Use of Depleted Oil and Gas Reservoirs as Bioreactors to Produce Hydrogen and Capture Carbon Dioxide
Abstract
1. Introduction
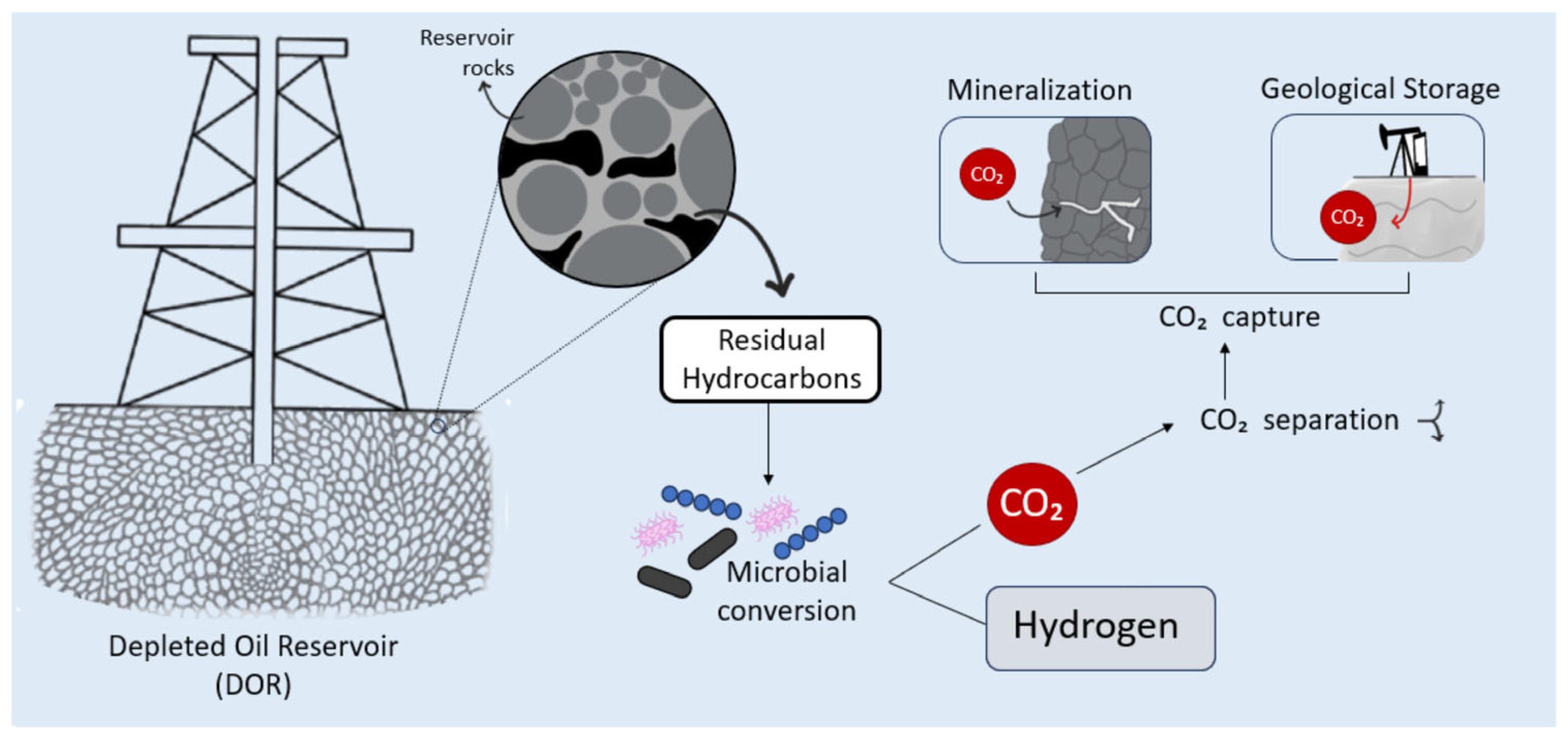
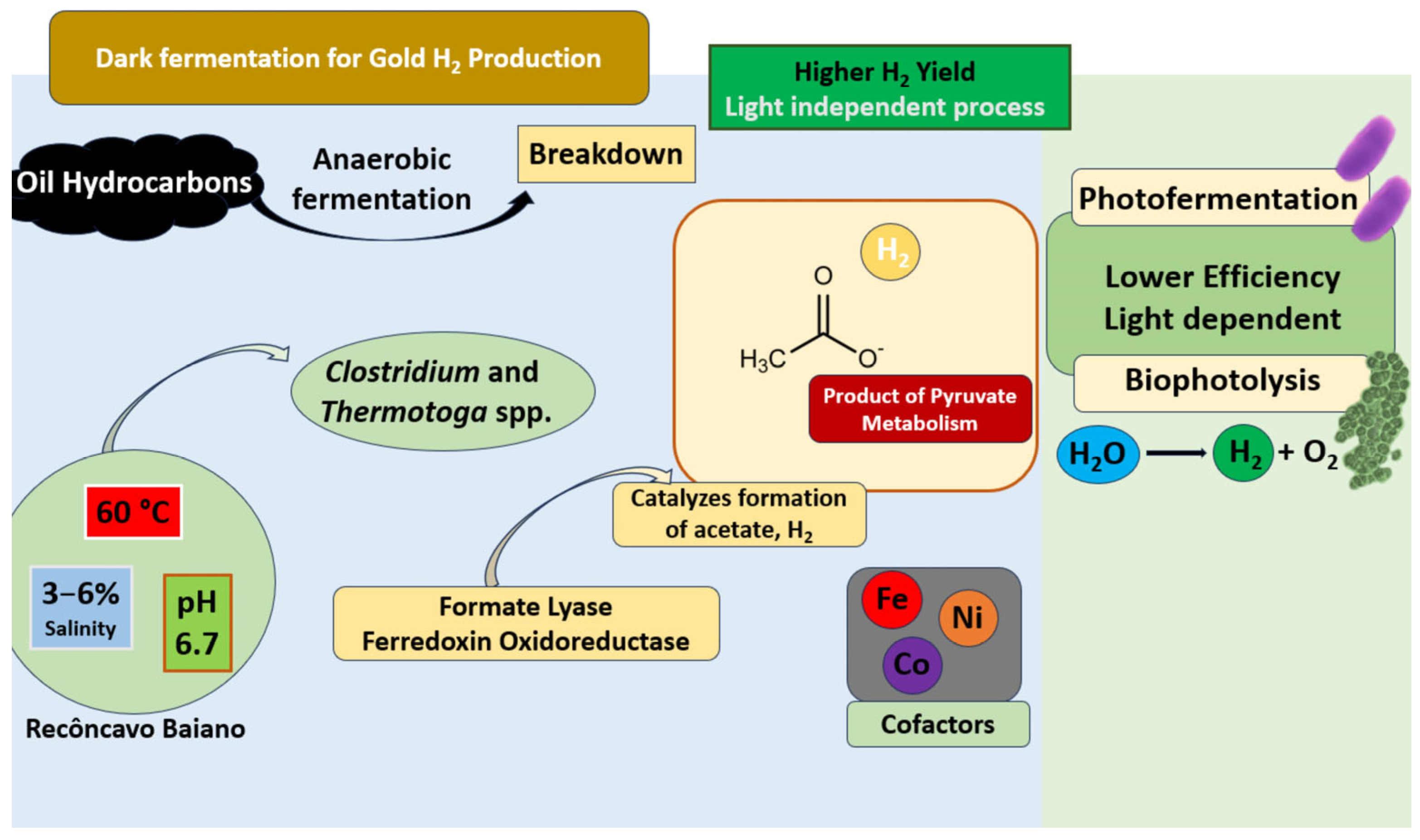
2. Microbial Basis for H2 Production in Deplete Oil Reservoirs
3. Viability of Acidogenic H2-Producing Consortia in Depleted Oil Reservoirs
4. Depleted Oil Reservoir Conditions and Technological Challenges
5. Infrastructure Adaptation for H2 Production in DORs
6. Carbon Capture, Utilization and Storage
7. Safety Aspects of H2Au Production
8. Economic Aspects of the Process of Obtaining H2 Using DORs as Bioreactors
9. Pilot Projects and Case Studies
9.1. Cemvita—H2 Production from DORs—The “H2Au” Approach
9.2. Recôncavo Baiano Studies: Application Potential of Cemvita-Inspired H2Au Production
10. Relevance of H2 from DORs and Its Broader Impacts
11. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANP | Brazilian National Agency of Petroleum, Natural Gas and Biofuels |
| CO2 | Carbon dioxide |
| H2 | Molecular H2 |
| CH4 | Methane |
| VFAs | Volatile fatty acids |
| MECs | Microbial electrolysis cells |
| DORs | Depleted oil reservoirs |
| RH | Residual hydrocarbons |
| H2Au | Gold Hydrogen |
| CCUS | Carbon capture, utilization, and storage |
| DF | Dark fermentation |
| assA | alkyl succinate synthase |
| bssA | benzyl succinate synthase |
| assR | alkyl succinate regulator |
| bssR | benzyl succinate regulator |
| hydA | hydrogenase synthase |
| fdh | putative formate dehydrogenase synthase |
| TRL | Technology readiness level |
| H2S | Hydrogen sulfide |
| SRB | Sulfate reducing bacteria |
| CAPEX | Capital expenditures |
| OPEX | Operational expenditures |
| PNH2 | Brazil’s National H2 Program |
References
- Meherkotay, S.; Das, D. Biohydrogen as a Renewable Energy Resource—Prospects and Potentials. Int. J. Hydrogen Energy 2008, 33, 258–263. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen Energy Systems: A Critical Review of Technologies, Applications, Trends and Challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Piggot, G.; Verkuijl, C.; Van Asselt, H.; Lazarus, M. Curbing Fossil Fuel Supply to Achieve Climate Goals. Clim. Policy 2020, 20, 881–887. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Large-Scale Green Hydrogen Production Using Alkaline Water Electrolysis Based on Seasonal Solar Radiation. Energy Harvest. Syst. 2024, 11, 20230011. [Google Scholar] [CrossRef]
- Gouws, S.; Mackay, J. Production of Green Hydrogen through PEM Water Electrolysis. Pure Appl. Chem. 2024, 96, 1383–1401. [Google Scholar] [CrossRef]
- Filgueira, J.M.; Pereira Júnior, A.O.; Barbosa De Araújo, R.S.; Silva, N.F.D. Economic and Social Impacts of the Oil Industry on the Brazilian Onshore. Energies 2020, 13, 1922. [Google Scholar] [CrossRef]
- Jain, R.; Panwar, N.L.; Jain, S.K.; Gupta, T.; Agarwal, C.; Meena, S.S. Bio-Hydrogen Production through Dark Fermentation: An Overview. Biomass Conv. Bioref. 2022, 14, 12699–12724. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Liu, W.; Guo, J.; Xian, M. Debottlenecking the Biological Hydrogen Production Pathway of Dark Fermentation: Insight into the Impact of Strain Improvement. Microb. Cell Fact. 2022, 21, 166. [Google Scholar] [CrossRef]
- Albuquerque, M.M.; Martinez-Burgos, W.J.; De Bona Sartor, G.; Medeiros, A.B.P.; De Carvalho, J.C.; Soccol, C.R. Microbial Electrolysis Cells in Biohydrogen Production. In Biohydrogen-Advances and Processes; Soccol, C.R., Brar, S.K., Permaul, K., Pakshirajan, K., De Carvalho, J.C., Eds.; Biofuel and Biorefinery Technologies; Springer Nature: Cham, Switzerland, 2024; Volume 13, pp. 429–453. ISBN 978-3-031-49817-6. [Google Scholar]
- Logan, B. Novel Microbial Electrolysis Cell Design for Efficient Hydrogen Generation from Wastewaters. In Proceedings of the 2022 Annual Merit Review and Peer Evaluation Meeting, Online, 6–8 June 2022. [Google Scholar]
- Vieira Barboza, D.; Jasmim Meiriño, M.; Da Silveira Barros, S.R.; Fernandes Bella, R.L. Towards the Sustainable Decommissioning of Fixed Platforms by Aligning Ecosystem Services and Wind Generation: A Brazilian Case. Int. J. Energy Econ. Policy 2023, 13, 235–242. [Google Scholar] [CrossRef]
- Storey, B.M.; Worden, R.H.; McNamara, D.D.; Wheeler, J.; Parker, J.; Kristen, A. Reactivation of Abandoned Oilfields for Cleaner Energy Generation: Three-Dimensional Modelling of Reservoir Heterogeneity and Geometry. Processes 2024, 12, 2883. [Google Scholar] [CrossRef]
- Meenakshisundaram, A.; Tomomewo, O.S.; Aimen, L.; Bade, S.O. A Comprehensive Analysis of Repurposing Abandoned Oil Wells for Different Energy Uses: Exploration, Applications, and Repurposing Challenges. Clean. Eng. Technol. 2024, 22, 100797. [Google Scholar] [CrossRef]
- Oliver, D.S.; Fossum, K.; Bhakta, T.; Sandø, I.; Nævdal, G.; Lorentzen, R.J. 4D Seismic History Matching. J. Pet. Sci. Eng. 2021, 207, 109119. [Google Scholar] [CrossRef]
- Kang, W.-L.; Zhou, B.-B.; Issakhov, M.; Gabdullin, M. Advances in Enhanced Oil Recovery Technologies for Low Permeability Reservoirs. Pet. Sci. 2022, 19, 1622–1640. [Google Scholar] [CrossRef]
- Magill, J. Oil, Gas Companies Deploy AI in the fight to Reduce Carbon Emissions. Available online: https://www.forbes.com/sites/jimmagill/2021/03/28/oil-gas-companies-deploy-ai-in-the-fight-to-reduce-carbon-emissions/ (accessed on 30 May 2023).
- Gold Hydrogen | The Gold Standard for the Energy Transition. Available online: https://goldhydrogen.com/ (accessed on 28 June 2025).
- Jacobs, T. Firm Says Microbes Strike Hydrogen Gold in California Oil Field. Available online: https://jpt.spe.org/firm-says-microbes-strike-hydrogen-gold-in-california-oil-field (accessed on 13 July 2025).
- Wu, H.; Li, A.; Zhang, H.; Li, S.; Yang, C.; Lv, H.; Yao, Y. Microbial Mechanisms for Higher Hydrogen Production in Anaerobic Digestion at Constant Temperature versus Gradient Heating. Microbiome 2024, 12, 170. [Google Scholar] [CrossRef]
- Liang, K. Metabolic Pathways and Genetic Engineering of Anaerobic Bacteria for Biohydrogen Production. Biosci. Evid. 2024, 14, 81–92. [Google Scholar] [CrossRef]
- Universidade Federal Da Bahia: Isolamento e Identificação Molecular de Bactérias Redutoras de Sulfato de Amostras de Água Produzida Em Campo de Petróleo. Available online: https://repositorio.ufba.br/handle/ri/18451 (accessed on 18 March 2025).
- Myhr, S.; Lillebø, B.L.P.; Sunde, E.; Beeder, J.; Torsvik, T. Inhibition of Microbial H2S Production in an Oil Reservoir Model Column by Nitrate Injection. Appl. Microbiol. Biotechnol. 2002, 58, 400–408. [Google Scholar] [CrossRef]
- Deng, S.; Wang, B.; Su, S.; Sun, S.; She, Y.; Zhang, F. Dynamics of Microbial Community and Removal of Hydrogen Sulfide (H2 S) Using a Bio-Inhibitor and Its Application under the Oil Reservoir Condition. Energy Fuels 2022, 36, 14128–14135. [Google Scholar] [CrossRef]
- Broussard, Z.R.; Kincaid, K.P.; Karimi, M.; Karimi, T.; Da, S.M.L.B.; Gonçalves, R.A.; Harris, R.A.; Trevino, A.C.; Lahme, L.L.A.; Magalháes, B.D.F.; et al. Method. U.S. Patent US2023099645A1, 30 March 2023. [Google Scholar]
- Broussard, Z.R.; Kincaid, K.P.; Karimi, M.; Karimi, T.; Da, S.M.L.B.; Gonçalv, R.A.; Harris, R.A.; Trevino, A.C.; Lahme, L.L.A.; Magalhães, B.D.F.; et al. Method for Microbiological Production of Hydrogen. U.S. Patent US2024392324A1, 28 November 2024. [Google Scholar]
- Alkan, H.; Bauer, J.F.; Burachok, O.; Kowollik, P.; Olbricht, M.; Amro, M. Hydrogen from Depleted/Depleting Hydrocarbon Reservoirs: A Reservoir Engineering Perspective. Appl. Sci. 2024, 14, 6217. [Google Scholar] [CrossRef]
- Anaerobic Utilization of Hydrocarbons, Oils, and Lipids; Boll, M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-319-50390-5. [Google Scholar]
- Laczi, K.; Erdeiné Kis, Á.; Szilágyi, Á.; Bounedjoum, N.; Bodor, A.; Vincze, G.E.; Kovács, T.; Rákhely, G.; Perei, K. New Frontiers of Anaerobic Hydrocarbon Biodegradation in the Multi-Omics Era. Front. Microbiol. 2020, 11, 590049. [Google Scholar] [CrossRef]
- Beller, H.R.; Spormann, A.M. Analysis of the Novel Benzylsuccinate Synthase Reaction for Anaerobic Toluene Activation Based on Structural Studies of the Product. J. Bacteriol. 1998, 180, 5454–5457. [Google Scholar] [CrossRef]
- Grossi, V.; Cravo-Laureau, C.; Méou, A.; Raphel, D.; Garzino, F.; Hirschler-Réa, A. Anaerobic 1-Alkene Metabolism by the Alkane- and Alkene-Degrading Sulfate Reducer Desulfatibacillum Aliphaticivorans Strain CV2803T. Appl. Environ. Microbiol. 2007, 73, 7882–7890. [Google Scholar] [CrossRef]
- Wilkes, H.; Buckel, W.; Golding, B.T.; Rabus, R. Metabolism of Hydrocarbons in n-Alkane-Utilizing Anaerobic Bacteria. Microb. Physiol. 2016, 26, 138–151. [Google Scholar] [CrossRef]
- Cabrol, L.; Marone, A.; Tapia-Venegas, E.; Steyer, J.-P.; Ruiz-Filippi, G.; Trably, E. Microbial Ecology of Fermentative Hydrogen Producing Bioprocesses: Useful Insights for Driving the Ecosystem Function. FEMS Microbiol. Rev. 2017, 41, 158–181. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Fernandes, A.; Lopes, A.; Grasa, L.; Salafranca, J. Microbes and Parameters Influencing Dark Fermentation for Hydrogen Production. Appl. Sci. 2024, 14, 10789. [Google Scholar] [CrossRef]
- Von Netzer, F.; Pilloni, G.; Kleindienst, S.; Krüger, M.; Knittel, K.; Gründger, F.; Lueders, T. Enhanced Gene Detection Assays for Fumarate-Adding Enzymes Allow Uncovering of Anaerobic Hydrocarbon Degraders in Terrestrial and Marine Systems. Appl. Environ. Microbiol. 2013, 79, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Díez, M.P.; Villanueva-Galindo, E.; Moreno-Andrade, I.; Díaz, E.; De La Rubia, M.A.; Mohedano, A.F.; Perez-Rangel, M. Enhanced Hydrogen Production from Food Waste via Bioaugmentation with Clostridium and Lactobacillus. Biomass Conv. Bioref. 2025. [Google Scholar] [CrossRef]
- Muñoz-Páez, K.M.; Buitrón, G.; Vital-Jácome, M. Predicting Metabolic Pathways and Microbial Interactions in Dark Fermentation Systems Treating Real Cheese Whey Effluents. Bioresour. Technol. 2024, 413, 131536. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Spengler, K.; Terberger, K.; Boehm, M.; Appel, J.; Barske, T.; Timm, S.; Battchikova, N.; Hagemann, M.; et al. Pyruvate:Ferredoxin Oxidoreductase and Low Abundant Ferredoxins Support Aerobic Photomixotrophic Growth in Cyanobacteria. eLife 2022, 11, e71339. [Google Scholar] [CrossRef]
- Biswas, R.; Zheng, T.; Olson, D.G.; Lynd, L.R.; Guss, A.M. Elimination of Hydrogenase Active Site Assembly Blocks H2 Production and Increases Ethanol Yield in Clostridium thermocellum. Biotechnol. Biofuels 2015, 8, 20. [Google Scholar] [CrossRef]
- De Souza, L.C.; Herring, C.D.; Lynd, L.R. Genetic Investigation of Hydrogenases in Thermoanaerobacterium Thermosaccharolyticum Suggests That Redox Balance via Hydrogen Cycling Enables High Ethanol Yield. Appl. Environ. Microbiol. 2025, 91, e01109-24. [Google Scholar] [CrossRef]
- Yadav, S.; Haas, R.; Boydas, E.B.; Roemelt, M.; Happe, T.; Apfel, U.-P.; Stripp, S.T. Oxygen Sensitivity of [FeFe]-Hydrogenase: A Comparative Study of Active Site Mimics inside vs. Outside the Enzyme. Phys. Chem. Chem. Phys. 2024, 26, 19105–19116. [Google Scholar] [CrossRef]
- Schuchmann, K.; Chowdhury, N.P.; Müller, V. Complex Multimeric [FeFe] Hydrogenases: Biochemistry, Physiology and New Opportunities for the Hydrogen Economy. Front. Microbiol. 2018, 9, 2911. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Lubitz, W.; Higuchi, Y. Structure and Function of [NiFe] Hydrogenases. J. Biochem. 2016, 160, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Fleitas García, A.-R.; Guez, J.-S.; Dussap, C.G.; Fontanille, P.; Christophe, G. Effect of Redox Potential on Biohydrogen Production during Dark Fermentation of Food Wastes in Bioreactor. Int. J. Hydrogen Energy 2025, 141, 1199–1210. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Li, X.; Qin, Z.; Su, Y.; Freguia, S.; Feng, L.; Chen, Y. Phenanthrene Regulates Metabolic Pathways for Hydrogen Accumulation in Sludge Alkaline Dark Fermentation. Bioresour. Technol. 2023, 384, 129311. [Google Scholar] [CrossRef]
- Li, X.; Sui, K.; Zhang, J.; Liu, X.; Xu, Q.; Wang, D.; Yang, Q. Revealing the Mechanisms of Rhamnolipid Enhanced Hydrogen Production from Dark Fermentation of Waste Activated Sludge. Sci. Total Environ. 2022, 806, 150347. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, S.; Dong, Z.; Cao, S.; Yuan, A.; Sha, H.; Chen, N. Enhancing Dark Fermentative Hydrogen Production from Wheat Straw through Synergistic Effects of Active Electric Fields and Enzymatic Hydrolysis Pretreatment. Bioresour. Technol. 2024, 406, 130993. [Google Scholar] [CrossRef]
- Xue, S.; Liang, D.; Yan, J.; Xu, Y.; Wang, F.; Lv, G. Geographic Scale-Based Analysis of Hydrogen Production Efficiency and Mechanism in Dark Fermentation Utilizing Diverse Inoculums. Int. J. Hydrogen Energy 2024, 89, 390–400. [Google Scholar] [CrossRef]
- Fu, Y.; Song, Y.; Chen, H.; Chen, H.; Li, Y.; Wu, Q. Effects of Biological Carrier Optimization on Hydrogen Production and Biofilm Formation in Anaerobic Circulating Fluidized Bed Reactor via Dark Fermentation. Int. J. Hydrogen Energy 2025, 144, 85–95. [Google Scholar] [CrossRef]
- Sun, H.; Shen, J.; Hu, M.; Zhang, J.; Cai, Z.; Zang, L.; Zhang, F.; Ji, D. Manganese Ferrite Nanoparticles Enhanced Biohydrogen Production from Mesophilic and Thermophilic Dark Fermentation. Energy Rep. 2021, 7, 6234–6245. [Google Scholar] [CrossRef]
- Rodríguez-Reyes, J.J.; García-Depraect, O.; Cantera, S.; Mena-Navarro, V.; León-Becerril, E. Assessment of the Recovery of Hydrogen Production Activity in Dark Fermentation Reactors after a Long Period of Shutdown. Int. J. Hydrogen Energy 2025, 144, 1134–1146. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Sneha, N.P.; Rafi, S.M.; Sarkar, O. Dark Fermentative Hydrogen Production: Potential of Food Waste as Future Energy Needs. Sci. Total Environ. 2023, 888, 163801. [Google Scholar] [CrossRef] [PubMed]
- Ouafa, A.; Kerroum, D.; Antonio, P.; Lamis, R.; Chaima, B.; Rania, Z.-Z.; Mossaab, B.-L. Coffee Grounds and Fruit and Vegetable Waste Co-Digestion in Dark Fermentation: Evaluation of Mixing Ratio and Hybrid Pretreatments Impact on Bio-Hydrogen Production. Biomass Bioenergy 2025, 199, 107896. [Google Scholar] [CrossRef]
- Wei, Y.; Jiao, Y.; Chen, H. Polydimethyldiallylammonium Chloride Inhibits Dark Fermentative Hydrogen Production from Waste Activated Sludge. Bioresour. Technol. 2024, 393, 130003. [Google Scholar] [CrossRef]
- Feng, S.; Guo, W.; Zhang, S.; Luo, G.; Nguyen, H.T.; Nguyen, N.C.; Cheng, D.; Ye, Y.; Ngo, H.H. Optimization of Hydraulic Retention Time in Continuous Orange Peel Crude Enzyme-Mediated Dark Fermentation for Sustainable Biohydrogen Production from Synthetic Swine Wastewater. J. Water Process Eng. 2025, 73, 107714. [Google Scholar] [CrossRef]
- Srivastava, P.; García-Quismondo, E.; Palma, J.; González-Fernández, C. Coupling Dark Fermentation and Microbial Electrolysis Cells for Higher Hydrogen Yield: Technological Competitiveness and Challenges. Int. J. Hydrogen Energy 2024, 52, 223–239. [Google Scholar] [CrossRef]
- Hussien, M.; Mohamed, H.O.; Jadhav, D.A.; Bahaa, A.; Jo, S.-M.; Jang, J.-H.; Kim, J.-H.; Kwon, J.-Y.; Sayed, E.T.; Abdelkareem, M.A.; et al. Synergistic Integration of Dark Fermentation and Microbial Electrolysis Cells for Hydrogen Production and Sustainable Swine Manure Treatment. Int. J. Hydrogen Energy 2025, 115, 299–309. [Google Scholar] [CrossRef]
- Jalil, A.; Yu, Z. Impact of Substrates, Volatile Fatty Acids, and Microbial Communities on Biohydrogen Production: A Systematic Review and Meta-Analysis. Sustainability 2024, 16, 10755. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, B.; Zhang, H.; Qian, S.; Wei, T.; Zhang, Z.; Song, L.; Yang, X. Fermentative Hydrogen Production from Lignocellulose by Mesophilic Clostridium populeti FZ10 Newly Isolated from Microcrystalline Cellulose-Acclimated Compost. Appl. Sci. 2022, 12, 9562. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chang, C.-Y.; Cheng, C.-L.; Lee, D.-J.; Chang, J.-S. Fermentative Hydrogen Production by Clostridium butyricum CGS5 Using Carbohydrate-Rich Microalgal Biomass as Feedstock. Int. J. Hydrogen Energy 2012, 37, 15458–15464. [Google Scholar] [CrossRef]
- Veshareh, M.J.; Poulsen, M.; Nick, H.M.; Feilberg, K.L.; Eftekhari, A.A.; Dopffel, N. The Light in the Dark: In-Situ Biorefinement of Crude Oil to Hydrogen Using Typical Oil Reservoir Thermotoga Strains. Int. J. Hydrogen Energy 2022, 47, 5101–5110. [Google Scholar] [CrossRef]
- Jannat, F.T.; Aftab, K.; Kalsoom, U.; Baig, M.A. A Bibliometric Analysis of the Role of Nanotechnology in Dark Fermentative Biohydrogen Production. Environ. Sci. Pollut. Res. 2024, 31, 24815–24835. [Google Scholar] [CrossRef]
- Kottenhahn, P.; Philipps, G.; Jennewein, S. Hexanol Biosynthesis from Syngas by Clostridium carboxidivorans P7-Product Toxicity, Temperature Dependence and in Situ Extraction. Heliyon 2021, 7, e07732. [Google Scholar] [CrossRef] [PubMed]
- Maintinguer, S.; Fernandes, B.; Duarte, I.; Saavedra, N.; Adorno, M.; Varesche, M. Fermentative Hydrogen Production by Microbial Consortium. Int. J. Hydrogen Energy 2008, 33, 4309–4317. [Google Scholar] [CrossRef]
- Černý, M.; Vítězová, M.; Vítěz, T.; Bartoš, M.; Kushkevych, I. Variation in the Distribution of Hydrogen Producers from the Clostridiales Order in Biogas Reactors Depending on Different Input Substrates. Energies 2018, 11, 3270. [Google Scholar] [CrossRef]
- Han, H.; Cui, M.; Wei, L.; Yang, H.; Shen, J. Enhancement Effect of Hematite Nanoparticles on Fermentative Hydrogen Production. Bioresour. Technol. 2011, 102, 7903–7909. [Google Scholar] [CrossRef]
- Zheng, X.-J.; Wei, L.-F.; Zhang, Z.-H.; Jiang, Q.-J.; Wei, Y.-J.; Xie, B.; Wei, M.-B. Research on Photocatalytic H2 Production from Acetic Acid Solution by Pt/TiO2 Nanoparticles under UV Irradiation. Int. J. Hydrogen Energy 2009, 34, 9033–9041. [Google Scholar] [CrossRef]
- Beschkov, V.; Parvanova-Mancheva, T.; Vasileva, E. Experimental Study of Bio-Hydrogen Production by Clostridium beijerinckii from Different Substrates. Energies 2023, 16, 2747. [Google Scholar] [CrossRef]
- Martins, M.; Mourato, C.; Pereira, I.A.C. Desulfovibrio Vulgaris Growth Coupled to Formate-Driven H2 Production. Environ. Sci. Technol. 2015, 49, 14655–14662. [Google Scholar] [CrossRef]
- Lackner, N.; Hintersonnleitner, A.; Wagner, A.O.; Illmer, P. Hydrogenotrophic Methanogenesis and Autotrophic Growth of Methanosarcina Thermophila. Archaea 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Saranya, S.; Thamanna, L.; Sreekutty, V.P.; Dhayanithi, S.; Chellapandi, P. Bioremediation of Oil and Natural Gas Industry Waste Using Methanogens: Current Status and Future Perspective to Biohythane Production. Arab. J. Sci. Eng. 2025, 50, 4457–4475. [Google Scholar] [CrossRef]
- Martins, M.; Pereira, I.A.C.; Pita, M.; De Lacey, A.L. Biological Production of Hydrogen. In Enzymes for Solving Humankind’s Problems; Moura, J.J.G., Moura, I., Maia, L.B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 247–273. ISBN 978-3-030-58314-9. [Google Scholar]
- Van Der Kraan, G.M.; Bruining, J.; Lomans, B.P.; Van Loosdrecht, M.C.M.; Muyzer, G. Microbial Diversity of an Oil-Water Processing Site and Its Associated Oil Field: The Possible Role of Microorganisms as Information Carriers from Oil-Associated Environments. FEMS Microbiol. Ecol. 2010, 71, 428–443. [Google Scholar] [CrossRef]
- Xuan, J.; He, L.; Wen, W.; Feng, Y. Hydrogenase and Nitrogenase: Key Catalysts in Biohydrogen Production. Molecules 2023, 28, 1392. [Google Scholar] [CrossRef]
- Mbow, F.T.; Akbari, A.; Dopffel, N.; Schneider, K.; Mukherjee, S.; Meckenstock, R.U. Insights into the Effects of Anthropogenic Activities on Oil Reservoir Microbiome and Metabolic Potential. New Biotechnol. 2024, 79, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.E.; Brant, J.L.; Campo, P.; Clark, D.R.; Coulon, F.; Gregson, B.H.; McGenity, T.J.; McKew, B.A. Effects of Dispersants and Biosurfactants on Crude-Oil Biodegradation and Bacterial Community Succession. Microorganisms 2021, 9, 1200. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, C.; Gutierrez, T. Biosurfactants and Their Applications in the Oil and Gas Industry: Current State of Knowledge and Future Perspectives. Front. Bioeng. Biotechnol. 2021, 9, 626639. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, J.; Xia, R.; Dong, H.; Zhou, J. Electron Syntrophy between Mixed Hydrogenogens and Geobacter Metallireducens Boosted Dark Hydrogen Fermentation: Clarifying Roles of Electroactive Extracellular Polymeric Substances. Bioresour. Technol. 2024, 395, 130350. [Google Scholar] [CrossRef]
- Head, I.M.; Jones, D.M.; Röling, W.F.M. Marine Microorganisms Make a Meal of Oil. Nat. Rev. Microbiol. 2006, 4, 173–182. [Google Scholar] [CrossRef]
- Dolfing, J.; Larter, S.R.; Head, I.M. Thermodynamic Constraints on Methanogenic Crude Oil Biodegradation. ISME J. 2008, 2, 442–452. [Google Scholar] [CrossRef]
- Pannekens, M.; Kroll, L.; Müller, H.; Mbow, F.T.; Meckenstock, R.U. Oil Reservoirs, an Exceptional Habitat for Microorganisms. New Biotechnol. 2019, 49, 1–9. [Google Scholar] [CrossRef]
- Joye, S.; Kleindienst, S.; Gilbert, J.; Handley, K.; Weisenhorn, P.; Overholt, W.; Kostka, J. Responses of Microbial Com-munities to Hydrocarbon Exposures. Oceanog 2016, 29, 136–149. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, H.; Liu, J.; Hou, X.; Zhang, Y.; Liu, X.; Wei, S.; Cui, Q. Isolation and Characterization of Biosurfactant-Producing Bacteria for Enhancing Oil Recovery. Processes 2024, 12, 2575. [Google Scholar] [CrossRef]
- Vilcáez, J.; Chowdhury, E. Biogenic Hydrogen Production from Oil Hydrocarbons at Geological Carbon Storage Conditions. Energy Convers. Manag. 2025, 325, 119438. [Google Scholar] [CrossRef]
- Wentzel, A.; Lewin, A.; Cervantes, F.J.; Valla, S.; Kotlar, H.K. Deep Subsurface Oil Reservoirs as Poly-Extreme Habitats for Microbial Life. A Current Review. In Polyextremophiles; Seckbach, J., Oren, A., Stan-Lotter, H., Eds.; Cellular Origin, Life in Extreme Habitats and Astrobiology; Springer: Dordrecht, The Netherlands, 2013; Volume 27, pp. 439–466. ISBN 978-94-007-6487-3. [Google Scholar]
- McInerney, M.J.; Sieber, J.R.; Gunsalus, R.P. Syntrophy in Anaerobic Global Carbon Cycles. Curr. Opin. Biotechnol. 2009, 20, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Volpi, M.P.C.; Adarme, O.F.H.; Araújo, M.F.; Bella, T.R.; Procópio, P.F.; De Abreu, L.G.F.; Carazzolle, M.F.; Pereira, G.A.G.; Mockaitis, G. Biohydrogen and Methane Production via Silage-Based Dark Co-Fermentation Using Vinasse and Filter Cake. Bioresour. Technol. Rep. 2024, 27, 101927. [Google Scholar] [CrossRef]
- Tang, T.; Chen, Y.; Liu, M.; Du, Y.; Tan, Y. Effect of pH on the Performance of Hydrogen Production by Dark Fermentation Coupled Denitrification. Environ. Res. 2022, 208, 112663. [Google Scholar] [CrossRef]
- Dauptain, K.; Trably, E.; Santa-Catalina, G.; Carrere, H. Biomass Acid Pretreatment Impacts on Metabolic Routes and Bacterial Composition of Dark Fermentation Process. Waste Manag. 2024, 181, 211–219. [Google Scholar] [CrossRef]
- Barca, C.; Ranava, D.; Bauzan, M.; Ferrasse, J.-H.; Giudici-Orticoni, M.-T.; Soric, A. Fermentative Hydrogen Production in an Up-Flow Anaerobic Biofilm Reactor Inoculated with a Co-Culture of Clostridium acetobutylicum and Desulfovibrio vulgaris. Bioresour. Technol. 2016, 221, 526–533. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, L.; Dong, W.; Mo, H.; Ba, T.; Li, T.; Guan, D.; Zhao, W.; Wang, N.; Ma, Z.; et al. Revealing the Mechanisms of Alkali-Based Magnetic Nanosheets Enhanced Hydrogen Production from Dark Fermentation: Comparison between Mesophilic and Thermophilic Conditions. Bioresour. Technol. 2022, 343, 126141. [Google Scholar] [CrossRef]
- Gurubel Tun, K.J.; León-Becerril, E.; García-Depraect, O. Optimal Control Strategy Based on Artificial Intelligence Applied to a Continuous Dark Fermentation Reactor for Energy Recovery from Organic Wastes. Green Energy Resour. 2025, 3, 100112. [Google Scholar] [CrossRef]
- Tian, Y.; Xue, S.; Ma, Y. Comparative Analysis of Bacterial Community and Functional Species in Oil Reservoirs with Different in Situ Temperatures. Int. Microbiol. 2020, 23, 557–563. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Liu, X.; Wang, J.; Xu, J.; Chen, X.; Liu, X.; Hu, B.; Nie, Y.; Wu, X.-L. Global Diversity and Ecological Functions of Viruses Inhabiting Oil Reservoirs. Nat. Commun. 2024, 15, 6789. [Google Scholar] [CrossRef] [PubMed]
- Sherry, A.; Gray, N.; Aitken, C.; Dolfing, J. Microbial Oil Degradation Under Methanogenic Conditions. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3905–3917. ISBN 978-3-540-77584-3. [Google Scholar]
- Hamilton, J.J.; Calixto Contreras, M.; Reed, J.L. Thermodynamics and H2 Transfer in a Methanogenic, Syntrophic Community. PLoS Comput. Biol. 2015, 11, e1004364. [Google Scholar] [CrossRef] [PubMed]
- Kadnikov, V.V.; Ravin, N.V.; Sokolova, D.S.; Semenova, E.M.; Bidzhieva, S.K.; Beletsky, A.V.; Ershov, A.P.; Babich, T.L.; Khisametdinov, M.R.; Mardanov, A.V.; et al. Metagenomic and Culture-Based Analyses of Microbial Communities from Petroleum Reservoirs with High-Salinity Formation Water, and Their Biotechnological Potential. Biology 2023, 12, 1300. [Google Scholar] [CrossRef]
- Varjani, S.J. Microbial Degradation of Petroleum Hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Hsia, I.C.C.; Shouki, I.A.; Li, J. Feasibility of Rejuvenating Depleted Oil Fields with New Energy: Biohydrogen. In Proceedings of the Offshore Technology Conference, Asia, Kuala Lumpur, Malaysia, 22 February 2024; OTC: Houston, TX, USA, 2024; p. D021S005R004. [Google Scholar]
- Madirisha, M.M.; Ikotun, B.D. Hydrogen Storage in Depleted Geological Hydrocarbon Reservoirs: Enhancing Wellbore Integrity and Sustainability with Geopolymer Cement-Review. J. Energy Storage 2024, 84, 110834. [Google Scholar] [CrossRef]
- Chaubey, T.; Grover, B.S.; Tessier, P. Staged Membrane Process for High Pressure Hydrogen Production. US9114352B2, 2014. [Google Scholar]
- Edlund, D.J.; Pledger, W.A.; Studebaker, R.T. Hydrogen Purification Membranes, Components and Fuel Processing Systems Containing the Same. U.S. Patent US6723156B2, 20 April 2004. [Google Scholar]
- Edlund, D.J.; Hoshi, K.; Studebaker, R.T.; Studebaker, R.J. Hydrogen Purification Devices. U.S. Patent US10717040B2, 21 July 2020. [Google Scholar]
- Edlund, D.J. Hydrogen Generation Assemblies and Hydrogen Purification Devices. U.S. Patent US9616389B2, 23 May 2017. [Google Scholar]
- Li, Y.; Zhang, Q.; Zhang, Z.; Jing, Y.; Zhang, H.; Yue, J.; Jiang, D.; Lu, C.; Zhang, Y.; Wang, C. Biological Hydrogen-Alkane Co-Production Fermentation System and Method with Negative Carbon Emission. 2022.
- Gillick, S.R.; Babaei, M. In-Situ Hydrogen Production from Natural Gas Wells with Subsurface Carbon Retention. SPE J. 2024, 29, 2119–2129. [Google Scholar] [CrossRef]
- Abraham, J.J.; Carvero, A.; Teodoriu, C.; AlMujalhem, M.; Amani, M. Evaluating Potential Near Wellbore Integrity Issues in Cement and Casing Layers During Subsurface Hydrogen Storage and Production. In Proceedings of the GOTECH, Dubai City, United Arab Emirates, 21 April 2025; SPE: Richardson, TX, USA, 2025; p. D031S044R001. [Google Scholar]
- H2FIT Tubes: Paving the Way for Hydrogen Production, Storage, and Transport. Available online: https://www.centravis.com/en/news/h2fit-tubes-paving-the-way-for-hydrogen-production-storage-and-transport/ (accessed on 28 June 2025).
- Naquash, A.; Riaz, A.; Yehia, F.; Chaniago, Y.D.; Lim, H.; Lee, M. Hydrogen Purification through a Membrane–Cryogenic Integrated Process: A 3 E’s (Energy, Exergy, and Economic) Assessment. Gases 2023, 3, 92–105. [Google Scholar] [CrossRef]
- Yang, M.; Hunger, R.; Berrettoni, S.; Sprecher, B.; Wang, B. A Review of Hydrogen Storage and Transport Technologies. Clean Energy 2023, 7, 190–216. [Google Scholar] [CrossRef]
- Massarweh, O.; Abushaikha, A.S. CO2 Sequestration in Subsurface Geological Formations: A Review of Trapping Mechanisms and Monitoring Techniques. Earth-Sci. Rev. 2024, 253, 104793. [Google Scholar] [CrossRef]
- Santos, H.S.; Nguyen, H.; Venâncio, F.; Ramteke, D.; Zevenhoven, R.; Kinnunen, P. Mechanisms of Mg Carbonates Precipitation and Implications for CO2 Capture and Utilization/Storage. Inorg. Chem. Front. 2023, 10, 2507–2546. [Google Scholar] [CrossRef]
- Tay, W.H.; Lau, K.K.; Shariff, A.M. High Frequency Ultrasonic-Assisted Chemical Absorption of CO2 Using Monoethanolamine (MEA). Sep. Purif. Technol. 2017, 183, 136–144. [Google Scholar] [CrossRef]
- Sai Bhargava Reddy, M.; Ponnamma, D.; Sadasivuni, K.K.; Kumar, B.; Abdullah, A.M. Carbon Dioxide Adsorption Based on Porous Materials. RSC Adv. 2021, 11, 12658–12681. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yu, W.; Wu, A.; Shu, W.; Zhang, Y. Recent Progress on CO2 Separation Membranes. RSC Adv. 2024, 14, 20714–20734. [Google Scholar] [CrossRef] [PubMed]
- De Morais, M.G.; De Morais, E.G.; Duarte, J.H.; Deamici, K.M.; Mitchell, B.G.; Costa, J.A.V. Biological CO2 Mitigation by Microalgae: Technological Trends, Future Prospects and Challenges. World J. Microbiol. Biotechnol. 2019, 35, 78. [Google Scholar] [CrossRef]
- Case Studies of CO2 Storage in Depleted Oil and Gas Fields. IEAGHG. Available online: https://ieaghg.org/publications/case-studies-of-co2-storage-in-depleted-oil-and-gas-fields/ (accessed on 24 June 2025).
- Shakor, Z.M.; Parsafard, N.; Al-Shafei, E. Adsorption and Separation Modeling of CO2, Hydrogen, and Biogas: A Mathematical Review. J. Mater. Sci. 2025, 60, 6850–6876. [Google Scholar] [CrossRef]
- Kamolov, A.; Turakulov, Z.; Rejabov, S.; Díaz-Sainz, G.; Gómez-Coma, L.; Norkobilov, A.; Fallanza, M.; Irabien, A. Decarbonization of Power and Industrial Sectors: The Role of Membrane Processes. Membranes 2023, 13, 130. [Google Scholar] [CrossRef]
- Joachimski, M.M.; Müller, J.; Gallagher, T.M.; Mathes, G.; Chu, D.L.; Mouraviev, F.; Silantiev, V.; Sun, Y.D.; Tong, J.N. Five Million Years of High Atmospheric CO2 in the Aftermath of the Permian-Triassic Mass Extinction. Geology 2022, 50, 650–654. [Google Scholar] [CrossRef]
- Kumar, R.; Chung, W.J.; Khan, M.A.; Son, M.; Park, Y.-K.; Lee, S.S.; Jeon, B.-H. Breakthrough Innovations in Carbon Dioxide Mineralization for a Sustainable Future. Rev. Environ. Sci. Biotechnol. 2024, 23, 739–799. [Google Scholar] [CrossRef]
- Wang, N.; Wang, D.; Krook-Riekkola, A.; Ji, X. MEA-Based CO2 Capture: A Study Focuses on MEA Concentrations and Process Parameters. Front. Energy Res. 2023, 11, 1230743. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of Microalgae in Global CO2 Sequestration: Physiological Mechanism, Recent Development, Challenges, and Future Prospective. Sustainability 2021, 13, 13061. [Google Scholar] [CrossRef]
- Kiseleva, S.V.; Chernova, N.I.; Vlaskin, M.S.; Grigorenko, A.V.; Chunzhuk, E.A.; Malaniy, S.Y.; Bakumenko, E.A.; Rositskaya, T.V. Carbon Dioxide Absorption by Microalgae: Analysis of Technologies and Energy Costs. Therm. Eng. 2024, 71, 1038–1048. [Google Scholar] [CrossRef]
- Costs of CO2 Sequestration. Thunder Said Energy. Available online: https://thundersaidenergy.com/downloads/co2-disposal-in-geologic-formations-the-economics/ (accessed on 24 June 2025).
- Mazzotti, M.; Abanades, J.C.; Allam, R.; Lackner, K.S.; Meunier, F.; Rubin, E.; Sanchez, J.C.; Yogo, K.; Zevenhoven, R. Mineral Carbonation and Industrial Uses of Carbon Dioxide. In IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Raksajati, A.; Ho, M.T.; Wiley, D.E. Reducing the Cost of CO2 Capture from Flue Gases Using Aqueous Chemical Absorption. Ind. Eng. Chem. Res. 2013, 52, 16887–16901. [Google Scholar] [CrossRef]
- Cost of Carbon Capture by Approach or Technology. Available online: https://www.statista.com/statistics/1304575/global-carbon-capture-cost-by-technology/ (accessed on 24 June 2025).
- Is Carbon Capture Too Expensive?—Analysis. Available online: https://www.iea.org/commentaries/is-carbon-capture-too-expensive (accessed on 24 June 2025).
- Meng, J.; Liao, W.; Zhang, G. Emerging CO2-Mineralization Technologies for Co-Utilization of Industrial Solid Waste and Carbon Resources in China. Minerals 2021, 11, 274. [Google Scholar] [CrossRef]
- Zeng, H.; Qu, X.; Xu, D.; Luo, Y. Porous Adsorption Materials for Carbon Dioxide Capture in Industrial Flue Gas. Front. Chem. 2022, 10, 939701. [Google Scholar] [CrossRef]
- Ruales, E.; Gómez-Serrano, C.; Morillas-España, A.; González-López, C.; Escolà Casas, M.; Matamoros, V.; Garfí, M.; Ferrer, I. Resource Recovery and Contaminants of Emerging Concern Mitigation by Microalgae Treating Wastewater. J. Environ. Manag. 2024, 367, 121950. [Google Scholar] [CrossRef]
- Yuen, Y.T.; Sharratt, P.N.; Jie, B. Carbon Dioxide Mineralization Process Design and Evaluation: Concepts, Case Studies, and Considerations. Environ. Sci. Pollut. Res. 2016, 23, 22309–22330. [Google Scholar] [CrossRef]
- François, M.H.J.-J.; Grimstvedt, A.; Knuutila, H.K. Iron Solubility Measurements in Aqueous MEA for CO2 Capture. Ind. Eng. Chem. Res. 2025, 64, 2318–2328. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of Carbon Dioxide for Post-Combustion Capture: A Review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Castel, C.; Bounaceur, R.; Favre, E. Membrane Processes for Direct Carbon Dioxide Capture From Air: Possibilities and Limitations. Front. Chem. Eng. 2021, 3, 668867. [Google Scholar] [CrossRef]
- IEA Greenhouse Gas R&D Programme; Stalker, L.; Michael, K.; Jenkins, C.; Holland, K.; Peeters, L.; Myers, M.; Ross, A. Reviewing the Implications of Unlikely but Potential CO2 Migration to the Surface or Shallow Subsurface; IEAGHG: Cheltenham, UK, 2025. [Google Scholar] [CrossRef]
- Karimi, M.; Shirzad, M.; Silva, J.A.C.; Rodrigues, A.E. Carbon Dioxide Separation and Capture by Adsorption: A Review. Environ. Chem. Lett. 2023, 21, 2041–2084. [Google Scholar] [CrossRef]
- Ray, S.; Kuppam, C.; Pandit, S.; Kumar, P. Biogas Upgrading by Hydrogenotrophic Methanogens: An Overview. Waste Biomass Valorization 2023, 14, 537–552. [Google Scholar] [CrossRef]
- Barbier, S.; Huang, F.; Andreani, M.; Tao, R.; Hao, J.; Eleish, A.; Prabhu, A.; Minhas, O.; Fontaine, K.; Fox, P.; et al. A Review of H2, CH4, and Hydrocarbon Formation in Experimental Serpentinization Using Network Analysis. Front. Earth Sci. 2020, 8, 209. [Google Scholar] [CrossRef]
- Kanaujiya, R.; Metya, A.K.; Choudhary, N.; Kumar, R.; Patra, T.K. Molecular Dynamics Insights into Tetrahydrofuran-Assisted Formation of CH4, CO2, and H2 Gas Hydrates. Phys. Chem. Chem. Phys. 2025, 27, 13991–13999. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.; Khan, M.R. Carbonate and Sandstone Reservoirs in CO2 Sequestration: Assessing Porosity and Permeability for Enhanced Storage Potential. Pet. Petrochem. Eng. J. 2024, 8, 1–12. [Google Scholar] [CrossRef]
- Ni, S.; Lv, W.; Ji, Z.; Wang, K. CO2 Mineralized Sequestration and Assistance by Microorganisms in Reservoirs: Development and Outlook. Energies 2023, 16, 7571. [Google Scholar] [CrossRef]
- Askarova, A.; Mukhametdinova, A.; Markovic, S.; Khayrullina, G.; Afanasev, P.; Popov, E.; Mukhina, E. An Overview of Geological CO2 Sequestration in Oil and Gas Reservoirs. Energies 2023, 16, 2821. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, S.; Li, D.; Jiang, X. Density-Driven Convection for CO2 Solubility Trapping in Saline Aquifers: Modeling and Influencing Factors. Geotechnics 2023, 3, 70–103. [Google Scholar] [CrossRef]
- Johnson, J.W.; Nitao, J.J.; Knauss, K.G. Reactive Transport Modelling of CO2 Storage in Saline Aquifers to Elucidate Fundamental Processes, Trapping Mechanisms and Sequestration Partitioning. Geol. Soc. Spec. Publ. 2004, 233, 107–128. [Google Scholar] [CrossRef]
- Chen, Q.; Ramdin, M.; Vlugt, T.J.H. Solubilities of CO2, CH4, C2H6, CO, H2, N2, N2O, and H2 S in Commercial Physical Solvents from Monte Carlo Simulations. Mol. Simul. 2023, 49, 1341–1349. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Lim, B. Hydrogen Separation Membranes: A Material Perspective. Molecules 2024, 29, 4676. [Google Scholar] [CrossRef]
- Yang, G.-C.; Zhou, L.; Mbadinga, S.M.; Liu, J.-F.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Formate-Dependent Microbial Conversion of CO2 and the Dominant Pathways of Methanogenesis in Production Water of High-Temperature Oil Reservoirs Amended with Bicarbonate. Front. Microbiol. 2016, 7, 365. [Google Scholar] [CrossRef] [PubMed]
- Tyne, R.L.; Barry, P.H.; Lawson, M.; Byrne, D.J.; Warr, O.; Xie, H.; Hillegonds, D.J.; Formolo, M.; Summers, Z.M.; Skinner, B.; et al. Rapid Microbial Methanogenesis during CO2 Storage in Hydrocarbon Reservoirs. Nature 2021, 600, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Huang, Y.-J.; Chang, J.-S. Boosting CO2 Capture and Conversion to Biohydrogen through Enhanced Microalgal Biomass Yield. Int. J. Hydrogen Energy 2025, 139, 998–1007. [Google Scholar] [CrossRef]
- Ashour, M.; Mansour, A.T.; Alkhamis, Y.A.; Elshobary, M. Usage of Chlorella and Diverse Microalgae for CO2 Capture-towards a Bioenergy Revolution. Front. Bioeng. Biotechnol. 2024, 12, 1387519. [Google Scholar] [CrossRef]
- Maghzian, A.; Aslani, A.; Zahedi, R. A Comprehensive Review on Effective Parameters on Microalgae Productivity and Carbon Capture Rate. J. Environ. Manag. 2024, 355, 120539. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kanai, D.; Sugiyama, K.; Fujii, K. Biogas Upgrading by Wild Alkaliphilic Microalgae and the Application Potential of Their Biomass in the Carbon Capture and Utilization Technology. Fermentation 2024, 10, 134. [Google Scholar] [CrossRef]
- King, G.E.; King, D.E. Environmental Risk Arising From Well-Construction Failure—Differences Between Barrier and Well Failure, and Estimates of Failure Frequency Across Common Well Types, Locations, and Well Age. SPE Prod. Oper. 2013, 28, 323–344. [Google Scholar] [CrossRef]
- Jackson, R.B. The Integrity of Oil and Gas Wells. Proc. Natl. Acad. Sci. USA 2014, 111, 10902–10903. [Google Scholar] [CrossRef]
- Laumb, J.D.; Glazewski, K.A.; Hamling, J.A.; Azenkeng, A.; Watson, T.L. Wellbore Corrosion and Failure Assessment for CO2 EOR and Storage: Two Case Studies in the Weyburn Field. Int. J. Greenh. Gas Control 2016, 54, 479–489. [Google Scholar] [CrossRef]
- Cirimello, P.G.; Otegui, J.L.; Carfi, G.; Morris, W. Failure and Integrity Analysis of Casings Used for Oil Well Drilling. Eng. Fail. Anal. 2017, 75, 1–14. [Google Scholar] [CrossRef]
- Yousuf, N.; Olayiwola, O.; Guo, B.; Liu, N. A Comprehensive Review on the Loss of Wellbore Integrity Due to Cement Failure and Available Remedial Methods. J. Pet. Sci. Eng. 2021, 207, 109123. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, Z.; Fu, X. A Review on Well Integrity Issues for CO 2 Geological Storage and Enhanced Gas Recovery. Renew. Sustain. Energy Rev. 2016, 59, 920–926. [Google Scholar] [CrossRef]
- Al-Shehri, D.A. Oil and Gas Wells: Enhanced Wellbore Casing Integrity Management through Corrosion Rate Prediction Using an Augmented Intelligent Approach. Sustainability 2019, 11, 818. [Google Scholar] [CrossRef]
- Wu, L.; Hou, Z.-M.; Luo, Z.-F.; Fang, Y.-L.; Huang, L.-C.; Wu, X.-N.; Chen, Q.-J.; Wang, Q.-C. Impacts of Microbial Interactions on Underground Hydrogen Storage in Porous Media: A Comprehensive Review of Experimental, Numerical, and Field Studies. Pet. Sci. 2024, 21, 4067–4099. [Google Scholar] [CrossRef]
- Opoku Duartey, K.; Ampomah, W.; Rahnema, H.; Mehana, M. Underground Hydrogen Storage: Transforming Subsurface Science into Sustainable Energy Solutions. Energies 2025, 18, 748. [Google Scholar] [CrossRef]
- Zappone, A.; Rinaldi, A.P.; Grab, M.; Wenning, Q.C.; Roques, C.; Madonna, C.; Obermann, A.C.; Bernasconi, S.M.; Brennwald, M.S.; Kipfer, R.; et al. Fault Sealing and Caprock Integrity for CO2 Storage: An in Situ Injection Experiment. Solid Earth 2021, 12, 319–343. [Google Scholar] [CrossRef]
- IEA Greenhouse Gas R&D Programme; Bason, D.; Nourollah, H.; De Morton, S.; Watson, M.; Petho, G.; Maffeis, I.; Rieger, M.; O’Brien, G. Geological Storage of CO2: Seal Integrity Review; IEAGHG: Cheltenham, UK, 2024. [Google Scholar]
- Dopffel, N.; An-Stepec, B.A.; De Rezende, J.R.; Sousa, D.Z.; Koerdt, A. Editorial: Microbiology of Underground Hydrogen Storage. Front. Energy Res. 2023, 11, 1242619. [Google Scholar] [CrossRef]
- Sugai, Y.; Owaki, Y.; Sasaki, K. Simulation Study on Reservoir Souring Induced by Injection of Reservoir Brine Containing Sulfate-Reducing Bacteria. Sustainability 2020, 12, 4603. [Google Scholar] [CrossRef]
- Hu, J.; Li, C.; Zhang, Q.; Guo, Q.; Zhao, S.; Wang, W.; Lee, D.-J.; Yang, Y. Using Chemical Looping Gasification with Fe2O3/Al2O3 Oxygen Carrier to Produce Syngas (H2+CO) from Rice Straw. Int. J. Hydrogen Energy 2019, 44, 3382–3386. [Google Scholar] [CrossRef]
- Hagar, H.S.; Foroozesh, J.; Kumar, S.; Zivar, D.; Banan, N.; Dzulkarnain, I. Microbial H2S Generation in Hydrocarbon Reservoirs: Analysis of Mechanisms and Recent Remediation Technologies. J. Nat. Gas Sci. Eng. 2022, 106, 104729. [Google Scholar] [CrossRef]
- McMahon, M.J.L.; Daugulis, A.J. Gas Phase H2S Product Recovery in a Packed Bed Bioreactor with Immobilized Sulfate-Reducing Bacteria. Biotechnol. Lett. 2008, 30, 467–473. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Kong, F.; Sun, R.; Li, R.; Yang, J.; Min, Q. Experimental Study on Dynamic Response Performance of Hydrogen Sensor in Confined Space under Ceiling. Front. Energy Res. 2024, 12, 1456316. [Google Scholar] [CrossRef]
- Se, H.; Jiang, J.; Sun, C.; Song, K. Hydrogen Leakage Detection in Confined Spaces with Drift Suppression Based on Subspace Alignment. In Proceedings of the 10th Hydrogen Technology Convention, Volume 1; Sun, H., Pei, W., Dong, Y., Yu, H., You, S., Eds.; Springer Proceedings in Physics; Springer Nature: Singapore, 2024; Volume 393, pp. 180–186. ISBN 978-981-99-8630-9. [Google Scholar]
- Sheng, J.J. Techno-Economic Analysis of Hydrogen Generation in Hydrocarbon Reservoirs. SPE J. 2024, 29, 5752–5760. [Google Scholar] [CrossRef]
- Sundeep, S.; Sethuraman, L.; Akindipe, D.; Fingersh, L.; Wenrick, Z.; Munoz, A. Repurposing Inactive Oil and Gas Wells for Energy Storage: Maximizing the Potential via Optimal Drivetrain Control. IET Conf. Proc. 2023, 2023, 486–493. [Google Scholar] [CrossRef]
- TresCantos CAPEX y OPEX en Proyectos de Hidrógeno. ARIEMA. Available online: https://www.ariema.com/capex-opex-proyectos-hidrogeno-ariema (accessed on 28 June 2025).
- Ganguly, A.; Sun, P.; Liu, X.; Delgado, H.E.; Sun, L.; Elgowainy, A. Techno-Economic and Life Cycle Analysis of Bio-Hydrogen Production Using Bio-Based Waste Streams through the Integration of Dark Fermentation and Microbial Electrolysis. Green Chem. 2025, 27, 6213–6231. [Google Scholar] [CrossRef]
- Teke, G.M.; Anye Cho, B.; Bosman, C.E.; Mapholi, Z.; Zhang, D.; Pott, R.W.M. Towards Industrial Biological Hydrogen Production: A Review. World J. Microbiol. Biotechnol. 2024, 40, 37. [Google Scholar] [CrossRef]
- Trade, N.Z.M. of F.A. and Japan: Strategic Hydrogen Roadmap—30 October 2020. Available online: https://www.mfat.govt.nz/en/trade/mfat-market-reports/japan-strategic-hydrogen-roadmap-30-october-2020 (accessed on 28 June 2025).
- Financial Incentives for Hydrogen and Fuel Cell Projects. Available online: https://www.energy.gov/eere/fuelcells/financial-incentives-hydrogen-and-fuel-cell-projects (accessed on 28 June 2025).
- Fujii-Rajani, R.; Patnaik, S. What Will Happen to the Inflation Reduction Act Under a Republican Trifecta? Brookings. Available online: https://www.brookings.edu/articles/what-will-happen-to-the-inflation-reduction-act-under-a-republican-trifecta/ (accessed on 13 July 2025).
- Cemvita Launches the Gold Hydrogen Program for Subsurface Biomanufacturing of Hydrogen | Gold Hydrogen. Available online: https://goldhydrogen.com/cemvita-launches-the-gold-hydrogen-program-for-subsurface-biomanufacturing-of-hydrogen/ (accessed on 28 June 2025).
- Adomavicius, V.; Simkoniene, G. Overview of Innovations and Recommendations for Efficient Operation of RES-Based Power Plants. In Proceedings of the 22nd International Scientific Conference “Engineering for Rural Development”, Jelgava, Latvia, 24–26 May 2023. [Google Scholar]
- Adeli, K.; Nachtane, M.; Faik, A.; Saifaoui, D.; Boulezhar, A. How Green Hydrogen and Ammonia Are Revolutionizing the Future of Energy Production: A Comprehensive Review of the Latest Developments and Future Prospects. Appl. Sci. 2023, 13, 8711. [Google Scholar] [CrossRef]
- Cemvita’s Successful Field Test Demonstrates Gold HydrogenTM Production in Situ | Gold Hydrogen. Available online: https://goldhydrogen.com/cemvitas-successful-field-test-demonstrates-gold-hydrogen-production-in-situ/ (accessed on 28 June 2025).
- Grinstein, J.D. Fighting Climate Change with Synthetic Biology: Cemvita Factory Generates Cost-Effective, Low-Carbon Solutions That Net Climate-Positive Results. Genet. Eng. Biotechnol. News 2022, 42, 12–13. [Google Scholar] [CrossRef]
- Ni, S.; Lv, W.; Ji, Z.; Wang, K.; Mei, Y.; Li, Y. Progress of Crude Oil Gasification Technology Assisted by Microorganisms in Reservoirs. Microorganisms 2024, 12, 702. [Google Scholar] [CrossRef]
- Head, I.M.; Gray, N.D.; Larter, S.R. Life in the Slow Lane; Biogeochemistry of Biodegraded Petroleum Containing Reservoirs and Implications for Energy Recovery and Carbon Management. Front. Microbiol. 2014, 5, 566. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Rafa, N.; Mofijur, M.; Badruddin, I.A.; Inayat, A.; Ali, M.S.; Farrok, O.; Yunus Khan, T.M. Biohydrogen Production From Biomass Sources: Metabolic Pathways and Economic Analysis. Front. Energy Res. 2021, 9, 753878. [Google Scholar] [CrossRef]
- Szymczak, P.D. Biotech Firm To Create Gold Hydrogen by Injecting Microbes into Depleted Oil Reservoirs. Available online: https://jpt.spe.org/biotech-firm-to-create-gold-hydrogen-by-injecting-microbes-into-depleted-oil-reservoirs (accessed on 28 June 2025).
- H2-View News: Start-Up Plans to Generate Low-Cost Hydrogen from Old Oil Wells with Microbes|H2 Energy Group 2024. Available online: https://h2eg.com/h2-view-news-start-up-plans-to-generate-low-cost-hydrogen-from-old-oil-wells-with-microbes/ (accessed on 28 June 2025).
- Microbes That Eat Oil and Excrete Cheap, Clean Hydrogen. GlobalSpec. Available online: https://insights.globalspec.com/article/19402/microbes-that-eat-oil-and-excrete-cheap-clean-hydrogen (accessed on 28 June 2025).
- Kevin, P.K.; Marcio, L.B.D.S.; Mojtaba, K.; Renata, A.G.; Roger, A.H.; Aaron, C.T.; Zachary, R.B.; Tahereh, K.; Luiza, L.A.L.; Barbara, D.F.M.; et al. Method. UK Patent GB2613039A, 24 May 2023. [Google Scholar]
- Zachary, R.B.; Tj, T.; Michael, S.; Edward, J.K.; Raja, S.R.; Renata, A.G.; Aaron, C.T.; Addien, W.; Barbara, D.F.M.; Edris, T. Process and Plant. UK Patent GB2629486A, 30 October 2024. [Google Scholar]
- Broussard, Z.R.; Tidwell, T.; Samuel, M.; Koch, E.J.; Ramanathan, R.S.; Gonçalves, R.A.; Trevino, A.; Wray, A.; Magalhães, B.D.F. Process and Plant. PCT Patent Application WO2024206528A2, 3 October 2024. [Google Scholar]
- Karimi, T.; Karimi, M. Methods and Systems for Producing Organic Compounds in a Subterranean Environment. U.S. Patent US2022282604A1, 8 September 2022. [Google Scholar]
- Shen, L.; Zhao, Q.; Wu, X.; Li, X.; Li, Q.; Wang, Y. Interspecies Electron Transfer in Syntrophic Methanogenic Consortia: From Cultures to Bioreactors. Renew. Sustain. Energy Rev. 2016, 54, 1358–1367. [Google Scholar] [CrossRef]
- Hu, B.; Chen, S. Pretreatment of Methanogenic Granules for Immobilized Hydrogen Fermentation. Int. J. Hydrogen Energy 2007, 32, 3266–3273. [Google Scholar] [CrossRef]
- Ghignone, J.I.; Andrade, G.D. General Geology and Major Oil Fields of Recôncavo Basin, Brazil. In Geology of Giant Petroleum Fields; American Association of Petroleum Geologists: Tulsa, OK, USA, 1970; ISBN 978-1-62981-225-0. [Google Scholar]
- Sarma, S.; Ortega, D.; Minton, N.P.; Dubey, V.K.; Moholkar, V.S. Homologous Overexpression of Hydrogenase and Glycerol Dehydrogenase in Clostridium pasteurianum to Enhance Hydrogen Production from Crude Glycerol. Bioresour. Technol. 2019, 284, 168–177. [Google Scholar] [CrossRef]
- Meshulam-Simon, G.; Behrens, S.; Choo, A.D.; Spormann, A.M. Hydrogen Metabolism in Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 2007, 73, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Programa Nacional de Hidrogênio-PNH2—Ministério de Minas e Energia. Available online: https://www.gov.br/mme/pt-br/programa-nacional-do-hidrogenio-1 (accessed on 1 July 2025).
| Challenge | Description | Strategies/Technologies |
|---|---|---|
| Substrate Availability /Toxicity | Hydrocarbons are hard to degrade due to low solubility and chemical stability. | Use engineered microbes for hydrocarbon breakdown; apply surfactants to improve hydrocarbon solubility [82,97]. |
| Hydrocarbon Activation | Hydrocarbons need activation to start microbial degradation. | Use assA for alkanes; use bssA for aromatics; syntrophic consortia to produce fermentation intermediates [78,79]. |
| Thermodynamic Constraints | High H2 pressure inhibits hydrogenase and fermentation. | Use hydrogen transfer between species to lower H2 levels; use biofilm reactors or MECs to reduce H2 pressure [85]. |
| Methanogen Competition | Methanogens reduce hydrogen yield by consuming H2. | Inhibit methanogens via heat shock or chemical inhibitors; adjust retention time to favor hydrogen producers [85]. |
| Microbial Diversity /Ecological Interactions | Success relies on microbial cooperation within consortia. | Use mixed consortia (e.g., Clostridium, Thermotoga) for better degradation; promote interactions between fermentative and hydrogen-producing microbes [78,79]. |
| Field-scale Validation | Scaling up hydrogen production faces practical challenges. | Conduct field trials to validate microbial hydrogen production (e.g., Cemvita Factory); use real-time monitoring to optimize hydrogen production [60]. |
| Infrastructure Component | Existing in Oil & Gas Fields | Required Modifications for H2 Production | New Installations for H2 Bioproduction |
|---|---|---|---|
| Wells and Boreholes | Vertical and directional production/injection wells | Casing reinforcement; corrosion-resistant linings; zonal isolation with packers/plugs [105] | New wells if reservoir geometry or access is insufficient |
| Well Cement and Casings | Standard steel casing and Portland cement | Replacement or coating with corrosion-resistant alloys; acid-tolerant cement materials [106] | — |
| Subsurface Infrastructure | Reservoir access through perforated casings | Isolation of fermentation zones; installation of retrievable plugs [105] | Bioreactor adaptation: enhanced sealing for anaerobic containment |
| Injection Systems | Water/gas/polymer injection systems | Modified for microbial inoculum and nutrient solution delivery | Modular microbial and nutrient injection skids |
| Production Tubing | Hydrocarbon production tubing | Material upgrade to resist H2S, CO2, and acids [107] | — |
| Surface Separation Units | Oil/gas/water separators | Adapted to separate and handle H2-rich biogas [108] | H2-specific gas–liquid separators |
| Gas Processing Units | Natural gas dehydration, compression, and sweetening | Integration of H2-compatible compressors and piping | H2 purification systems (pressure swing adsorption, membrane units) |
| Monitoring Equipment | Pressure, temperature, flow rate sensors | Additional sensors for pH, redox, microbial activity, gas composition (H2, CO2, H2S) | Real-time bioprocess monitoring modules |
| Control Systems | Present for process automation and remote monitoring | Integration with new sensors and fermentation specific controls | AI-enabled microbial fermentation control algorithms |
| Storage Tanks | Crude oil, water, and gas storage facilities | H2 compatible materials for gas storage (e.g., high-alloy steel) [109] | H2-specific pressurized storage tanks or absorption beds |
| Pipelines | Steel pipelines for oil/gas transport | Retrofitting or replacement with H2 compatible materials | New dedicated H2 pipelines (short range/local) |
| Waste Management | Produced water treatment, gas flaring | Treatment of microbial byproducts and acidic effluents | Biosludge and fermentation waste handling units |
| Laboratory Facilities | On-site labs for chemical analysis | Capability expansion for microbial, gas, and fermentation monitoring | Mobile genetic and microbial culture labs |
| Parameter | Geological (In Situ) | Mineralization (In Situ) | Chemical Absorption | Adsorption | Membrane Separation | Microalgae-Based Sequestration |
|---|---|---|---|---|---|---|
| Mechanism | CO2 is trapped in porous rocks via structural, capillary, solubility, or residual trapping [110] | CO2 reacts with minerals (Ca, Mg silicates) to form stable carbonates [111] | CO2 chemically reacts with monoethanolamine [112] | CO2 adheres to solid porous materials under pressure [113] | Selective diffusion of CO2 through polymer/inorganic membranes [114] | CO2 fixed by photosynthesis into algal biomass [115] |
| Maturity/TRL | High (especially for DORs) [116] | Moderate to high | High | Moderate to high [117] | Moderate [118] | Moderate (higher for closed photobioreactors) |
| CO2 Permanence | Very High (millions of years) [119] | Very High (solid carbonates are stable) [120] | Low to Moderate [121] | Low to Moderate [117] | Low to Moderate | Moderate [122] |
| Energy Requirement | Low | Moderate | High | Moderate | Low to Moderate | Moderate [123] |
| Cost (USD/ton CO2) | ~$10–20 [124] | ~$50–100 [125] | ~$62–80 [126] | ~$15–130 [127] | ~$30–80 [127] | ~$30–200 [122] |
| Scalability | High [128] | Moderate | High | Moderate | High | Moderate |
| Co-benefits | Enhanced oil/H2 recovery | Heavy metal stabilization [129] | None | May support pressure swing adsorption for H2 purification [130] | Energy-efficient for H2 purification | Biomass for fuels, wastewater treatment |
| Key Limitations | Risk of leakage, site-specific [131] | Slow natural kinetics [132] | Solvent degradation [133] | Sorbent regeneration [134] | Fouling/selectivity [135] | Growth depends on conditions |
| Environmental Impact | Low (if monitored) [136] | Low to moderate [136] | Chemical waste | Sorbent disposal [137] | Low | Low; improves air/water quality [131] |
| Application in H2 | Explored in microbial H2 | Emerging for microbial mineralization | Used in gas purification | Used in downstream cleanup | Growing interest | Promising for integrated systems |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampaio, I.C.F.; de Moura, I.V.L.; Matos, J.B.T.L.; Jones, C.M.; de Almeida, P.F. Use of Depleted Oil and Gas Reservoirs as Bioreactors to Produce Hydrogen and Capture Carbon Dioxide. Fermentation 2025, 11, 490. https://doi.org/10.3390/fermentation11090490
Sampaio ICF, de Moura IVL, Matos JBTL, Jones CM, de Almeida PF. Use of Depleted Oil and Gas Reservoirs as Bioreactors to Produce Hydrogen and Capture Carbon Dioxide. Fermentation. 2025; 11(9):490. https://doi.org/10.3390/fermentation11090490
Chicago/Turabian StyleSampaio, Igor Carvalho Fontes, Isabela Viana Lopes de Moura, Josilene Borges Torres Lima Matos, Cleveland Maximino Jones, and Paulo Fernando de Almeida. 2025. "Use of Depleted Oil and Gas Reservoirs as Bioreactors to Produce Hydrogen and Capture Carbon Dioxide" Fermentation 11, no. 9: 490. https://doi.org/10.3390/fermentation11090490
APA StyleSampaio, I. C. F., de Moura, I. V. L., Matos, J. B. T. L., Jones, C. M., & de Almeida, P. F. (2025). Use of Depleted Oil and Gas Reservoirs as Bioreactors to Produce Hydrogen and Capture Carbon Dioxide. Fermentation, 11(9), 490. https://doi.org/10.3390/fermentation11090490







