Recent Advances in Microbial Bioconversion as an Approach to Boost Hydroxytyrosol Recovery from Olive Mill Wastewater
Abstract
1. Introduction
2. Phytochemical Characteristics of Olive Mill Wastewater (OMWW) and Target Bioactive Constituents
3. Microbial Communities in OMWW
| Microbial Group | Dominant Taxa | Key Functions/Roles | References |
|---|---|---|---|
| Bacteria | Gammaproteobacteria (Enterobacteriaceae, Moraxellaceae, Pseudomonadaceae, Xanthomonadaceae) | Dominant group (~30%), biodegradation, opportunistic pathogens | [70,72] |
| Betaproteobacteria (Oxalobacteraceae and related genera) | Relevant in detoxification, co-dominant in microbial communities | ||
| Actinobacteria (Micrococcaceae, Propionibacteriaceae, Microbacteriaceae) | Minor fraction, involved in nutrient cycling | [80] | |
| Bacteroidia (Prevotellaceae), Gammaproteobacteria (Acinetobacter, Enterobacter, Escherichia, Klebsiella), Clostridia (Clostridium) | Potential harmful pathogens with health/environmental risk | [73] | |
| Yeasts | Microbotryomycetes (Rhodotorula) | Biodegradation of phenols and sugars, detoxification | [74] |
| Saccharomycetes (Geotrichum candidum) | Ability to grow on OMWW, reducing COD, phenols, and antimicrobial compounds | [71] | |
| Saccharomycetes (Pichia, Candida, Saccharomyces) | Biodegradation, high enzymatic activity (pectolytic, xylanolytic, β-glucosidase, etc.) | [75] | |
| Fungi (molds) | Eurotiomycetes (Penicillium, Aspergillus), Dothideomycetes (Alternaria), Agaricomycetes (Phanerochaete, Trametes, Pleurotus) | Strong biodegraders, phenol reduction, and detoxification | [17,75] |
| Eurotiomycetes (Penicillium, Aspergillus), Dothideomycetes (Alternaria), Agaricomycetes (Phanerochaete, Trametes, Pleurotus) | Lignocellulolytic enzyme producers (low frequency) | ||
| Others: Acremonium, Fusarium, Paecilomyces, Byssochlamys, etc. | Contribute to degradation, phenol reduction | ||
| Aspergillus, Penicillium, Paecilomyces, Fusarium, Alternaria, Scopulariopsis | Multiple hydrolytic activities (lipase, protease, amylase, cellulase, pectinase, and tannase) are useful for biotechnological and environmental applications such as bioremediation and bioconversion | [79] | |
| Chalara, Lecythophora, Phoma, Rhinocladiella, Bionectria, Cerrena, Phycomyces | Ligninolytic and/or cellulolytic activity | ||
| Acremonium, Scopulariopsis, Rhinocladiella | Selective proteolytic/lipolytic activity (i.e., specialized for specific protein or lipid substrates) |
4. Recovery Strategies and Commercial Relevance of Hydroxytyrosol (HT)
5. Hydrolytic Bioconversion of Olive Mill Wastewater (OMWW) for Hydroxytyrosol Production
5.1. Spontaneous Fermentation in OMWW
5.2. Driven Microbial Fermentation
5.3. Microbiological and Regulatory Assessment of OMWW for Food Applications
6. OMWW as Functional Foods and Beverages
6.1. Impact on Food Sensory Characteristics
6.2. Effect of Storage of Olive Mill Wastewater on Hydroxytyrosol Concentration
6.3. Feasibility and Viability of OMWW Application in Food
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EFSA | European Food Safety Authority |
| OMWW | Olive Mill Wastewater |
| BHT | Butylated Hydroxytoluene |
| OLE | Oleuropein |
| HT | Hydroxytyrosol |
| HPLC | High-Performance Liquid Chromatography |
| COD | Chemical Oxygen Demand |
| LAB | Lactic Acid Bacteria |
| RFLP | Restriction Fragment Length Polymorphism |
| T2D | Type 2 Diabetes |
| TSS | Total Suspended Solids |
| VOO | Virgin Olive Oil |
References
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Kour, N.; Gulshan, B.; Singh, S.; Bhatti, S.S.; Arora, S.; Singh, B.; Vhatia, A. Polyphenols mediated attenuation of diabetes associated cardiovascular complications: A comprehensive review. J. Diabetes Metab. Disord. 2023, 23, 73–99. [Google Scholar] [CrossRef]
- Farhat, G. Polyphenols in obesity and weight management: Are they worth further research? An umbrella review. Nutr. Bull. 2024, 49, 126–131. [Google Scholar] [CrossRef]
- Kannan, K.; George, J.A.; Sahadevan, R.; Kothari, M.; Sadhukhan, S. Insights into one drug, multi-target aspects of polyphenols for diabetes management: In vitro, in vivo, and clinical evidence. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: Recent advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef]
- Hafezi, K.; Hemmati, A.A.; Abbaszadeh, H.; Valizadeh, A.; Makvandi, M. Anticancer activity and molecular mechanisms of α-conidendrin, a polyphenolic compound present in Taxus yunnanensis, on human breast cancer cell lines. Phytother. Res. 2020, 34, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The two-way polyphenols-microbiota interactions and their effects on obesity and related metabolic diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar]
- Qi, Y.Y.; Gong, T.; Zhao, P.T.; Niu, Y.J.; Hu, Y.Y.; Hu, C.Y.; Zhang, S.; Meng, Y.H. Hydroxytyrosyl oleate is a promising safe additive to inhibit the oxidation of olive oil. Food Control 2023, 153, 109895. [Google Scholar] [CrossRef]
- Cedola, A.; Cardinali, A.; D’antuono, I.; Conte, A.; Del Nobile, M.A. Cereal foods fortified with by-products from the olive oil industry. Food Biosci. 2020, 33, 100490. [Google Scholar] [CrossRef]
- Fki, I.; Allouche, N.; Sayadi, S. The use of polyphenolic extract, purified hydroxytyrosol and 3,4-dihydroxyphenyl acetic acid from olive mill wastewater for the stabilization of refined oils: A potential alternative to synthetic antioxidants. Food Chem. 2005, 93, 197–204. [Google Scholar] [CrossRef]
- Wang, E.; Jiang, Y.; Zhao, C. Hydroxytyrosol isolation, comparison of synthetic routes and potential biological activities. Food Sci. Nutr. 2024, 12, 6899–6912. [Google Scholar] [CrossRef]
- Fernández-Bolaños, J.; Rodriguez, G.; Rodriguez, R.; Heredia, A.; Guillén, R.; Jiménez, A. Production in large quantities of highly purified hydroxytyrosol from liquid-solid waste of two-phase olive oil processing or “alperujo”. J. Agric. Food Chem. 2002, 50, 6804–6811. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Soares, T.F.; Alves, R.C.; Oliveira, M.B.P.P. From olive oil production to by-products: Emergent technologies to extract bioactive compounds. Food Rev. Int. 2024, 40, 3342–3369. [Google Scholar] [CrossRef]
- Romeo, F.V.; Ganuzzo, G.; Foti, P.; Balistreri, G.; Caggia, C.; Rapisarda, P. Microbial application to improve olive mill wastewater phenolic extracts. Molecules 2021, 26, 1944. [Google Scholar] [CrossRef] [PubMed]
- Ntougias, S.; Bourtzis, K.; Tsiamis, G. The microbiology of olive mill wastes. Biomed. Res. Int. 2013, 2013, 784591. [Google Scholar] [CrossRef]
- Koutsos, T.M.; Chatzistathis, T.; Balampekou, E.I. A new framework proposal, towards a common EU agricultural policy, with the best sustainable practices for the re-use of olive mill wastewater. Sci. Total Environ. 2018, 622–623, 942–953. [Google Scholar] [CrossRef]
- Fernandes, M.J.; Gomes, G.; Carvalho, P.; Martins, R.C.; Domingues, E. Phenolic compounds recovery to treat and valorize olive mill wastewater: Technologies overview. Chem. Eng. Sci. 2025, 305, 121145. [Google Scholar] [CrossRef]
- Shabir, S.; Noshin, I.; Maimona, S.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment technologies for olive mill wastewater with impacts on plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef]
- Foti, P.; Romeo, F.V.; Russo, N.; Pino, A.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive mill wastewater as renewable raw materials to generate high added-value ingredients for agro-food industries. Appl. Sci. 2021, 11, 7511. [Google Scholar] [CrossRef]
- Weyh, C.; Kruger, K.; Peeling, P.; Castell, L. The role of minerals in the optimal functioning of the immune system. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef] [PubMed]
- Zahi, M.; Zam, W.; El Hattab, M. State of knowledge on chemical, biological and nutritional properties of olive mill wastewater. Food Chem. 2022, 381, 132238. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.; Occhipinti, P.S.; Romeo, F.V.; Timpanaro, N.; Musumeci, T.; Randazzo, C.L.; Caggia, C. Phenols recovered from olive mill wastewater as natural booster to fortify blood orange juice. Food Chem. 2022, 393, 133428. [Google Scholar] [CrossRef]
- Zbakh, H.; El Abbassi, A. Potential use of olive mill wastewater in the preparation of functional beverages: A review. J. Funct. Foods 2012, 4, 53–65. [Google Scholar] [CrossRef]
- Galanakis, C.; Tornberg, E.; Gekas, V. The effect of heat processing on the functional properties of pectin contained in olive mill wastewater. LWT Food Sci. Technol. 2010, 43, 1001–1008. [Google Scholar] [CrossRef]
- Aiello, F.; Malivindi, R.; Motta, M.F.; Crupi, P.; Nicoletti, R.; Benincasa, C.; Clodoveo, M.L.; Rago, V.; Spizzirri, U.G.; Restuccia, D. Synthesis and characterization of a biopolymer pectin/ethanolic extract from olive mill wastewater: In vitro safety and efficacy tests on skin wound healing. Int. J. Mol. Sci. 2023, 24, 15075. [Google Scholar] [CrossRef]
- Otero, P.; Garcia-Olivera, P.; Carpena, M.; Barral-Martinez, M.; Chamorro, F.; Echave, J.; Garcia-Perez, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; et al. Applications of by-products from the olive oil processing: Revalorization strategies based on target molecules and green extraction technologies. Trends Food Sci. Technol. 2021, 116, 1084–1104. [Google Scholar] [CrossRef]
- De Bruno, A.; Romeo, R.; Piscopo, A.; Poiana, M. Antioxidant quantification in different portions obtained during olive oil extraction process in an olive oil press mill. J. Sci. Food Agric. 2021, 101, 1119–1126. [Google Scholar] [CrossRef]
- Foti, P.; Occhipinti, P.S.; Russo, N.; Scilimati, A.; Miciaccia, M.; Caggia, C.; Perrone, M.G.; Randazzo, C.L.; Romeo, F.V. Olive mill wastewater fermented with microbial pools as a new potential functional beverage. Molecules 2023, 28, 646. [Google Scholar] [CrossRef]
- Cuffaro, D.; Bertolini, A.; Silva, A.M.; Rodrigues, F.; Gabbia, D.; De Martin, S.; Saba, A.; Bertini, S.; Digiacomo, M.; Maccjia, M. Comparative analysis on polyphenolic composition of different olive mill wastewater and related extra virgin olive oil extracts and evaluation of nutraceutical properties by cell-based studies. Foods 2024, 13, 3312. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Conidi, C.; Loizzo, M.R.; Sicari, V.; Cassano, A. Olive mill wastewater polyphenol-enriched fractions by integrated membrane process: A promising source of antioxidant, hypolipidemic and hypoglycaemic compounds. Antioxidants 2020, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Chemical screening of olive biophenol extracts by hyphenated liquid chromatography. Anal. Chim. Acta 2007, 603, 176–189. [Google Scholar] [CrossRef]
- D’Antuono, I.; Kontogianni, V.G.; Kotsiou, K.; Linsalata, V.; Logrieco, A.F.; Tasioula-Margari, M.; Cardinali, A. Polyphenolic characterization of olive mill wastewaters, coming from Italian and Greek olive cultivars, after membrane technology. Food Res. Int. 2014, 65, 301–310. [Google Scholar] [CrossRef]
- Romero, C.; Brenes, M.; Garcia, P.; Garrido, A. Hydroxytyrosol 4-β-D-glucoside, an important phenolic compound in olive fruits and derived products. J. Agric. Food Chem. 2002, 50, 3835–3839. [Google Scholar] [CrossRef]
- Peeters, K.; Visnjevec, A.M.; Esakkimuthu, E.S.; Schwarzkopf, M.; Tavzes, C. The valorisation of olive mill wastewater from Slovenian Istria by Fe3O4 particles to recover polyphenolic compounds for the chemical specialties sector. Molecules 2021, 26, 6946. [Google Scholar] [CrossRef]
- Castejón, M.; Montoya, T.; Ortega-Vidal, J.; Altarejos, J.; Alarcon-de-la-Lastra, C. Ligstroside aglycon, an extra virgin olive oil secoiridoid, prevents inflammation by regulation of MAPKs, JAK/STAT, NF-κB, Nrf2/HO-1, and NLRP3 inflammasome signaling pathways in LPS-stimulated murine peritoneal macrophages. Food Funct. 2022, 13, 10200–10209. [Google Scholar] [CrossRef]
- Huertas-Alonso, A.J.; Gonzalez-Serrano, D.J.; Hadidi, M.; Salgado-Ramos, M.; Orellana-Palacios, J.C.; Sánchez-Verdú, M.P.; Xia, Q.; Simirgiotis, M.J.; Barba, F.J.; Dar, B.N.; et al. Table olive wastewater as a potential source of biophenols for valorization: A Mini Review. Fermentation 2022, 8, 215. [Google Scholar] [CrossRef]
- Servili, M.; Baldioli, M.; Selvaggi, R.; Miniati, E.; Macchioni, A.; Montedoro, G. High-performance liquid chromatography evaluation of phenols in olive fruit, virgin olive oil, vegetation waters, and pomace and 1D-and 2D-nuclear magnetic resonance characterization. J. Am. Oil Chem. Soc. 1999, 76, 873–882. [Google Scholar] [CrossRef]
- Saha, R.; Majie, A.; Baidya, R.; Sarkar, B. Verbascoside: Comprehensive review of a phenylethanoid macromolecule and its journey from nature to bench. Inflammopharmacology 2024, 32, 2729–2751. [Google Scholar] [CrossRef]
- Chen, L.; Gnanaraj, C.; Arulselvan, P.; El-seedi, H.; Teng, H. A review on advanced microencapsulation technology to enhance bioavailability of phenolic compounds: Based on its activity in the treatment of Type 2 Diabetes. Trends Food Sci. Technol. 2019, 85, 149–162. [Google Scholar] [CrossRef]
- Kaya, S.; Yilmaz, D.E.; Akmayan, I.; Egri, O.; Arasoglu, T.; Derman, S. Caffeic acid phenethyl ester loaded electrospun nanofibers for wound dressing application. J. Pharm. Sci. 2022, 111, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Alrowais, R.; Yousef, R.S.; Ahmed, O.K.; Mahmoud-Aly, M.; Daiem, M.M.A.; Said, N. Enhanced detoxification methods for the safe reuse of treated olive mill wastewater in irrigation. Environ. Sci. Eur. 2023, 35, 95. [Google Scholar] [CrossRef]
- Jia, K.; Shi, P.; Zhang, L.; Yan, X.; Xu, J.; Liao, K. Trans-cinnamic acid alleviates high-fat diet-induced renal injury via JNK/ERK/P38 MAPK pathway. J. Nutr. Biochem. 2025, 135, 109769. [Google Scholar] [CrossRef]
- Deeb, A.A.; Fayyad, M.K.; Alawi, M.A. Separation of polyphenols from Jordanian olive oil mill wastewater. Chromatogr. Res. Int. 2012, 2012, 812127. [Google Scholar] [CrossRef]
- Souza, T.N.; Santos, F.M.; Alves, P.R.; Ferro, J.N.; Correia, A.C.C.; Melo, T.S.; Soares, W.R.; Andrade, B.S.; Lagente, V.; Barreto, E. Local administration of p-coumaric acid decreases lipopolysaccharide-induced acute lung injury in mice: In vitro and in silico studies. Eur. J. Pharmacol. 2021, 897, 173929. [Google Scholar] [CrossRef]
- Kumar, M.; Kaushik, D.; Shubham, S.; Kumar, A.; Kumar, V.; Oz, E.; Brennan, C.; Zeng, M.; Proestos, C.; Çadırcı, K.; et al. Ferulic acid: Extraction, estimation, bioactivity and applications for human health and food. J. Sci. Food Agric. 2025, 105, 4168–4177. [Google Scholar] [CrossRef]
- Pitsillou, E.; Liang, J.; Hung, A.; Karagiannis, T.C. The SARS-CoV-2 helicase as a target for antiviral therapy: Identification of potential small molecule inhibitors by in silico modelling. J. Mol. Graph. Model. 2022, 114, 108193. [Google Scholar] [CrossRef]
- Bartel, I.; Mandryk, I.; Horbanczuk, J.O.; Wierzbicka, A.; Koszarska, M. Nutraceutical properties of syringic acid in civilization diseases-Review. Nutrients 2024, 16, 10. [Google Scholar] [CrossRef]
- Yalameha, B.; Nejabati, H.R.; Nouri, M. Cardioprotective potential of vanillic acid. Clin. Exp. Pharmacol. Physiol. 2023, 50, 193–204. [Google Scholar] [CrossRef]
- Gueboudji, Z.; Addad, D.; Kadi, K.; Nagaz, K.; Secrafi, M.; Yahya, L.B.; Lachehib, B.; Abdelmalek, A. Biological activities and phenolic compounds of olive oil mill wastewater from Abani, endemic Algerian variety. Sci. Rep. 2022, 12, 6042. [Google Scholar] [CrossRef]
- Shetti, P.; Gudasi, S.; Kubade, M. Apigenin in apigenin nanoliposomes: Development and validation of an analytical technique based on HPTLC. Res. J. Biotechnol. 2025, 20, 13–19. [Google Scholar] [CrossRef]
- Obied, H.; Bedgood, D.; Mailer, R.; Prenzler, P.D.; Robards, K. Impact of cultivar, harvesting time, and seasonal variation on the content of biophenols in olive mill waste. J. Agric. Food Chem. 2008, 56, 8851–8858. [Google Scholar] [CrossRef] [PubMed]
- Garbinato, C.; Lima-Rezende, C.A.; Schneider, S.E.; Pedroso, J.; Dos Santos, A.E.; Petry, F.; Aguiar, G.P.S.; Müller, L.G.; Lanza, M.; Piato, A.; et al. Investigation on the anticonvulsant potential of luteolin and micronized luteolin in adult zebrafish (Danio rerio). Neurochem. Res. 2021, 46, 3025–3034. [Google Scholar] [CrossRef] [PubMed]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovanelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Benardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and active biological properties of the plant-derived bioactive compounds luteolin and luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef]
- Peng, Y.; Qu, R.; Xu, S.; Bi, H.; Guo, D. Regulatory mechanism and therapeutic potentials of naringin against inflammatory disorders. Heliyon 2024, 10, e24619. [Google Scholar] [CrossRef]
- Cai, J.; Wen, H.; Zhou, H.; Zhang, D.; Lan, D.; Liu, S.; Li, C.; Dai, X.; Song, T.; Wang, X.; et al. Naringenin: A flavanone with anti-inflammatory and anti-infective properties. Biomed. Pharmacother. 2023, 164, 114990. [Google Scholar] [CrossRef]
- Klen, T.; Wondra, A.G.; Vrhovsek, U.; Vodopivec, B.M. Phenolic Profiling of Olives and Olive Oil Process-Derived Matrices Using UPLC-DAD-ESI-QTOF-HRMS Analysis. J. Agric. Food Chem. 2015, 63, 3859–3872. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, pharmacokinetics, pharmacological activities, and toxicity of Quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef]
- Jang, W.; Kim, M.; Cho, J. Antioxidant, anti-inflammatory, anti-menopausal, and anti-cancer effects of lignans and their metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef]
- Aggoun, M.; Arhab, R.; Cornu, A.; Portelli, J.; Barkat, M.; Graulet, B. Olive mill wastewater microconstituents composition according to olive variety and extraction process. Food Chem. 2016, 209, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Burri, B.J.; La Frano, M.R.; Zhu, C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutr. Rev. 2016, 74, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and zeaxanthin and their roles in age-related macular degeneration-neurodegenerative disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Navarro, D.; Maunier, S.; Sigoillot, J.; Lorquin, J.; Delattre, M.; Simon, J.; Asther, M.; Labat, M. Simple phenolic content in olive oil residues as a function of extraction systems. Food Chem. 2001, 75, 501–507. [Google Scholar] [CrossRef]
- Lafka, T.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic and antioxidant potential of olive oil mill wastes. Food Chem. 2011, 125, 92–98. [Google Scholar] [CrossRef]
- Visioli, F.; Romani, A.; Mulinacci, N.; Zarini, S.; Conte, D.; Vincieri, F.F.; Galli, C. Antioxidant and other biological activities of olive mill waste waters. J. Agric. Food Chem. 1999, 47, 3397–3401. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Kavroulakis, N.; Ntougias, S. Bacterial and β-proteobacterial diversity in Olea europaea var. mastoidis- and O. europaea var. koroneiki-generated olive mill wastewaters: Influence of cultivation and harvesting practice on bacterial community structure. World J. Microbiol. Biotechnol. 2011, 27, 57–66. [Google Scholar]
- Bleve, G.; Lezzi, C.; Chiriatti, M.A.; D’Ostuni, I.; Tristezza, M.; Di Venere, D.; Sergio, L.; Mita, G.; Grieco, F. Selection of non-conventional yeasts and their use in immobilized form for the bioremediation of olive oil mill wastewaters. Bioresour. Technol. 2011, 102, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Tsiamis, G.; Tzagkaraki, G.; Chamalaki, A.; Xypteras, N.; Andersen, G.; Vayenas, D.; Bourtzis, K. Olive-mill wastewater bacterial communities display a cultivar specific profile. Curr. Microbiol. 2012, 64, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Venieri, D.; Rouvalis, A.; Iliopoulou-Georgudaki, J. Microbial and toxic evaluation of raw and treated olive oil mill wastewaters. J. Chem. Technol. Biotechnol. 2010, 85, 1380–1388. [Google Scholar] [CrossRef]
- Sinigaglia, M.; Di Benedetto, N.; Bevilacqua, A.; Corbo, M.R.; Capece, A.; Romano, P. Yeasts isolated from olive mill wastewaters from southern Italy: Technological characterization and potential use for phenol removal. Appl. Microbiol. Biotechnol. 2010, 87, 2345–2354. [Google Scholar] [CrossRef]
- Ben Sassi, A.; Ouazzani, N.; Walker, G.M.; Ibnsouda, S.; El Mzibri, M.; Boussaid, A. Detoxification of olive mill wastewaters by Moroccan yeast isolates. Biodegradation 2008, 19, 337–346. [Google Scholar] [CrossRef]
- Karpouzas, D.G.; Rousidou, C.; Papadopoulou, K.K.; Bekris, F.; Zervakis, G.I.; Singh, B.K.; Ehaliotis, C. Effect of continuous olive mill wastewater applications, in the presence of nitrogen fertilization, on the structure of rhizosphere-soil fungal communities. FEMS Microbiol. Ecol. 2009, 70, 388–401. [Google Scholar] [CrossRef]
- Ipsilantis, I.; Karpouzas, D.G.; Papadopoulou, K.K.; Ehaliotis, C. Effects of soil application of olive mill wastewater on the structure and function of the community of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2009, 41, 2466–2476. [Google Scholar] [CrossRef]
- Mann, J.; Markham, J.L.; Peiris, P.; Nair, N.; Spooner-Hart, R.N.; Holford, P. Screening and selection of fungi for bioremediation of olive mill wastewater. World J. Microbiol. Biotechnol. 2010, 26, 567–571. [Google Scholar] [CrossRef]
- Zaier, H.; Maktouf, S.; Roussos, S.; Rhouma, A. Filamentous fungi isolated from Tunisian olive mill wastes use of solid-state fermentation for enzyme production. Not. Bot. Horti Agrobot. 2021, 49, 12125. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Saadaoui, N.; Kiai, H.; Raiti, J.; Hafidi, A. Potential applications of olive mill wastewater as biopesticide for crops protection. Sci. Total Environ. 2017, 576, 10–21. [Google Scholar] [CrossRef]
- Wang, Z.; Lei, Z.; Zhang, H.; Liu, Z.; Chen, W.; Jia, Y.; Shi, R.; Wang, C. From biosynthesis to legislation: A review of hydroxytyrosol’s biological functions and safety. Int. J. Mol. Sci. 2025, 26, 4470. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.F.; Resende, D.; Monteiro, M.; Coimbra, M.A.; Silva, A.M.; Cardoso, S.M. Application of hydroxytyrosol in the functional foods field: From ingredient to dietary supplements. Antioxidants 2020, 9, 1246. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cruz, I.; del Mar Contreras, M.; Romero, I.; Ribeiro, B.; Roseiro, L.B.; Duarte, L.C.; Carvalheiro, F.; Castro, E. Strategies for the purification of hydroxytyrosol-rich extracts obtained from exhausted olive pomace. Sep. Purif. Technol. 2023, 325, 124664. [Google Scholar] [CrossRef]
- Ballesteros-Gomez, A.; Serrano-Crespin, A.; Rubio, S. Supramolecular-solvent based extraction of hydroxytyrosol from brines of the processing of table olives. Sep. Purif. Technol. 2023, 322, 124351. [Google Scholar] [CrossRef]
- Allouche, N.; Fki, I.; Sayadi, S. Toward a high yield recovery of antioxidants and purified hydroxytyrosol from olive mill wastewaters. J. Agric. Food Chem. 2004, 52, 267–273. [Google Scholar] [CrossRef]
- Hadrich, F.; Geißen, S.U.; Chamkha, M.; Sayadi, S. Optimizing the extraction conditions of hydroxytyrosol from olive leaves using a modified spherical activated carbon: A new experimental design. BioMed Res. Int. 2022, 2022, 6199627. [Google Scholar] [CrossRef]
- Xynos, N.; Abatis, D.; Argyropoulou, A.; Polychronopoulos, P.; Aligiannis, N.; Skaltsounis, A.L. Development of a sustainable procedure for the recovery of hydroxytyrosol from table olive processing wastewater using adsorption resin technology and centrifugal partition chromatography. Planta Med. 2015, 81, 1621–1627. [Google Scholar] [CrossRef]
- Xiao, R.; Gao, D.; Xie, W.; Fu, Q.; Wang, L.; Zhang, K.; Zeng, J. Nonlinear behavior in preparative liquid chromatography: A method-development case study for hydroxytyrosol purification. J. Sep. Sci. 2021, 44, 973–980. [Google Scholar] [CrossRef]
- Britton, J.; Davis, R.; O’Connor, K.E. Chemical, physical and biotechnological approaches to the production of the potent antioxidant hydroxytyrosol. Appl. Microbiol. Biotechnol. 2013, 103, 5957–5974. [Google Scholar] [CrossRef]
- Deri-Zenaty, B.; Bachar, S.; Rebroš, M.; Fishman, A. A coupled enzymatic reaction of tyrosinase and glucose dehydrogenase for the production of hydroxytyrosol. Appl. Microbiol. Biotechnol. 2020, 104, 4945–4955. [Google Scholar] [CrossRef]
- Liu, W.K.; Su, B.M.; Xu, X.Q.; Xu, L.; Lin, J. Multienzymatic cascade for synthesis of hydroxytyrosol via two-stage biocatalysis. J. Agric. Food Chem. 2024, 72, 15293–15300. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Hu, H.; Ng, K.R.; Yang, R.; Lyu, X. De novo production of hydroxytyrosol by metabolic engineering of Saccharomyces cerevisiae. J. Agric. Food Chem. 2022, 70, 7490–7499. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Chen, J.; Hu, M.; Fang, F.; Zhou, J. Promoting FADH2 regeneration of hydroxylation for high-level production of hydroxytyrosol from glycerol in Escherichia coli. J. Agric. Food Chem. 2023, 71, 16681–16690. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Mitchell, A. Reducing phenolics related to bitterness in table olives. J. Food Qual. 2018, 2018, 3193185. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Gong, P.; Zhang, H.; Zhang, M.; Qi, C.; Chen, G.; Wang, C.; Chen, W. Biosynthesis and biotechnological synthesis of hydroxytyrosol. Foods 2024, 13, 1694. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.; Sayadi, S. High production of Aspergillus niger -glucosidase at pilot-scale and application for hydroxytyrosol release from olive by-product. Int. J. Food Sci. Technol. 2015, 50, 1882–1890. [Google Scholar] [CrossRef]
- Macedo, G.A.; Barbosa, P.d.P.M.; Dias, F.F.G.; Crawford, L.M.; Wang, S.C.; Bell, J.M.L.N.D.M. Optimizing the integration of microwave processing and enzymatic extraction to produce polyphenol-rich extracts from olive pomace. Foods 2023, 12, 3754. [Google Scholar] [CrossRef]
- Catinella, G.; Donzella, S.; Borgonovo, G.; Dallavalle, S.; Contente, M.L.; Pinto, A. Efficient 2-step enzymatic cascade for the bioconversion of oleuropein into hydroxytyrosol. Antioxidants 2022, 11, 260. [Google Scholar] [CrossRef]
- Dammak, I.; Khoufi, S.; Sayadi, S. A performance comparison of olive oil mill wastewater enzymatic treatments. Food Bioprod. Process. 2016, 100, 61–71. [Google Scholar] [CrossRef]
- Giannakopoulou, A.; Chatzikonstantinou, A.V.; Chalmpes, N.; Tsapara, G.; Gournis, D.; Polydera, A.C.; Stamatis, H. Development of a novel bi-enzymatic nanobiocatalyst for the efficient bioconversion of oleuropein to hydroxytyrosol. Catalysts 2021, 11, 749. [Google Scholar] [CrossRef]
- Mazzei, R.; Bazzarelli, F.; Terholsen, H.; Nardi, M.; Piacentini, E.; Procopio, A.; Bornscheuer, U.T.; Giorno, L. Triple enzymatic cascade reaction to produce hydroxytyrosol acetate from olive leaves using integrated membrane bioreactors. ChemSusChem 2025, 18, e202401707. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, E.; Botsaris, G.; Athanasiadi, E.; Mantzouridou, F.T. Processing wastewaters from Spanish-style cv. chalkidiki green olives: A potential source Enterococcus casseliflavus and hydroxytyrosol. Microorganisms 2020, 8, 1274. [Google Scholar] [CrossRef] [PubMed]
- Feki, M.; Allouche, N.; Bouaziz, M.; Gargoubi, A.; Sayadi, S. Effect of storage of olive mill wastewaters on hydroxytyrosol concentration. Eur. J. Lipid Sci. Technol. 2006, 108, 1021–1027. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.A. Microbiological activity in stored olive oil. Int. J. Food Microbiol. 2002, 75, 111–118. [Google Scholar] [CrossRef]
- Nanis, I.; Hatzikamari, M.; Katharopoulos, E.; Boukouvala, E.; Ekateriniadou, L.; Litopoulou-Tzanetaki, E.; Gerasopoulos, D. Microbiological and physicochemical changes during fermentation of solid residue of olive mill wastewaters: Exploitation towards the production of an olive paste-type product. LWT 2020, 117, 108671. [Google Scholar] [CrossRef]
- Rebollo-Romero, I.; Fernández-Cruz, E.; Carrasco-Galán, F.; Valero, E.; Cantos-Villar, E.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrillaet, C. Factors influencing the production of the antioxidant hydroxytyrosol during alcoholic fermentation: Yeast strain, initial tyrosine concentration and initial must. LWT 2020, 130, 109631. [Google Scholar] [CrossRef]
- Pino, A.; Vaccalluzzo, A.; Solieri, L.; Romeo, F.V.; Todaro, A.; Caggia, C.; Arroyo-Lòper, F.N.; Bautista-Gallego, J.; Randazzo, C.L. Effect of sequential inoculum of beta-glucosidase positive and probiotic strains on brine fermentation to obtain low salt Sicilian table olives. Front. Microbiol. 2019, 10, 174. [Google Scholar] [CrossRef]
- Hamza, M.; Sayadi, S. Valorisation of olive mill wastewater by enhancement of natural hydroxytyrosol recovery. Int. J. Food Sci. Technol. 2015, 50, 826–833. [Google Scholar] [CrossRef]
- Vaccalluzzo, A.; Pino, A.; De Angelis, M.; Bautista-Gallego, J.; Romeo, F.V.; Foti, P.; Caggia, C.; Randazzo, C.L. Effects of different stress parameters on growth and on oleuropein-degrading abilities of Lactiplantibacillus plantarum strains selected as tailored starter cultures for naturally table olives. Microorganisms 2020, 8, 1607. [Google Scholar] [CrossRef]
- Hamza, M.; Khoufi, S.; Sayadi, S. Changes in the content of bioactive polyphenolic compounds of olive mill wastewater by the action of exogenous enzymes. J. Agric. Food Chem. 2012, 60, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.; Sami, S. The possibility of recovering hydroxytyrosol from olive milling wastewater by enzymatic bioconversion, in products from olive tree. In Products from Olive Tree; Boskou, D., Clodove, M.L., Eds.; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Lemes, A.C.; Silvério, S.C.; Rodrigues, S.; Rodrigues, L.R. Integrated strategy for purification of esterase from Aureobasidium pullulans. Sep. Purif. Technol. 2019, 209, 409–418. [Google Scholar] [CrossRef]
- Bouallagui, Z.; Sayadi, S. Bioconversion of p-tyrosol into hydroxytyrosol under bench-scale fermentation. BioMed Res. Int. 2018, 2018, 7390751. [Google Scholar] [CrossRef] [PubMed]
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste and repealing certain Directives. Off. J. Eur. Union 2008, L312, 3–30.
- Regulation (EC) No. 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Communities 2002, L31, 1–24.
- Regulation (EC) No. 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs. Off. J. Eur. Union 2004, L139, 1–54.
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the regions—A New Circular Economy Action Plan for a Cleaner and More Competitive Europe. Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en#documents (accessed on 30 August 2024).
- Arienzo, A.; Murgia, L.; Fraudentali, I.; Gallo, V.; Angelini, R.; Antonini, G. Microbiological quality of ready-to-eat leafy green salads during shelf-life and home-refrigeration. Foods 2020, 9, 1421. [Google Scholar] [CrossRef]
- Sciurba, L.; Indelicato, S.; Gaglio, R.; Barbera, M.; Marra, F.P.; Bongiorno, D.; Davino, S.; Piazzese, D.; Settanni, L.; Avellone, G. Analysis of olive oil mill wastewater from conventionally farmed olives: Chemical and microbiological safety and polyphenolic profile for possible use in food product functionalization. Foods 2025, 14, 449. [Google Scholar] [CrossRef]
- Restivo, I.; Sciurba, L.; Indelicato, S.; Allegra, M.; Lino, C.; Garofalo, G.; Bongiorno, D.; Davino, S.; Avellone, G.; Settanni, L.; et al. Repurposing olive oil mill wastewater into a valuable ingredient for functional bread production. Foods 2025, 14, 1945. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2020, 131, 108940. [Google Scholar] [CrossRef]
- Maccarronello, A.E.; Cardullo, N.; Silva, A.M.; Di Francesco, A.; Costa, P.C.; Rodrigues, F.; Muccilli, V. Unlocking the nutraceutical potential of Corylus avellana L. shells: Microwave-assisted extraction of phytochemicals with antiradical and anti-diabetic properties. J. Sci. Food Agric. 2024, 104, 9472–9485. [Google Scholar] [CrossRef]
- Maccarronello, A.E.; Cardullo, N.; Silva, A.M.; Di Francesco, A.; Costa, P.C.; Rodrigues, F.; Muccilli, V. From waste to bioactive compounds: A response surface methodology approach to extract antioxidants from Pistacia vera shells for postprandial hyperglycaemia management. Food Chem. 2024, 443, 138504. [Google Scholar] [CrossRef]
- Muccilli, V.; Maccarronello, A.E.; Rosanandrasana, C.; Cardullo, N.; de Luna, M.S.; Pittalà, M.G.G.; Riccobene, P.M.; Carroccio, S.C.; Scamporrino, A.A. Green3: A green extraction of green additives for green plastics. Heliyon 2024, 10, e24469. [Google Scholar] [CrossRef]
- Signorello, L.; Arena, M.P.; Brugnoli, M.; Romeo, F.V.; Gullo, M. Valorization of olive mill wastewater by selective sequential fermentation. Foods 2025, 14, 2170. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Russo, N.; Romeo, A.; Carbone, C.; Grimaudo, M.A.; Alvarez-Lorenzo, C.; Randazzo, C.; Musumeci, T.; Caggia, C. Coating Lacticaseibacillus rhamnosus GG in alginate systems: An emerging strategy towards improved viability in orange juice. AAPS PharmSciTech 2021, 22, 123. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.D.; Robards, K. Potent antioxidant biophenols from olive mill waste. Food Chem. 2008, 111, 171–178. [Google Scholar] [CrossRef]
- Serra, A.T.; Matias, A.A.; Nunes, A.V.M.; Leitão, M.C.; Brito, D.; Bronze, R.; Silva, S.; Pires, A.; Crespo, M.T.; San Romão, M.V.; et al. In vitro evaluation of olive- and grape-based natural extracts as potential preservatives for food. IFSET 2008, 9, 311–319. [Google Scholar] [CrossRef]
- Tafesh, A.; Najami, N.; Jadoun, J.; Halahlih, F.; Riepl, H.; Azaizeh, H. Synergistic antibacterial effects of polyphenolic compounds from olive mill wastewater. Evid. Based Complement. Alternat. Med. 2011, 2011, 431021. [Google Scholar] [CrossRef] [PubMed]
- Fasolato, L.; Cardazzo, B.; Balzan, S.; Carraro, L.; Taticchi, A.; Montemurro, F.; Novelli, E. Minimum bactericidal concentration of phenols extracted from oil vegetation water on spoilers, starters and food-borne bacteria. Ital. J. Food Saf. 2015, 4, 4519. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Phenols recovered from olive mill wastewater as additives in meat products. IFSET 2018, 79, 98–105. [Google Scholar] [CrossRef]
- Chaves-López, C.; Serio, A.; Mazzarrino, G.; Martuscelli, M.; Scarpone, E.; Paparella, A. Control of household mycoflora in fermented sausages using phenolic fractions from olive mill wastewaters. Int. J. Food Microbiol. 2015, 207, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; He, G.; Guo, X.; Hu, Y.; Shen, Y.; Gou, X. Antioxidant activity of olive wine, a byproduct of olive mill wastewater. Pharm. Biol. 2016, 54, 2276–2281. [Google Scholar] [CrossRef] [PubMed]
- Tarchi, I.; Bouaziz, M.; Bhat, Z.F.; Aït-Kaddour, A. Effect of olive leaf extract on the quality of Cantal cheese. Food Chem. X 2024, 24, 101966. [Google Scholar] [CrossRef] [PubMed]
- Bellumori, M.; Cecchi, L.; Romani, A.; Mulinacci, N.; Innocenti, M. Recovery and stability over time of phenolic fractions by an industrial filtration system of olive mill wastewater: A three-year study. J. Sci. Food Agric. 2018, 98, 2761–2769. [Google Scholar] [CrossRef]
- He, J.; Alister-Briggs, M.; de Lyster, T.; Jones, G.P. Stability and antioxidant potential of purified olive mill wastewater extracts. Food Chem. 2012, 131, 1312–1321. [Google Scholar] [CrossRef]
- Gonzalez-Ortega, R.; Di Mattia, C.D.; Pittia, P.; Natasa, P.U. Effect of heat treatment on phenolic composition and radical scavenging activity of olive leaf extract at different pH conditions: A spectroscopic and kinetic study. J. Sci. Food Agric. 2023, 103, 2047–2056. [Google Scholar] [CrossRef]
- Ahmed, P.; Fernández, P.; Figueroa, L.; Pajot, H. Exploitation alternatives of olive mill wastewater: Production of value-added compounds useful for industry and agriculture. Biofuel Res. J. 2019, 6, 980–994. [Google Scholar] [CrossRef]
- Di Mauro, M.; Fava, G.; Spampinato, M.; Aleo, D.; Melilli, B.; Saita, M.; Centonze, G.; Maggiore, R.; D’Antona, N. Polyphenolic fraction from olive mill wastewater: Scale-up and in vitro studies for ophthalmic nutraceutical applications. Antioxidants 2019, 8, 462. [Google Scholar] [CrossRef]
- Carmona, I.; Aguirre, I.; Griffith, D.; García-Borrego, A. Towards a circular economy in virgin olive oil production: Valorization of the olive mill waste (OMW) “alpeorujo” through polyphenol recovery with natural deep eutectic solvents (NADESs) and vermicomposting. Sci. Total Environ. 2023, 872, 162198. [Google Scholar] [CrossRef]
- Enaime, G.; Dababat, S.; Wichern, M.; Lübken, M. Olive mill wastes: From wastes to resources. Environ. Sci. Pollut. Res. Int. 2024, 31, 20853–20880. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.R. Enzymatic hydrolysis of non-animal proteins for improving nutritional and sensory properties of foods. J. Food Biochem. 2021, 45, e13891. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Gao, Z.; Li, S.; Chen, X.; Guo, J. Assessment of chemical constitution and aroma properties of kiwi wines obtained from pure and mixed fermentation with Wickerhamomyces anomalus and Saccharomyces cerevisiae. J. Sci. Food Agric. 2022, 102, 175–184. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.; Mei, W.; Li, T.; Tao, Y. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef]
- Jiang, X.; Peng, D.; Zhang, W.; Duan, M.; Ruan, Z.; Huang, S.; Zhou, S.; Fang, Q. Effect of aroma-producing yeasts in high-salt liquid-state fermentation soy sauce and the biosynthesis pathways of the dominant esters. Food Chem. 2021, 344, 128681128681. [Google Scholar] [CrossRef]
- Szudera-Kończal, K.; Myszka, K.; Kubiak, P.; Drabińska, N.; Majcher, M. The combined effect of lactic acid bacteria and Galactomyces geotrichum fermentation on the aroma composition of sour whey. Molecules 2023, 28, 4308. [Google Scholar] [CrossRef]
- Rao, M.; Bast, A.; De Boer, A. Valorized food processing by-products in the EU: Finding the balance between safety, nutrition, and sustainability. Sustainability 2021, 13, 4428. [Google Scholar] [CrossRef]
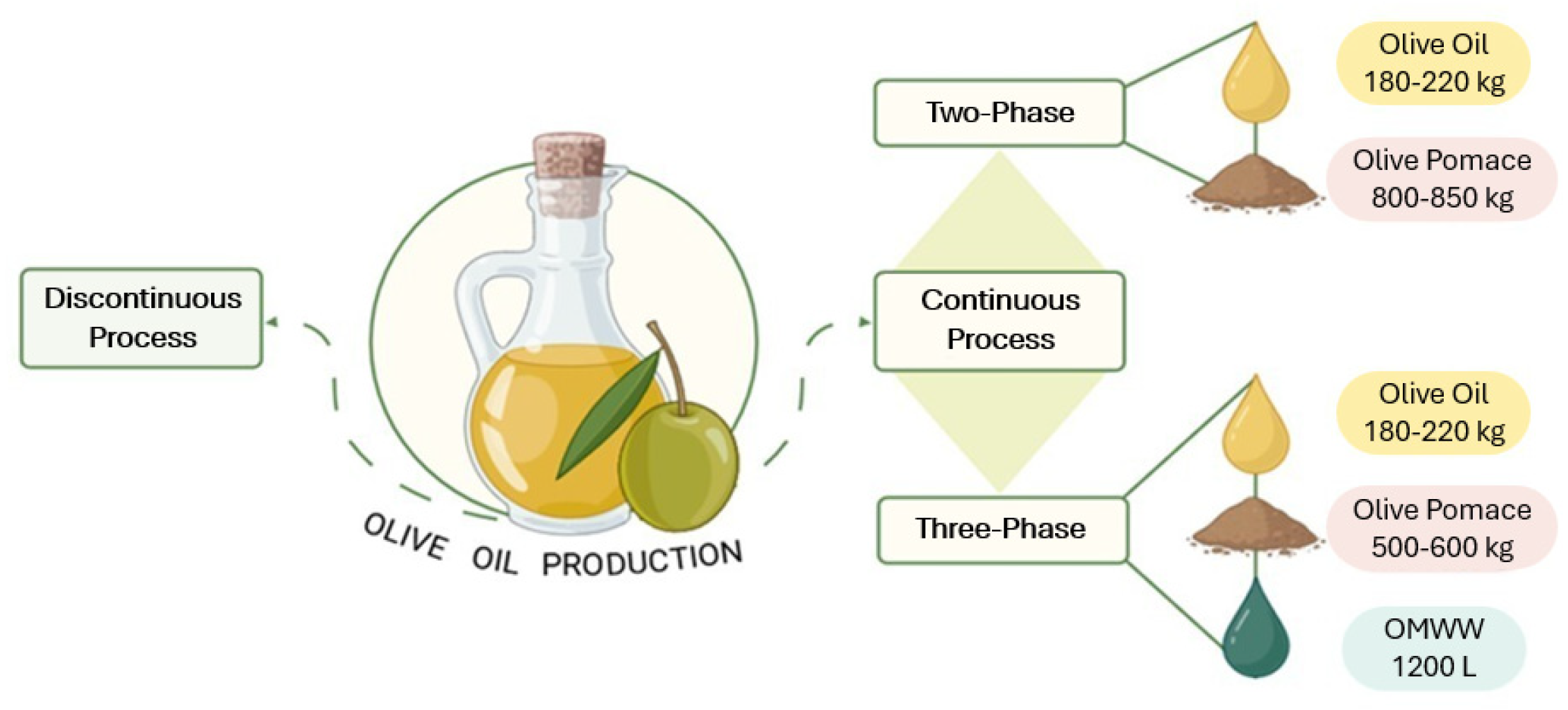
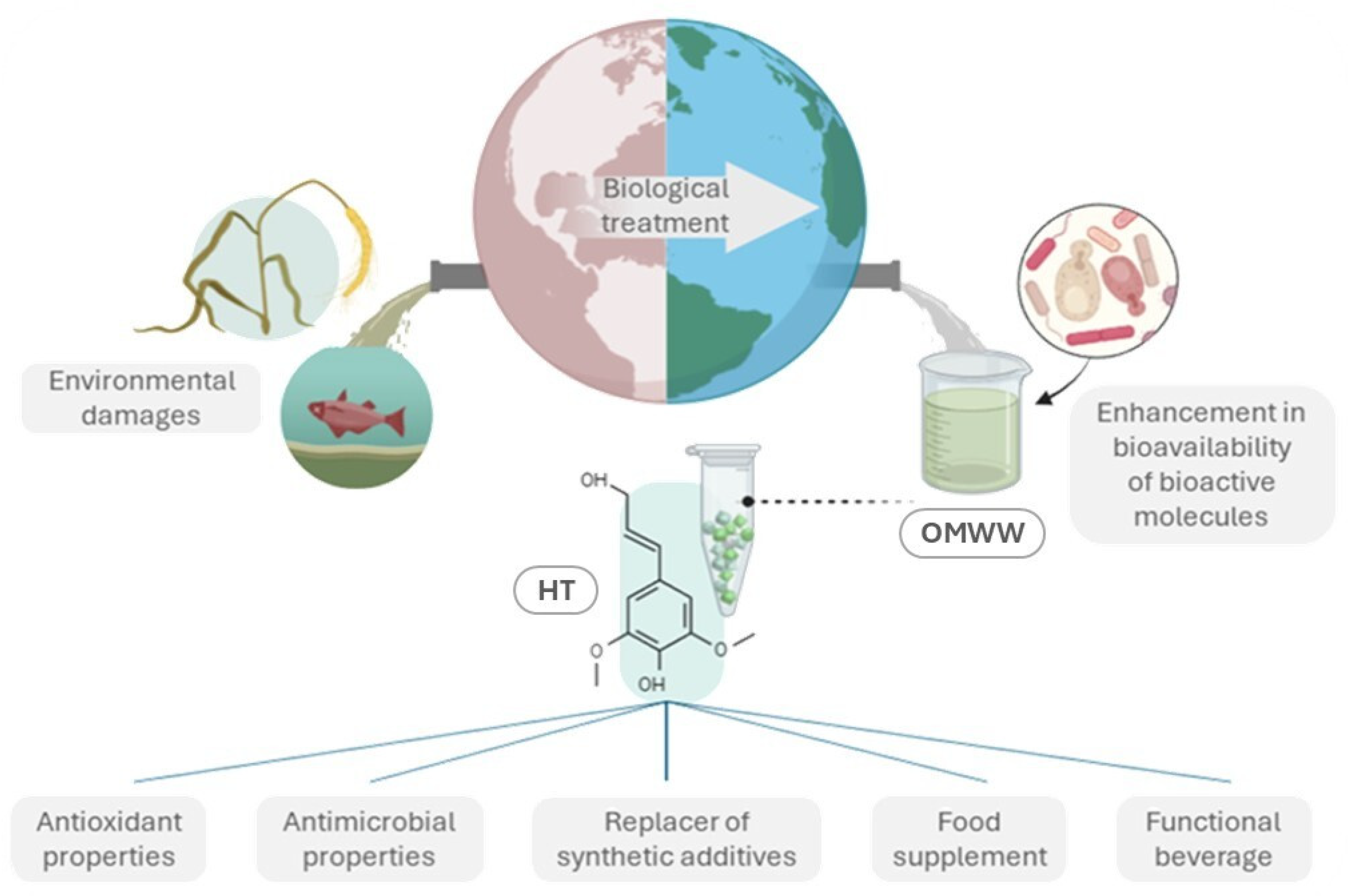
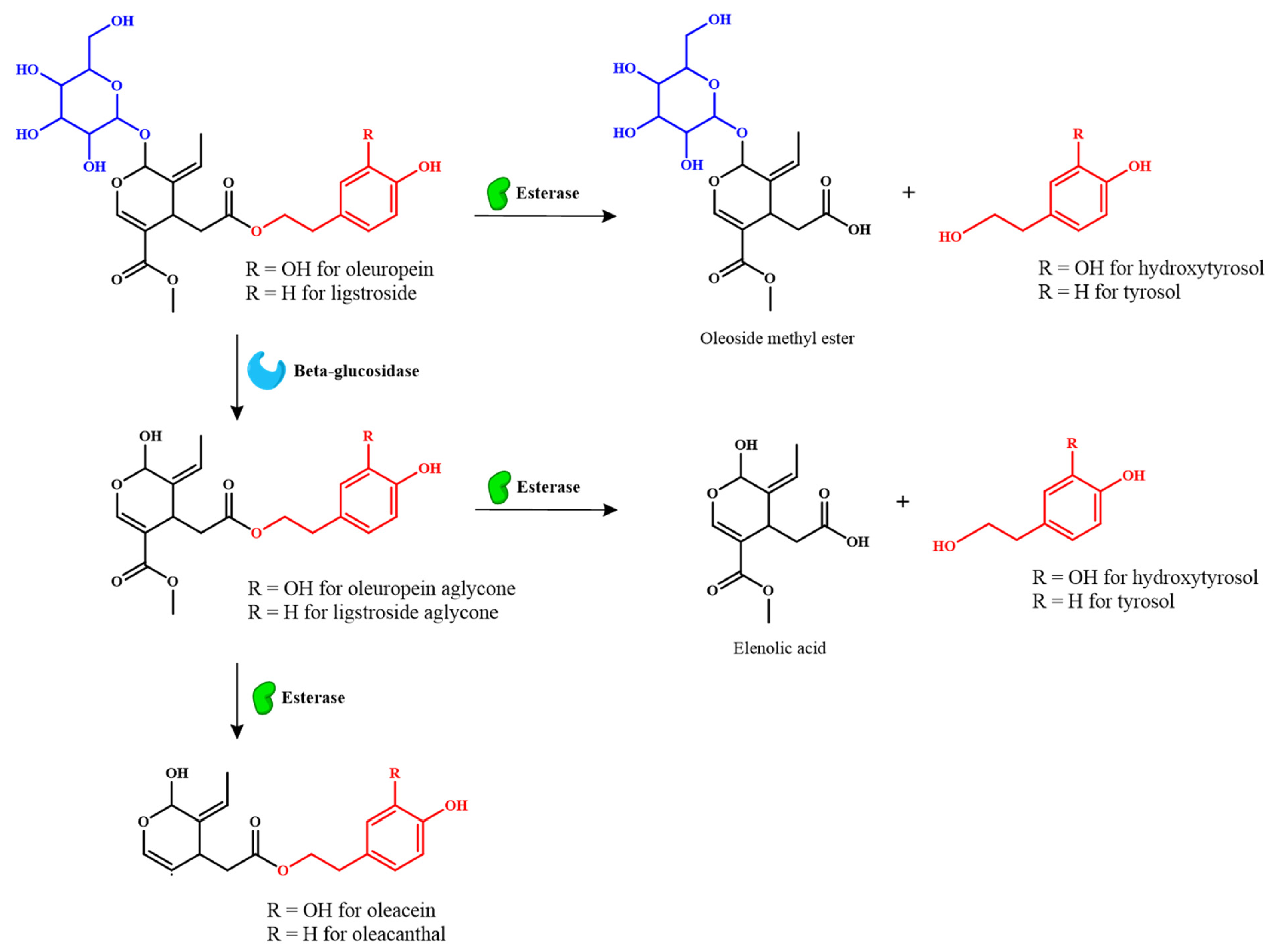

| Bioactive Compound | Content Range a | Biological Properties | Reference |
|---|---|---|---|
| Secoiridoids and derivatives | |||
| Hydroxytyrosol | 110.2–469.1 | Antioxidant, anti-inflammatory, chemopreventive, antimicrobial, skin bleaching, cardioprotective, and antiatherogenic | [29,30,31,32,33] |
| Hydroxytyrosol glucoside | 78–1522 | Antioxidant, antimicrobial | [34,35,36] |
| Ligstroside | 23.8–35.0 | Antioxidant, anti-inflammatory | [37,38] |
| Oleuropein | 1.5–36.1 | Antioxidant, neuroprotective, cardioprotective, antiatherogenic, hypoglycemic, antimicrobial, and antiviral | [29,31,32] |
| Tyrosol | 9.5–89.7 | Antioxidant, anti-inflammatory, antidepressive-like activity, chemoprotective, skin-protective | [29,32,39] |
| Verbascoside | 49.0–271.3 | Antioxidant, neuroprotective, chemoprotective | [31,32,40,41] |
| Phenolic acids | |||
| 4-hydroxyphenyl acetic acid | 8.5–274.0 | Antioxidant, hypoglycemic | [11,32,42] |
| Caffeic acid | 12.9–321.0 | Antioxidant, antimicrobial, wound healing promoting | [11,31,43] |
| Cinnamic acid | 1.2–4.8 | Antioxidant, anti-inflammatory | [39,44,45] |
| p-coumaric acid | 117.0–298.0 | Antioxidant, anti-inflammatory, chemopreventive | [11,46,47] |
| Ferulic acid | 70.2–95.0 | Antioxidant, antimicrobial, photoprotective, hypoglycemic | [11,46,48] |
| Homovanillic acid | 3.2–56.9 | Antimicrobial | [29,49] |
| Syringic acid | 10.3–30.6 | Neuroprotective, hepatoprotective, cardioprotective, hypoglycemic | [39,44,50] |
| Vanillic acid | 1.5–7.0 | Cardioprotective | [29,31,39,51] |
| Flavonoids | |||
| Apigenin | 0.1–1.9 | Antioxidant, chemopreventive, hypoglycemic, antimicrobial, and antiviral | [29,52,53] |
| Luteolin | 13.7–15.2 | Antioxidant, cardioprotective, anti-inflammatory, anticonvulsant | [32,54,55] |
| Luteolin-7-O-glucoside | 8.7–26.6 | Antioxidant, anti-inflammatory | [29,56] |
| Naringin | INq | Antioxidant, anti-inflammatory, anti-ulcerative | [52,57] |
| Naringenin | INq | Antioxidant, anti-inflammatory, anti-infective, cardioprotective | [52,58] |
| Rutin | 7.2–32.4 | Antioxidant, chemopreventive, hepatoprotective | [29,33,44] |
| Quercitrin | INq | Antioxidant, anti-inflammatory, antimicrobial, analgesic | [59,60] |
| Lignans | |||
| Pinoresinol | 13.1–78.7 | Antioxidant, anti-inflammatory, chemopreventive | [29,31,61] |
| Carotenoids b | |||
| β-cryptoxanthin | 0.1–0.6 | Protective against osteoporosis, chemopreventive | [62,63] |
| Zeaxanthin | 0.4–1.9 | Protective against ocular diseases | [62,64] |
| Tocopherols b | |||
| α-tocopherol | 15.6–39.1 | Antioxidant, anti-aging, skin-protective | [62,65] |
| γ-tocopherol | 2.6–5.6 | Antioxidant, anti-aging, skin-protective | [62,65] |
| Unit Operations | Description | Typical Outcomes (Yield/Purity) | References |
|---|---|---|---|
| Liquid–liquid extraction | Counter-current or batch solvent extraction with organic (e.g., ethyl acetate, methanol, ethanol) or supramolecular solvents from olive waste and brines. Conditions (pH, solvent ratio, temperature) are optimized for maximum HT recovery. | Up to 88–90% recovery | [27,84,85,86] |
| Membrane filtration | Nanofiltration and reverse osmosis concentrate HT from aqueous extracts, separating it from sugars and other small molecules. | Concentration factors 7–9, stable solutions | [30,85] |
| Adsorption/desorption | Use of activated carbon or non-ionic resins to selectively adsorb HT, followed by elution (often with ethanol or water). | Up to 97% purity, 73–92% recovery | [24,87] |
| Chromatographic purification | Preparative liquid chromatography (C18, C8 columns), centrifugal partition chromatography, or HPLC to achieve high-purity HT. | >95–98% purity, not easily scalable | [16,88,89,90] |
| Product | OMWW Use | Main Characteristics | Applications | References |
|---|---|---|---|---|
| Natural preservatives | Phenolic extracts from OMWW | Antimicrobial, antifungal, antioxidant activity | Shelf life extension of food; alternative to synthetic preservatives | [127,128,129,130] |
| Encapsulated phenolics | OMWW bioactives encapsulated for food use | Stabilized, protected from degradation, controlled release | Functional ingredients in food matrices | [131] |
| Meat additives | OMWW phenols in raw meat products | Improved hygiene, oxidation prevention | Meat preservation and quality enhancement | [132] |
| Fermented sausages | OMWW extracts in fermented meat products | Antifungal activity against spoilage molds and spores | Clean-label antifungal agents | [133] |
| Functional beverages | Fermented OMWW with selected microorganisms | Increased HT and total phenolics; antioxidant and anti-inflammatory properties | Functional drinks with health benefits | [30,134] |
| Enriched fruit juice | Blood orange juice fortified with OMWW polyphenol concentrate | High HT content; EFSA-compatible levels after storage | Nutraceutical beverage; sensory acceptable | [8,24] |
| Acetic beverages | OMWW fermented into vinegar-like drinks (static and submerged) | Rich in acetic/gluconic acid; high antioxidant activity | Low-alcohol functional beverage | [134] |
| Olive wine | Spontaneous fermentation of OMWW | Contains HT, flavonoids, polyphenols; antioxidant activity in vitro and in vivo | Beverage with potential oxidative stress prevention abilities in animal models | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zingale, I.M.; Maccarronello, A.E.; Carbone, C.; Randazzo, C.L.; Musumeci, T.; Caggia, C. Recent Advances in Microbial Bioconversion as an Approach to Boost Hydroxytyrosol Recovery from Olive Mill Wastewater. Fermentation 2025, 11, 477. https://doi.org/10.3390/fermentation11080477
Zingale IM, Maccarronello AE, Carbone C, Randazzo CL, Musumeci T, Caggia C. Recent Advances in Microbial Bioconversion as an Approach to Boost Hydroxytyrosol Recovery from Olive Mill Wastewater. Fermentation. 2025; 11(8):477. https://doi.org/10.3390/fermentation11080477
Chicago/Turabian StyleZingale, Irene Maria, Anna Elisabetta Maccarronello, Claudia Carbone, Cinzia Lucia Randazzo, Teresa Musumeci, and Cinzia Caggia. 2025. "Recent Advances in Microbial Bioconversion as an Approach to Boost Hydroxytyrosol Recovery from Olive Mill Wastewater" Fermentation 11, no. 8: 477. https://doi.org/10.3390/fermentation11080477
APA StyleZingale, I. M., Maccarronello, A. E., Carbone, C., Randazzo, C. L., Musumeci, T., & Caggia, C. (2025). Recent Advances in Microbial Bioconversion as an Approach to Boost Hydroxytyrosol Recovery from Olive Mill Wastewater. Fermentation, 11(8), 477. https://doi.org/10.3390/fermentation11080477










