Probiotic Potential and Characterization of Enterococcus faecium Strains Isolated from Camel Milk: Implications for Animal Health and Dairy Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Bacterial Strains
2.2. Molecular Identification Using 16S rRNA Gene
2.3. Assessment of Probiotic Potential
2.3.1. Tolerance to pH and Bile Salts
2.3.2. Assessment of Adhesion Properties
- a.
- Adhesion to Hydrocarbons
- b.
- Bacterial Adhesion to Gastric Mucin
- c.
- Adhesion to Intestinal Cell Line (STC-1)
2.3.3. Auto-Aggregation Test
2.3.4. Co-Aggregation with Saccharomyces Cerevisiae
2.3.5. Survival Under Simulated In Vitro Digestion Conditions
2.3.6. Antagonistic Activity
2.3.7. Safety Assessment of Enterococcus faecium Strains
- a.
- Antibiotic Susceptibility
- b.
- Cytotoxic Assay on STC-1 Cells
2.4. Statistical Analysis
3. Results and Discussion
3.1. Recovery and Preliminary Identification of Lactic Acid Bacteria
3.2. Functional Screening: Acid and Bile Salt Resistance
3.3. Genetic Characterization of Isolates by 16S rRNA Sequencing
3.4. Evaluation of Adhesion to Organic Solvents, Gastric Mucin, and Intestinal Cells
3.4.1. Bacterial Adhesion to Hydrocarbons (BATH) Assay of E. faecium Strains
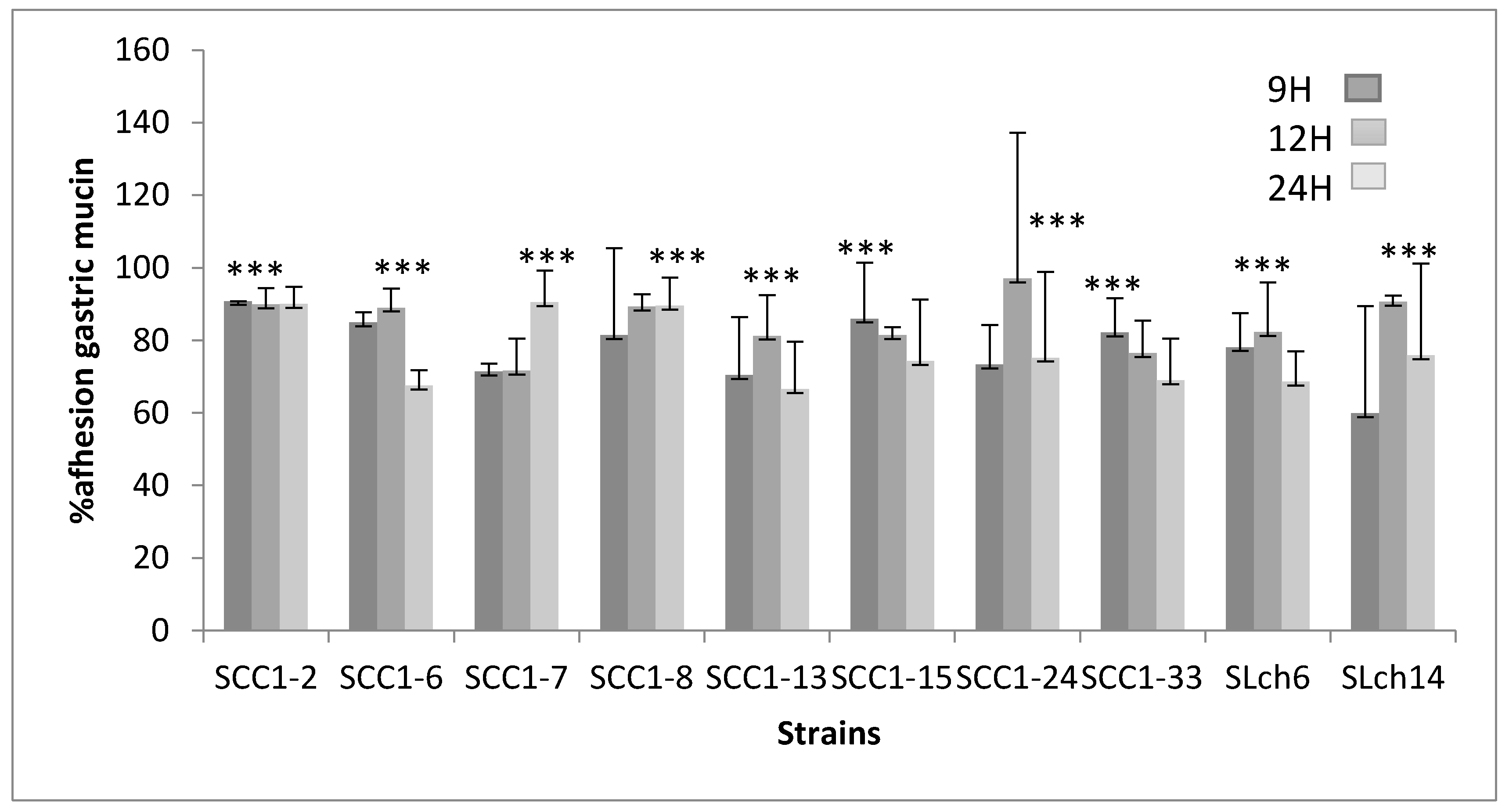
3.4.2. Adhesion to Gastric Mucin and STC-1 Cells
3.5. Auto-Aggregation and Co-Aggregation
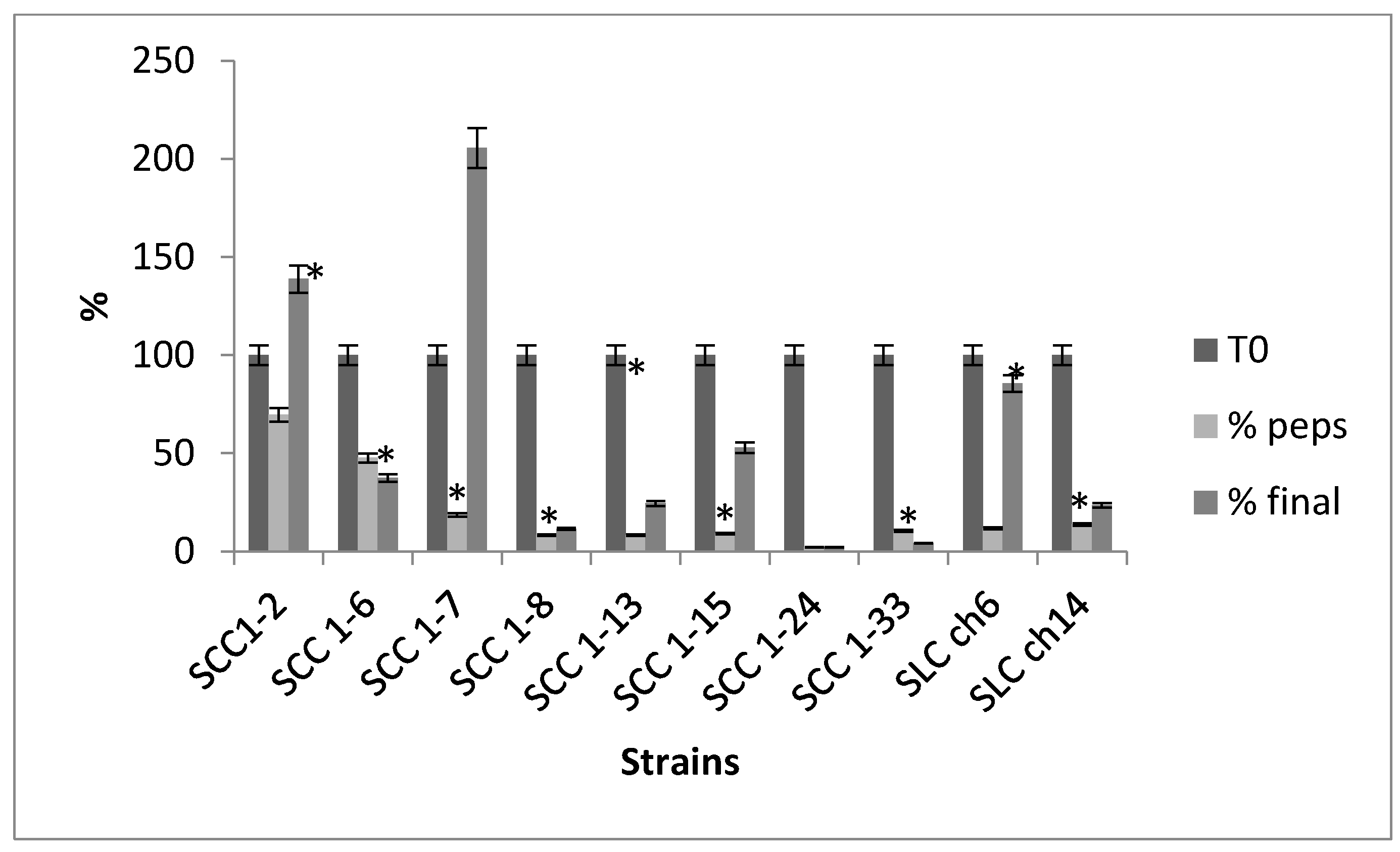
3.6. Survival in Simulated In Vitro Digestion
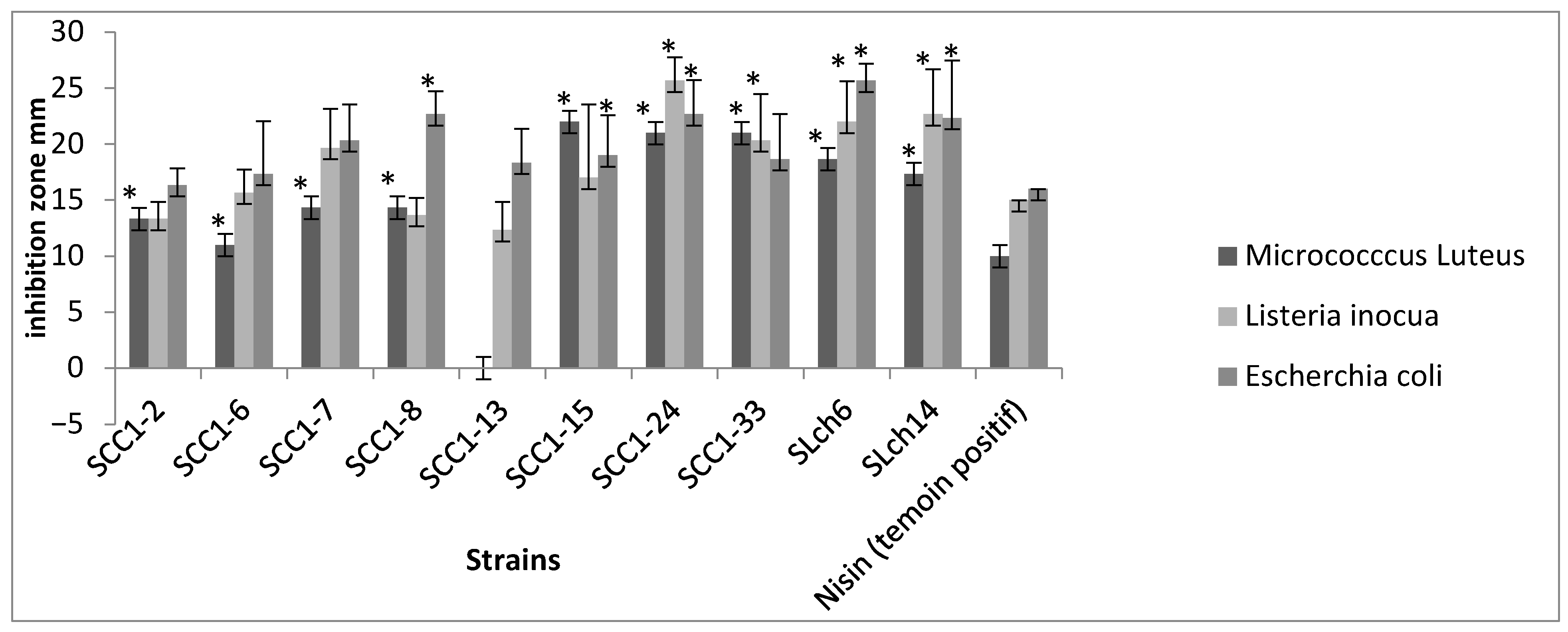
3.7. Antagonistic Effect
3.8. Safety Evaluation of E. faecium Strains
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LAB | Lactic Acid Bacteria |
| BATH | Bacterial Adhesion to hydrocarbons test |
| SCC | Strain Colostrum Camel |
| SLCh | Strain Milk Chenchou |
References
- Homayouni, A.; Alizadeh, M.; Alikhah, H.; Zijah, V. Factors influencing probiotic survival in ice cream. Int. J. Dairy Sci. 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Rivera-Espinoza, Y.; Gallardo-Navarro, Y. Non-dairy probiotic products. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2018, 16, 605–616. [Google Scholar] [CrossRef]
- McFarland, L.V. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J. Gastroenterol. 2010, 16, 2202–2222. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, A.; Azizi, A.; Ehsani, M.R.; Yarmand, M.S.; Razavi, S.H. Effect of microencapsulation and resistant starch on the probiotic survival and sensor properties of symbiotic ice cream. Food Chem 2008, 111, 50–55. [Google Scholar] [CrossRef]
- Lankaputhra, W.E.V.; Shah, N.P. Survival of Lactobacillus acidophilus and Bifidobacterium ssp. in the presence of acid and bile salts. Cult. Dairy Prod. J. 1995, 30, 2–7. [Google Scholar]
- Gupta, M.; Bajaj, B.K. Functional characterization of potential probiotic lactic acid bacteria isolated from Kalarei and development of probiotic fermented oat flour. Prob. Antimicrob Prot. 2018, 10, 654–661. [Google Scholar] [CrossRef]
- Kadri, Z.; Vandamme, P.; Ouadghiri, M.; Cnockaert, M.; Aerts, M.; Elfahime, E.M.; Amar, M. Streptococcus tangierensis sp. Nov. And Streptococcus cameli sp. Nov., two novel Streptococcus species isolated from raw camel milk in Morocco. Antonie Van Leeuwenhoek 2014, 107, 503–510. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B.; Loiseau, G.; Levieux, D. Lactoferrin and immunoglobulin contents in camel’s milk (Camelus bactrianus, Camelus dromedarius, and hybrids) from Kazakhstan. J. Dairy Sci. 2007, 90, 38–46. [Google Scholar] [CrossRef]
- Agrawal, M.; Arora, S.; Li, J.; Rahmani, R.; Sun, L.; Steinlauf, A.F.; Mechanick, J.I.; Zaidi, M. Bone, inflammation, and inflammatory bowel disease. Curr. Osteoporos. Rep. 2011, 9, 251–257. [Google Scholar] [CrossRef]
- Tanhaeian, A.; Shahriari Ahmadi, F.; Sekhavati, M.H.; Mamarabadi, M. Expression and purification of the main component contained in camel milk and its antimicrobial activities against bacterial plant pathogens. Prob. Antimicrob Prot. 2018, 10, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Al-Otaibi, M.M.; Al-Zoreky, N.S.; El-Dermerdash, H.A. Camel’s milk as a natural source for probiotics. Res. J. Microbiol. 2013, 8, 70. [Google Scholar] [CrossRef]
- Prasad, J.; Gill, H.; Smart, J.; Gopal, P.K. Selection and characterization of Lactobacillus and Bifidobacterium strains for use as probiotics. Int. Dairy J. 1998, 8, 993–1002. [Google Scholar] [CrossRef]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 2001. [Google Scholar]
- ISO 15214:1998; Microbiology of food and animal feeding stuffs—Horizontal method for the enumeration of mesophilic lactic acid bacteria—Colony-count technique at 30 degrees C. International Organization for Standardization: Geneva, Switzerland, 1998.
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J. Appl. Microbiol. 1998, 84, 759–768. [Google Scholar] [CrossRef]
- Walter, J. Ecological role of Lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G.; Vermeulen, N.; Vogel, R.F. Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiol. 2007, 24, 128–138. [Google Scholar] [CrossRef]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technol. 2008, 226, 1065–1073. [Google Scholar] [CrossRef]
- Kos, B.; Suskovic, J.; Vukovic, S.; Simpraga, M.; Frece, J.; Matosic, S. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2008, 94, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Del Re, B.; Sgorbati, B.; Miglioli, M.; Palenzola, D. Adhesion, auto-aggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 2000, 31, 438–442. [Google Scholar] [CrossRef]
- De Gregorio, P.; Colomba, L.; Malfa, P.; La Mantia, T.; Potortì, A.G.; Martorana, M.; D’Oca, M.C.; Settanni, L. Co-aggregation and hydrophobicity of lactic acid bacteria isolated from traditional fermented foods. LWT—Food Sci. Technol. 2014, 59, 1151–1158. [Google Scholar] [CrossRef]
- Seiquer, I.; Aspe, T.; Vaquero, P.; Navarro, P. Effects of heat treatment of casein in the presence of reducing sugars on calcium bioavailability: In vitro and in vivo assays. J. Agric. Food Chem. 2001, 49, 1049–1055. [Google Scholar] [CrossRef]
- Schillinger, U.; Lucke, F.K. Antimicrobial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 1989, 55, 1901–1906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yavuzdurmaz, H. Isolation, Characterization, Determination of Probiotic Properties of Lactic Acid Bacteria from Human Milk. Master’s Thesis, Izmir Institute of Technology, Lzmir, Turkey, 2007; pp. 1–69. [Google Scholar]
- CLSI Document M100-S18; Performance Standards for Antimicrobial Susceptibility Testing Eighteenth Informational Supplement. Clinical and Laboratory Standards Institute Press: Wayne, Pennsylvania, PA, USA, 2008; Volume 28, pp. 46–52.
- Rindi, G.; Grant, S.G.; Yiangou, Y.; Ghatei, M.A.; Bloom, S.R.; Bautch, V.L.; Solcia, E.; Polak, J.M. Development of neuroendocrine tumors in the gastrointestinal tract of transgenic mice. Heterogeneity of hormone expression. Am. J. Pathol. 1990, 136, 1349–1363. [Google Scholar] [PubMed] [PubMed Central]
- Çakır, I. Determination of Some Probiotic Properties on Lactobacilli and Bifidobacteria. Ph.D. Thesis, Ankara University, Ankara, Turkey, 2003. [Google Scholar]
- Chou, L.S.; Weimer, B. Isolation and characterization of acid and bile tolerant isolates from strains of Lactobacillus acidophilus. J. Dairy Sci. 1999, 82, 23–31. [Google Scholar] [CrossRef]
- Ayyash, M.; Abushelaibi, A.; Al-Mahadin, S.; Enan, M.; El-Tarabily, K.; Shah, N. In-vitro investigation into probiotic characterisation of Streptococcus and Enterococcus isolated from camel milk. LWT 2018, 87, 478–487. [Google Scholar] [CrossRef]
- Hamed, E.; Elattar, A. Identification and some probiotic potential of lactic acid bacteria isolated from Egyptian camels milk. Life Sci. J. 2013, 10, 1952–1961. [Google Scholar]
- Akhmetsadykova, S.; Baubelkopva, A.; Konuspayeva, G.; Akhmetsadykov, N.; Loiseau, G. Microflora identification of fresh and fermented camel milk from Kazakhstan. Emir. J. Food Agric. 2014, 26, 327–332. [Google Scholar] [CrossRef]
- Pelletier, C.; Bouley, C.; Cayuela, C.; Bouttier, S.; Bourlioux, P.; Bellon-Fontaine, M.N. Cell surface characteristics of Lactobacillus casei subsp. casei, Lactobacillus paracasei subsp. paracasei, and Lactobacillus rhamnosus strains. Appl. Environ. Microbiol. 1997, 63, 1725–1731. [Google Scholar] [CrossRef]
- Xu, H.; Jeong, H.S.; Lee, H.Y.; Ahn, J. Assessment of cell surface properties and adhesion potential of selected probiotic strains. LAM 2009, 49, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016, 206, 1–11. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; Huch, M.; Abriouel, H.; Holzapfel, W.; Gálvez, A. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Sistek, V.; Rolinec, M.; Marcinčák, S.; Nagy, J.; Bujňák, L. Probiotic potential of Enterococcus faecium strains isolated from raw milk. Czech J. Food Sci. 2012, 30, 221–226. [Google Scholar]
- Angmo, K.; Kumari, A.; Bhalla, C. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT-Food Sci. Technol. 2015, 66, 428–435. [Google Scholar] [CrossRef]
- Bernet-Carnard, M.F.; Lievin, V.; Brassart, D.; Neeser, J.R.; Servin, A.L.H. The human L. acidophilus strain LA1 secretes a non bacteriocin anti-bacterial substance(s) active in vitro and in vivo. Appl. Environ. Microbiol. 1997, 63, 2747–2753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alander, M.; Satokari, R.; Korpela, R.; Saxelin, M.; Vilpponen-Salmela, T.; Mattila-Sandholm, T.; von-Wright, A. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosu GG, after oral consumption. Appl. Environ. Microbiol. 1999, 65, 351–354. [Google Scholar] [CrossRef]
- Schiffrin, E.J.; Rochat, F.; Link-Amster, H.; Aeschlimann, J.M.; Donnet-Hughes, A. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J. Dairy Sci. 1995, 78, 491–497. [Google Scholar] [CrossRef]
- Coconnier, M.H.; Bernet, M.F.; Kernéis, S.; Chauvière, G.; Fourniat, J.; Servin, L. Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol. Lett. 1993, 110, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; D’Auria, A.; Linsalata, A.; Lippolis, G.; Di Leo, G. Adhesion of Enterococcus faecalis to Caco-2 cell monolayers: A possible role in inflammatory bowel disease. World J. Gastroenterol. 2005, 11, 5986–5991. [Google Scholar][Green Version]
- Laparra, A.; Sanz, Y. Comparison of different in vitro models to study bacterial adhesion to the intestinal epithelium. Lett. Appl. Microbiol. 2009, 49, 695–701. [Google Scholar] [CrossRef]
- Duary, R.K.; Batish, V.K.; Grover, S. Probiotic properties of lactic acid bacteria isolated from food and evaluated in vitro for cholesterol-lowering potential. J. Food Saf. 2011, 31, 158–169. [Google Scholar]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S.; et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp. Diabetes Res. 2012, 2012, 902917. [Google Scholar] [CrossRef] [PubMed]
- Kotikalapudi, B.L. Characterization and Encapsulation of Probiotic Bacteria Using a Pea-Protein Alginate Matrix. Ph.D. Thesis, University of Saskatchewan, Saskatchewan, SK, Canada, 2009; pp. 62–138. [Google Scholar]
- Jensen, H.; Grimmer, S.; Naterstad, K.; Axelsson, L. In vitro testing of commercial and potential probiotic lactic acid bacteria. Inter. J. Food Microbiol. 2012, 153, 216–222. [Google Scholar] [CrossRef]
- Nascimento, L.C.S. Probiotic and Substance of Production of Antimicrobial Cultures are Capable of Lactating Acid and Application in the Leite Fermentado. Ph.D. Thesis, Programa de Pós-Graduação em Ciência e Tecnologia de Alimentos, Instituto de Biociências, Letras e Ciências Exatas, Universidade Estadual Paulista, Sao Paulo, Brazil, 2015. [Google Scholar]
- Ghrairi, T.; Frere, J.; Berjeaud, J.M.; Manai, M. Purification and Characterisation of Bacteriocins Produced by Enterococcus faecium from Tunisian Rigouta Cheese. Food Control 2008, 19, 162–169. [Google Scholar] [CrossRef]
- Aguilar-Galvez, A.; Dubois-Dauphin, R.; Campos, D.; Thonart, P. Genetic determination and localization of multiple bacteriocins produced by Enterococcus faecium CWBI-B1430 and Enterococcus mundtii CWBI-B1431. Food Sci. Biotechnol. 2011, 20, 289–296. [Google Scholar] [CrossRef]
- Vimont, A.; Fernandez, B.; Hammami, R.; Ababsa, A.; Daba, H.; Fliss, I. Bacteriocin-producing Enterococcus faecium LCW 44: A high potential probiotic candidate from raw camel milk. Front. Microbiol 2017, 8, 865. [Google Scholar] [CrossRef] [PubMed]
- Hasman, H.; Villadsen, A.G.; Aarestrup, F.M. Diversity and stability of plasmids from glycopeptide-resistant Enterococcus faecium (GRE) isolated from pigs in Denmark. Microb Drug Resis. 2009, 11, 178–184. [Google Scholar] [CrossRef]
- CLSI M100, 2023; Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023.
- De Souza, B.M.S.; Borgonovi, T.F.; Casarotti, S.N.; Todorov, S.D.; Barretto Penna, A.L. Lactobacillus casei and Lactobacillus fermentum strains isolated from Mozzarella cheese: Probiotic potential, safety, acidifying kinetic parameters and viability under gastrointestinal tract conditions. Prob. Antimicrob. Prot. 2019, 11, 382–396. [Google Scholar] [CrossRef]
- Leblanc, D.J. Enterococcus. In The Prokaryote, 3rd ed.; Dworkin, M., Ed.; Springer: New York, NY, USA, 2006; Volume 4, pp. 175–204. [Google Scholar]
- Banwo, K.; Sanni, A.; Tan, H. Functional properties of Pediococcus species isolated from traditional fermented cereal gruel and milk in Nigeria. Food Biotechnol. 2013, 27, 14–38. [Google Scholar] [CrossRef]
| Isolate Code | Closest Species Match | % of Similarity | Closest GenBank Accession Number |
|---|---|---|---|
| SCC1-2 | E. faecium | 99% | JN560903.1 |
| SCC1-6 | E. faecium | 99% | KF149320.1 |
| SCC1-7 | E. faecium | 99% | JX847611.1 |
| SCC1-8 | E. faecium | 99% | JQ726533.1 |
| SCC1-13 | E. faecium | 99% | EU878170.1 |
| SCC1-15 | E. faecium | 99% | KC422716.1 |
| SCC1-24 | E. faecium | 99% | JN560911.1 |
| SCC1-33 | E. faecium | 99% | JN560898.1 |
| SLch6 | E. faecium | 99% | HM162421.1 |
| SLch14 | E. faecium | 99% | AY587799.1 |
| Strains | Adhesion to Ethyl Acetate | Adhesion to Chloroform | Co-Aggregation with S. cerevisae | Auto-Aggregation | |
|---|---|---|---|---|---|
| 20 °C | 37 °C | ||||
| SCC1-2 | 16 b ± 5.66 | 12 b ± 5.66 | 61.77 a ± 11.05 | 46.78 a ± 2.16 | 41.03 b ± 11.1 |
| SCC1-6 | 26.0 ab ± 2.83 | 16.7 ab ± 2.62 | 58.10 ab ± 13.38 | 45.07 a ± 2.23 | 62.76 a± 3.0 |
| SCC1-7 | 20.7 ab ± 9.75 | 14.4 ab ± 4.08 | 48.74 b ± 1.99 | 43.31 ab ± 12.29 | 59.66 a± 6.3 |
| SCC1-8 | 31.8 a ± 10.71 | 18.8 ab ± 2.95 | 48.43 b ± 6.93 | 44.94 ab ± 18.76 | 41.7 b ± 9.6 |
| SCC1-13 | 00 c ± 00 | 30.4 a ± 7.58 | 62.59 a ± 0.68 | 43.41 ab ± 0.1 | 49.1 a ± 10.0 |
| SCC1-15 | 25.0 ab ± 8.16 | 28.6 a ± 5.05 | 55.28 ab ± 4.13 | 43.98 ab ± 0.29 | 43.0 b ± 5.0 |
| SCC1-24 | 34.5 a ± 4.88 | 14.8 ab ± 5.24 | 44.40 b ± 7.2 | 43.46 ab ± 2.08 | 59.3 a ± 3.4 |
| SCC1-33 | 18 b ± 2.83 | 18.5 ab ± 00 | 48.64 b ± 2.6 | 41.03 b ± 19.46 | 47.0 b ± 10.5 |
| SLch6 | 22 ab ± 2.83 | 25.5 a ± 0.76 | 50.00 ab ± 1.57 | 42.15 ab ± 8.19 | 63.1 b ± 5.0 |
| SLch14 | 15.4 b ± 5.44 | 18.8 ab ± 4.42 | 55.15 ab ± 14.34 | 41.18 b ± 10.72 | 60.0 a ± 5.0 |
| Strains | T30 | R30 | V30 | E15 | A10 | K1000 |
|---|---|---|---|---|---|---|
| SCC1-2 | S | R | S | R | S | S |
| SCC 1-6 | S | R | S | S | S | S |
| SCC 1-7 | S | R | S | S | S | S |
| SCC 1-8 | S | R | S | S | S | S |
| SCC 1-13 | S | R | S | S | R | S |
| SCC 1-15 | S | R | S | S | S | S |
| SCC 1-24 | S | R | R | S | S | S |
| SCC 1-33 | S | R | S | S | S | S |
| SLC ch6 | S | R | S | S | S | S |
| SLC ch14 | S | R | S | S | S | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fguiri, I.; Ziadi, M.; Arroum, S.; Khorchani, T.; Mohamed, H. Probiotic Potential and Characterization of Enterococcus faecium Strains Isolated from Camel Milk: Implications for Animal Health and Dairy Products. Fermentation 2025, 11, 444. https://doi.org/10.3390/fermentation11080444
Fguiri I, Ziadi M, Arroum S, Khorchani T, Mohamed H. Probiotic Potential and Characterization of Enterococcus faecium Strains Isolated from Camel Milk: Implications for Animal Health and Dairy Products. Fermentation. 2025; 11(8):444. https://doi.org/10.3390/fermentation11080444
Chicago/Turabian StyleFguiri, Imen, Manel Ziadi, Samira Arroum, Touhami Khorchani, and Hammadi Mohamed. 2025. "Probiotic Potential and Characterization of Enterococcus faecium Strains Isolated from Camel Milk: Implications for Animal Health and Dairy Products" Fermentation 11, no. 8: 444. https://doi.org/10.3390/fermentation11080444
APA StyleFguiri, I., Ziadi, M., Arroum, S., Khorchani, T., & Mohamed, H. (2025). Probiotic Potential and Characterization of Enterococcus faecium Strains Isolated from Camel Milk: Implications for Animal Health and Dairy Products. Fermentation, 11(8), 444. https://doi.org/10.3390/fermentation11080444





