Assessing the Fermentation Quality, Bacterial Composition and Ruminal Degradability of Caragana korshinskii Ensiled with Oat Grass
Abstract
1. Introduction
2. Materials and Methods
2.1. Harvesting of Fresh Materials and Silage Preparation
2.2. Analysis of Chemical Composition and Fermentation Characteristics
2.3. Evaluation of Rumen Degradation Characteristics of the Optimized C. korshinskii Silage
2.4. Microbial Community Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Fresh C. korshinskii and Oat Grass
3.2. The Fermentation Parameters of C. korshinskii Silage
3.3. The Chemical Composition of C. korshinskii Silage
3.4. Bacterial Community Profiles During Ensiling
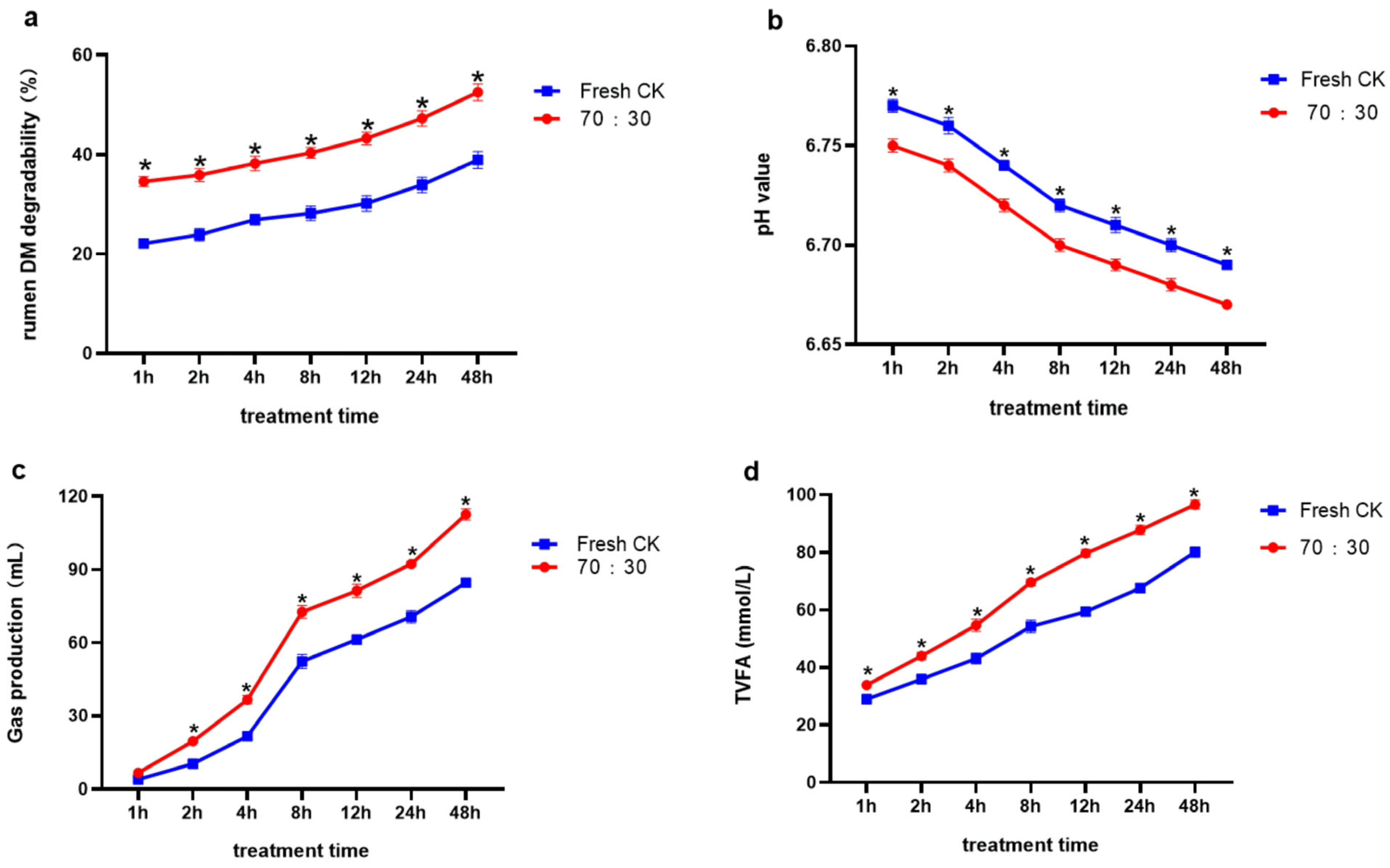
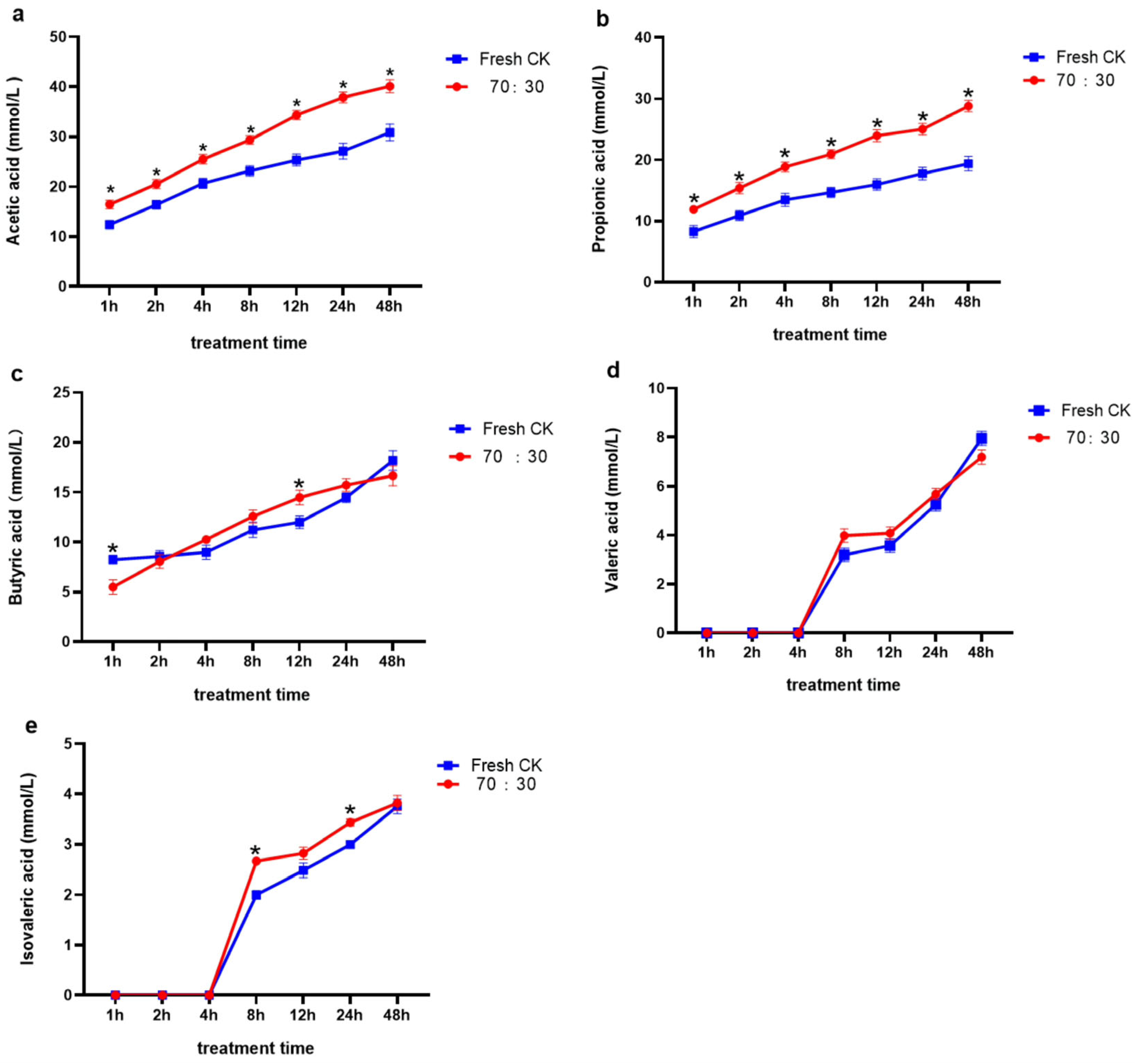
3.5. In Situ Rumen Degradability and In Vitro Rumen Fermentation
3.6. Bacterial Community Profiles During In Vitro Rumen Fermentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WSCs | Water-soluble carbohydrates |
| DM | Dry matter |
| CP | Crude protein |
| NDF | Neutral detergent fiber |
| ADF | Acid detergent fiber |
| EE | Ether extract |
| VFAs | Volatile fatty acids |
References
- Wang, S.; Ding, C.; Tian, J.; Cheng, Y.; Xu, N.; Zhang, W.; Wang, X.; Nazar, M.; Liu, B. An evaluation of storage length on ensiling characteristics, bacterial community compositions, co-occurrence networks, and their functional shifts and pathogenic risk in high-moisture oat silage. Chem. Biol. Technol. Agric. 2024, 11, 173. [Google Scholar] [CrossRef]
- Xia, T.; Tahir, M.; Wang, T.; Wang, Y.; Zhang, X.; Liu, S.; Teng, K.; Fu, Z.; Yun, F.; Wang, S.; et al. Lactobacillus cocktail and cellulase synergistically improve the fiber transformation rate in Sesbania cannabina and sweet sorghum mixed silage. Chem. Biol. Technol. Agric. 2024, 11, 81. [Google Scholar] [CrossRef]
- Jayanegara, A.; Sujarnoko, T.U.; Ridla, M.; Kondo, M.; Kreuzer, M. Silage quality as influenced by concentration and type of tannins present in the material ensiled: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2019, 103, 456–465. [Google Scholar] [CrossRef]
- Yu, H.; Hu, R.; Jia, Y.; Xiao, Y.; Du, S. Novel mechanistic understanding that Lactiplantibacillus plantarum is more capable of improving the ensiling performance of wheat straw silage than xylanase by driving certain key metabolites. Chem. Biol. Technol. Agric. 2024, 11, 155. [Google Scholar] [CrossRef]
- Jayanegara, A.; Wardiman, B.; Kondo, M.; Ridla, M.; Laconi, E. Fermentative quality of silage as affected by protein level in the ensiled material: A meta-analysis. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Palu, Indonesia, 25–26 July 2020; p. 012001. [Google Scholar]
- Sun, Z.Q.; Li, Y.; Liu, G.B.; Gao, R.; Bao, J.Z.; Wang, L.; Wu, Z.; Yu, Z. Associative effects of ensiling mixtures of sweet sorghum and korshinsk pea shrub on fermentation quality, chemical composition, and in vitro rumen digestion characteristics. Anim. Sci. J. 2022, 93, e13700. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, G.; Yuan, X.J.; Zhang, J.; Wen, A.Y.; Sun, X.H.; Shao, T. Effect of ensiling whole crop oat with lucerne in different ratios on fermentation quality, aerobic stability and in vitro digestibility on the Tibetan plateau. J. Anim. Physiol. Anim. Nutr. 2017, 101, e144–e153. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Qiu, R.; Sun, L.; Bao, J.; Liu, Y.; Ge, G.; Jia, Y.; Wang, Z. Effect isolated lactic acid bacteria inoculation on the quality, bacterial composition and metabolic characterization of Caragana korshinskii silage. Chem. Biol. Technol. Agric. 2024, 11, 67. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, H.; Gao, Y.; Diao, Q. Dynamic profiles of fermentation characteristics and bacterial community composition of Broussonetia papyrifera ensiled with perennial ryegrass. Bioresour. Technol. 2020, 310, 123396. [Google Scholar] [CrossRef]
- Helrich, K. (Ed.) Official Methods of Analysis of the AOAC, 15th ed.; Association of Official Analytical Chemists Inc.: Arlington, DC, USA, 1990; Volume 2. [Google Scholar]
- Wang, K.; Nan, X.; Chu, K.; Tong, J.; Yang, L.; Zheng, S.; Zhao, G.; Jiang, L.; Xiong, B. Shifts of Hydrogen Metabolism from Methanogenesis to Propionate Production in Response to Replacement of Forage Fiber with Non-forage Fiber Sources in Diets in vitro. Front. Microbiol. 2018, 9, 2764. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media1. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Mehrez, A.; Ørskov, E. A study of artificial fibre bag technique for determining the dig estibility of feeds in the rumen. J. Agric. Sci. 1977, 88, 645–650. [Google Scholar] [CrossRef]
- Merry, R.J.; Smith, R.H.; McAllan, A.B. Studies of rumen function in an in vitro continuous culture system. Arch. Anim. Nutr. 1987, 37, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, H.; Zi, X.; Cai, Y. Silage fermentation and ruminal degradation of stylo prepared with lactic acid bacteria and cellulase. Anim. Sci. J. 2017, 88, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X. Evaluation of maifanite and silage as amendments for green waste composting. Waste Manag. 2018, 77, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wu, N.; Na, N.; Sun, J.; Sun, L.; Qili, M.; Li, D.; Li, E.; Yang, B. Dynamics of gas and greenhouse gases production during fermentation of barley silage with lactic acid bacteria. Chem. Biol. Technol. Agric. 2024, 11, 82. [Google Scholar] [CrossRef]
- Kung Jr, L.; Shaver, R.; Grant, R.; Schmidt, R. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Brink, M.; Janssens, G.P.J.; Demeyer, P.; Bağci, Ö.; Delezie, E. Ammonia concentrations, litter quality, performance and some welfare parameters of broilers kept on different bedding materials. Br. Poult. Sci. 2022, 63, 768–778. [Google Scholar] [CrossRef]
- He, L.; Lv, H.; Xing, Y.; Wang, C.; You, X.; Chen, X.; Zhang, Q. The nutrients in Moringa oleifera leaf contribute to the improvement of stylo and alfalfa silage: Fermentation, nutrition and bacterial community. Bioresour. Technol. 2020, 301, 122733. [Google Scholar] [CrossRef]
- Chen, L.; Qu, H.; Bai, S.; Yan, L.; You, M.; Gou, W.; Li, P.; Gao, F. Effect of wet sea buckthorn pomace utilized as an additive on silage fermentation profile and bacterial community composition of alfalfa. Bioresour. Technol. 2020, 314, 123773. [Google Scholar] [CrossRef]
- Velarde-Guillén, J.; Sainz-Ramírez, A.; Celis-Álvarez, M.D.; Arriaga-Jordán, C.M.; Martínez-García, C.G. Characterisation of landrace ‘criollo’ maize silage from the highlands of Mexico in terms of starch content. Trop. Anim. Health Prod. 2022, 54, 283. [Google Scholar] [CrossRef]
- Elferink, S.; Driehuis, F.; Gottschal, J.C.; Spoelstra, S.F. Silage Fermentation Processes and Their Manipulation; FAO Plant Production and Protection Paper: Rome, Italy, 2000; pp. 17–30. [Google Scholar]
- Ni, K.; Zhao, J.; Zhu, B.; Su, R.; Pan, Y.; Ma, J.; Zhou, G.; Tao, Y.; Liu, X.; Zhong, J. Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 2018, 265, 563–567. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Guan, H.; Huang, L.; Ma, X.; Peng, Y.; Li, Z.; Nie, G.; Zhou, J.; Yang, W.; et al. Microbial community and fermentation characteristic of Italian ryegrass silage prepared with corn stover and lactic acid bacteria. Bioresour. Technol. 2019, 279, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Qiu, R.; Wang, Z.; Liu, Y.; Bao, J.; Sun, L.; Liu, T.; Ge, G.; Jia, Y. Effects of Cellulase and Lactic Acid Bacteria on Ensiling Performance and Bacterial Community of Caragana korshinskii Silage. Microorganisms 2023, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Cueva, S.F.; Harper, M.; Roth, G.W.; Wells, H.; Canale, C.; Gallo, A.; Masoero, F.; Hristov, A.N. Effects of ensiling time on corn silage starch ruminal degradability evaluated in situ or in vitro. J. Dairy Sci. 2023, 106, 3961–3974. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Wang, Q.; Wang, Y.; Zhang, Z. Effects of cellulase and xylanase on fermentative profile, bacterial diversity, and in vitro degradation of mixed silage of agro-residue and alfalfa. Chem. Biol. Technol. Agric. 2023, 10, 40. [Google Scholar] [CrossRef]
- Graf, J. The Family Rikenellaceae. Prokaryotes; Rosenberg, E., DeLong, E., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer Nature: London, UK, 2014. [Google Scholar]
- Qiu, M.; Hu, J.; Peng, H.; Li, B.; Xu, J.; Song, X.; Yu, C.; Zhang, Z.; Du, X.; Bu, G.; et al. Research Note: The gut microbiota varies with dietary fiber levels in broilers. Poult. Sci. 2022, 101, 101922. [Google Scholar] [CrossRef]
- Wei, X.; Ouyang, K.; Long, T.; Liu, Z.; Li, Y.; Qiu, Q. Dynamic Variations in Rumen Fermentation Characteristics and Bacterial Community Composition during In Vitro Fermentation. Fermentation 2022, 8, 276. [Google Scholar] [CrossRef]


| Items | C. korshinskii | Oat Grass |
|---|---|---|
| Dry matter (% FM 1) | 67.40 ± 1.55 | 20.63 ± 1.03 |
| Crude protein (% DM 2) | 14.31 ± 0.93 | 11.24 ± 0.75 |
| Neutral detergent fiber (% DM) | 69.67 ± 2.78 | 62.40 ± 1.20 |
| Acid detergent fiber (% DM) | 51.14 ± 1.70 | 35.24 ± 1.28 |
| Water-soluble carbohydrate (% DM) | 4.25 ± 0.21 | 21.46 ± 1.16 |
| Ether extraction (% DM) | 3.51 ± 0.13 | 3.91 ± 0.11 |
| Ash (% DM) | 4.31 ± 0.08 | 6.95 ± 0.04 |
| Lactic acid bacteria (Log10 cfu/g FM) | 4.45 ± 0.38 | 4.75 ± 0.36 |
| Item | 100:0 | 90:10 | 80:20 | 70:30 | 60:40 | 50:50 1 | p-Value 2 |
|---|---|---|---|---|---|---|---|
| Ensiled for 7 d | |||||||
| pH | 4.89 ± 0.09 a | 4.38 ± 0.05 b | 4.22 ± 0.06 b | 4.06 ± 0.04 c | 4.40 ± 0.07 b | 4.31 ± 0.13 b | <0.001 |
| NH3-N (g/kg TN 3) | 2.16 ± 0.44 d | 6.08 ± 0.27 c | 6.61 ± 0.15 c | 11.70 ± 0.85 a | 6.37 ± 0.50 c | 7.67 ± 0.68 b | <0.001 |
| Lactic acid (mmol/g) | 6.31 ± 0.16 f | 36.15 ± 1.47 c | 44.82 ± 1.84 b | 49.29 ± 2.03 a | 24.18 ± 1.13 e | 30.53 ± 1.53 d | <0.001 |
| Acetate acid (g/kg DM 4) | 3.33 ± 0.63 d | 8.92 ± 1.05 c | 11.47 ± 0.56 b | 13.92 ± 1.38 a | 11.18 ± 0.71 b | 11.59 ± 1.14 b | <0.001 |
| Ensiled for 14 d | |||||||
| pH | 4.78 ± 0.21 a | 4.21 ± 0.06 b | 4.11 ± 0.03 b | 4.03 ± 0.03 b | 4.23 ± 0.05 b | 4.20 ± 0.04 b | <0.001 |
| NH3-N (g/kg TN) | 2.77 ± 0.36 d | 6.82 ± 0.51 c | 9.85 ± 0.80 b | 13.70 ± 0.56 a | 7.52 ± 0.82 c | 8.76 ± 0.68 b | <0.001 |
| Lactic acid (mmol/g) | 8.30 ± 0.35 d | 53.23 ± 3.64 b | 62.24 ± 3.59 a | 64.30 ± 4.58 a | 45.29 ± 2.97 c | 52.14 ± 2.44 b | <0.001 |
| Acetate acid (g/kg DM) | 4.14 ± 0.49 c | 11.43 ± 0.61 b | 12.63 ± 1.46 b | 18.28 ± 1.74 a | 12.66 ± 1.27 b | 12.85 ± 1.77 b | <0.001 |
| Ensiled for 30 d | |||||||
| pH | 4.53 ± 0.18 a | 4.14 ± 0.03 b | 4.07 ± 0.03 b | 4.02 ± 0.02 b | 4.13 ± 0.06 b | 4.12 ± 0.06 b | <0.001 |
| NH3-N (g/kg TN) | 5.05 ± 0.46 e | 13.46 ± 0.84 c | 16.37 ± 0.38 b | 18.32 ± 0.82 a | 12.05 ± 0.63 d | 13.04 ± 0.53 cd | <0.001 |
| Lactic acid (mmol/g) | 14.29 ± 0.55 d | 57.26 ± 1.73 b | 65.13 ± 2.56 a | 68.12 ± 3.08 a | 50.92 ± 2.21 c | 54.53 ± 2.85 bc | <0.001 |
| Acetate acid (g/kg DM) | 8.36 ± 0.81 c | 16.90 ± 1.94 b | 16.19 ± 1.87 b | 20.29 ± 1.03 a | 13.84 ± 0.49 b | 14.76 ± 1.27 b | <0.001 |
| Ensiled for 45 d | |||||||
| pH | 4.40 ± 0.17 a | 4.13 ± 0.02 b | 4.04 ± 0.04 b | 4.02 ± 0.03 b | 4.12 ± 0.09 b | 4.11 ± 0.07 b | 0.002 |
| NH3-N (g/kg TN) | 5.19 ± 0.42 d | 14.99 ± 0.93 c | 18.88 ± 1.09 b | 21.91 ± 1.35 a | 13.09 ± 1.02 c | 13.75 ± 0.60 c | <0.001 |
| Lactic acid (mmol/g) | 22.25 ± 1.50 d | 60.00 ± 1.52 b | 68.12 ± 3.89 a | 70.73 ± 3.71 a | 53.26 ± 2.47 c | 56.64 ± 1.97 bc | <0.001 |
| Acetate acid (g/kg DM) | 9.32 ± 0.53 d | 17.98 ± 1.71 c | 21.39 ± 1.21 b | 24.84 ± 1.38 a | 16.36 ± 1.32 c | 17.72 ± 1.26 c | <0.001 |
| Ensiled for 60 d | |||||||
| pH | 4.36 ± 0.09 a | 4.09 ± 0.02 b | 4.02 ± 0.03 b | 3.97 ± 0.01 b | 4.06 ± 0.04 b | 4.03 ± 0.09 b | <0.001 |
| NH3-N (g/kg TN) | 5.54 ± 0.27 e | 15.47 ± 0.57 c | 19.31 ± 0.89 b | 22.85 ± 1.37 a | 13.50 ± 0.63 d | 14.92 ± 0.73 cd | <0.001 |
| Lactic acid (mmol/g) | 27.62 ± 1.84 c | 62.91 ± 1.59 b | 70.63 ± 2.78 a | 71.28 ± 1.61 a | 68.41 ± 2.55 a | 71.62 ± 2.43 a | <0.001 |
| Acetate acid (g/kg DM) | 10.11 ± 0.76 d | 20.27 ± 1.75 b | 23.04 ± 1.64 b | 27.18 ± 1.55 a | 19.79 ± 1.51 b | 20.77 ± 1.25 b | <0.001 |
| Item | 100:0 | 90:10 | 80:20 | 70:30 | 60:40 | 50:50 1 | p-Value 2 |
|---|---|---|---|---|---|---|---|
| Ensiled for 7 d | |||||||
| DM 3 | 66.39 ± 1.62 a | 61.87 ± 0.79 b | 57.37 ± 1.80 c | 52.37 ± 1.89 d | 47.93 ± 1.43 e | 43.27 ± 1.58 f | <0.001 |
| CP | 14.14 ± 0.08 a | 13.96 ± 0.06 a | 13.56 ± 0.10 b | 13.36 ± 0.11 c | 13.01 ± 0.14 d | 12.70 ± 0.13 e | <0.001 |
| NDF | 69.47 ± 0.99 a | 68.30 ± 1.04 ab | 67.97 ± 1.07 ab | 65.18 ± 0.94 b | 66.29 ± 2.15 ab | 65.43 ± 1.72 b | 0.014 |
| ADF | 50.20 ± 1.82 a | 48.88 ± 1.29 ab | 47.04 ± 1.24 b | 43.19 ± 0.74 c | 44.16 ± 1.35 c | 42.82 ± 1.32 c | <0.001 |
| Ensiled for 14 d | |||||||
| DM | 65.52 ± 0.91 a | 60.13 ± 1.34 b | 55.88 ± 1.46 c | 50.23 ± 1.28 d | 46.14 ± 1.26 e | 42.50 ± 1.48 f | <0.001 |
| CP | 14.09 ± 0.09 a | 13.90 ± 0.06 b | 13.49 ± 0.16 c | 13.32 ± 0.10 c | 12.93 ± 0.13 d | 12.64 ± 0.06 e | <0.001 |
| NDF | 68.96 ± 1.79 a | 67.52 ± 1.24 ab | 66.58 ± 0.83 ab | 64.81 ± 0.30 b | 65.83 ± 1.49 b | 65.19 ± 1.32 b | 0.014 |
| ADF | 49.03 ± 1.57 a | 47.06 ± 0.53 ab | 45.92 ± 2.71 ab | 41.06 ± 0.88 c | 43.67 ± 1.42 bc | 41.19 ± 1.84 c | <0.001 |
| Ensiled for 30 d | |||||||
| DM | 63.82 ± 0.93 a | 58.97 ± 0.71 b | 53.90 ± 1.74 c | 48.36 ± 0.99 d | 44.86 ± 1.95 e | 40.40 ± 1.14 f | <0.001 |
| CP | 14.01 ± 0.08 a | 13.85 ± 0.07 ab | 13.43 ± 0.16 abc | 13.29 ± 0.09 abc | 12.88 ± 0.10 bc | 12.59 ± 0.08 c | 0.010 |
| NDF | 67.45 ± 2.50 a | 66.31 ± 1.89 ab | 65.46 ± 1.54 ab | 62.72 ± 0.36 b | 64.78 ± 0.98 ab | 63.02 ± 0.82 b | 0.016 |
| ADF | 47.61 ± 1.05 a | 45.71 ± 1.46 ab | 44.28 ± 0.42 bc | 40.26 ± 0.91 d | 42.16 ± 2.38 cd | 40.39 ± 1.06 d | <0.001 |
| Ensiled for 45 d | |||||||
| DM | 62.75 ± 1.00 a | 57.21 ± 1.23 b | 52.88 ± 2.14 c | 46.29 ± 1.69 d | 43.18 ± 1.09 e | 39.12 ± 1.34 f | <0.001 |
| CP | 13.95 ± 0.07 a | 13.79 ± 0.05 b | 13.38 ± 0.08 c | 13.24 ± 0.10 c | 12.83 ± 0.08 d | 12.54 ± 0.11 e | <0.001 |
| NDF | 66.99 ± 0.16 a | 65.90 ± 1.91 ab | 65.14 ± 0.36 b | 62.18 ± 0.30 c | 64.16 ± 0.32 b | 62.61 ± 0.09 c | <0.001 |
| ADF | 46.46 ± 1.08 a | 44.37 ± 0.64 b | 43.19 ± 1.27 bc | 39.33 ± 0.70 d | 41.59 ± 1.04 c | 39.14 ± 0.76 d | <0.001 |
| Ensiled for 60 d | |||||||
| DM | 62.04 ± 1.06 a | 56.69 ± 0.70 b | 52.07 ± 1.28 c | 45.74 ± 1.44 d | 42.21 ± 1.46 e | 38.06 ± 2.25 f | <0.001 |
| CP | 13.91 ± 0.06 a | 13.73 ± 0.02 b | 13.35 ± 0.07 c | 13.21 ± 0.07 d | 12.79 ± 0.13 e | 12.50 ± 0.05 f | <0.001 |
| NDF | 66.78 ± 0.74 a | 65.36 ± 0.69 ab | 65.02 ± 0.47 ab | 61.16 ± 1.32 d | 63.60 ± 1.67 bc | 61.72 ± 1.18 cd | <0.001 |
| ADF | 46.18 ± 0.28 a | 43.93 ± 1.92 b | 43.01 ± 0.52 b | 38.86 ± 0.73 c | 40.73 ± 1.42 c | 38.65 ± 1.33 c | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Wang, K.; Xiong, B.; Xue, F.; Kang, Y.; Liu, S.; Yang, L. Assessing the Fermentation Quality, Bacterial Composition and Ruminal Degradability of Caragana korshinskii Ensiled with Oat Grass. Fermentation 2025, 11, 420. https://doi.org/10.3390/fermentation11070420
Shen Y, Wang K, Xiong B, Xue F, Kang Y, Liu S, Yang L. Assessing the Fermentation Quality, Bacterial Composition and Ruminal Degradability of Caragana korshinskii Ensiled with Oat Grass. Fermentation. 2025; 11(7):420. https://doi.org/10.3390/fermentation11070420
Chicago/Turabian StyleShen, Yao, Kun Wang, Benhai Xiong, Fuguang Xue, Yajie Kang, Shichao Liu, and Liang Yang. 2025. "Assessing the Fermentation Quality, Bacterial Composition and Ruminal Degradability of Caragana korshinskii Ensiled with Oat Grass" Fermentation 11, no. 7: 420. https://doi.org/10.3390/fermentation11070420
APA StyleShen, Y., Wang, K., Xiong, B., Xue, F., Kang, Y., Liu, S., & Yang, L. (2025). Assessing the Fermentation Quality, Bacterial Composition and Ruminal Degradability of Caragana korshinskii Ensiled with Oat Grass. Fermentation, 11(7), 420. https://doi.org/10.3390/fermentation11070420






