Anti-Obesity and Hepatoprotective Effects of Probiotic Goat Milk in Mice: Insights from Hepatic Proteomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Culture Conditions, and Formulation of Fermented Goat Milk (FGM)
2.2. Animals, Diets, and Experimental Design
2.3. Body Weight Monitoring and Sample Collection
2.4. Metabolic and Histological Aznalyses
2.5. Proteomic Analysis
2.5.1. Protein Extraction and Sample Preparation
2.5.2. Protein Identification and Mass Spectrometry Data Analysis
2.5.3. Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
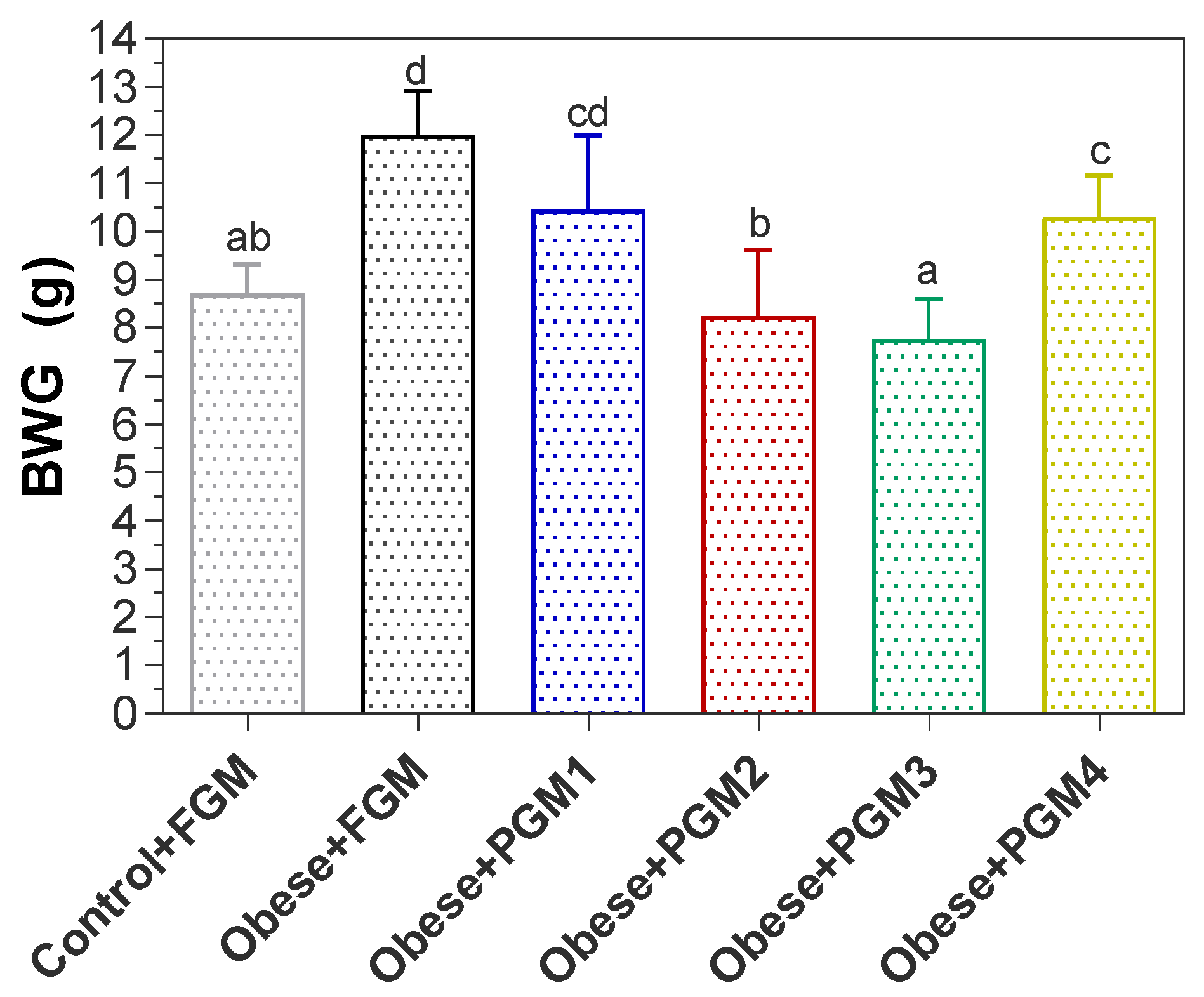
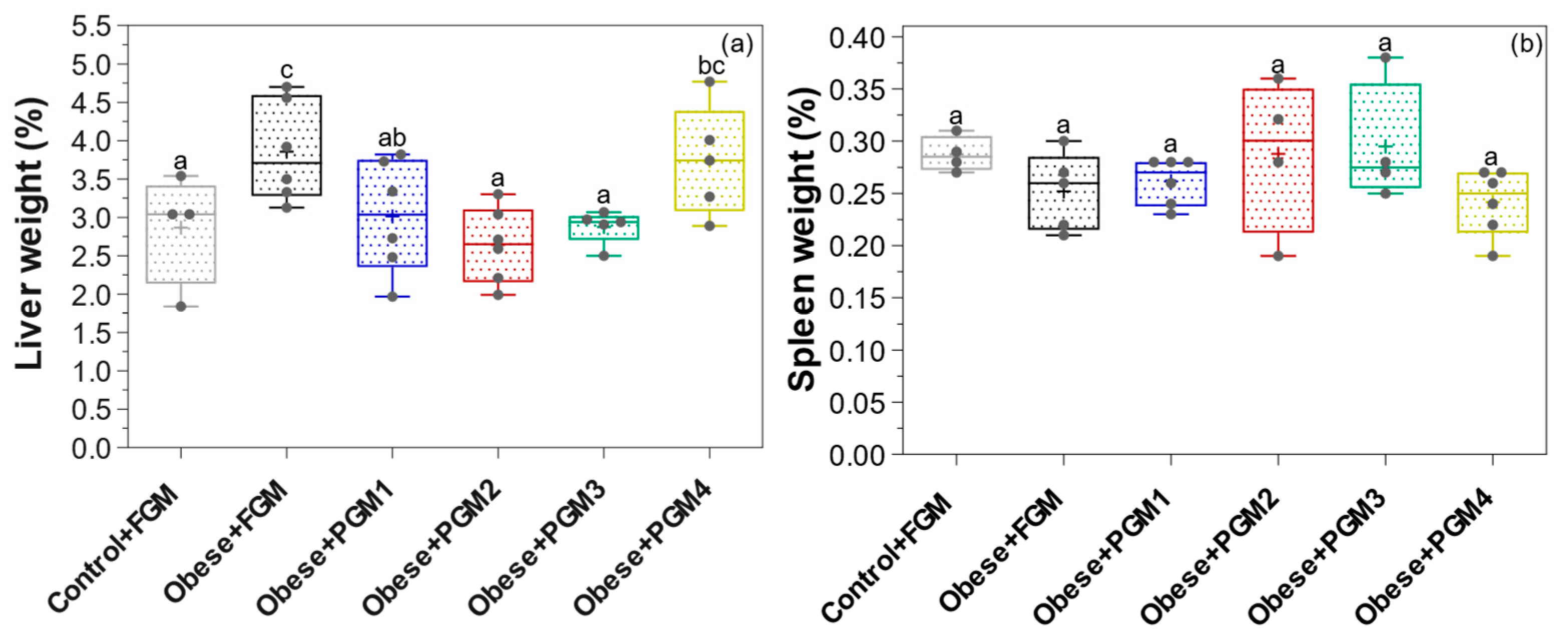
3.1. Effect of PGM on Body, Liver, and Spleen Weight
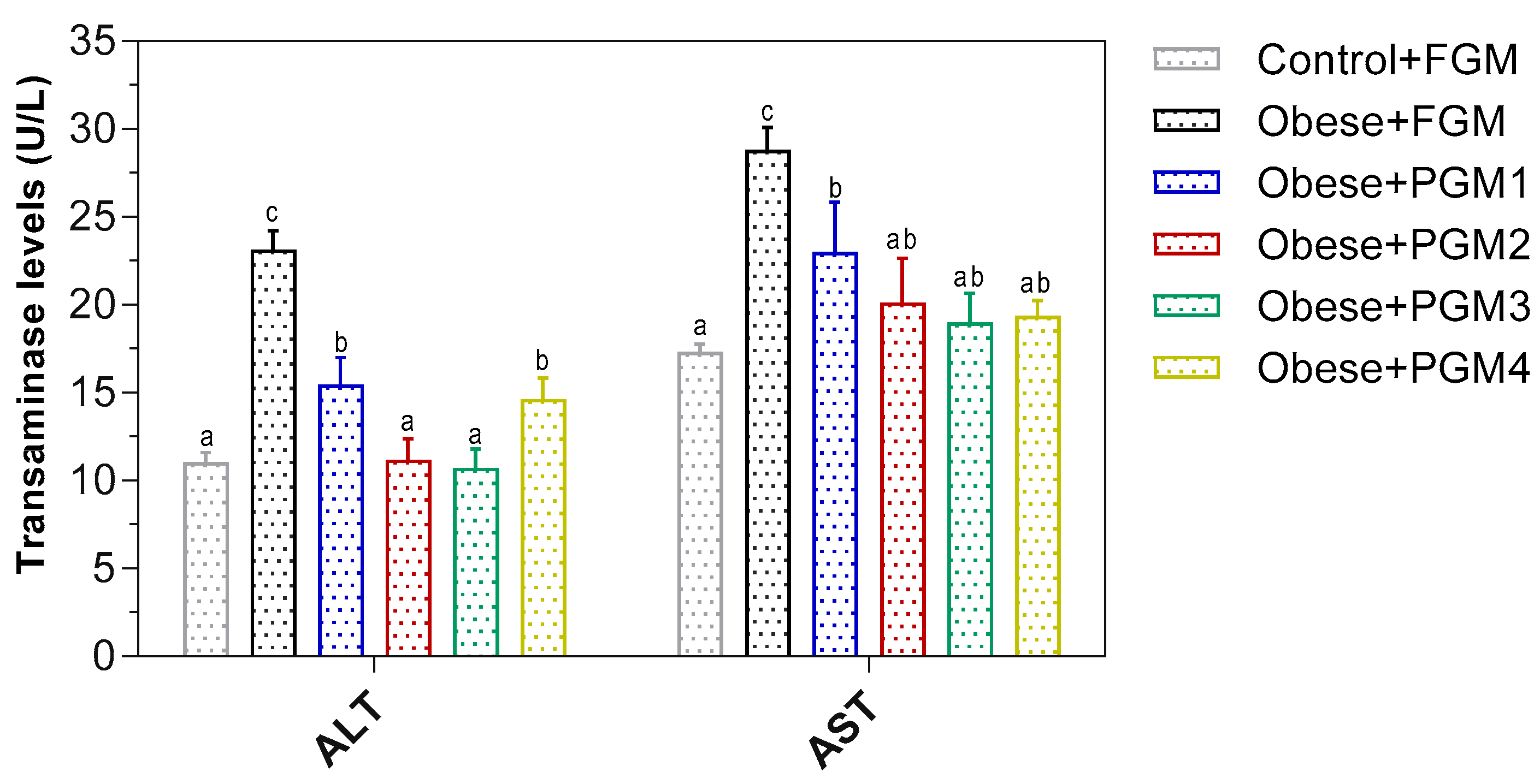
3.2. Evaluation of PGM on Transaminase Activities and the Structure of Hepatocytes in Mice
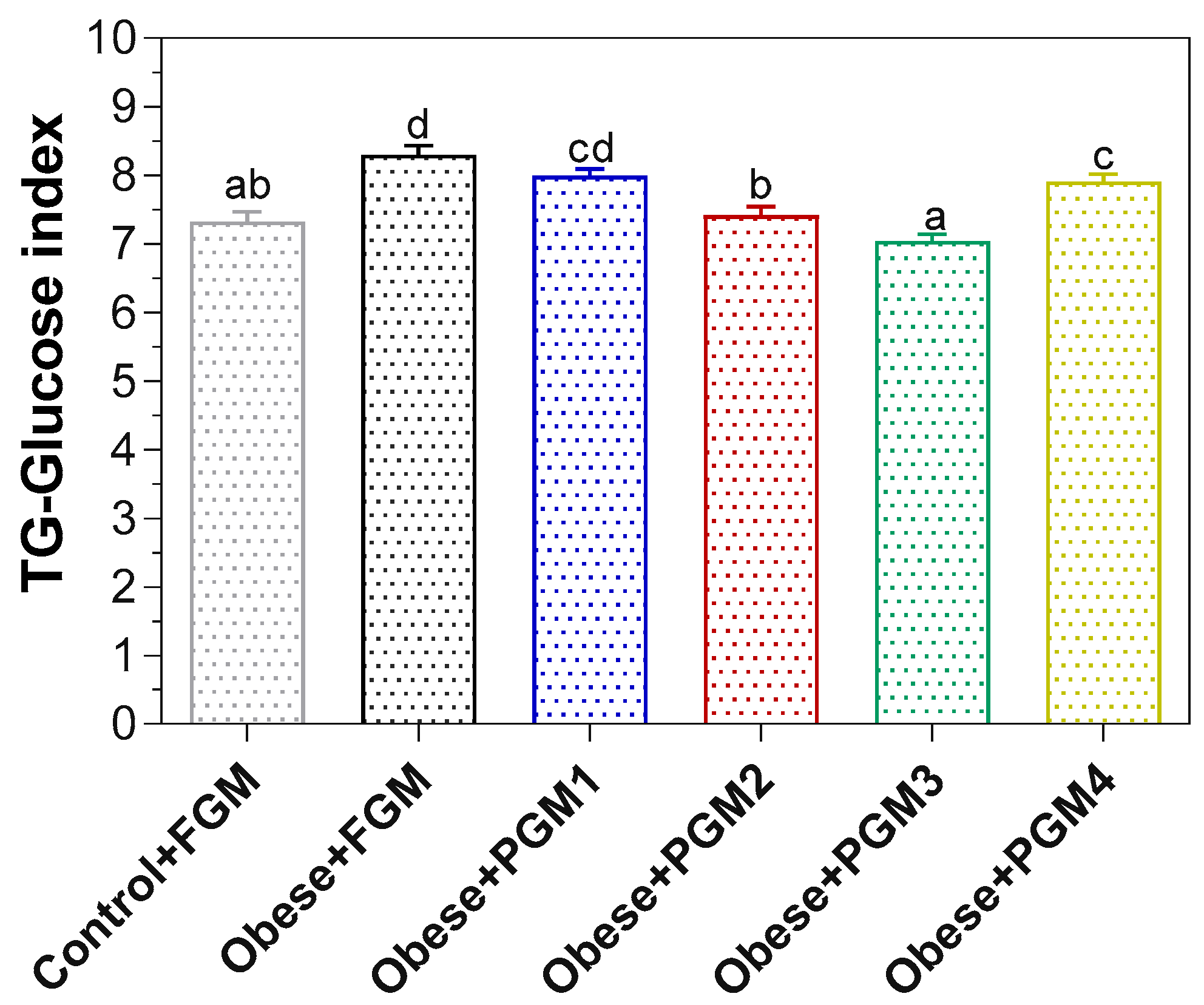
3.3. Effect of Administration of PGM on TG/Glucose Index, Lipid Profile, and Cardiovascular Risk Indicators
3.4. Evaluation of Liver Functionality by Proteomic Analysis
3.4.1. Quantitative Analysis of Proteins in Livers of Mice Fed HFD Supplemented with PGM3
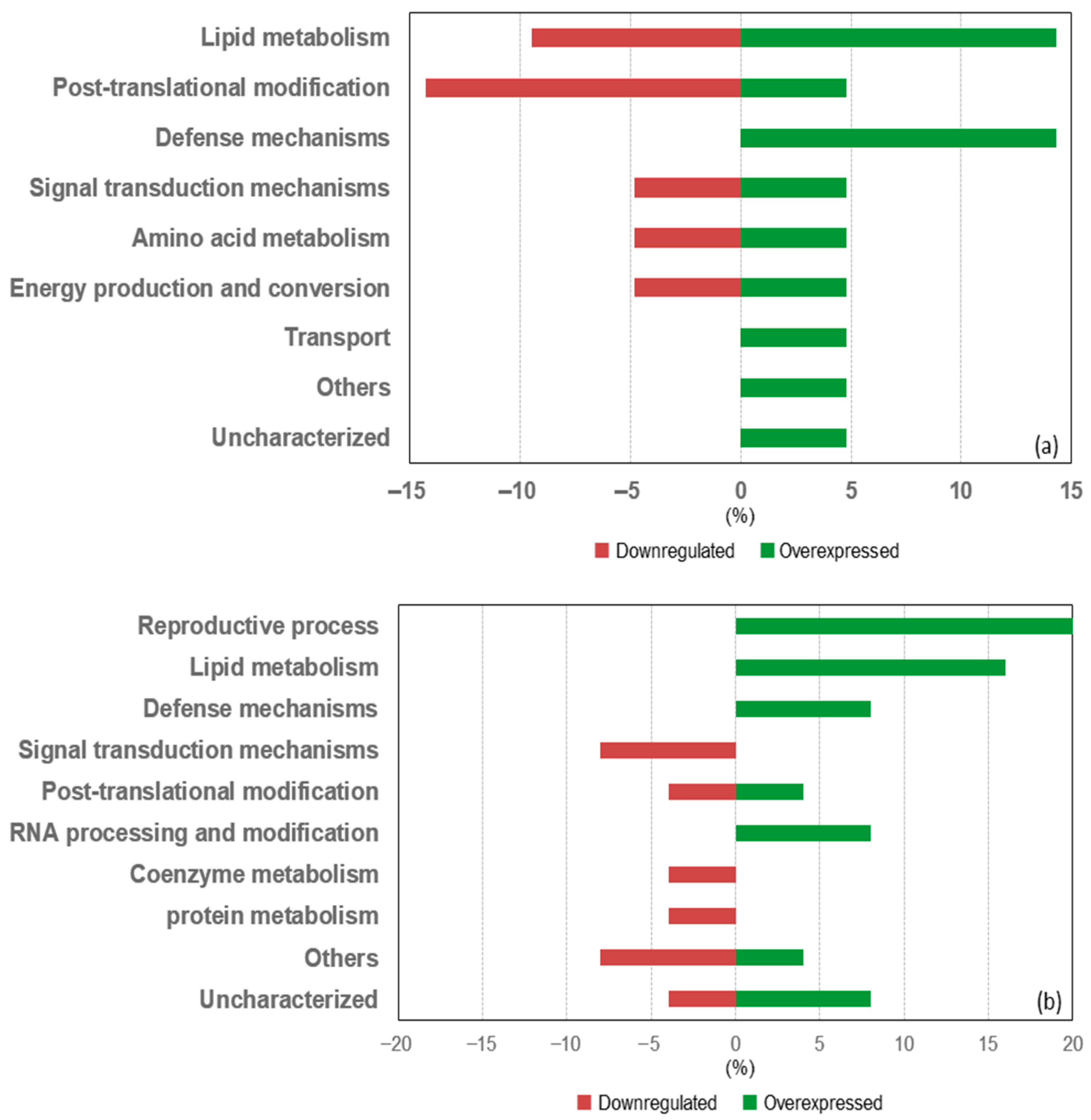
3.4.2. Differentially Expressed Proteins in the Livers of Mice from the Obese + FGM Group Compared to the Control + FGM Group
3.4.3. Differentially Expressed Proteins in the Livers of Mice from the Obese + PGM3 Group Compared to the Obese + FGM Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 June 2025).
- Vulchi, J.; Suryadevara, V.; Mohan, P.; Kamalanathan, S.; Sahoo, J.; Naik, D.; Selvarajan, S. Obesity and Metabolic Dysfunction-Associated Fatty Liver Disease: Understanding the Intricate Link. J. Transl. Gastroenterol. 2023, 1, 74–86. [Google Scholar] [CrossRef]
- Li, H.; Huang, W.; Wang, M.; Chen, P.; Chen, L.; Zhang, X. Tandem Mass Tag-Based Quantitative Proteomics Analysis of Metabolic Associated Fatty Liver Disease Induced by High Fat Diet in Mice. Nutr. Metab. 2020, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Mengos, A.E.; Stubblefield, T.M.; Mandarino, L.J. High Fat Diet-Induced Changes in Hepatic Protein Abundance in Mice. J. Proteomics Bioinform. 2012, 5, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.C.; Bhat, A.; Maras, J.S. Early Hepatic Proteomic Signatures Reveal Metabolic Changes in High-Fat-Induced Obesity in Rats. Br. J. Nutr. 2024, 131, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Nardo, W.D.; Lee, O.; Johari, Y.; Bayliss, J.; Pensa, M.; Miotto, P.M.; Keenan, S.N.; Ryan, A.; Rucinski, A.; Svinos, T.M.; et al. Integrated Liver-Secreted and Plasma Proteomics Identify a Predictive Model That Stratifies MASH. Cell Rep. Med. 2025, 6, 102085. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Ferreira, T.; Nascimento-Gonçalves, E.; Castro-Ribeiro, C.; Lemos, S.; Rosa, E.; Antunes, L.M.; Oliveira, P.A. Obesity Rodent Models Applied to Research with Food Products and Natural Compounds. Obesities 2022, 2, 171–204. [Google Scholar] [CrossRef]
- De Moura, E.; Dias, M.; Dos Reis, S.A.; Da Conceição, L.L.; Sediyama, C.M.N.D.O.; Pereira, S.S.; De Oliveira, L.L.; Gouveia Peluzio, M.D.C.; Martinez, J.A.; Milagro, F.I. Diet-Induced Obesity in Animal Models: Points to Consider and Influence on Metabolic Markers. Diabetol. Metab. Syndr. 2021, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Yadav, M.; Duary, R.K.; Shukla, P. Gut Microbiota Modulation Through Dietary Approaches Targeting Better Health During Metabolic Disorders. Mol. Nutr. Food Res. 2025, e70033. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, X.; Sun, J.; Pang, X.; Sun, Q.; Lu, Y. Regulatory Mechanisms of the Probiotic-Targeted Gut–Liver Axis for the Alleviation of Alcohol-Related Liver Disease: A Review. Crit. Rev. Food Sci. Nutr. 2025, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Su, Y.; Wang, Q.; Lv, L.; Xue, C.; Xu, L.; Li, L. Multi-Omics Analysis of the Effects of Dietary Changes and Probiotics on Diet-Induced Obesity. Curr. Res. Food Sci. 2023, 6, 100435. [Google Scholar] [CrossRef] [PubMed]
- Marquez, A.; Andrada, E.; Russo, M.; Bolondi, M.L.; Fabersani, E.; Medina, R.; Gauffin-Cano, P. Characterization of Autochthonous Lactobacilli from Goat Dairy Products with Probiotic Potential for Metabolic Diseases. Heliyon 2022, 8, e10462. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Q.; Xiang, M.; Zhang, F.; Wei, D.; Wen, Z.; Zhou, Y. Astragaloside Alleviates Hepatic Fibrosis Function via PAR2 Signaling Pathway in Diabetic Rats. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 41, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Low, S.; Khoo, K.C.J.; Irwan, B.; Sum, C.F.; Subramaniam, T.; Lim, S.C.; Wong, T.K.M. The Role of Triglyceride Glucose Index in Development of Type 2 Diabetes Mellitus. Diabetes Res. Clin. Pract. 2018, 143, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, T.; Goswami, K. Castelli Risk Index-1 and Atherogenic Coefficient Are Better Predictors of Cardiometabolic Risk in Patients with Hypothyroidism. Int. J. Clin. Biochem. Res. 2020, 7, 254–259. [Google Scholar] [CrossRef]
- Yang, L.; Vaitheesvaran, B.; Hartil, K.; Robinson, A.J.; Hoopmann, M.R.; Eng, J.K.; Kurland, I.J.; Bruce, J.E. The Fasted/Fed Mouse Metabolic Acetylome: N6-Acetylation Differences Suggest Acetylation Coordinates Organ-Specific Fuel Switching. J. Proteome Res. 2011, 10, 4134–4149. [Google Scholar] [CrossRef] [PubMed]
- La Greca, A.; Solari, C.; Furmento, V.; Lombardi, A.; Biani, M.C.; Aban, C.; Moro, L.; García, M.; Guberman, A.S.; Sevlever, G.E.; et al. Extracellular Vesicles from Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Acquire a Stromal Modulatory Proteomic Pattern during Differentiation. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING V10: Protein-Protein Interaction Networks, Integrated over the Tree of Life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Devaraj, S.; Jialal, I. The Role of the Triglyceride-Glucose Index as a Biomarker of Cardio-Metabolic Syndromes. Lipids Health Dis. 2024, 23, 416. [Google Scholar] [CrossRef] [PubMed]
- Avagimyan, A.; Pogosova, N.; Fogacci, F.; Aghajanova, E.; Djndoyan, Z.; Patoulias, D.; Sasso, L.L.; Bernardi, M.; Faggiano, A.; Mohammadifard, N.; et al. Triglyceride-Glucose Index (TyG) as a Novel Biomarker in the Era of Cardiometabolic Medicine. Int. J. Cardiol. 2025, 418, 132663. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ji, H.; Sun, W.; An, X.; Lian, F. Triglyceride Glucose (TyG) Index: A Promising Biomarker for Diagnosis and Treatment of Different Diseases. Eur. J. Intern. Med. 2025, 131, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bagnicka, E.; Chen, H.; Shu, G. Health Potential of Fermented Goat Dairy Products: Composition Comparison with Fermented Cow Milk, Probiotics Selection, Health Benefits and Mechanisms. Food Funct. 2023, 14, 3423–3436. [Google Scholar] [CrossRef] [PubMed]
- Andrada, E.; Marquez, A.; Russo, M.; Gauffin-Cano, P.; Medina, R. Fermented Goat Milk as a Functional Food for Obesity Prevention or Treatment: A Narrative Review. Front. Food Sci. Technol. 2024, 3. [Google Scholar] [CrossRef]
- Marquez, A.; Russo, M.; Tomei, C.; Castellano, P.; Puglisi, E.; Medina, R.; Gauffin-Cano, P. Effects of Fermented Goat Milk on Adiposity and Gut Microbiota in a Diet-Induced Obesity Murine Model. Fermentation 2024, 10, 155. [Google Scholar] [CrossRef]
- Russo, M.; Marquez, A.; Abeijón-Mukdsi, M.C.; Santacruz, A.; López-Malo, A.; Gauffin-Cano, P.; Medina, R. Microencapsulated Feruloyl Esterase-Producing Lactobacilli Ameliorate Lipid Profile and Glycaemia in High Fat Diet-Induced Obese Mice. Benef. Microbes 2019, 10, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Marquez, A.; Herrera, H.; Abeijon-Mukdsi, C.; Saavedra, L.; Hebert, E.; Gauffin-Cano, P.; Medina, R. Oral Administration of Lactobacillus Fermentum CRL1446 Improves Biomarkers of Metabolic Syndrome in Mice Fed a High-Fat Diet Supplemented with Wheat Bran. Food Funct. 2020, 11, 3879–3894. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Li, Z.; Yu, L.; Liu, Y.; Hu, Y.; Bi, Y.; Cheng, M. Integrative Proteomic and Lipidomic Analysis of Kaili Sour Soup-Mediated Attenuation of High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in a Rat Model. Nutr. Metab. 2021, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Yu, H.; Gu, R.; Chen, D.; Chen, X.; Huang, Y.; Xi, W.; Huang, Y. Proteomics for Studying the Effects of L. Rhamnosus LV108 against Non-Alcoholic Fatty Liver Disease in Rats. RSC Adv. 2018, 8, 38517–38528. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.M.; Chong, E.-J.; Mah, W.Y.; Pan, Y.; Fang, C.M.; Murugaiah, C.; Alshagga, M. Exploring the Anti-Obesity Effects of Lactobacillus in C57BL/6 Mice: Mechanisms, Interventions, and Future Directions. Lett. Appl. Microbiol. 2025, 78, ovaf024. [Google Scholar] [CrossRef] [PubMed]
- Madaudo, C.; Bono, G.; Ortello, A.; Astuti, G.; Mingoia, G.; Galassi, A.R.; Sucato, V. Dysfunctional High-Density Lipoprotein Cholesterol and Coronary Artery Disease: A Narrative Review. J. Pers. Med. 2024, 14, 996. [Google Scholar] [CrossRef] [PubMed]
- Galindev, U.; Erdenebold, U.; Batnasan, G.; Ganzorig, O.; Batdorj, B. Anti-Obesity Effects of Potential Probiotic Lactobacillus Strains Isolated from Mongolian Fermented Dairy Products in High-Fat Diet-Induced Obese Rodent Model. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2024, 55, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Fan, H.; Zhang, S.; Chen, C.; You, Y.; Wang, C.; Li, J.; Luo, L.; Cheng, Y.; Zhou, M.; et al. Association of LDL-C/HDL-C Ratio with Coronary Heart Disease: A Meta-Analysis. Indian Heart J. 2024, 76, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, Y.; Li, S.; Ma, Y.; Lin, J.; Wan, J.; Zhao, M. The Atherogenic Index of Plasma (AIP) Is a Predictor for the Severity of Coronary Artery Disease. Front. Cardiovasc. Med. 2023, 10, 1140215. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Ko, C.-W.; Tso, P.; Bhargava, A. Apolipoprotein A-IV: A Multifunctional Protein Involved in Protection against Atherosclerosis and Diabetes. Cells 2019, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- San Mauro Martín, I.; Collado Yurrita, L.; Cuadrado Cenzual, M.Á.; Ciudad Cabañas, M.J.; Mendive Dubourdieu, P. Role of ApoA1 on High-Density Lipoprotein: An Intervention with Plant Sterols in Patients with Hypercholesterolemia. Nutr. Hosp. 2014, 31, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- Flores Lopez, A.; Quiros-Tejeira, R.; Lyden, E.; McGill, B.; Dike, C. Association between BMI Change, Transaminases, and Other Metabolic Parameters in Children with Nonalcoholic Fatty Liver Disease. J. Obes. 2024, 2024, 6997280. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, H.; Zhuo, W.; Chen, Y.; Huang, Y.; Chen, X.; Lu, T.; Xu, Z. U-Shaped Associations between Body Mass Index and Serum Liver Enzyme Levels. Sci. Rep. 2025, 15, 443. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tan, J.K.; Liu, J.; Goon, J.A. Roles of Traditional and Next-Generation Probiotics on Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review and Network Meta-Analysis. Antioxidants 2024, 13, 329. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Hu, P.; Feng, L.-P.; Huang, L.-L.; Wang, Y.; Yan, X.; Xiong, J.; Xia, H.-L. Protective Effects of Ferulic Acid on Metabolic Syndrome: A Comprehensive Review. Molecules 2022, 28, 281. [Google Scholar] [CrossRef] [PubMed]
- Münte, E.; Hartmann, P. The Role of Short-Chain Fatty Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease and Other Metabolic Diseases. Biomolecules 2025, 15, 469. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Chen, M.; He, B.; Chen, Y.; Zheng, Y. Role of Short-Chain Fatty Acids in Non-Alcoholic Fatty Liver Disease and Potential Therapeutic Targets. Front. Microbiol. 2025, 16, 1539972. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Chang, C.-C.; Wang, C.-P.; Hung, W.-C.; Tsai, I.-T.; Tang, W.-H.; Wu, C.-C.; Wei, C.-T.; Chung, F.-M.; Lee, Y.-J.; et al. Circulating Fatty Acid-Binding Protein 1 (FABP1) and Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus. Int. J. Med. Sci. 2020, 17, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Newberry, E.P.; Xie, Y.; Kennedy, S.M.; Luo, J.; Davidson, N.O. Protection against Western Diet-Induced Obesity and Hepatic Steatosis in Liver Fatty Acid-Binding Protein Knockout Mice. Hepatology 2006, 44, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Lecchi, C. The Immune Functions of A1 Acid Glycoprotein. Curr. Protein Pept. Sci. 2019, 20, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Teng, Y.; Lan, Y.; Wang, M.; Zhang, T.; Wang, D.; Qi, F. The Association between Fat Distribution and A1-Acid Glycoprotein Levels among Adult Females in the United States. Lipids Health Dis. 2024, 23, 235. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, E.; Kwon, E.-Y.; Jang, H.-S.; Hur, C.-G.; Choi, M.-S. Network Analysis of Hepatic Genes Responded to High-Fat Diet in C57BL/6J Mice: Nutrigenomics Data Mining from Recent Research Findings. J. Med. Food 2010, 13, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, K.H.G.; Blanchet, C.; Felix, J.; Dansercoer, A.; De Vos, D.; Bloch, Y.; Van Beeumen, J.; Svergun, D.; Gutsche, I.; Savvides, S.N.; et al. Structure of ATP Citrate Lyase and the Origin of Citrate Synthase in the Krebs Cycle. Nature 2019, 568, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, J.; Guo, Y.; Ye, H.; Yu, C.; Xu, C.; Xu, L.; Wu, S.; Sun, W.; Wei, H.; et al. Functional Proteomic Analysis of Nonalcoholic Fatty Liver Disease in Rat Models: Enoyl-Coenzyme a Hydratase down-Regulation Exacerbates Hepatic Steatosis. Hepatology 2010, 51, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical Study of Oxidative Stress Markers in the Liver, Kidney and Heart of High Fat Diet Induced Obesity in Rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.; Catalán, V.; Rodríguez, A.; Rotellar, F.; Valentí, V.; Silva, C.; Salvador, J.; Frühbeck, G.; Gómez-Ambrosi, J.; Roca, P.; et al. Identification of Liver Proteins Altered by Type 2 Diabetes Mellitus in Obese Subjects. Liver Int. Off. J. Int. Assoc. Study Liver 2012, 32, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, L.; Rui, L. Identification of MUP1 as a Regulator for Glucose and Lipid Metabolism in Mice. J. Biol. Chem. 2009, 284, 11152–11159. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Zhu, W.; Wang, Y.; Lam, K.S.L.; Zhang, J.; Wu, D.; Kraegen, E.W.; Li, Y.; Xu, A. Major Urinary Protein-1 Increases Energy Expenditure and Improves Glucose Intolerance through Enhancing Mitochondrial Function in Skeletal Muscle of Diabetic Mice. J. Biol. Chem. 2009, 284, 14050–14057. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, W.; Li, S.; Guo, D.; He, J.; Wang, Y. Acetyl-CoA Carboxylases and Diseases. Front. Oncol. 2022, 12, 836058. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zhang, Y.; Fu, M.; Pei, Y.; Wu, L.; Wang, R.; Yang, G. Cystathionine Gamma-Lyase/H2S System Suppresses Hepatic Acetyl-CoA Accumulation and Nonalcoholic Fatty Liver Disease in Mice. Life Sci. 2020, 252, 117661. [Google Scholar] [CrossRef] [PubMed]
- Juwono, J.; Martinus, R.D. Does Hsp60 Provide a Link between Mitochondrial Stress and Inflammation in Diabetes Mellitus? J. Diabetes Res. 2016, 2016, 8017571. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Park, S.; Sin, H.-S.; Jang, S.-H.; Lee, S.-W.; Kim, S.-Y.; Kwon, B.; Yu, K.-Y.; Kim, S.; Yang, D. Hypocholesterolemic Effects of Probiotic Mixture on Diet-Induced Hypercholesterolemic Rats. Nutrients 2017, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Dore, R.; Levata, L.; Lehnert, H.; Schulz, C. Nesfatin-1: Functions and Physiology of a Novel Regulatory Peptide. J. Endocrinol. 2017, 232, R45–R65. [Google Scholar] [CrossRef] [PubMed]
- Öztürk Özkan, G. Effects of Nesfatin-1 on Food Intake and Hyperglycemia. J. Am. Coll. Nutr. 2020, 39, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Tekin, T.; Cicek, B.; Konyaligil, N. Turkey Regulatory Peptide Nesfatin-1 and Its Relationship with Metabolic Syndrome. Eurasian J. Med. 2019, 51, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, Z.; Gao, L.; Li, Y.; Zhao, J.; Zhang, W. AMPK-Dependent Modulation of Hepatic Lipid Metabolism by Nesfatin-1. Mol. Cell. Endocrinol. 2015, 417, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Li, Z.; Zhang, Y.; Yang, L.; Pan, Y.; Wang, Z.; Feng, G.-S.; Chen, Y. Apolipoprotein A-I Possesses an Anti-Obesity Effect Associated with Increase of Energy Expenditure and up-Regulation of UCP1 in Brown Fat. J. Cell. Mol. Med. 2011, 15, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Kwon, Y.; Yea, K.; Moon, H.-Y.; Yoon, J.H.; Ghim, J.; Hyun, H.; Kim, D.; Koh, A.; Berggren, P.-O.; et al. Apolipoprotein A1 Increases Mitochondrial Biogenesis through AMP-Activated Protein Kinase. Cell. Signal. 2015, 27, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Jang, H.B.; Park, J.E.; Park, K.-H.; Kang, J.H.; Park, S.I.; Song, J. Relationship between Serum Levels of Body Iron Parameters and Insulin Resistance and Metabolic Syndrome in Korean Children. Osong Public Health Res. Perspect. 2014, 5, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-Q.; Wu, H.; Meng, R.; Du, S.; Tao, S.-J. Distribution of Serum Transferrin, and Its Associations with Metabolic Disorders among Chinese: A Nation-Wide, Health and Nutrition Survey. Mol. Nutr. Food Res. 2015, 59, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Unoki-Kubota, H.; Shimizu, Y.; Okamura, T.; Iwata, W.; Kajio, H.; Yamamoto-Honda, R.; Shiga, T.; Yamashita, S.; Tobe, K.; et al. Proteomic Analysis of Serum Biomarkers for Prediabetes Using the Long-Evans Agouti Rat, a Spontaneous Animal Model of Type 2 Diabetes Mellitus. J. Diabetes Investig. 2017, 8, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Kirpich, I.A.; Gobejishvili, L.N.; Homme, M.B.; Waigel, S.; Cave, M.; Arteel, G.; Barve, S.S.; McClain, C.J.; Deaciuc, I.V. Integrated Hepatic Transcriptome and Proteome Analysis of Mice with High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease. J. Nutr. Biochem. 2011, 22, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Flores, J.; Flores-Pérez, E.; Hernández-Ortiz, M.; Vargas-Ortiz, K.; Ramírez-Emiliano, J.; Encarnación-Guevara, S.; Pérez-Vázquez, V. Protein Expression Profile of Twenty-Week-Old Diabetic Db/Db and Non-Diabetic Mice Livers: A Proteomic and Bioinformatic Analysis. Biomolecules 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]

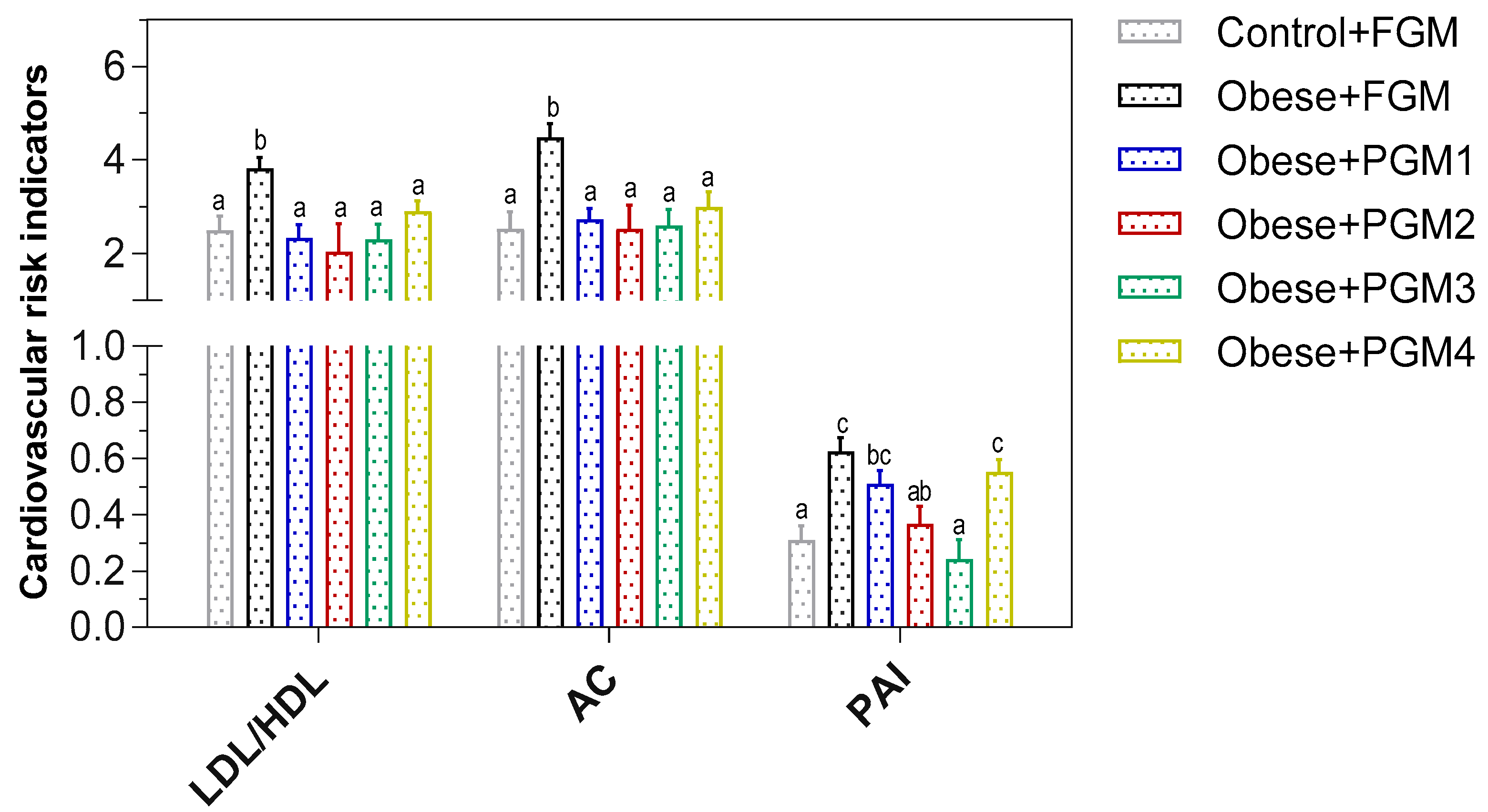
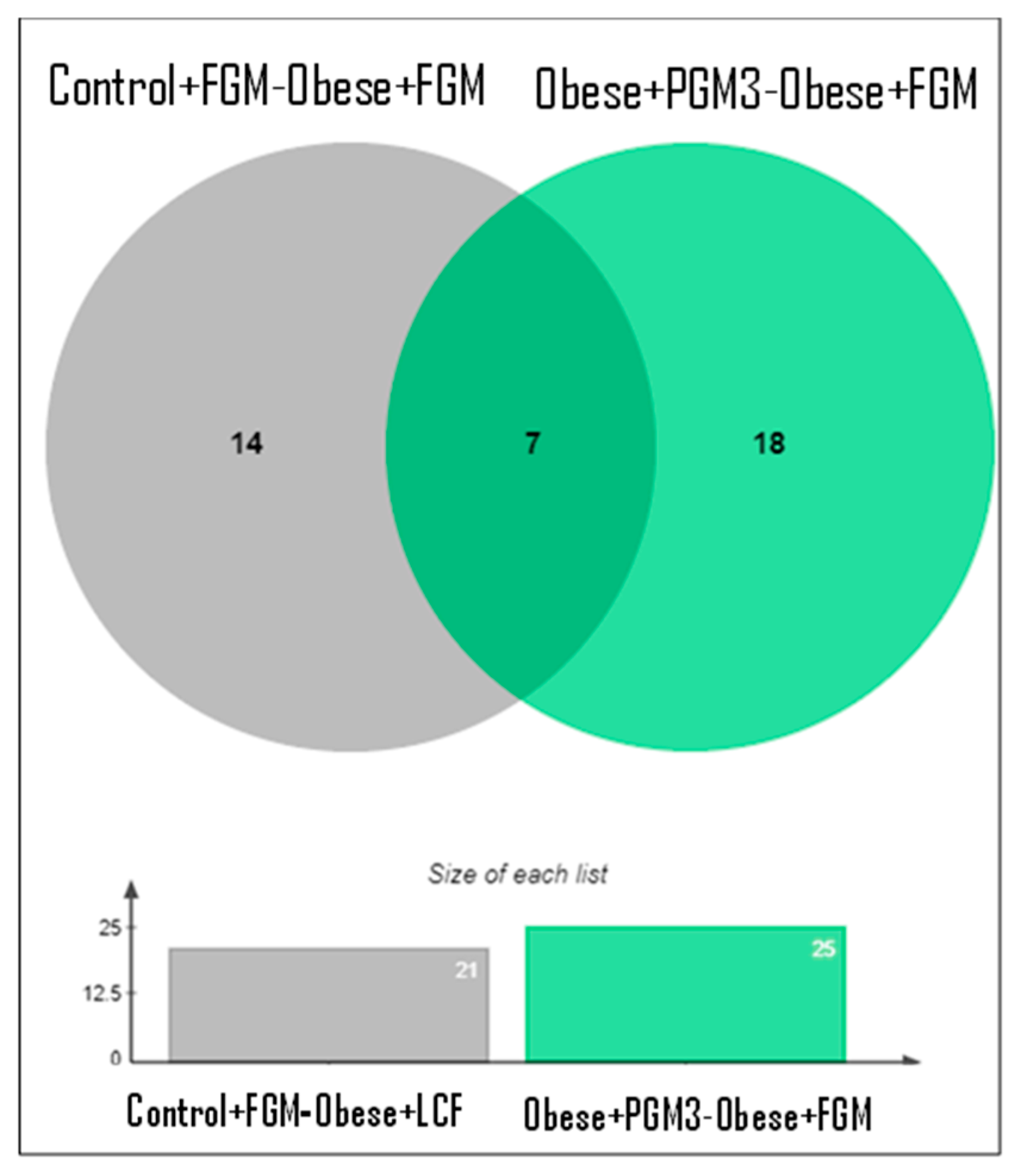

| Groups | TG | TC | LDL-c | HDL-c |
|---|---|---|---|---|
| Control + FGM | 0.23 ± 0.02 a | 0.46 ± 0.01 a | 0.32 ± 0.02 a | 0.14 ± 0.01 b |
| Obese + FGM | 0.45 ± 0.05 c | 0.54 ± 0.03 a | 0.38 ± 0.03 ab | 0.10 ± 0.01 a |
| Obese + PGM1 | 0.50 ± 0.05 c | 0.57 ± 0.03 a | 0.36 ± 0.05 ab | 0.15 ± 0.01 bc |
| Obese + PGM2 | 0.39 ± 0.03 ab | 0.57 ± 0.07 a | 0.32 ± 0.08 ab | 0.16 ± 0.01 c |
| Obese + PGM3 | 0.29 ± 0.04 bc | 0.59 ± 0.04 a | 0.37 ± 0.05 ab | 0.16 ± 0.01 c |
| Obese + PGM4 | 0.46 ± 0.04 c | 0.57 ± 0.04 a | 0.40 ± 0.04 b | 0.13 ± 0.01 b |
| Access No. | Gene | Description | Category/Function | Expression Changes |
|---|---|---|---|---|
| P12710 | Fabp1 | Fatty acid-binding protein 1 | Lipid metabolism | 3.73 |
| P07361 | Agp2 | Alpha-1-acid glycoprotein 2 | Signal transduction mechanisms | 3.45 |
| Q8VCH0 | Acaa1b | Acetyl-CoA acyltransferase | Lipid metabolism | 2.91 |
| Q9DCX2 | Atp5h | ATP synthase subunit d | Energy production and conversion | 2.86 |
| Q3U2G2 | Hspa4 | Heat shock protein 70 | Post-translational modification | 2.34 |
| P70362 | Ufd1 | Ubiquitin recognition factor | Post-translational modification | 2.22 |
| P05201 | Got1 | Aspartate aminotransferase | Amino acid metabolism | 2.17 |
| P63038 | Hspd1 | Heat shock protein 60 | Post-translational modification | 2.03 |
| P17563 | Selenbp1 | Methanethiol oxidase | Transport | −2.01 |
| Q9DA03 | Lyrm7 | Complex III assembly factor LYRM7 | Energy production and conversion | −2.12 |
| Q5SWU9 | Acaca | Acetyl-CoA carboxylase | Lipid metabolism | −2.15 |
| Q03734 | Serpina3m | Serine protease inhibitor A3M | Defense mechanisms | −2.28 |
| O08692 | Ngp | Neutrophilic granule protein | Defense mechanisms | −2.51 |
| Q3UH59 | Myh10 | Myosin X | Cytoskeletal organization | −2.64 |
| F7AA26 | Pakap | Protein kinase A-anchoring proteins | Signal transduction mechanisms | −3.69 |
| Q8VCN5 | Cth | Cystathionine gamma-lyase | Amino acid metabolism | −4.36 |
| P18419 | Svs4 | Seminal vesicle secretory protein 4 | Uncharacterized | −4.43 |
| G3X8T9 | Serpina3n | Serine protease inhibitor A3N | Defense mechanisms | −4.77 |
| P11588 | Mup1 | Major urinary protein 1 | Post-translational modification | −4.84 |
| P19157 | Gstp1 | Glutathione S-transferase P1 | Lipid metabolism | −4.86 |
| Q91V92 | Acly | ATP-citrate synthase | Lipid metabolism | −4.93 |
| Access No. | Gene | Description | Category/Function | Expression Changes |
|---|---|---|---|---|
| Q8BS30 | Svs2 | Seminal vesicle secretory protein 2 | Reproductive process | 5.44 |
| P18419 | Svs4 | Seminal vesicle secretory protein 4 | Reproductive process | 4.95 |
| P81117 | Nucb2 | Nucleobindin-2 | Lipid metabolism | 4.51 |
| Q00623 | Apoa1 | Apolipoprotein A-I | Lipid metabolism | 3.15 |
| O08692 | Ngp | Neutrophilic granule protein | Defense mechanisms | 3.15 |
| G3X8T9 | Serpina3n | Serine protease inhibitor A3N | Defense mechanisms | 3.08 |
| P06728 | Apoa4 | Apolipoprotein A-IV | Lipid metabolism | 3.39 |
| A0A5F8MPB9 | Rdx | Radixin | Uncharacterized | 2.88 |
| Q9R0U0 | Srsf10 | Serine/arginine-rich splicing factor 10 | RNA processing and modification | 2.80 |
| Q8C845 | Efhd2 | EF-hand domain-containing protein D2 | Uncharacterized | 2.54 |
| P11588 | Mup22 | Major urinary protein 1 | Post-translational modification | 2.32 |
| P19157 | Gstp1 | Glutathione S-transferase P1 | Lipid metabolism | 2.15 |
| Q9WVB0 | Rbpms | RNA-binding protein | RNA processing and modification | 2.10 |
| P43275 | H1-1 | Histone H1 | Chromatin structure | 2.01 |
| P30933 | Svs5 | Seminal vesicle secretory protein 5 | Reproductive process | 2.23 |
| Q64356 | Svs6 P | Seminal vesicle secretory protein 6 | Reproductive process | 2.34 |
| Q8BZH8 | Svs3b | Seminal vesicle secretory protein 3B | Reproductive process | 2.46 |
| Q01149 | Col1a2 | Collagen type I alpha 2 chain | Extracellular structures | −2.36 |
| Q9QXS1 | Plec | Plectin | Cytoskeletal organization | −2.40 |
| D3YZD8 | Aamdc | Mth938 domain-containing protein | Uncharacterized | −2.51 |
| P0CG50 | Ubc | Polyubiquitin-C | Protein metabolism | −3.11 |
| Q3U2G2 | Hspa4 | Heat shock protein | Post-translational modification | −3.38 |
| P07361 | Agp2 / | Alpha-1-acid glycoprotein | Signal transduction mechanisms | −3.77 |
| Q921I1 | Orm2 | Serotransferrin | Signal transduction mechanisms | −3.98 |
| Q9R1Z7 | Tf | 6-pyruvoyltetrahydropterin synthase | Coenzyme metabolism | −4.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marquez, A.; Andrada, E.; Russo, M.; Babot, J.D.; Medina, R.; Gauffin-Cano, P. Anti-Obesity and Hepatoprotective Effects of Probiotic Goat Milk in Mice: Insights from Hepatic Proteomics. Fermentation 2025, 11, 419. https://doi.org/10.3390/fermentation11070419
Marquez A, Andrada E, Russo M, Babot JD, Medina R, Gauffin-Cano P. Anti-Obesity and Hepatoprotective Effects of Probiotic Goat Milk in Mice: Insights from Hepatic Proteomics. Fermentation. 2025; 11(7):419. https://doi.org/10.3390/fermentation11070419
Chicago/Turabian StyleMarquez, Antonela, Estefanía Andrada, Matias Russo, Jaime Daniel Babot, Roxana Medina, and Paola Gauffin-Cano. 2025. "Anti-Obesity and Hepatoprotective Effects of Probiotic Goat Milk in Mice: Insights from Hepatic Proteomics" Fermentation 11, no. 7: 419. https://doi.org/10.3390/fermentation11070419
APA StyleMarquez, A., Andrada, E., Russo, M., Babot, J. D., Medina, R., & Gauffin-Cano, P. (2025). Anti-Obesity and Hepatoprotective Effects of Probiotic Goat Milk in Mice: Insights from Hepatic Proteomics. Fermentation, 11(7), 419. https://doi.org/10.3390/fermentation11070419









