Optimization of Monascus purpureus Culture Conditions in Rice Bran for Enhanced Monascus Pigment Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
2.2. Determination of Color Value and Biomass
- OD: optical density.
2.3. Single-Factor Experiment
2.4. Steepest Ascent Design
2.5. Experimental Design for Response Surface Methodology (RSM)
2.6. Determination of MP Constituents and Citrinin (CIT) Content
2.7. Observation of Colony Morphology and Measurement of Colony Diameter
2.8. Determination of Biomass and Spore Number
2.9. Observation of Spore Micromorphology
2.10. Observation of Mycelia Morphology
2.11. Data Analysis
3. Results
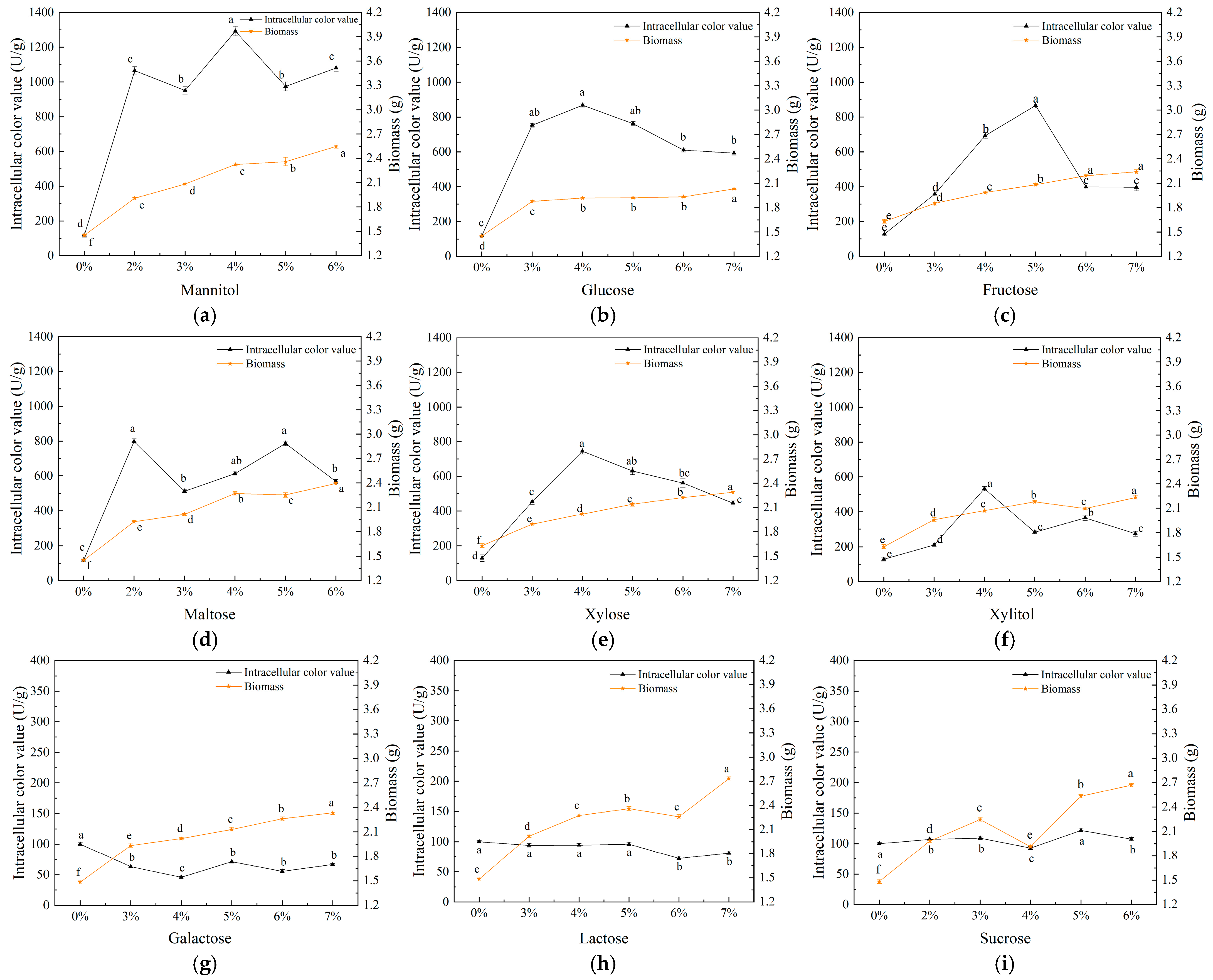
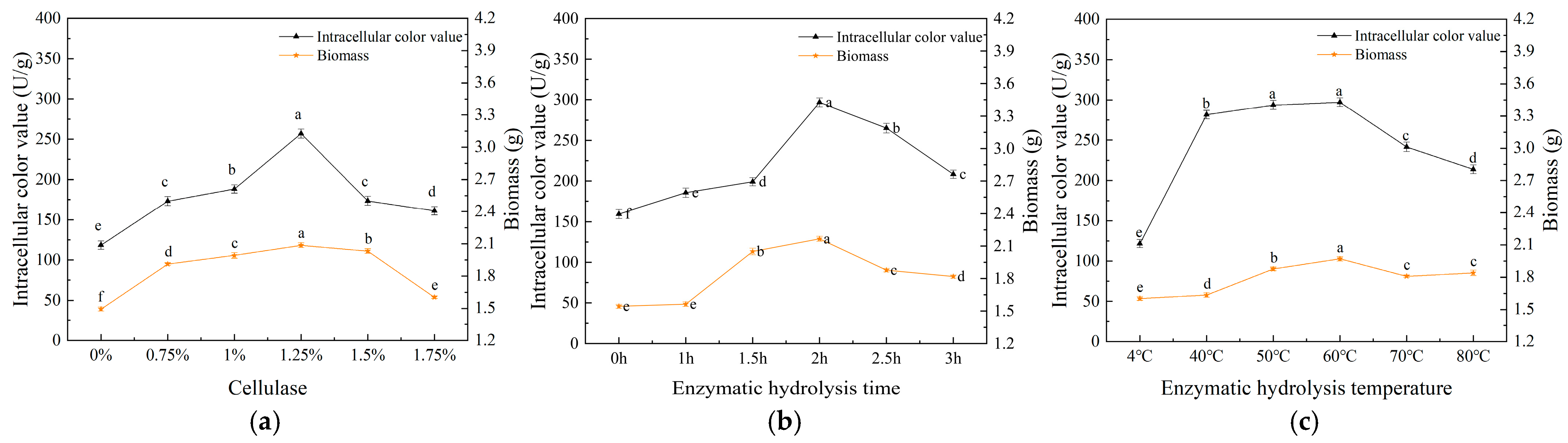
3.1. Single-Factor Experimental Analysis
3.1.1. Effect of Carbon Source on Biomass and Intracellular Pigments
3.1.2. Effect of Cellulase on MP Production and Intracellular Pigments
3.2. Steepest Ascent Experiment
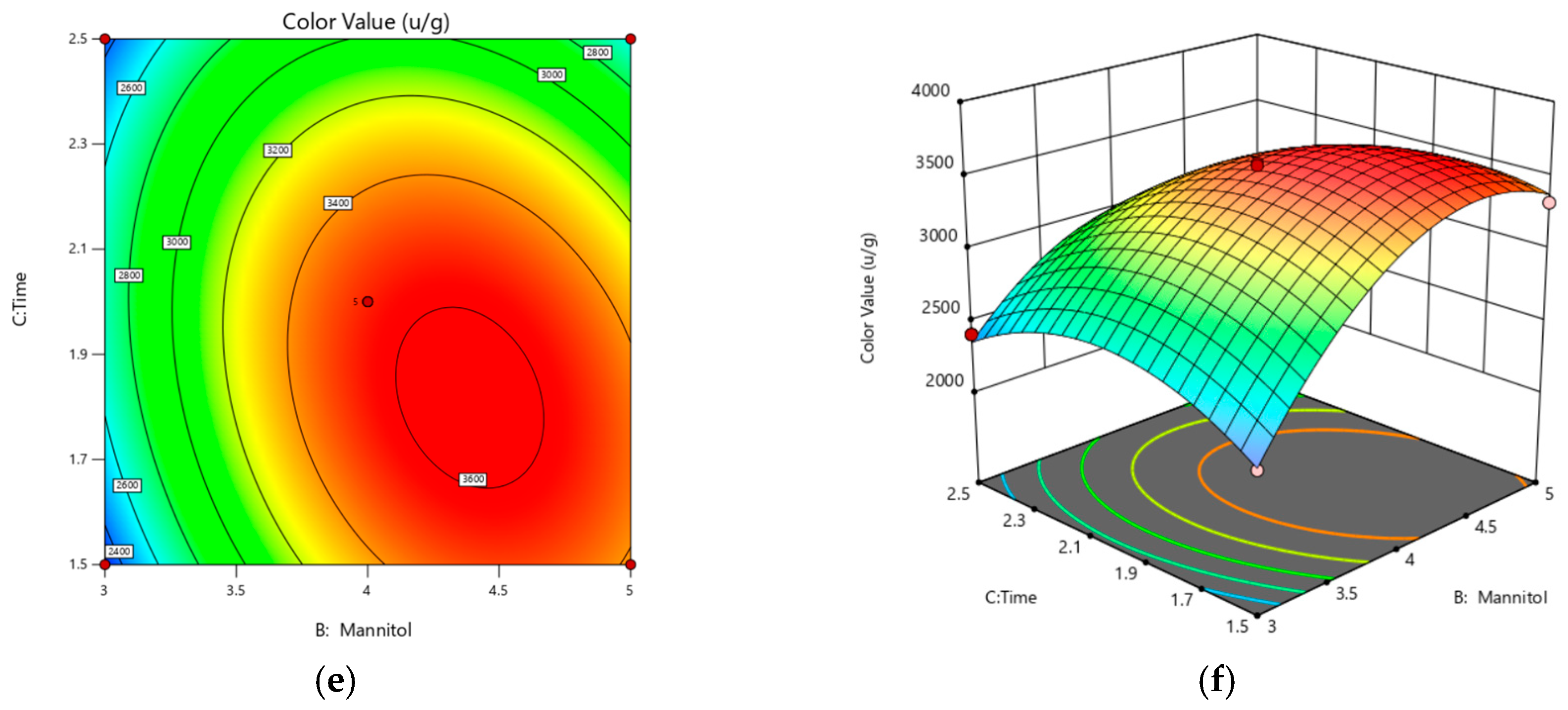
3.3. Response Surface Results and Analysis
3.3.1. Response Surface Experiments and Results
3.3.2. Model Fitting
3.3.3. Response Surface Analysis
3.4. Effect of Optimization Culture Conditions on Monascus Pigment Production, and Growth and Development
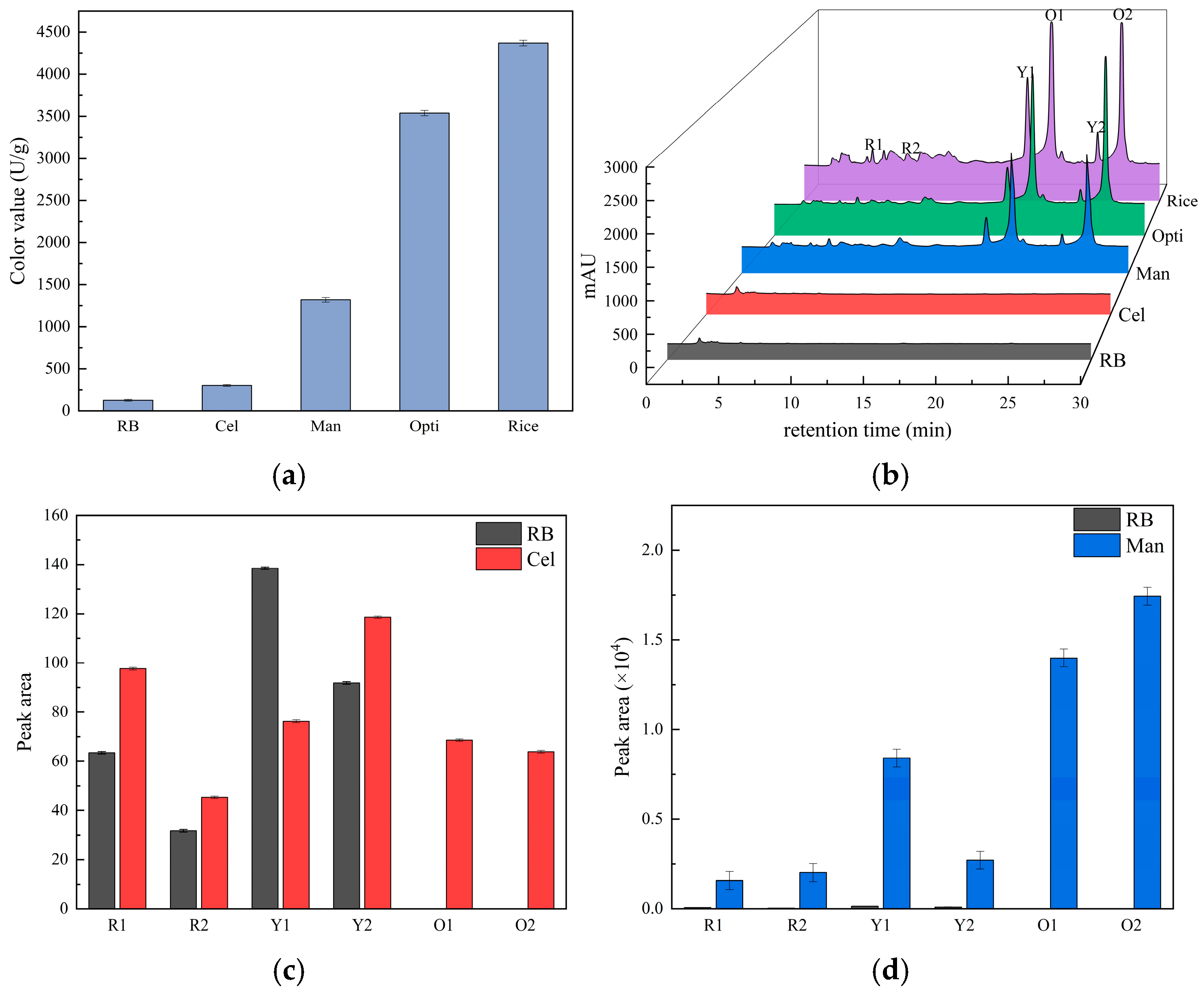
3.4.1. Effect of Optimization Culture Conditions on Monascus Pigment Production
3.4.2. Effect of Optimization Culture Conditions on Pigment Compositions and Citrinin Content
3.4.3. Effect of Optimization Culture Conditions on the Morphology of Colonies and Spores
3.4.4. Effect of Optimization Culture Conditions on Monascus Growth and Development
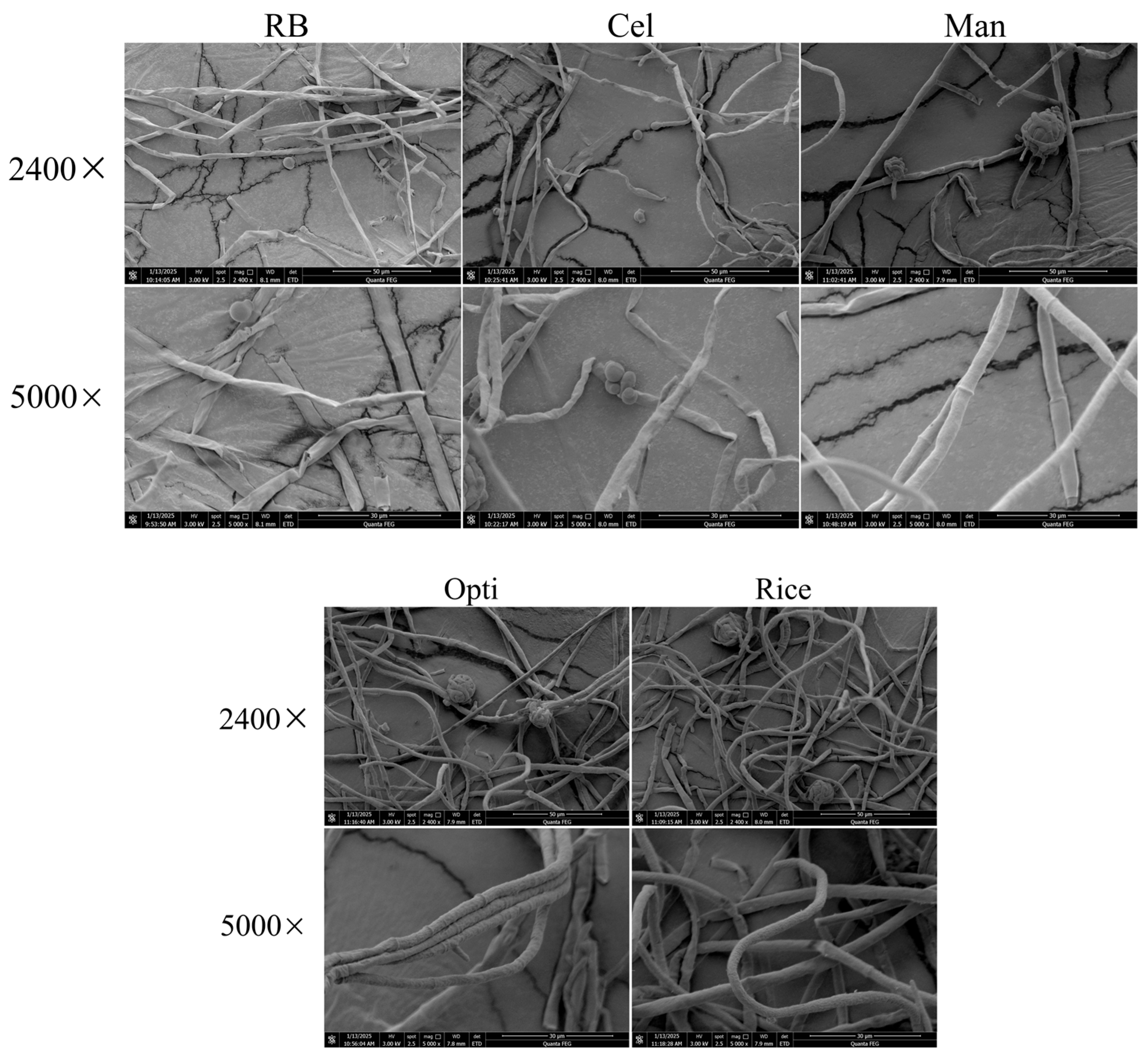
3.4.5. Effect of Optimization Culture Conditions on the Morphology of Mycelia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- He, S.S.; Liu, X.; Wang, Y.L.; Xie, J.H.; Gao, H.; Li, X.J.; Huang, Z.B. Metabolomics analysis based on UHPLC-Q-TOF-MS/MS reveals effects of genistein on reducing mycotoxin citrinin production by Monascus aurantiacus Li AS3.4384. LWT-Food Sci. Technol. 2020, 130, 109613. [Google Scholar] [CrossRef]
- Feng, Y.L.; Shao, Y.C.; Zhou, Y.X.; Chen, W.P.; Chen, F.S. Monascus pigments. Appl. Microbiol. Biotechnol. 2012, 96, 1421–1440. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Qin, X.L.; Zhao, Y.R.; Sun, X.X.; Yu, X.; Feng, Y.L. Strategies to enhance the production efficiency of Monascus pigments and control citrinin contamination. Process Biochem. 2022, 117, 19–29. [Google Scholar] [CrossRef]
- Koli, S.H.; Suryawanshi, R.K.; Mohite, B.V.; Patil, S.V. Prospective of Monascus pigments as an additive to commercial sunscreens. Nat. Prod. Commun. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Lee, C.L.; Wen, J.Y.; Hsu, Y.W.; Pan, T.M. The blood lipid regulation of Monascus-produced monascin and ankaflavin via the suppression of low-density lipoprotein cholesterol assembly and stimulation of apolipoprotein A1 expression in the liver. J. Microbiol. Immunol. Infect. 2018, 51, 27–37. [Google Scholar] [CrossRef]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Kiyota, A.; Yasukawa, K.; Sakamoto, N.; Kimura, Y.; Suzuki, T.; Takayasu, J.; Nishino, H. Anti-tumor-initiating effects of Monascin, an azaphilonoid pigment from the extract of Monascus pilosus fermented rice (red-mold rice). Chem. Biodivers. 2005, 2, 1305–1309. [Google Scholar] [CrossRef]
- Hsu, L.C.; Hsu, Y.W.; Liang, Y.H.; Liaw, C.C.; Kuo, Y.H.; Pan, T.M. Induction of apoptosis in human breast adenocarcinoma cells MCF-7 by Monapurpyridine A, a new azaphilone derivative from Monascus purpureus NTU 568. Molecules 2012, 17, 664–673. [Google Scholar] [CrossRef]
- Meinicke, R.M.; Vendruscolo, F.; Moritz, D.E.; Oliveira, D.D.; Schmidell, W.; Samohyl, R.W.; Ninow, J.L. Potential use of glycerol as substrate for the production of red pigments by Monascus ruber in submerged fermentation. Biocatal. Agric. Biotechnol. 2012, 1, 238–242. [Google Scholar] [CrossRef]
- Guo, X.Y.; Atehli, D.; Chen, M.H.; Chen, D.; Wang, Y.R. A Zn (II)(2) Cys (6) transcription factor MPsGeI suppresses pigment biosynthesis in Monascus. Int. J. Biol. Macromol. 2023, 233, 123504. [Google Scholar] [CrossRef]
- Terán Hilares, R.; De Souza, R.A.; Marcelino, P.F.; Da Silva, S.S.; Dragone, G.; Mussatto, S.I.; Santos, J.C. Sugarcane bagasse hydrolysate as a potential feedstock for red pigment production by Monascus ruber. Food Chem. 2018, 245, 786–791. [Google Scholar] [CrossRef]
- Silbir, S.; Goksungur, Y. Natural red pigment production by Monascus purpureus in submerged fermentation systems using a food industry waste: Brewer’s spent grain. Foods 2019, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Kantifedaki, A.; Kachrimanidou, V.; Mallouchos, A.; Papanikolaou, S.; Koutinas, A.A. Orange processing waste valorisation for the production of bio-based pigments using the fungal strains Monascus purpureus and Penicillium purpurogenum. J. Cleaner. Prod. 2018, 185, 882–890. [Google Scholar] [CrossRef]
- ElSayed, E.S.R.; Gach, J.; Olejniczak, T.; Boratyński, F. A new endophyte Monascus ruber SRZ112 as an efficient production platform of natural pigments using agro-industrial wastes. Sci. Rep. 2022, 12, 12611. [Google Scholar] [CrossRef] [PubMed]
- Asghari, M.; Jahadi, M.; Hesam, F.; GhasemiSepro, N. Optimization of Monascus pigment production on date waste substrates using solid state fermentation. Appl. Food Biotechnol. 2021, 8, 247–254. [Google Scholar]
- Arslan, N.P.; Yazici, A.; Komesli, S.; Esim, N.; Ortucu, S. Direct conversion of waste loquat kernels to pigments using Monascus purpureus ATCC16365 with proteolytic and amylolytic activity. Biomass Convers. Biorefin. 2021, 11, 2191–2199. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, W.; Nkechi, O.; Lu, P.X.; Bai, J.; Lin, Q.L.; Liu, J. Utilization of low-cost agricultural by product rice husk for Monascus pigments production via submerged batch-fermentation. J. Sci. Food Agric. 2022, 102, 2454–2463. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Y.C.; Guo, T.; Tang, C.G.; Chai, X.Y.; Zhao, W.; Bai, J.; Lin, Q.L. Cost-effective pigment production by Monascus purpureus using rice straw hydrolysate as substrate in submerged fermentation. J. Biosci. Bioeng. 2020, 129, 229–236. [Google Scholar] [CrossRef]
- Chen, X.J.; Chen, M.M.; Wu, X.F.; Li, X.J. Cost-effective process for the production of Monascus pigments using potato pomace as carbon source by fed-batch submerged fermentation. Food Sci. Nutr. 2021, 9, 5415–5427. [Google Scholar] [CrossRef]
- Pourali, O.; Asghari, F.S.; Yoshida, H. Production of phenolic compounds from rice bran biomass under subcritical water conditions. Chem. Eng. J. 2010, 160, 259–266. [Google Scholar] [CrossRef]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice bran: A novel functional ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816. [Google Scholar] [CrossRef]
- Xu, Z.M.; Godber, J.S. Purification and identification of components of γ-oryzanol in rice bran oil. J. Agric. Food. Chem. 1999, 47, 2724–2728. [Google Scholar] [CrossRef]
- Cao, T.C.; Nguyen, T.P.; Nguyen, S.N.; Tran, T.T.T.; Ton, N.M.N.; Le, V.V.M. Cellulase-treated deoiled rice bran: Effects of treatment conditions on dietary fiber content and utilization for formulation of cookies. J. Food Meas. Charact. 2022, 16, 840–848. [Google Scholar] [CrossRef]
- Gong, P.F.; Shi, R.Y.; Liu, Y.T.; Luo, Q.Q.; Wang, C.G.; Chen, W. Recent advances in Monascus pigments produced by Monascus purpureus: Biosynthesis, fermentation, function, and application. Lwt-Food Sci. Technol. 2023, 185, 115162. [Google Scholar] [CrossRef]
- Li, L.; Zhu, L.; Liu, X.; Guo, H.; Huang, N.; Wang, Z.; Dai, L. Multiomics comparative analysis reveals the role of carbon metabolism in enhancing monascin and ankaflavin production in Monascus purpureus. Ind. Crop. Prod. 2025, 228, 120938. [Google Scholar] [CrossRef]
- Qin, X.; Han, H.; Zhang, J.; Xie, B.; Zhang, Y.; Liu, J.; Dong, W.; Hu, Y.; Yu, X.; Feng, Y. Transcriptomic and metabolomic analyses of soybean protein isolate on Monascus pigments and Monacolin K production. J. Fungi. 2024, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Joyce, A.P.; Leung, S.S. Use of response surface methods and path of steepest ascent to optimize ligand-binding assay sensitivity. J. Immunol. Methods. 2023, 392, 12–23. [Google Scholar] [CrossRef]
- Lee, D.; Kim, S.; Byun, J. A method of steepest ascent for multiresponse surface optimization using a desirability function method. Qual. Reliab. Eng. lnt. 2020, 36, 1931–1948. [Google Scholar] [CrossRef]
- Chen, D.; Li, H. Mannitol improves Monascus pigment biosynthesis with rice bran as a substrate in Monascus purpureus. Front. Microbiol. 2023, 14, 1300461. [Google Scholar] [CrossRef]
- Shi, R.; Luo, Q.; Liu, Y.; Chen, W.; Wang, C. Effect of γ-heptalactone on the morphology and production of Monascus pigments and Monacolin K in Monascus purpureus. J. Fungi. 2022, 8, 179. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, J.; Li, M.; Shao, Y.; Chen, F. MrGcn5 is required for the mycotoxin production, sexual and asexual development in Monascus ruber. Food Biosci. 2021, 43, 101304. [Google Scholar] [CrossRef]
- Zeng, H.W.; Wang, C.T.; Qiao, J.; Zhang, B.J.; Zhao, B.; Dai, C.Y. Determining a suitable carbon source for the production of intracellular pigments from Monascus purpureus HBSD 08. Pigment. Resin. Technol. 2019, 48, 547–554. [Google Scholar]
- Moussa, S.; Abdou, D.; Mohamed, G.A.; Abo-El-Seoud, M.A.; Karam Eldin, A.Z.A.; El-mehalawy, A.A. Production of red pigment by Monascus purpureus Nrrl 1992 under submerged and solid-state fermentation. Egypt. J. Microbiol. 2018, 53, 83–94. [Google Scholar]
- Omamor, I.B.; Eziashi, E.I.; Adekunle, A.A. Carbon nutrition in relation to growth of three Monascus species isolated from decaying date fruits. Afr. J. Microbiol. Res. 2008, 2, 153–155. [Google Scholar]
- Lima, A.G.; Dantas, L.A.; Egea, M.B. Mannitol-based media and static pH are efficient conditions for red pigment production from Monascus purpureus ATCC 36928 in submerged culture. Processes 2023, 11, 633. [Google Scholar] [CrossRef]
- Da Silva, M.F.; Flaibam, B.; De Mélo, A.H.F.; Sampaio, U.; Clerici, M.T.P.S.; Goldbeck, R. Optimization of enzymatic hydrolysis of cellulose extracted from bamboo culm for bioethanol production by Saccharomyces cerevisiae modified via CRISPR/Cas9. Food Res. Int. 2024, 192, 114768. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.; Gaikwad, A. Optimization of enzymatic hydrolysis of bamboo biomass for enhanced saccharification of cellulose through taguchi orthogonal design. J. Environ. Chem. Eng. 2021, 9, 104807. [Google Scholar] [CrossRef]
- Ruan, L.G.; Wu, H.F.; Wu, S.Y.; Zhou, L.F.; Wu, S.X.; Shang, C.H. Optimizing the conditions of pretreatment and enzymatic hydrolysis of sugarcane bagasse for bioethanol production. ACS Omega 2024, 9, 29566–29575. [Google Scholar] [CrossRef]
- Dashtban, M.; Maki, M.; Leung, K.T.; Mao, C.; Qin, W. Cellulase activities in biomass conversion: Measurement methods and comparison. Crit. Rev. Biotechnol. 2010, 30, 302–309. [Google Scholar] [CrossRef]
- Abdel-Raheam, H.E.F.; Alrumman, S.A.; Gadow, S.I.; El-Sayed, M.H.; Hikal, D.M.; Hesham, A.E.L.; Ali, M.M.A. Optimization of Monascus purpureus for natural food pigments production on potato wastes and their application in ice lolly. Front. Microbiol. 2022, 13, 862080. [Google Scholar] [CrossRef]
- Chen, D.; Xue, Y.; Chen, M.H.; Li, Z.J.; Wang, C.L. Optimization of submerged fermentation medium for citrinin-free monascin production by Monascus. Prep. Biochem. Biotechnol. 2016, 46, 772–779. [Google Scholar] [CrossRef]
- Yang, S.Z.; Huang, Z.F.; Liu, H.Q.; Hu, X.; Wu, Z.Q. Improving mycelial morphology and adherent growth as well as metabolism of Monascus yellow pigments using nitrate resources. Appl. Microbiol. Biotechnol. 2020, 104, 9607–9617. [Google Scholar] [CrossRef]
- Li, T.; Zhao, W.Q.; Wang, C.T.; Shi, K.; Chen, G. Regulated synthesis and metabolism of Monascus pigments in a unique environment. World J. Microbiol. Biotechnol. 2023, 39, 16129. [Google Scholar] [CrossRef]





| Count | Enzymatic Hydrolysis Temperature | Mannitol Additive Amount | Enzymatic Hydrolysis Time | Color Value (U/g) |
|---|---|---|---|---|
| 1 | 40 °C | 2% | 1.0 h | 1398 |
| 2 | 50 °C | 3% | 1.5 h | 2475 |
| 3 | 60 °C | 4% | 2.0 h | 3575 |
| 4 | 70 °C | 5% | 2.5 h | 3000 |
| 5 | 80 °C | 6% | 3.0 h | 2705 |
| Run | A: Enzymatic Hydrolysis Temperature (°C) | B: Mannitol Additive Amount (%) | C: Enzymatic Hydrolysis Time (hour) | Color Value (U/g) |
|---|---|---|---|---|
| 1 | 60 | 4 | 2.0 | 3575 |
| 2 | 50 | 4 | 1.5 | 3175 |
| 3 | 60 | 5 | 1.5 | 3325 |
| 4 | 60 | 4 | 2.0 | 3540 |
| 5 | 60 | 4 | 2.0 | 3505 |
| 6 | 60 | 5 | 2.5 | 2625 |
| 7 | 60 | 3 | 1.5 | 2285 |
| 8 | 70 | 3 | 2.0 | 2250 |
| 9 | 60 | 4 | 2.0 | 3590 |
| 10 | 70 | 4 | 2.5 | 2705 |
| 11 | 50 | 5 | 2.0 | 3190 |
| 12 | 60 | 3 | 2.5 | 2410 |
| 13 | 50 | 4 | 2.5 | 2625 |
| 14 | 70 | 4 | 1.5 | 3000 |
| 15 | 60 | 4 | 2.0 | 3500 |
| 16 | 70 | 5 | 2.0 | 2925 |
| 17 | 50 | 3 | 2.0 | 2440 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 3,715,000.00 | 9 | 412,800.00 | 81.50 | <0.0001 | ** |
| A | 37,812.50 | 1 | 37,812.50 | 7.47 | 0.0009 | ** |
| B | 897,800.00 | 1 | 897,800.00 | 177.26 | <0.0001 | ** |
| C | 252,100.00 | 1 | 252,100.00 | 49.76 | 0.0002 | ** |
| AB | 1406.25 | 1 | 1406.25 | 0.28 | 0.6145 | N |
| AC | 16,256.25 | 1 | 16,256.25 | 3.21 | 0.1163 | N |
| BC | 170,200.00 | 1 | 170,200.00 | 33.59 | 0.0007 | ** |
| AA | 412,200.00 | 1 | 412,200.00 | 81.38 | <0.0001 | ** |
| BB | 1,173,000.00 | 1 | 1,173,000.00 | 231.64 | <0.0001 | ** |
| CC | 524,300.00 | 1 | 524,300.00 | 103.51 | <0.0001 | ** |
| Residual | 35,455.00 | 7 | 5065.00 | |||
| Lack of Fit | 28,925.00 | 3 | 9641.67 | 5.91 | 0.0596 | N |
| Pure Error | 6530.00 | 4 | 1632.50 | |||
| Cor Total | 3,750,000.00 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Xu, Y.; Li, H.; Zhu, X. Optimization of Monascus purpureus Culture Conditions in Rice Bran for Enhanced Monascus Pigment Biosynthesis. Fermentation 2025, 11, 505. https://doi.org/10.3390/fermentation11090505
Chen D, Xu Y, Li H, Zhu X. Optimization of Monascus purpureus Culture Conditions in Rice Bran for Enhanced Monascus Pigment Biosynthesis. Fermentation. 2025; 11(9):505. https://doi.org/10.3390/fermentation11090505
Chicago/Turabian StyleChen, Di, Yanping Xu, Han Li, and Xuemin Zhu. 2025. "Optimization of Monascus purpureus Culture Conditions in Rice Bran for Enhanced Monascus Pigment Biosynthesis" Fermentation 11, no. 9: 505. https://doi.org/10.3390/fermentation11090505
APA StyleChen, D., Xu, Y., Li, H., & Zhu, X. (2025). Optimization of Monascus purpureus Culture Conditions in Rice Bran for Enhanced Monascus Pigment Biosynthesis. Fermentation, 11(9), 505. https://doi.org/10.3390/fermentation11090505





