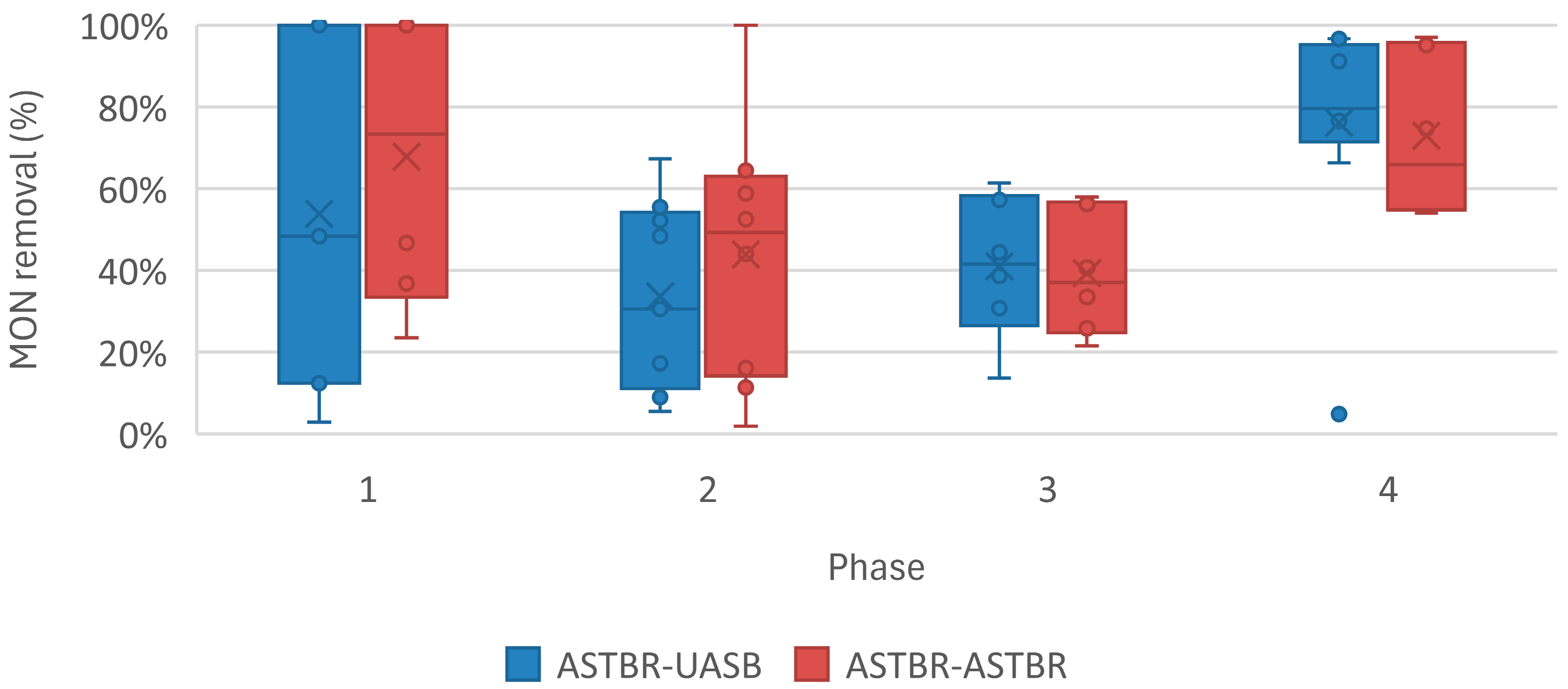
| MON Removal (%) | - | 96% (9%) | 88% (1%) | 84% (19%) | 71% (23%) |
| COD Removal (%) | 2% (9%) | 2% (8%) | 3% (14%) | 3% (7%) | 7% (7%) |
| Phase 0 | Phase 1 | Phase 2 | Phase 3 | Phase 4 | |
|---|---|---|---|---|---|
| Average MON Load (ng·mL−1) | - | 7.25 (8.6) | 43.83 (13.54) | 56.14 (36.7) | 839.83 (583.31) |
| UASB | |||||
| COD Removal (%) | 47 (7) | 44 (15) | 44 (7) | 48 (6) | 30 (13) |
| Average COD Removed (g·L−1) | 13.11 (1.49) | 10.88 (2.45) | 9.93 (2) | 10.37 (1.92) | 7.05 (2.92) |
| Monensin Removal (%) | - | 56 (38) | 21 (10) | 26 (13) | 54 (23) |
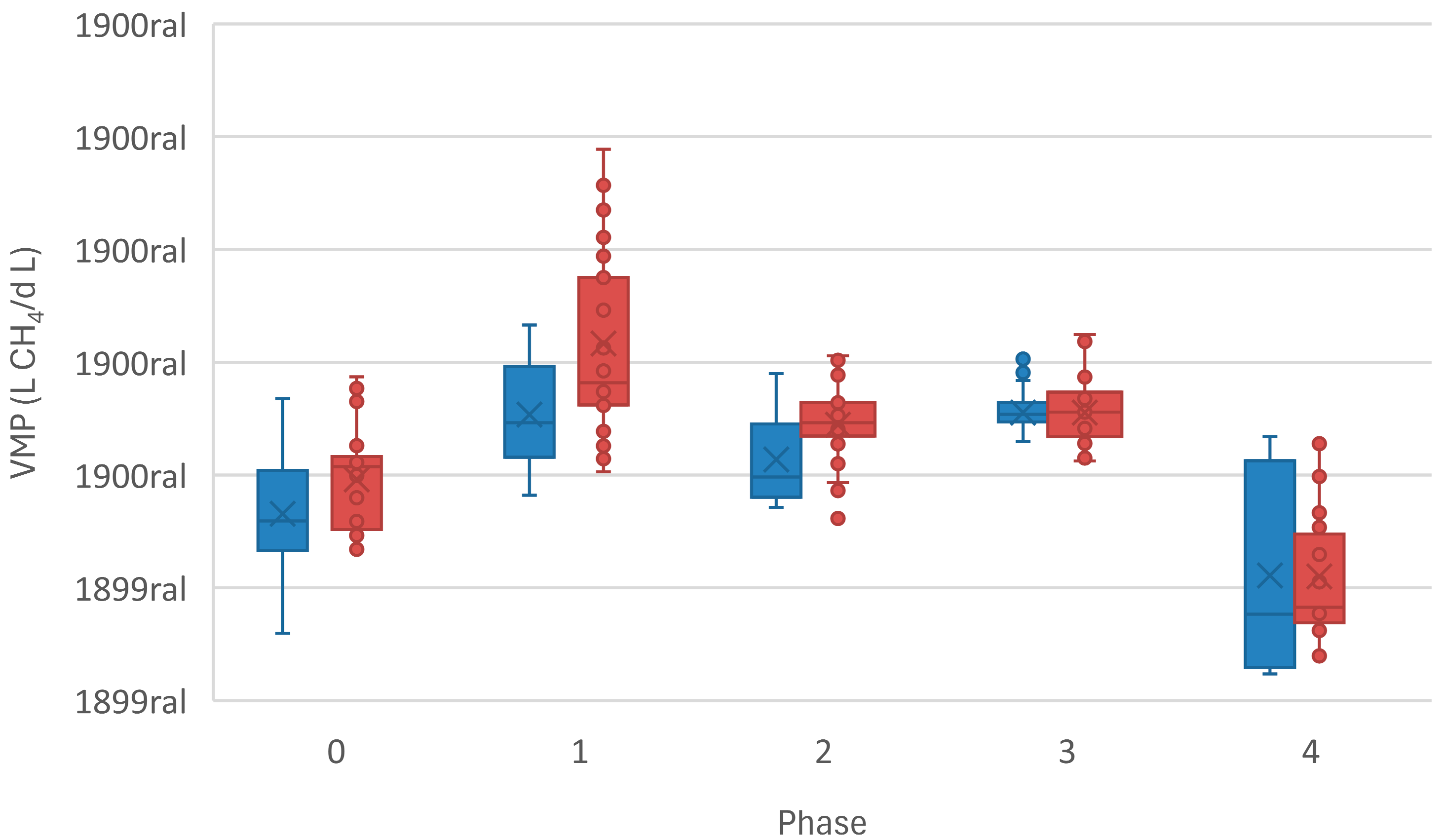
| VMP (L CH4·L−1·d−1) | 1.94 (0.58) | 2.54 (0.71) | 2.17 (0.78) | 2.6 (0.47) | 1.51 (1.01) |
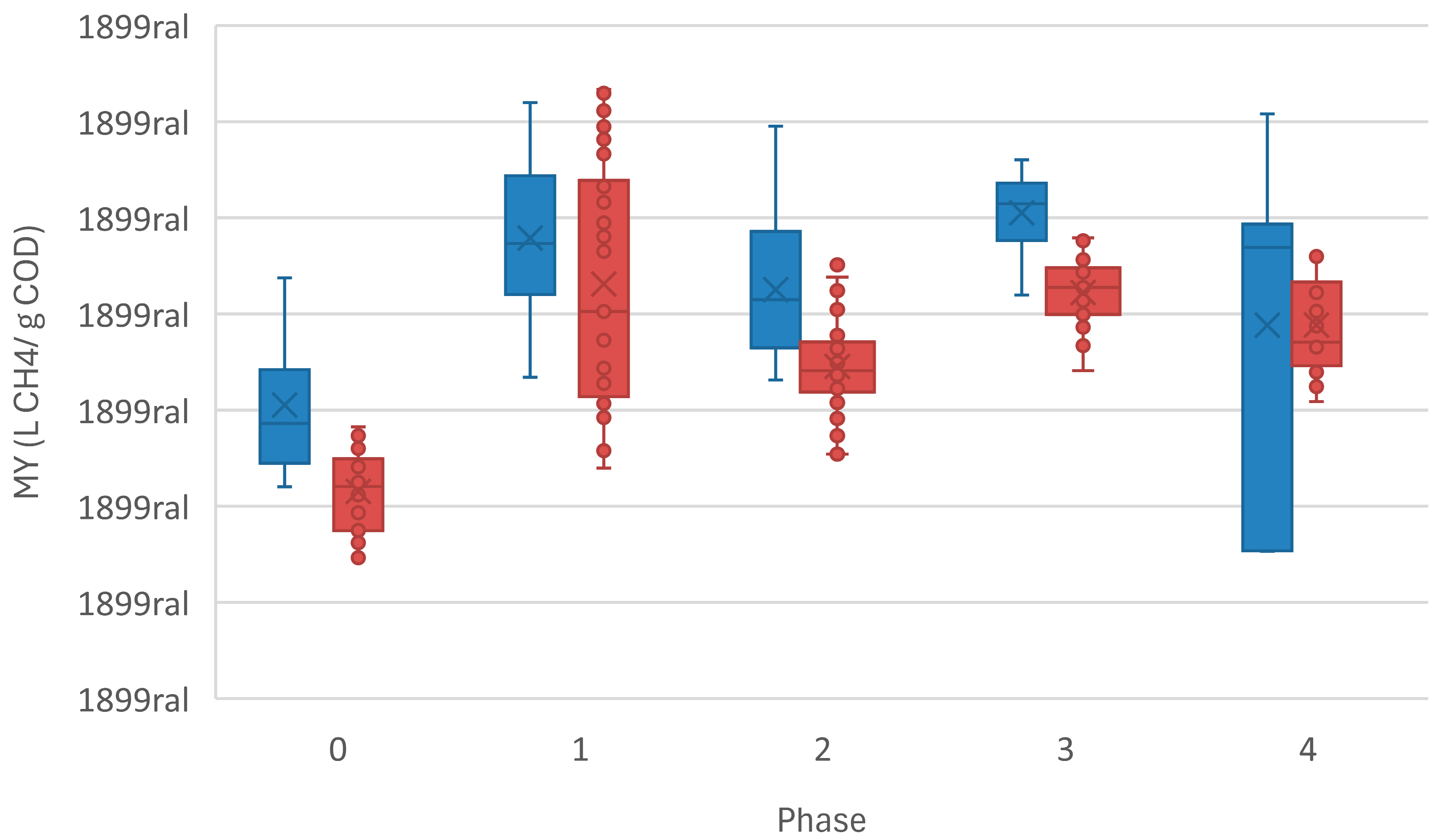
| MY (L CH4·g−1 COD Removed) | 0.148 | 0.233 | 0.219 | 0.25 | 0.214 |
| ASTBR | |||||
| COD Removal (%) | 68 (9) | 65 (10) | 63 (10) | 58 (8) | 33 (15) |
| Average COD Removed (g·L−1) | 18.92 (2.65) | 15.04 (2.85) | 14.32 (2.79) | 12.52 (2.23) | 7.63 (3.17) |
| Monensin Removal (%) | - | 59 (34) | 30 (28) | 20 (9) | 37 (13) |
| VMP (L CH4·L−1·d−1) | 1.98 (0.63) | 3.18 (1.1) | 2.44 (0.73) | 2.56 (0.56) | 1.42 (0.83) |
| MY (L CH4·g−1 COD Removed) | 0.105 | 0.211 | 0.17 | 0.205 | 0.186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatila, S.; Zaiat, M. Monensin Degradation and Methane Production from Sugarcane Vinasse in Two-Phase Thermophilic Anaerobic Fixed-Bed and Sludge Blanket Bioreactors. Fermentation 2025, 11, 518. https://doi.org/10.3390/fermentation11090518
Chatila S, Zaiat M. Monensin Degradation and Methane Production from Sugarcane Vinasse in Two-Phase Thermophilic Anaerobic Fixed-Bed and Sludge Blanket Bioreactors. Fermentation. 2025; 11(9):518. https://doi.org/10.3390/fermentation11090518
Chicago/Turabian StyleChatila, Sami, and Marcelo Zaiat. 2025. "Monensin Degradation and Methane Production from Sugarcane Vinasse in Two-Phase Thermophilic Anaerobic Fixed-Bed and Sludge Blanket Bioreactors" Fermentation 11, no. 9: 518. https://doi.org/10.3390/fermentation11090518
APA StyleChatila, S., & Zaiat, M. (2025). Monensin Degradation and Methane Production from Sugarcane Vinasse in Two-Phase Thermophilic Anaerobic Fixed-Bed and Sludge Blanket Bioreactors. Fermentation, 11(9), 518. https://doi.org/10.3390/fermentation11090518






