Production Technology of Fermented Distiller’s Grains and Its Effect on Production Performance and Egg Quality of Laying Hens
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of FDG
2.2. Chemical Analysis of DG and FDG
2.3. In Vitro Digestion Analysis of DG and FDG
2.4. Birds and Feeding Management
2.5. Sample Collection in Animal Feeding Trial
2.6. Egg Quality Determination
2.7. Statistical Analysis
3. Results
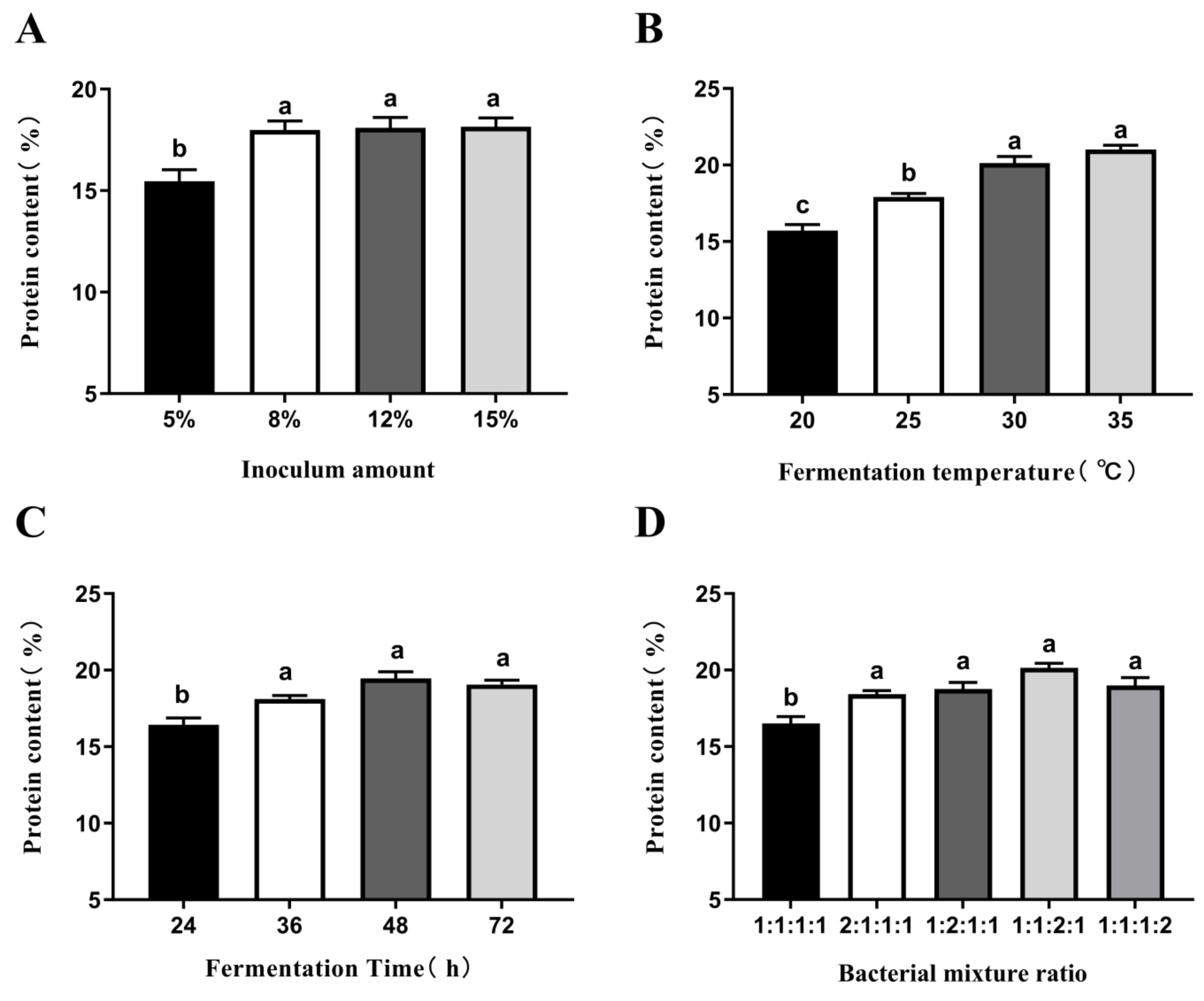
3.1. Confirmation of DG Fermentation Conditions
3.2. Chemical Analysis of DG and FDG
3.3. In Vitro Evaluation of DG and FDG
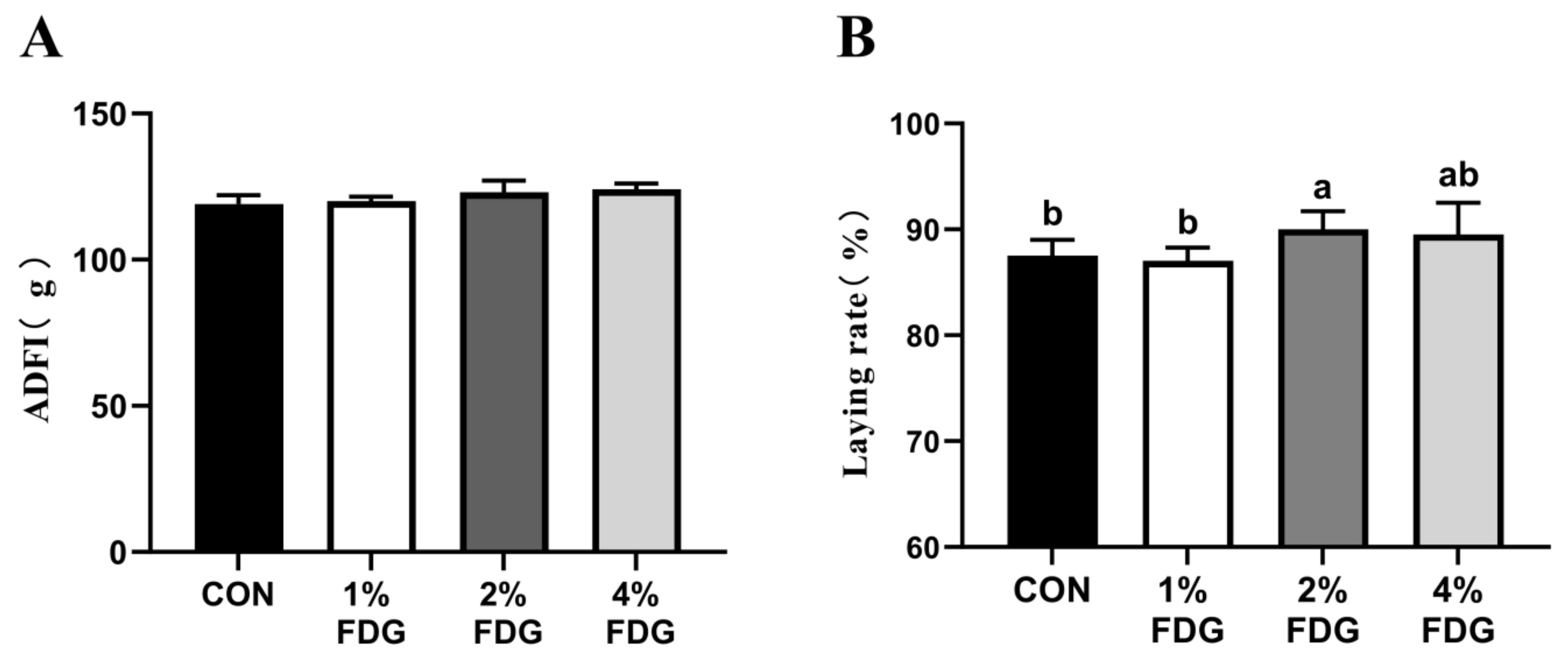
3.4. Effects of FDG on Laying Performance
3.5. Effects of FDG on Egg Quality
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shad, Z.M.; Venkitasamy, C.; Wen, Z. Corn Distillers Dried Grains with Solubles: Production, Properties, and Potential Uses. Cereal Chem. 2021, 98, 999–1019. [Google Scholar] [CrossRef]
- Liu, K. Chemical Composition of Distillers Grains, a Review. J. Agric. Food Chem. 2011, 59, 1508–1526. [Google Scholar] [CrossRef]
- Roth, M.; Jekle, M.; Becker, T. Opportunities for Upcycling Cereal Byproducts with Special Focus on Distiller’s Grains. Trends Food Sci. Technol. 2019, 91, 282–293. [Google Scholar] [CrossRef]
- Villegas-Torres, M.F.; Ward, J.M.; Lye, G.J. The Protein Fraction from Wheat-Based Dried Distiller’s Grain with Solubles (DDGS): Extraction and Valorization. New Biotechnol. 2015, 32, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, D.; Li, Y. Proteins in Dried Distillers’ Grains with Solubles: A Review of Animal Feed Value and Potential Non-Food Uses. J. Am. Oil Chem. Soc. 2021, 98, 957–968. [Google Scholar] [CrossRef]
- Tao, X.; Lv, J.; Xu, H.; Xiong, X.; Jin, S. Research Progress on Comprehensive Utilization of Distillers’ Grains and Practices of Moutai. China Brew. 2023, 42, 22–27. [Google Scholar]
- Wang, C.; Liu, Y.; Xu, L.; Xin, C.; Tan, Z.; Zhang, X.; Ma, C.; Chen, S.; Li, H. Changes of the Main Components, Physicochemical Properties of Distiller’s Grains after Extrusion Processing with Focus on Modification Mechanism. Food Chem. 2022, 390, 133187. [Google Scholar] [CrossRef]
- Pack, E.D.; Weiland, S.; Musser, R.; Schmale, D.G. Survey of Zearalenone and Type-B Trichothecene Mycotoxins in Swine Feed in the USA. Mycotoxin Res. 2021, 37, 297–313. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and Future Strategies for Wine Yeast Lees Valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel Application and Industrial Exploitation of Winery By-Products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Lee, M.-S.; Hoadley, A.; Patel, J.; Lim, S.; Kozielski, K.; Li, C. Techno-Economic Analysis for Direct Processing of Wet Solid Residues Originated from Grain and Inedible Plant Wastes. Bioenerg. Res. 2023, 16, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.S.; Rosendahl, L.; Nielsen, M.P.; Glasius, M.; Rudolf, A.; Iversen, S.B. Continuous Production of Bio-Oil by Catalytic Liquefaction from Wet Distiller’s Grain with Solubles (WDGS) from Bio-Ethanol Production. Biomass Bioenerg. 2012, 36, 327–332. [Google Scholar] [CrossRef]
- Fan, W.; Sun, X.; Cui, G.; Li, Q.; Xu, Y.; Wang, L.; Li, X.; Hu, B.; Chi, Z. A Strategy of Co-Fermentation of Distillers Dried Grains with Solubles (DDGS) and Lignocellulosic Feedstocks as Swine Feed. Crit. Rev. Biotechnol. 2023, 43, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.; Huang, C.; Ge, X.; Xi, B.; Mao, J. Chinese Baijiu Distiller’s Grains Resourcing: Current Progress and Future Prospects. Resour. Conserv. Recycl. 2022, 176, 105900. [Google Scholar] [CrossRef]
- Iram, A.; Cekmecelioglu, D.; Demirci, A. Distillers’ Dried Grains with Solubles (DDGS) and Its Potential as Fermentation Feedstock. Appl. Microbiol. Biotechnol. 2020, 104, 6115–6128. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Abdelrahman, M.M.; Alatiyat, R.M.; Aljumaah, M.R.; Al Jassim, R.; Stanley, D. Effect of Dietary Inclusion of Graded Levels of Distillers Dried Grains with Solubles on the Performance, Blood Profile and Rumen Microbiota of Najdi Lambs. Heliyon 2021, 7, e05683. [Google Scholar] [CrossRef]
- Van Emon, M.L.; Gunn, P.J.; Neary, M.K.; Lemenager, R.P.; Schultz, A.F.; Lake, S.L. Effects of Added Protein and Dietary Fat on Lamb Performance and Carcass Characteristics When Fed Differing Levels of Dried Distiller’s Grains with Solubles. Small Rumin. Res. 2012, 103, 164–168. [Google Scholar] [CrossRef]
- Huang, J.; Wu, T.; Sun, X.; Zou, C.; Yang, Y.; Cao, Y.; Yang, Y.; Wasim Iqbal, M.; Lin, B. Effect of Replacing Conventional Feeds with Tropical Agricultural By-Products on the Growth Performance, Nutrient Digestibility and Ruminal Microbiota of Water Buffaloes. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1034–1042. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Rajendran, A.; Hu, B. New Technologies in Value Addition to the Thin Stillage from Corn-to-Ethanol Process. Rev. Environ. Sci. Bio/Technol. 2017, 16, 175–206. [Google Scholar] [CrossRef]
- Salim, H.M.; Kruk, Z.A.; Lee, B.D. Nutritive Value of Corn Distillers Dried Grains with Solubles as an Ingredient of Poultry Diets: A Review. World Poultry Sci. J. 2010, 66, 411–432. [Google Scholar] [CrossRef]
- Lei, H.; Yang, C.; Chen, S.; He, Y.; Bin, D. Comparison of Nutritional Value among White, Beer and Rice Distiller’s Grains for Broilers. Chin. J. Anim. Nutr. 2017, 29, 4131–4136. [Google Scholar]
- Cordeiro, D.A.; dos Santos, F.R.; dos Santos, H.B.; Silva, M.R.S.; de Oliveira, N.F.; Minafra, C.S. Enzymatic Complex for Broilers Fed on a Diet Containing Different Levels of Distiller Dried Grains with Solubles. Food Chem. 2022, 386, 132761. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Park, J.; Lee, H.-G.; Song, J.-Y.; Kim, D.-H.; Ji, W.; Joo, S.S.; Kim, M.; Jung, J.Y.; Kim, M.; et al. Dietary Probiotic Lacticaseibacillus Paracasei NSMJ56 Modulates Gut Immunity and Microbiota in Laying Hens. Poult. Sci. 2024, 103, 103505. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Alhotan, R.A. Distiller’s Dried Grains Supplemented with Enzyme Cocktail and Yeast Affect Egg Quality, Reproductive Performance and Blood Profile of Breeding Hens. J. Anim. Plant. Sci. 2023, 34, 260–275. [Google Scholar] [CrossRef]
- Bland, K.; Utterback, P.; Koelkebeck, K.; Parsons, C. Evaluation of Feeding Various Sources of Distillers Dried Grains with Solubles in Non-Feed-Withdrawal Molt Programs for Laying Hens. Poult. Sci. 2014, 93, 1421–1427. [Google Scholar] [CrossRef]
- Koenig, K.M.; Chibisa, G.E.; Penner, G.B.; Beauchemin, K.A. Optimum Roughage Proportion in Barley-Based Feedlot Cattle Diets: Growth Performance, Feeding Behavior, and Carcass Traits. J. Anim. Sci. 2020, 98, skaa299. [Google Scholar] [CrossRef]
- Böttger, C.; Südekum, K.-H. Within Plant Variation of Distillers Dried Grains with Solubles (DDGS) Produced from Multiple Raw Materials in Varying Proportions: Chemical Composition and in Vitro Evaluation of Feeding Value for Ruminants. Anim. Feed. Sci. Technol. 2017, 229, 79–90. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Koreleski, J. The Use of Distillers Dried Grains with Solubles (DDGS) in Poultry Nutrition. World Poult. Sci. J. 2008, 64, 257–266. [Google Scholar] [CrossRef]
- Official Methods of Analysis. Official Methods of Analysis of AOAC International, 22nd ed.; Latimer, G.W., Jr., Ed.; Oxford University Press: Oxford, UK, 2023; ISBN 978-0-19-761013-8. [Google Scholar]
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Worldwide Occurrence of Mycotoxins in Commodities, Feeds and Feed Ingredients. Anim. Feed. Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Liu, C.; Liang, Y.; Zhou, Z.; Liu, D. Absorption, Distribution, Metabolism, and in Vitro Digestion of Beta-Cypermethrin in Laying Hens. J. Agric. Food Chem. 2017, 65, 7647–7652. [Google Scholar] [CrossRef]
- NY/T 823-2020; Performance Terminology and Measurements for Poultry. China Standards Press: Beijing, China, 2020.
- Ge, Y.; Yu, X.; Zhao, X.; Liu, C.; Li, T.; Mu, S.; Zhang, L.; Chen, Z.; Zhang, Z.; Song, Z.; et al. Fermentation Characteristics and Postacidification of Yogurt by Streptococcus Thermophilus CICC 6038 and Lactobacillus Delbrueckii Ssp. Bulgaricus CICC 6047 at Optimal Inoculum Ratio. J. Dairy Sci. 2024, 107, 123–140. [Google Scholar] [CrossRef]
- Chen, K.; Deng, X.; Jiang, D.; Qin, L.; Lu, M.; Jiang, W.; Yang, M.; Zhang, L.; Jiang, J.; Lu, L. Efficient Conversion of Distillers Grains as Feed Ingredient by Synergy of Probiotics and Enzymes. Front. Microbiol. 2024, 15, 1403011. [Google Scholar] [CrossRef] [PubMed]
- Rho, Y.; Wey, D.; Zhu, C.; Kiarie, E.; Moran, K.; van Heugten, E.; de Lange, C.F.M. Growth Performance, Gastrointestinal and Digestibility Responses in Growing Pigs When Fed Corn–Soybean Meal-Based Diets with Corn DDGS Treated with Fiber Degrading Enzymes with or without Liquid Fermentation1. J. Anim. Sci. 2018, 96, 5188–5197. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, Y.; Zhao, J.; Zhou, Z.; Fan, H. Consuming Fermented Distillers’ Dried Grains with Solubles (DDGS) Feed Reveals a Shift in the Faecal Microbiota of Growing and Fattening Pigs Using 454 Pyrosequencing. J. Integr. Agric. 2017, 16, 900–910. [Google Scholar] [CrossRef]
- Sun, X.; Tiffany, D.G.; Urriola, P.E.; Shurson, G.G.; Hu, B. Nutrition Upgrading of Corn-Ethanol Co-Product by Fungal Fermentation: Amino Acids Enrichment and Anti-Nutritional Factors Degradation. Food Bioprod. Process. 2021, 130, 1–13. [Google Scholar] [CrossRef]
- Shurson, G.C. Yeast and Yeast Derivatives in Feed Additives and Ingredients: Sources, Characteristics, Animal Responses, and Quantification Methods. Anim. Feed. Sci. Technol. 2018, 235, 60–76. [Google Scholar] [CrossRef]
- Sun, X.; Dou, Z.; Shurson, G.C.; Hu, B. Bioprocessing to Upcycle Agro-Industrial and Food Wastes into High-Nutritional Value Animal Feed for Sustainable Food and Agriculture Systems. Resour. Conserv. Recycl. 2024, 201, 107325. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Chicilo, F.; Purdy, S.K.; Reaney, M.J.T. Value-Added Products from Ethanol Fermentation—A Review. Fermentation 2021, 7, 267. [Google Scholar] [CrossRef]
- Jia, R.; Cao, L.; Liu, W.; Shen, Z. Detoxification of Deoxynivalenol by Bacillus Subtilis ASAG 216 and Characterization the Degradation Process. Eur. Food Res. Technol. 2021, 247, 67–76. [Google Scholar] [CrossRef]
- Dzuman, Z.; Stranska-Zachariasova, M.; Vaclavikova, M.; Tomaniova, M.; Veprikova, Z.; Slavikova, P.; Hajslova, J. Fate of Free and Conjugated Mycotoxins within the Production of Distiller’s Dried Grains with Solubles (DDGS). J. Agric. Food Chem. 2016, 64, 5085–5092. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, G.H.; Han, G.P.; Kil, D.Y. Effect of Feeding Corn Distillers Dried Grains with Solubles Naturally Contaminated with Deoxynivalenol on Growth Performance, Meat Quality, Intestinal Permeability, and Utilization of Energy and Nutrients in Broiler Chickens. Poult. Sci. 2021, 100, 101215. [Google Scholar] [CrossRef]
- Barnharst, T.; Sun, X.; Rajendran, A.; Urriola, P.; Shurson, G.; Hu, B. Enhanced Protein and Amino Acids of Corn–Ethanol Co-Product by Mucor Indicus and Rhizopus Oryzae. Bioprocess. Biosyst. Eng. 2021, 44, 1989–2000. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Cui, L.; Li, J.; Wang, B.; Guo, L.; Wu, Z.; Zhu, W.; Wu, G. Fermentation Techniques in Feed Production. In Animal Agriculture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 407–429. ISBN 978-0-12-817052-6. [Google Scholar]
- Vuong, M.-D.; Thanh, N.-T.; Son, C.-K.; Yves, W. Protein Enrichment of Cassava-Based Dried Distiller’s Grain by Solid State Fermentation Using Trichoderma Harzianum and Yarrowia Lipolytica for Feed Ingredients. Waste Biomass Valori. 2021, 12, 3875–3888. [Google Scholar] [CrossRef]
- Sun, X.; Urriola, P.E.; Shurson, G.; Tiffany, D.; Hu, B. Enhancing Feeding Value of Corn Distiller’s Grains with Solubles via Fungal Co-Cultured Solid-State Fermentation for Monogastric Animal Nutrition. Anim. Feed. Sci. Technol. 2023, 303, 115673. [Google Scholar] [CrossRef]
- Yang, G.; Yang, D.Q.; Cao, W.T.; Wang, X.D. Screening of Cellulose Degrading Bacteria in Distiller’s Grains and Degradation Technology of Distiller’s Grains. Trans. Chin. Soc. Agric. Eng. 2020, 36, 212–221. [Google Scholar]
- Zeng, Z.K.; Jang, J.C.; Shurson, G.C.; Thakral, S.; Urriola, P.E. Ammonia Fiber Expansion Increases in Vitro Digestibility and Fermentability of Corn Distillers Dried Grains with Solubles with or without Carbohydrases. Anim. Feed. Sci. Technol. 2021, 273, 114824. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Qin, G.; Pan, L.; Sun, H.; Bao, N.; Hasham, M.M.; Farouk, M.H. Physicochemical Properties of Dietary Protein as Predictors for Digestibility or Releasing Percentage of Amino Acids in Monogastrics under In-Vitro Conditions. Ital. J. Anim. Sci. 2022, 21, 507–521. [Google Scholar] [CrossRef]
- Roberson, K.D.; Kalbfleisch, J.L.; Pan, W.; Charbeneau, R.A. Effect of Corn Distiller’s Dried Grains with Solubles at Various Levels on Performance of Laying Hens and Egg Yolk Color. Int. J. Poult. Sci. 2005, 4, 44–51. [Google Scholar]
- Abd El-Hac, M.E.; Alagawany, M.; Ragab Fara, M.; Dhama, K. Use of Maize Distiller’s Dried Grains with Solubles (DDGS) in Laying Hen Diets: Trends and Advances. Asian J. Anim. Vet. Adv. 2015, 10, 690–707. [Google Scholar] [CrossRef]
- Ruan, D.; Fouad, A.M.; Fan, Q.L.; Chen, W.; Xia, W.G.; Wang, S.; Cui, Y.Y.; Wang, Y.; Yang, L.; Zheng, C.T. Effects of Corn Dried Distillers’ Grains with Solubles on Performance, Egg Quality, Yolk Fatty Acid Composition and Oxidative Status in Laying Ducks. Poult. Sci. 2018, 97, 568–577. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, L.; Shan, A. The Effect of Vitamin E on Laying Performance and Egg Quality in Laying Hens Fed Corn Dried Distillers Grains with Solubles. Poult. Sci. 2013, 92, 2956–2964. [Google Scholar] [CrossRef]
- Purdum, S.; Hanford, K.; Kreifels, B. Short-Term Effects of Lower Oil Dried Distillers Grains with Solubles in Laying Hen Rations. Poult. Sci. 2014, 93, 2592–2595. [Google Scholar] [CrossRef]

| Item (% Unless Note) | Percentage (%) | Nutritional Component | Content |
|---|---|---|---|
| Ingredients | Metabolic energy, MJ/kg 3 | 14.95 | |
| Corn | 56.80 | Crude protein, % 3 | 15.80 |
| Soybean meal (44.2%) | 24.80 | Calcium, % 3 | 3.91 |
| Peanut meal | 7.00 | Total phosphorus, % 3 | 3.25 |
| Limestone | 7.74 | Available phosphorus, % 3 | 0.38 |
| Soybean oil | 1.50 | Methionine, % 4 | 0.35 |
| Dicalcium phosphate | 1.00 | Methionine + cysteine, % 4 | 0.67 |
| Salt | 0.30 | Lysine (Lys), % 4 | 1.05 |
| Lysine (98.5%) | 0.18 | Threonine (Thr), % 4 | 0.68 |
| DL-methionine | 0.25 | ||
| Vitamin premix 1 | 0.03 | ||
| Choline chloride | 0.10 | ||
| Mineral premix 2 | 0.30 | ||
| Total | 100 |
| Item | Item | DG 2 | FDG 2 |
|---|---|---|---|
| Regular nutrients (%) | Dry matter | 87.51 ± 0.62 | 89.28 ± 0.53 |
| Crude protein | 14.92 ± 0.49 b | 20.44 ± 0.76 a | |
| Crude fiber | 17.64 ± 0.58 a | 12.91 ± 0.33 b | |
| Crude ash | 12.59 ± 0.19 a | 7.46 ± 0.09 b | |
| Mycotoxin content | Aflatoxin (μg/kg) | 8.93 ± 0.31 | 3.21 ± 0.54 |
| Zearalenone (μg/kg) | 314.72 ± 30.18 a | 171.25 ± 20.03 b | |
| Deoxynivalenol (mg/kg) | 2.51 ± 0.31 a | 0.79 ± 0.19 b |
| Item | DG 2 | FDG 2 |
|---|---|---|
| Threonine (%) | 0.34 ± 0.09 b | 0.54 ± 0.12 a |
| Serine (%) | 0.42 ± 0.02 | 0.47 ± 0.07 |
| Proline (%) | 0.88 ± 0.11 | 1.44 ± 0.13 |
| Glycine (%) | 0.64 ± 0.10 | 0.59 ± 0.07 |
| Glutamate (%) | 2.57 ± 0.18 b | 3.79 ± 0.21 a |
| Cystine (%) | 0.93 ± 0.12 | 0.89 ± 0.09 |
| Valine (%) | 0.88 ± 0.16 | 1.12 ± 0.15 |
| Methionine (%) | 0.12± 0.04 | 0.13 ± 0.06 |
| Isoleucine (%) | 0.51 ± 0.02 | 0.52 ± 0.01 |
| Leucine (%) | 1.31 ± 0.11 | 1.39 ± 0.12 |
| Tyrosine (%) | 0.27 ± 0.11 | 0.56 ± 0.14 |
| Phenylalanine (%) | 0.64 ± 0.05 | 0.89 ± 0.13 |
| Lysine (%) | 0.28 ± 0.09 b | 0.49 ± 0.12 a |
| Histidine (%) | 0.32± 0.08 | 0.41± 0.04 |
| Arginine (%) | 0.51 ± 0.12 | 0.49 ± 0.01 |
| Alanine (%) | 0.78 ±0.08 | 1.33 ±0.09 |
| Tryptophan (%) | 0.15 ± 0.06 | 0.13 ± 0.04 |
| Total amino acid (%) | 11.75 ± 0.34 b | 15.82 ± 0.17 a |
| Item | DG 2 | FDG 2 |
|---|---|---|
| Dry matter digestibility (%) | 37.82 ± 1.93 b | 43.96 ± 1.52 a |
| Protein digestibility (%) | 55.61 ± 0.22 b | 63.14 ± 0.28 a |
| Crude fiber digestibility (%) | 13.95 ± 0.57 b | 22.89 ± 0.54 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, R.; Lu, S.; Li, T.; Li, M.; Zhang, G.; Wang, L.; Huang, S. Production Technology of Fermented Distiller’s Grains and Its Effect on Production Performance and Egg Quality of Laying Hens. Fermentation 2025, 11, 492. https://doi.org/10.3390/fermentation11090492
Jia R, Lu S, Li T, Li M, Zhang G, Wang L, Huang S. Production Technology of Fermented Distiller’s Grains and Its Effect on Production Performance and Egg Quality of Laying Hens. Fermentation. 2025; 11(9):492. https://doi.org/10.3390/fermentation11090492
Chicago/Turabian StyleJia, Ru, Simeng Lu, Tao Li, Meng Li, Guohua Zhang, Lan Wang, and Shimeng Huang. 2025. "Production Technology of Fermented Distiller’s Grains and Its Effect on Production Performance and Egg Quality of Laying Hens" Fermentation 11, no. 9: 492. https://doi.org/10.3390/fermentation11090492
APA StyleJia, R., Lu, S., Li, T., Li, M., Zhang, G., Wang, L., & Huang, S. (2025). Production Technology of Fermented Distiller’s Grains and Its Effect on Production Performance and Egg Quality of Laying Hens. Fermentation, 11(9), 492. https://doi.org/10.3390/fermentation11090492





