Abstract
Tailoring culture media and supplementation strategies to the specific requirements of a target product is essential for enhancing microbial production efficiency. This work addresses an unexplored aspect of K. phaffii cultivation: optimizing culture media for metabolite production from xylose, diverging from the conventional focus on recombinant protein expression and the use of glycerol or methanol as primary substrates. Ethylene glycol biosynthesis in an engineered K. phaffii strain was improved by evaluating media and nutrient supplementation. Among the seven evaluated formulations, FM22 and d’Anjou were the most effective, with inositol and thiamine dichloride playing key roles in enhancing production. Salt concentrations in both media were optimized using Central Composite Design (CCD), reducing complexity while increasing yields. Ethylene glycol production increased by 54% in FM22 and 21% in d’Anjou, accompanied by a threefold and 26% reduction in the total salt content, respectively. The vitamin solution was streamlined from seven to two components, each at half the standard concentration. Trace element solutions were reduced to 25% of the original volume without compromising productivity. These findings underscore the dual benefit of culture medium optimization: improved ethylene glycol yields and simplified formulations, establishing a foundation for the development of more efficient and cost-effective bioprocesses using K. phaffii.
1. Introduction
Ethylene glycol (EG) is a key component in numerous industrial applications, particularly in polymer synthesis such as polyethylene terephthalate (PET) and polyurethane [1,2]. It is considered one of the most promising bio-based chemicals [3] due to its growing market and the limitations of conventional production, which is based on a petrochemical derivative in a high-temperature, water-intensive process with significant by-product formation [1,2,4].
Exploring this opportunity, K. phaffii, a prominent yeast in modern biotechnology, has been engineered with four heterologous genes for converting xylose into EG via the Dahms pathway [5]. Nevertheless, converting this biotechnological potential into efficient production requires tailoring process parameters to the specific characteristics of the strain, product, and production process [6,7,8]. In this context, culture medium formulation is critical, as it must address the cells’ nutritional requirements while also optimizing cost, stability, consistency, and process performance [9,10,11]. Notably, the culture medium can contribute up to 30% of total production costs [12].
While rich complex media are commonly used at the laboratory scale due to their ability to enhance cell growth and productivity by providing readily available precursors that reduce microbial energy demand [12,13], chemically defined media are generally preferred for high-cell-density cultures in industrial applications, where reproducibility and cost-effectiveness are critical [14,15].
Among complex media, the yeast extract peptone (YP) and buffered glycerol complex medium (BMGY) [16] are commonly used for K. phaffii cultivation, primarily for cell propagation when large inoculum is required [12,17]. Similarly, the minimal medium with a defined nitrogen source (YNB), which can be enriched to meet the auxotrophic requirements of yeast mutants used in metabolic engineering, is also frequently used [12]. Within the defined media, the basal salt medium (BSM), proposed by the Invitrogen Corporation (2002), is the most recommended medium for the high-cell-density fermentation of K. phaffii [18,19]. Although considered a standard medium, it may not be optimal for all heterologous products due to limitations such as slower growth compared to nutrient-rich complex media, precipitation at pH above 5.5, an unbalanced composition, and high ionic strength [19,20]. Another basal salt medium with a similar composition and limitations, frequently used for protein expression in K. phaffii, is FM22 [21]. Since studies suggest that productivity in these media can be enhanced by nutrient supplementation, modified formulations such as the modified basal salt medium (MBSM) [22] and d’Anjou medium [23] have been developed to improve K. phaffii performance for multiple applications. Additionally, the addition of vitamins to these salt-based media has been shown to positively influence growth and productivity in K. phaffii [14,20,22].
Despite growing evidence that no single medium composition is universally effective [24,25], the development of tailored formulations for K. phaffii remains underexplored [18,20]. Media formulations optimized for heterologous protein expression employing glucose, methanol, or glycerol as carbon sources may not be the best choice for metabolite production from non-traditional carbon sources (xylose and biomass hydrolysate) through synthetic metabolic pathways. To the best of our knowledge, no studies have yet evaluated culture media for the production of chemical compounds, rather than proteins, from xylose in K. phaffii. In this context, the present study aimed to identify the most suitable conventional medium for EG production and evaluate the required supplementation with vitamins, amino acids, and trace elements. Subsequently, a Central Composite Design (CCD) was applied to optimize salt concentrations in the two most promising media to enhance production yield, lower salt content, and simplify the formulation.
2. Materials and Methods
2.1. Organism and Inoculum Preparation
The engineered K. phaffii strain JA122 was preserved in 30% glycerol at −80 °C in the “Collection of Microorganisms and Microalgae Applied to Agroenergy and Biorefineries (CMMAABio)” at Embrapa Agroenergy. For culture initiation, the strain was streaked onto YPD agar plates (1% yeast extract, 2% peptone, 2% glucose, and 2% agar, yeast extract and peptone from Kasvi, Curitiba, PR, Brazil; glucose and agar from Sigma-Aldrich, St. Louis, MO, USA), supplemented with antibiotics (100 µg/mL zeocin, from Thermo Fisher Scientific, Waltham, MA, USA; 200 µg/mL geneticin, and 200 µg/mL hygromycin B, from Sigma-Aldrich, St. Louis, MO, USA), and incubated at 28 °C for 48 h. A single colony was transferred to 10 mL of the antibiotic-supplemented YPD medium and incubated at 28 °C for 24 h at 200 rpm. The pre-inoculum was then transferred to baffled Erlenmeyer flasks containing the YPDX medium (1% yeast extract, 2% peptone, 2% glucose, and 1% xylose from Sigma-Aldrich, St. Louis, MO, USA) and incubated overnight under the same conditions. Cells were centrifuged (5600× g, 5 min), washed with sterile water, and resuspended in an appropriate volume for inoculum quantification (OD600).
2.2. Cultivation Parameters
The experiments were carried out in triplicate (except when CCD techniques were applied) in 50 mL Erlenmeyer flasks with a 25 mL working volume. A substrate concentration of 20 g/L glucose and 20 g/L xylose was used in all conditions. Flasks were inoculated to an initial OD600 of 10 and incubated at 28 °C and 160 rpm. The low agitation and the use of half the nominal flask volume were selected to ensure a low oxygen transfer rate, previously identified as suitable for EG production.
Samples were collected at 24, 48, and 72 h to quantify substrates and products. Cell density measurements were omitted due to variations in turbidity levels across the evaluated media caused by salt precipitation.
2.3. Comparative Evaluation of Culture Media
A screening of seven widely used culture media, initially formulated for other applications in K. phaffii, was performed to identify the most suitable medium for EG production by K. phaffii JA122. Four were defined media composed exclusively of salts: FM22, basal salt medium (BSM), modified basal salt medium (MBSM), and d’Anjou. Additionally, two complex media and one minimal medium were evaluated: the buffered complex medium (BMY, an adaptation of BMGY with a substituted carbon source), yeast extract peptone medium (YP), and a minimal medium with a defined nitrogen source (YNB). The compositions of these media and their respective trace element solutions are detailed in Table 1 and Table 2.

Table 1.
The composition (g/L) of the culture media evaluated for EG production.

Table 2.
The composition (g/L) of trace element solutions evaluated to supplement the culture media.
Since vitamin supplementation has been reported to enhance K. phaffii cultivation, all seven media were evaluated with and without adding 0.25% (v/v) of the vitamin solution described in Table 3, which contains components known to support cell viability and growth [22]. For non-buffered media (FM22, BSM, MBSM, d’Anjou, and YP), pH was adjusted to 6.0 with 5 M KOH (Sigma-Aldrich, St. Louis, MO, USA) prior to inoculation. It is worth noting that the BSM formulation used in this study does not include a defined nitrogen source in its basal composition. In various BSM-based protocols, nitrogen is commonly introduced indirectly via pH control with ammonium hydroxide [14,20,22]; however, in the present study, KOH was used for pH adjustment, and no ammonium or other nitrogen-containing base was employed.

Table 3.
The composition (g/L) of the vitamin solution evaluated as a supplement to the culture media.
2.4. The Effects of Individual Vitamins, Non-Essential Amino Acids, and Trace Elements on EG Production
The beneficial effect of the vitamin solution was observed in some media for EG production. However, minimizing medium complexity and cost is desirable for large-scale applications. Each vitamin was individually evaluated to identify the key nutrients with significant effects on EG production. In the FM22 medium, EG production was measured under three conditions: without supplementation, with standard supplementation (0.25% v/v), and with double supplementation (0.5% v/v). Additionally, the effect of each vitamin was examined by either excluding or doubling its standard concentration while keeping all other vitamins constant. The effect of two non-essential amino acids, methionine (80 mg/L) and glutamic acid (100 mg/L), was also evaluated based on previous reports suggesting potential benefits for K. phaffii [20,26,27] and other yeast species [28,29,30]. Their effects were analyzed individually and in combination while maintaining complete vitamin supplementation.
Based on these results, further comparisons were carried out with FM22 and d’Anjou media. The standard formulations (Table 1 and Table 2), supplemented with the complete vitamin solution (Table 3), were compared to conditions in which only the vitamins that were shown to enhance EG production were retained. In addition, the effect of halving the concentration of these selected vitamins was evaluated. The omission of K2HPO4, originally present in the standard vitamin solution, was also assessed. Finally, the effect of varying the volume of the trace element solutions (PTM4 for FM22 and Trace Solution 2 for d’Anjou) was investigated at 2×, 0.5×, and 0.25× the standard volume.
2.5. Optimization of Salt Composition in Culture Media
To optimize EG production and reduce salt concentrations, a Central Composite Design (CCD) was applied to the two best-performing media, FM22 and d’Anjou. For FM22, the evaluated concentration ranges (g/L) were KH2PO4 (5–50), (NH4)2SO4 (1–10), CaSO4·2H2O (0–2), K2SO4 (2–20), and MgSO4·7H2O (2–20), with a rotatable alpha value of ±2.24. For d’Anjou, the ranges (in g/L) were KH2PO4 (2–20), CaCl2 (0–1), (NH4)2SO4 (5–30), and MgSO4·7H2O (2–10), with a rotatable alpha value of ±2. Three replicates at the central point were included to estimate response variability, requiring 45 experiments for FM22 and 27 experiments for d’Anjou.
The concentration ranges for both media were selected to enable reductions or, in some cases, the complete removal of specific components. For certain compounds, concentrations higher than those conventionally used were also tested, based on the maximum levels observed in other evaluated media, to explore potential benefits. Notably, the standard concentrations for both media were within the evaluated ranges. The standard vitamin and trace element solutions were used in all CCD runs.
2.6. Validation of Model Predictions and Feasibility of Optimized Media Conditions
Experiments were carried out in both culture media under the conditions predicted by the CCD to maximize EG production or reduce salt without compromising the EG yield to validate the model predictions. These conditions were also applied to confirm the feasibility of reducing vitamins and trace element concentrations, as previously established.
2.7. Analytical Methods
Glucose and EG were quantified by HPLC using a refractive index detector (RID) and a reverse-phase Aminex® HPX87H column (BioRad), with 5 mM H2SO4 as the mobile phase at 0.6 mL/min and 45 °C. Xylose and xylonic acid were analyzed via UPLC coupled with light scattering detection (ELSD), using an Acquity UPLC BEH Amide column. The mobile phases consisted of acetonitrile and water at ratios of 80:20 (A) and 40:60 (B), both containing 10 mM ammonium acetate and 0.2% (v/v) ammonium hydroxide. The gradient started with 100% phase A for 3 min, transitioning to 70% by 4 min, 25% by 5 min, and returning to 100% by the end of the run (11 min). The flow rate was 0.3 mL/min, and the column was maintained at 50 °C.
2.8. Statistical Analysis
After verifying the assumptions of analysis of variance (the normality of residuals and homoscedasticity), parametric tests were applied. Pairwise comparisons were carried out using the t-test, while Tukey’s test (p < 0.05) was employed for multiple comparisons. Statistical analyses were performed in Python 3.12 (Python Software Foundation, Wilmington, DE, USA) using the scipy and statsmodels libraries. The CCD data were analyzed in Statistica 12.0 (StatSoft Inc., Tulsa, OK, USA), with the significance level set at 90% (p < 0.1).
3. Results and Discussion
3.1. Comparative Evaluation of Culture Media
Several culture media have been developed for K. phaffii, primarily aiming to enhance heterologous protein production. As different bioproducts and processes impose distinct nutritional requirements, a systematic strategy was employed in this work to select and optimize a culture medium to improve EG production from xylose, while reducing both the number and concentration of medium components. Figure 1 summarizes the experimental workflow used to implement this strategy.
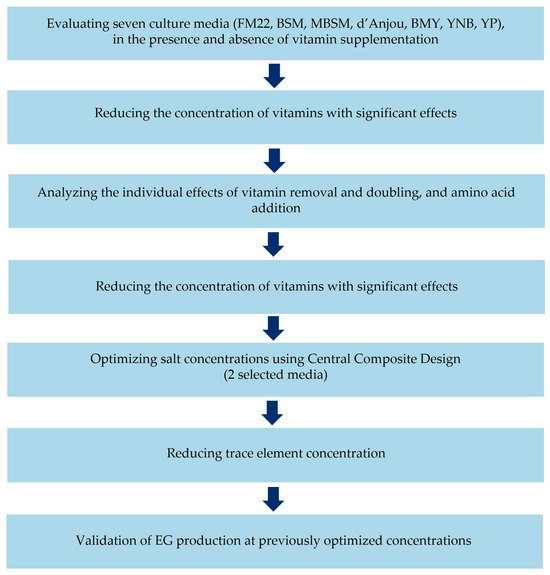
Figure 1.
A schematic representation of the experimental approach for optimizing EG production and refining culture medium composition.
Initially, the effect of seven different culture media (Table 1) on EG production was evaluated, maintaining the same initial concentrations of carbon sources (glucose and xylose) and cell density. In addition, the effect of vitamin solution supplementation was evaluated for each medium. The results showed that the medium composition strongly influenced EG production, with and without vitamin supplementation (Figure 2). Without vitamin supplementation, EG production ranged from 0.43 ± 0.14 g/L in MBSM to 3.33 ± 0.11 g/L in BSM after 72 h. BSM yielded the highest EG concentrations, followed by YP, YNB, and d’Anjou, with no statistically significant differences between the latter three media. In contrast, MBSM and BMY resulted in the lowest EG production (Figure 2A).
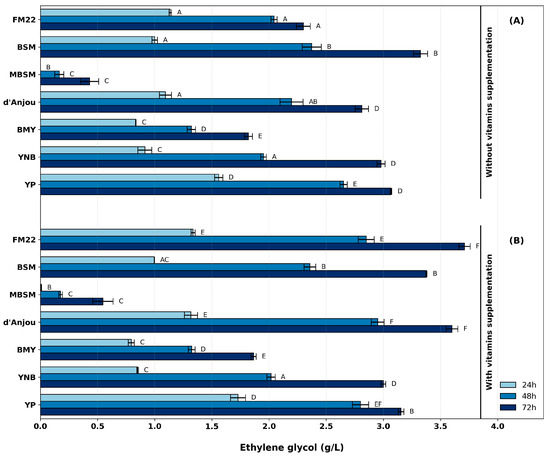
Figure 2.
EG production profiles in different culture media: (A) without and (B) with vitamin supplementation. Identical letters at the same sampling time indicate no significant difference according to Tukey’s test (p = 0.05). Error bars represent the standard error of the mean from independent triplicate experiments.
Among the evaluated media, BSM and FM22 are comparable in their high phosphorus and potassium content. BSM provides elevated concentrations of essential elements. Even if the BSM salt composition is considered unbalanced [14], any detrimental effects on EG production were observed. FM22 was formulated according to the proportions suggested by Wegner (1983) [31], who established the required concentration ranges and essential element ratios for K. phaffii growth at high cell densities [22]. However, this medium may not have provided all the nutrients required to support EG production at levels comparable to the top-performing media, potentially limiting its metabolic performance (Figure 2A). The observed differences in EG production between BSM and FM22 without vitamin supplementation contrasted with previous works that demonstrated better K. phaffii growth and protein production in FM22 than in BSM [14,20]. These studies attributed FM22’s superior performance to its lower concentrations of elements, which may favor a faster growth rate and consequently improve protein production performance. The intrinsic characteristic of basal salts was suggested to slow K. phaffii growth in the BSM [20].
Compared to BSM and FM22, d’Anjou [23] incorporated a nitrogen source and significantly reduced salt concentrations. Among the evaluated media, only d’Anjou fully aligns with the requirements suggested by Wegner (1983) [22,31]. Due to its lower salt content, the d’Anjou medium exhibits a reduced buffering capacity, which can be mitigated through active pH control in a bioreactor. During the EG production experiments, under the evaluated substrate concentration, the pH reduction was limited and did not compromise process performance. Indeed, EG production was better in d’Anjou (2.81 ± 0.10 g/L) than in FM22 (2.30 ± 0.11 g/L), suggesting that the lower salt concentration may have had a beneficial effect on EG production. In addition, analogous to FM22, an essential absence of nutrients or insufficient availability likely contributed to the reduced EG production compared to BSM (Table 2).
MBSM, an optimized version of BSM developed through linear optimization techniques, has shown beneficial effects on protein expression in K. phaffii [22]. However, it demonstrated the poorest EG production performance among the evaluated media. Compared to BSM, MBSM has a reduced number and concentration of trace elements, which may hinder cellular development and metabolic activity [14]. Thus, its poor performance may be attributed to the absence of essential components, such as nitrogen or specific trace elements (e.g., sodium iodide, cobalt chloride, and zinc chloride), or to the higher concentrations of sodium molybdate, ferrous sulfate, or H2SO4 relative to FM22.
The complex medium YP and the minimal medium with a defined nitrogen source (YNB) achieved yields exceeding 90% of the highest observed EG concentration (in BSM). This suggests that their complex components may provide essential nutrients that enhance EG production. This improved performance may also be attributed to the uptake of available glucose for growth, thereby maintaining specific productivity. However, it is important to note that these factors may not be directly linked, as EG production in this yeast is not growth-associated. The media that favor growth do not necessarily support the enhanced production of target compounds. For instance, the highest thaumatin production in K. phaffii occurred in BMGY, FM22, and BSM, yet these media did not support the highest cell growth [14]. In contrast to YP and YNB, BMY, essentially a combination of these two media, resulted in poor EG production, likely due to excess nutrients, which may hinder cellular development and metabolic activity [14].
The previously evaluated media were supplemented with a solution containing different vitamins (Table 3) to evaluate their effect on EG production using K. phaffii. The EG concentrations ranged from 0.55 ± 0.15 g/L in MBSM to 3.71 ± 0.09 g/L in FM22 (Figure 2B). Pairwise comparisons within each medium, with and without vitamin supplementation, revealed a significant increase in EG production in FM22 and d’Anjou across all time points and in YP at 24 and 48 h. For the remaining media, vitamin addition had no significant effect. The most prominent improvements were observed in FM22 and d’Anjou, where supplementation led to yield increases of over 61% and 28%, respectively, compared to the non-supplemented conditions. In addition, with vitamin supplementation, EG production in FM22 and d’Anjou was statistically equivalent (≈3.7 g/L), and significantly higher than in BSM (3.37 g/L).
The relevance of vitamin supplementation for cell growth and heterologous protein production in K. phaffii has already been demonstrated using various culture media [14,20,22]. For instance, Joseph et al. (2023) [14] reported that supplementation with a vitamin solution identical in composition to the one used in the present study improved thaumatin production in BSM, FM22, and BMGY, but had no effect on the d’Anjou medium.
This discrepancy highlights how the effect of vitamin supplementation can vary depending on the target product and the metabolic pathways involved. Moreover, the mechanisms through which vitamins influence EG production remain unclear. It may support yeast growth; however, no significant differences in sugar consumption rates were observed between vitamin-supplemented and non-supplemented conditions in the best-performing media (FM22 and d’Anjou), except for a slight increase in xylose consumption in FM22 at 48 h, from 14.17 ± 0.31 g/L (without vitamins) to 16.30 ± 0.35 g/L (with vitamins). Thus, vitamin supplementation may support yeast maintenance. Overall, the results indicate that FM22 and d’Anjou are versatile media suitable for different K. phaffii strains, provided that appropriate supplementation is applied. They further highlight that medium selection should consider not only the microorganism but also the target product. With the highest EG production seen in FM22 and d’Anjou, their complex compositions (often associated with salt precipitation), the observed effects of vitamin supplementation, and the lack of studies on component contributions during glucose and xylose metabolism, indicate that these media could be further optimized by evaluating the effect of individual vitamins, non-essential amino acids, trace elements, and salt concentrations on EG production.
3.2. Effect of Individual Vitamins, Non-Essential Amino Acids, and Trace Elements on EG Production
The addition of a commonly used vitamin solution (Table 3) improved EG production in some of the evaluated culture media, especially FM22 and d’Anjou (Figure 2B). However, identifying the most effective concentration and individual contribution of each vitamin is essential for reducing the complexity and costs of medium preparation. To investigate this, EG production in the FM22 medium was examined under three complete vitamin solution supplementation levels: no supplementation, the standard level previously applied (0.25% v/v), and a double level (0.5% v/v). In addition, the effect of excluding or doubling the concentration of individual vitamins (pantothenic acid, inositol, thiamine dichloride, pyridoxine hydrochloride, nicotinic acid, and biotin) was also examined, with all other vitamins maintained at their standard levels. Finally, since the non-essential amino acids methionine and glutamic acid were shown to benefit yeast physiological stability, their potential contributions to EG production were also evaluated. The effects of these variables on EG production are summarized in Figure 3.
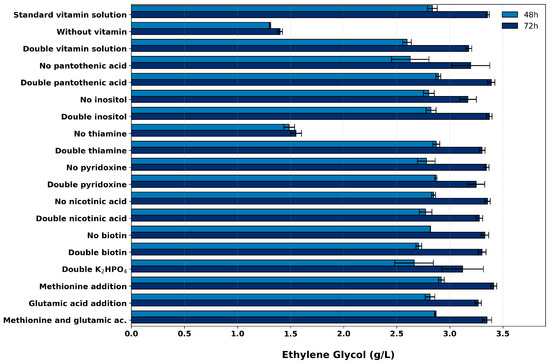
Figure 3.
EG production in the FM22 medium under various supplementation conditions: no vitamin supplementation, standard (0.25% v/v) and double (0.5% v/v) vitamin supplementation, the exclusion or doubling of individual vitamins, and addition of methionine and glutamic acid. Error bars represent the standard error of the mean from three independent replicates.
In this analysis, pairwise comparisons were performed to identify the effect of removing or increasing the concentration of each specific component. The heatmap (Figure 4) presents the p-values for each pairwise comparison of EG production at 72 h. While not all pairwise comparisons are meaningful or necessary, the heatmap reveals limited variability across most conditions. Higher p-values indicate no significant difference between pairs, suggesting that the evaluated modification did not significantly impact EG production, whereas lower p-values reflected the statistically significant effects of specific components. In this case, pairwise comparisons were preferred over multiple comparison procedures, as the analysis aimed to evaluate specific treatment contrasts. Moreover, multiple comparison procedures often reduce the sensitivity for detecting significant differences.

Figure 4.
Heatmap of p-values from pairwise comparisons of EG production after 72 h of cultivation under different supplementation conditions.
As previously observed, the addition of a vitamin solution increased EG production, whereas doubling its standard concentration had a negative impact compared to the initial supplementation level (Figure 3). In contrast, most individual vitamins did not affect EG production, except for thiamine dichloride and inositol, which showed a measurable influence (Figure 3 and Figure 4).
The most pronounced effect of individual vitamin supplementation on EG production was observed with thiamine dichloride. Its removal from the culture medium significantly reduced EG titers, resulting in concentrations comparable to those observed without any vitamin supplementation (Figure 3). In contrast, increasing the concentration of thiamine dichloride had no notable effect. Thiamine is critically involved in cellular energy metabolism, as its derivatives serve as essential cofactors for several enzymes involved in key metabolic pathways, including pyruvate decarboxylation, the tricarboxylic acid (TCA) cycle, and the pentose phosphate pathway [14,32]. The observed effect on EG production is likely linked to thiamine’s role in pyruvate decarboxylation via the thiamine-dependent pyruvate dehydrogenase complex. As EG production in K. phaffii is not growth-associated, maintaining a sufficient energy supply becomes particularly important to sustain metabolic activity and support carbon flux through the synthetic pathway. Under thiamine-limited conditions, impaired pyruvate processing may restrict acetyl-CoA generation and energy production, thereby constraining EG synthesis. Additionally, thiamine contributes to the cellular response to abiotic stress [24], which could enhance metabolic robustness under engineered conditions and indirectly improve productivity.
Inositol omission also resulted in a significant, though less pronounced, reduction in EG production, with a 5.5% decrease in the final concentration relative to the complete vitamin solution (Figure 3). This compound is associated with signaling and lipid metabolism, playing a pivotal role in phospholipid biosynthesis and lipid-mediated signaling [33]. Although EG production is not directly linked to lipid metabolism, inositol may help maintain membrane integrity and intracellular signaling under engineered conditions, indirectly supporting metabolic activity. Its role in stress regulation and transport processes could also contribute to preserving cellular homeostasis and sustaining productivity.
Doubling the concentration of nicotinic acid slightly reduced EG production (p = 0.09 compared to the complete vitamin solution). However, this variation (less than 3% in the final concentration) was minimal, and the observed statistical difference may be attributable to minor experimental variability under these conditions (Figure 3 and Figure 4). Nicotinic acid is a key precursor for NAD and NADP, which are essential cofactors in cellular metabolism [34]. This observation suggests that an excess of nicotinic acid may have affected the NAD/NADP balance, consequently influencing EG production.
The addition or removal of the other vitamins or potassium phosphate dibasic did not result in significant differences in EG production, despite their reported essential roles in other yeast-based production processes (Figure 4). For instance, calcium pantothenate, an essential growth factor, plays a pivotal role in carbohydrate and protein metabolism, contributing to shorter generation times [35]. Pyridoxine hydrochloride functions as a critical cofactor in various enzymatic processes [14,32]. Biotin, another indispensable growth factor, serves as a cofactor in carboxylation reactions involved in lipogenesis, gluconeogenesis, amino acid metabolism, and central carbon metabolism [24]. The response of K. phaffii to biotin limitations has been shown to depend strongly on the carbon source present in the medium [24]; however, no studies to date have evaluated this response under xylose metabolism. Notably, the PTM4 trace element solution used in the medium contains biotin, meaning that its removal from the vitamin solution did not lead to complete biotin depletion in the culture.
The addition of the amino acids glutamic acid and methionine, both reported to influence yeast’s chronological lifespan extension [28], had no effect on EG production (Figure 3 and Figure 4). In K. phaffii, methionine is primarily associated with the regulation of genes involved in cellular biosynthesis and fatty acid metabolism, and its effects have been reported to be substrate-dependent. In particular, methionine does not significantly affect growth in media containing glycerol but has shown a significant impact when methanol is used as the carbon source [26]. In the same study, glutamic acid, a precursor for the biosynthesis of several amino acids [20], was reported to provide the greatest benefit to growth in K. phaffii. While the effects of various amino acids on K. phaffii cultivation have been investigated using glucose [36] and methanol [20] as carbon sources, there is no available data regarding which amino acids may be most beneficial when xylose is used.
As inositol and thiamine dichloride had the most significant impact on EG production (Figure 3 and Figure 4), their single supplementation to FM22 and d’Anjou media was evaluated. The d’Anjou medium was included in the comparison due to its comparable EG production to FM22 and its positive response to vitamin supplementation (Figure 2B). To further confirm that the observed effects on EG production are due to inositol and thiamine dichloride supplementation, K2HPO4 was also removed from the vitamin solution. Thus, EG production in FM22 and d’Anjou media supplemented with the standard vitamin solution was compared to three alternative conditions: (1) a halved inositol concentration, with thiamine dichloride and K2HPO4 maintained at standard levels; (2) a halved thiamine dichloride concentration, with inositol and K2HPO4 unchanged; and (3) the removal of K2HPO4, with inositol and thiamine dichloride maintained at standard concentrations. The results are summarized in Figure 5.
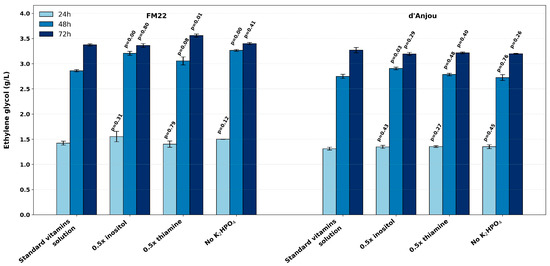
Figure 5.
Effect of inositol and thiamine dichloride concentrations and K2HPO4 removal on EG production in FM22 and d’Anjou media compared to standard vitamin solution supplementation. p-values correspond to pairwise comparisons with standard vitamin solution conditions at each sampling time.
In the FM22 medium, all three evaluated conditions significantly increased the EG production rate at 48 h, with concentrations exceeding those obtained under standard vitamin supplementation by 12.1%, 6.8%, and 14.2% for the reduced inositol level, reduced thiamine level, and K2HPO4 omission, respectively (Figure 5). However, after 72 h, neither halving the inositol concentration nor removing K2HPO4 from the vitamin solution affected the EG concentration (p = 0.80 and 0.41, respectively) compared to the standard condition. In contrast, reducing the thiamine dichloride concentration by half significantly increased the final EG concentration by 5.5%, from 3.37 ± 0.04 g/L to 3.56 ± 0.05 g/L (p = 0.008) (Figure 5). These results suggest that the inositol concentration could be reduced by half without compromising EG production, whereas halving thiamine dichloride may contribute to its improvement. In the d’Anjou medium, neither a reduction in thiamine dichloride concentrations nor the removal of K2HPO4 significantly affected the final EG levels. However, halving the inositol concentration significantly accelerated EG production, increasing it by approximately 6%, as indicated by the concentration measured at 48 h.
The evaluated media were also supplemented with trace element solutions (Table 2). Since their standard concentrations were optimized for other strains and carbon sources other than xylose, variations in their concentrations were also evaluated in this study. The cultivation results demonstrate that doubling, halving, or reducing the trace elements to one-fourth of the standard concentration (PTM4 solution in FM22 medium and Trace Solution 2 in d’Anjou medium) did not significantly impact EG production at any of the sampled time points (Figure 6). These results suggest that the current trace element concentrations can be reduced by at least fourfold without compromising production efficiency. The optimal concentration of trace elements in K. phaffii cultivation is thought to be product-dependent. This variability in required concentrations can be explained by the fact that trace metals are required as the structural components of proteins, enzyme cofactors, and active sites in cellular systems. Conversely, excess metal concentrations can inhibit metabolic processes [37]. The evaluation of trace element influence on cell growth and β-galactosidase production in K. phaffii revealed that, under optimal conditions, magnesium and zinc requirements could be reduced by 18- and 23-fold, respectively, compared to the concentrations used in FM22 and BSM formulations. In addition, supplementation with calcium, cobalt, iron, manganese, iodine, boron, and molybdenum had no significant impact on cell growth or enzyme production [37]. A higher yield of a malaria vaccine antigen was also observed when the PTM salt concentration was reduced to minimize metal-phosphate precipitation [38].
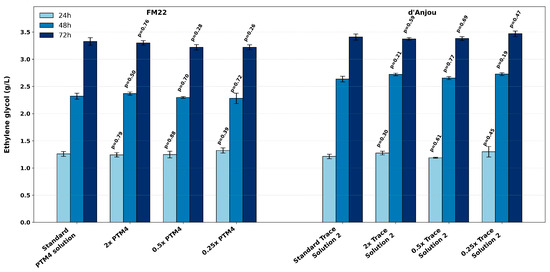
Figure 6.
The effect of trace element solution on EG production in FM22 and d’Anjou media compared to standard supplementation. p-values represent pairwise comparisons with the standard condition at the corresponding sampling times.
3.3. Optimization of Salt Composition in Culture Media
To improve EG production and reduce salt concentrations (and, consequently, salt precipitation), a Central Composite Design (CCD) was applied to optimize the salt composition of FM22 and d’Anjou media, which contain five and four salt components, respectively. Table 4 and Table 5 present the experimental runs and the corresponding EG concentrations obtained at 72 h for FM22 and d’Anjou media, respectively. Limited variability in EG production was observed across the experimental conditions for both media, with values ranging from 3.09 to 3.58 g/L in FM22 and from 2.47 to 3.53 g/L in d’Anjou after 72 h. The repetition of central points (included in the last three experiments of each table) highlights the good reproducibility of the experiment, with deviations below 3%.

Table 4.
Experimental runs, the observed and predicted EG concentrations, and relative errors from the CCD-based optimization of salt concentrations in the FM22 medium after 72 h of cultivation.

Table 5.
Experimental runs, the observed and predicted EG concentrations, and relative errors from the CCD-based optimization of salt concentrations in the d’Anjou medium after 72 h of cultivation.
Based on experimental data (Table 4 for FM22 and Table 5 for d’Anjou), predictive models were developed to describe the effects of salt concentrations in each medium. The regression equations for EG production, expressed in coded variables and including only statistically significant parameters (p < 0.1), are presented in Equations (1) and (2) for FM22 and d’Anjou media, respectively, at 72 h.
where X1 to X5 correspond to the coded concentrations of KH2PO4 (X1), (NH4)2SO4 (X2), CaSO4·2H2O (X3), K2SO4 (X4), and MgSO4·7H2O (X5) in Equation (1) and to KH2PO4 (X1), CaCl2 (X2), (NH4)2SO4 (X3), and MgSO4·7H2O (X4) in Equation (2).
EG (g/L) in FM22 medium = 3.412 − 0.092·X1 − 0.038·X4 − 0.027·X12 + 0.025·X2·X5 − 0.019·X3·X4 − 0.018·X1·X4 − 0.017·X3·X5 + 0.0167·X3 R2 = 0.83
EG (g/L) in d’Anjou medium = 3.421 + 0.161·X1 − 0.101·X12 − 0.061·X42 − 0.057·X1·X3 − 0.051·X4 R2 = 0.75
The ANOVA, performed at a 90% confidence level, confirmed that both models were statistically significant and suitable for predictive purposes. The regression F ratio was 11.9 for FM22 and 5.9 for the d’Anjou medium, with no significant lack of fit detected in either case. Although the coefficient of determination (R2) for the d’Anjou model was less robust, the predicted values closely matched the experimental results across the entire evaluated range, with deviations below 10%, as shown in Table 4 and Table 5.
The obtained models did not exhibit a maximum inflection point within the evaluated range. Therefore, the maximum of each regression equation was determined by applying a nonlinear iterative numerical optimization algorithm, using the models as objective functions and the experimental range as constraints. This method allows the identification of the maximum predicted value within the evaluated range.
For the FM22 medium, EG production was maximized using a formulation containing 17.3 g/L KH2PO4, 1 g/L (NH4)2SO4, 2 g/L CaSO4·2H2O, 2 g/L K2SO4, and 2 g/L MgSO4·7H2O (coded values: X1 = −1.02, X2 = −2.24, X3 = 2.24, X4 = −2.24, X5 = −2.24). In the d’Anjou medium, the maximum EG concentration was achieved with 17.3 g/L KH2PO4, 5 g/L (NH4)2SO4, and 5.2 g/L MgSO4·7H2O, without the addition of CaCl2 (coded values: X1 = 1.4, X2 = −2, X3 = −2, X4 = −0.4). Since variation in CaCl2 did not significantly affect the response, its concentration could be maintained at the lowest evaluated level.
3.4. Validation of Model Predictions and Feasibility of Optimized Media Conditions
To validate the model predictions for EG production in FM22 and d’Anjou media, experiments were carried out using salt concentrations identified as optimal for maximizing production. For FM22, the model predicted a concentration of 3.9 g/L under the optimized conditions; however, the experimentally observed value was 3.34 ± 0.05 g/L, corresponding to a deviation of approximately 15%. This discrepancy may be explained by the relatively low response variability within the tested range, which could have hindered the identification of a well-defined optimum. Moreover, the simultaneous evaluation of five variables increases the system complexity and requires a large number of experimental runs, potentially limiting the model’s ability to capture subtle interaction effects.
Nevertheless, it is noteworthy that the observed production under these conditions was statistically equivalent to that obtained with the standard FM22 formulation. Importantly, this result was achieved with a substantial reduction in salt concentrations: KH2PO4 from 42.9 to 17.3 g/L, (NH4)2SO4 from 5 to 1 g/L, K2SO4 from 14.3 to 2 g/L, and MgSO4·7H2O from 11.7 to 2 g/L, along with an increase in CaSO4·2H2O from 1 to 2 g/L. This represents a threefold reduction in total salt mass, from 74.9 to 24.3 g/L. For the conventional salt concentrations of the medium, the model predicted a 3.2 g/L EG production, while the experimentally observed concentration was 3.32 ± 0.12 g/L, corresponding to a relative error of 3.8%.
An additional analysis was carried out to evaluate the effects of a reduction in salt concentrations alongside the reduction in vitamin and trace element supplementation. For this, a medium with reduced salt concentrations, half the standard levels of thiamine dichloride and inositol (without any additional vitamin supplementation), and one-quarter of the standard volume of PTM4 solution was employed. Under these conditions, EG production (3.35 ± 0.04 g/L; p = 0.19) remained statistically equivalent to the optimized salt formulation supplemented with the complete vitamin solution and PTM4. This result, therefore, also validates all previous observations made throughout this work.
Following the optimization of vitamin and salt concentrations in the FM22 medium, additional experimental conditions were evaluated to explore alternative medium formulations. This approach was supported by previous experiments and several considerations: the low variability in EG production observed across the DCCR experiments; the recurrent precipitation of calcium salts, which could compromise medium stability and calcium availability as a nutrient; and the possible relevance of the nitrogen source for cellular maintenance. Considering these observations, three additional experimental conditions were evaluated, each using half the standard concentrations of thiamine dichloride and inositol and one-quarter of the standard volume of the PTM4 solution. The conditions used were as follows: (1) halving the concentrations of all salts; (2) halving the concentrations of all salts except (NH4)2SO4; and (3) eliminating CaSO4·2H2O while halving the concentrations of all other salts. These conditions resulted in EG concentrations of 3.53 ± 0.05, 3.59 ± 0.07, and 3.44 ± 0.03 g/L, respectively, after 72 h. The first two conditions yielded significantly higher EG concentrations than both the conventional FM22 formulation and the model-predicted maximum (p = 0.049 and 0.03, respectively), while the last condition resulted in production levels statistically equivalent to those of the conventional FM22 medium (p = 0.18). Notably, under these conditions, the observed EG concentration deviated by less than 2% from the model prediction, even though the model was developed using the full volume of PTM4 and the complete vitamin solution. These results further corroborate the effectiveness of reducing both vitamin and trace element supplementation in maintaining EG yield. They also highlight promising formulation strategies that can lower medium preparation costs while sustaining or even enhancing EG production.
For the d’Anjou medium, the model predicted an EG concentration of 3.6 g/L under optimal salt conditions. Experimentally, the observed concentration after 72 h of cultivation was 3.40 ± 0.10 g/L, representing a deviation of 5.5% from the predicted value. To validate additional findings for this medium, the same salt formulation was evaluated with only half the standard concentrations of thiamine dichloride and inositol (without any additional vitamin supplementation) and one-quarter of the standard concentration of Trace Solution 2. Under these conditions, EG production reached 3.31 ± 0.02 g/L, which was statistically equivalent to the previous condition (p = 0.33).
The optimized salt composition differed substantially from the original formulation: KH2PO4 was increased from 12 to 17.3 g/L, and (NH4)2SO4 increased from 0.36 to 5 g/L; in contrast, MgSO4·7H2O was reduced from 20 to 5.2 g/L, and CaCl2 was entirely removed (from 4.7 to 0 g/L). These modifications resulted in a total reduction in salt mass from 37 to 27.5 g/L, corresponding to a 26% decrease. Despite these changes, the new formulation yielded EG concentrations statistically equivalent to those obtained with the conventional d’Anjou medium (p = 0.32). For the conventional salt concentrations of the d’Anjou medium, the model predicted an EG production of 3.4 g/L. Experimentally, the observed concentration was 3.34 ± 0.05 g/L, corresponding to a relative error of 1.7%. These small deviations from the predicted values confirm the model’s accuracy in capturing the behavior of the system.
Comparing media development and supplementation strategies with data from the literature is not straightforward. Most available research focuses on the effects of adding individual components or small groups of additives to specific media formulations [17,24,25,26,37,39,40,41]. In addition, existing optimization studies often report highly specific findings tailored to particular products, which differ considerably from the present work in terms of substrate selection and product type [14,20,22].
In both media, it was possible to significantly reduce the quantity of salt used compared to the standard concentrations. This reduction is advantageous not only for lowering medium preparation costs but also for minimizing salt precipitation. Precipitate formation interferes with cell density measurements and may lead to several operational issues, including unbalanced nutrient supply, nutrient starvation, and even cell disruption due to its abrasive nature. It can also accelerate fermenter component deterioration and require additional steps in downstream processing [37,42].
In addition to the reduction in medium components, a significant increase in EG production was achieved based on the complete set of screening results. Initially, EG concentrations of 2.30 ± 0.11 g/L and 2.81 ± 0.10 g/L were obtained in FM22 and d’Anjou media, corresponding to 28% and 34% of the theoretical stoichiometric conversion, respectively. Through targeted adjustments to the culture media composition, these values increased to 3.59 ± 0.07 g/L and 3.41 ± 0.10 g/L, representing 43% and 41% of the theoretical yield and improvements of 54% and 21%, respectively. These enhancements were also accompanied by a reduction in the total salt mass used in the medium by threefold for FM22 and 26% for d’Anjou. Notably, these results were achieved solely through medium optimization, without altering any other process parameters, underscoring the importance of this research approach.
4. Conclusions
This study demonstrates the effectiveness of medium optimization strategies tailored to EG production in K. phaffii. Among the seven media evaluated, FM22 and d’Anjou stood out as the most suitable formulations. Inositol and thiamine dichloride were identified as critical supplements, contributing significantly to enhanced production. Targeted modifications, such as the simplification of the vitamin solution from seven to two components (each at half the standard concentration), and the reduction in trace element volumes, were implemented. As a result, EG yields increased by 54% in FM22 and 21% in d’Anjou, while the total salt content was reduced threefold and by 26%, respectively. These improvements were achieved without altering operational parameters, underscoring the central role of the medium composition in optimizing microbial production. Altogether, these findings provide useful insights that may contribute to the development of scalable and economically viable bioprocesses.
Author Contributions
T.F.P.: writing—original draft, visualization, investigation, data curation; J.R.M.d.A.: writing—review and editing, supervision, funding acquisition, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank CNPq, INCT Industrial Biotechnology, and the FAP-DF for financial support.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wong, M.K.; Lock, S.S.M.; Chan, Y.H.; Yeoh, S.J.; Tan, I.S. Towards Sustainable Production of Bio-Based Ethylene Glycol: Progress, Perspective and Challenges in Catalytic Conversion and Purification. Chem. Eng. J. 2023, 468, 143699. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Wang, Y.; Gu, J.; Lu, X.; Liao, X.; Shi, J.; Kim, C.H.; Lye, G.; Baganz, F.; et al. Ethylene Glycol and Glycolic Acid Production from Xylonic Acid by Enterobacter Cloacae. Microb. Cell Fact. 2020, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Jong, E.d.; Stichnothe, H.; Bell, G.; Jørgensen, H. Task 42-Bio-Based Chemicals: A 2020 Update; IEA Bioenergy: Vienna, Austria.
- Ding, L.; Tang, J.; Qiao, X.; Liu, C.; Xue, Y.; Wu, G. Design and Analysis of an Intensified Column with Side Reactor Configuration for Ethylene Glycol Production from Ethylene Oxide. Chem. Eng. Process. Process Intensif. 2020, 147, 107744. [Google Scholar] [CrossRef]
- Carneiro, C.V.G.C.; Trichez, D.; Bergmann, J.C.; Reis, V.C.B.; Wagner, N.; Walther, T.; Almeida, J.R.M.D. Engineering Komagataella Phaffii for Ethylene Glycol Production from Xylose. AMB Expr. 2024, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Safder, I.; Khan, S.; Islam, I.-U.; Ali, M.K.; Bibi, Z.; Waqas, M. Pichia pastoris Expression System: A Potential Candidate to Express Protein in Industrial and Biopharmaceutical Domains. Biomed. Lett. 2018, 4, 1–14. [Google Scholar]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A Highly Successful Expression System for Optimal Synthesis of Heterologous Proteins. J. Cell Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef] [PubMed]
- Looser, V.; Bruhlmann, B.; Bumbak, F.; Stenger, C.; Costa, M.; Camattari, A.; Fotiadis, D.; Kovar, K. Cultivation Strategies to Enhance Productivity of Pichia pastoris: A Review. Biotechnol. Adv. 2015, 33, 1177–1193. [Google Scholar] [CrossRef] [PubMed]
- Cankorur-Cetinkaya, A.; Narraidoo, N.; Kasavi, C.; Slater, N.K.H.; Archer, D.B.; Oliver, S.G. Process Development for the Continuous Production of Heterologous Proteins by the Industrial Yeast, Komagataella phaffii. Biotechnol. Bioeng. 2018, 115, 2962–2973. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Kim, H.U.; Na, J.-G.; Ko, Y.-S.; Cho, J.S.; Lee, S.Y. Factors Affecting the Competitiveness of Bacterial Fermentation. Trends Biotechnol. 2023, 41, 798–816. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Hashizume, T.; Ying, B.-W. A Data-Driven Approach for Cell Culture Medium Optimization. Biochem. Eng. J. 2025, 214, 109591. [Google Scholar] [CrossRef]
- Hahn-Hägerdal, B.; Karhumaa, K.; Larsson, C.U.; Gorwa-Grauslund, M.; Görgens, J.; van Zyl, W.H. Role of Cultivation Media in the Development of Yeast Strains for Large Scale Industrial Use. Microb. Cell Fact. 2005, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, J.P.; Oestreich, A.M.; Weidner, T.; Gerlach, D.; Czermak, P. Development of a Chemically Defined Fermentation Medium for the Production of a New Recombinant Fructosyltransferase. IJPMBS 2018, 7, 71–77. [Google Scholar] [CrossRef]
- Joseph, J.A.; Akkermans, S.; Van Impe, J.F. Macroscopic Modeling of the Growth and Substrate Consumption of Wild Type and Genetically Modified Pichia pastoris. Biotechnol. J. 2023, 18, 2300164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhou, H.; Zhang, J. Development of Wheat Bran Hydrolysate as Komagataella Phaffii Medium for Heterologous Protein Production. Bioprocess. Biosyst. Eng. 2021, 44, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.R.; Cregg, J.M. (Eds.) Pichia Protocols; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 1998; ISBN 978-0-89603-421-1. [Google Scholar]
- Charoenrat, T.; Khumruaengsri, N.; Promdonkoy, P.; Rattanaphan, N.; Eurwilaichitr, L.; Tanapongpipat, S.; Roongsawang, N. Improvement of Recombinant Endoglucanase Produced in Pichia pastoris KM71 through the Use of Synthetic Medium for Inoculum and pH Control of Proteolysis. J. Biosci. Bioeng. 2013, 116, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, C.V.G.C.; Serra, L.A.; Pacheco, T.F.; Ferreira, L.M.M.; Brandão, L.T.D.; Freitas, M.N.D.M.; Trichez, D.; Almeida, J.R.M.D. Advances in Komagataella Phaffii Engineering for the Production of Renewable Chemicals and Proteins. Fermentation 2022, 8, 575. [Google Scholar] [CrossRef]
- Ghosalkar, A.; Sahai, V.; Srivastava, A. Optimization of Chemically Defined Medium for Recombinant Pichia pastoris for Biomass Production. Bioresour. Technol. 2008, 99, 7906–7910. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.B.; Kuo, A.; Love, K.R.; Love, J.C. Development of a General Defined Medium for Pichia pastoris. Biotechnol. Bioeng. 2018, 115, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Stratton, J.; Chiruvolu, V.; Meagher, M. High Cell-Density Fermentation. In Pichia Protocols; Higgins, D.R., Cregg, J.M., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 1998; Volume 103, pp. 107–120. ISBN 978-0-89603-421-1. [Google Scholar]
- Pais-Chanfrau, J.M.; Trujillo-Toledo, L.E. Optimization of Culture Medium for Large-Scale Production of Heterologous Proteins in Pichia pastoris to Be Used in Nanoscience and Other Biotechnological Fields. Biol. Med. 2016, 8, 279–282. [Google Scholar] [CrossRef]
- D’anjou, M.C.; Daugulis, A.J. A Rational Approach to Improving Productivity in recombinant Pichia pastoris Fermentation. Biotechnol. Bioeng. 2001, 72, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Makeeva, A.S.; Sidorin, A.V.; Ishtuganova, V.V.; Padkina, M.V.; Rumyantsev, A.M. Effect of Biotin Starvation on Gene Expression in Komagataella Phaffii Cells. Biochemistry 2023, 88, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Taype, M.A.; Garcia-Ortega, X.; Albiol, J.; Montesinos-Seguí, J.L.; Valero, F. Continuous Cultivation as a Tool Toward the Rational Bioprocess Development With Pichia pastoris Cell Factory. Front. Bioeng. Biotechnol. 2020, 8, 632. [Google Scholar] [CrossRef] [PubMed]
- Ianshina, T.; Sidorin, A.; Petrova, K.; Shubert, M.; Makeeva, A.; Sambuk, E.; Govdi, A.; Rumyantsev, A.; Padkina, M. Effect of Methionine on Gene Expression in Komagataella Phaffii Cells. Microorganisms 2023, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Rajak, N.; Dey, T.; Sharma, Y.; Bellad, V.; Rangarajan, P.N. Unlocking Nature’s Toolbox: Glutamate-Inducible Recombinant Protein Production from the Komagatella Phaffii PEPCK Promoter. Microb. Cell Fact. 2024, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Song, L.; Liu, S.Q.; Huang, D. Independent and Additive Effects of Glutamic Acid and Methionine on Yeast Longevity. PLoS ONE 2013, 8, e79319. [Google Scholar] [CrossRef] [PubMed]
- Murakami, C.J.; Wall, V.; Basisty, N.; Kaeberlein, M. Composition and Acidification of the Culture Medium Influences Chronological Aging Similarly in Vineyard and Laboratory Yeast. PLoS ONE 2011, 6, e24530. [Google Scholar] [CrossRef] [PubMed]
- Koc, A.; Gasch, A.P.; Rutherford, J.C.; Kim, H.-Y.; Gladyshev, V.N. Methionine Sulfoxide Reductase Regulation of Yeast Lifespan Reveals Reactive Oxygen Species-Dependent and -Independent Components of Aging. Proc. Natl. Acad. Sci. USA 2004, 101, 7999–8004. [Google Scholar] [CrossRef] [PubMed]
- Wegner, E.H. Biochemical Conversions by Yeast Fermentation at High Cell Densities. U.S. Patent 4,414,329, 8 November 1983. [Google Scholar]
- Perli, T.; Wronska, A.K.; Ortiz-Merino, R.A.; Pronk, J.T.; Daran, J. Vitamin Requirements and Biosynthesis in Saccharomyces Cerevisiae. Yeast 2020, 37, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.A.; Gaspar, M.L.; Jesch, S.A. The Response to Inositol: Regulation of Glycerolipid Metabolism and Stress Response Signaling in Yeast. Chem. Phys. Lipids 2014, 180, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, I.; Alberghina, L.; Vai, M. Nicotinamide, Nicotinamide Riboside and Nicotinic Acid—Emerging Roles in Replicative and Chronological Aging in Yeast. Biomolecules 2020, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W.; Rucker, R.B. Pantothenic Acid. In Present Knowledge in Nutrition; Erdman, J.W., Macdonald, I.A., Zeisel, S.H., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 375–390. ISBN 978-0-470-95917-6. [Google Scholar]
- Heyland, J.; Fu, J.; Blank, L.M.; Schmid, A. Carbon Metabolism Limits Recombinant Protein Production in Pichia pastoris. Biotechnol. Bioeng. 2011, 108, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Plantz, B.A.; Nickerson, K.; Kachman, S.D.; Schlegel, V.L. Evaluation of Metals in a Defined Medium for Pichia pastoris Expressing Recombinant β-Galactosidase. Biotechnol. Prog. 2008, 23, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.P.; Shimp, R.L.; Miles, A.P.; Whitmore, M.; Stowers, A.W. High-Level Production and Purification of P30P2MSP119, an Important Vaccine Antigen for Malaria, Expressed in the Methylotropic Yeast Pichia pastoris. Protein Expr. Purif. 2001, 23, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Duman, Z.E.; Duraksoy, B.B.; Aktaş, F.; Woodley, J.M.; Binay, B. High-Level Heterologous Expression of Active Chaetomium Thermophilum FDH in Pichia pastoris. Enzyme Microb. Technol. 2020, 137, 109552. [Google Scholar] [CrossRef] [PubMed]
- Krahulec, J.; Šafránek, M. Impact of Media Components from Different Suppliers on Enterokinase Productivity in Pichia pastoris. BMC Biotechnol. 2021, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nguyen, V.; Glen, J.; Henderson, B.; Saul, A.; Miller, L.H. Improved Yield of Recombinant Merozoite Surface Protein 3 (MSP3) from Pichia pastoris Using Chemically Defined Media. Biotechnol. Bioeng. 2005, 90, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sinha, J.; Meagher, M.M. Glycerophosphate as a Phosphorus Source in a Defined Medium for Pichia pastoris Fermentation. Appl. Microbiol. Biotechnol. 2006, 72, 139–144. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).