Assisted Isolation of Camelliagenin B from Camellia oliefera Seed Cake Meal and Microbial Transformation by Bacillus subtilis ATCC 6633, Bacillus megaterium CGMCC 1.1741, and Streptomyces gresius ATCC 13273
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
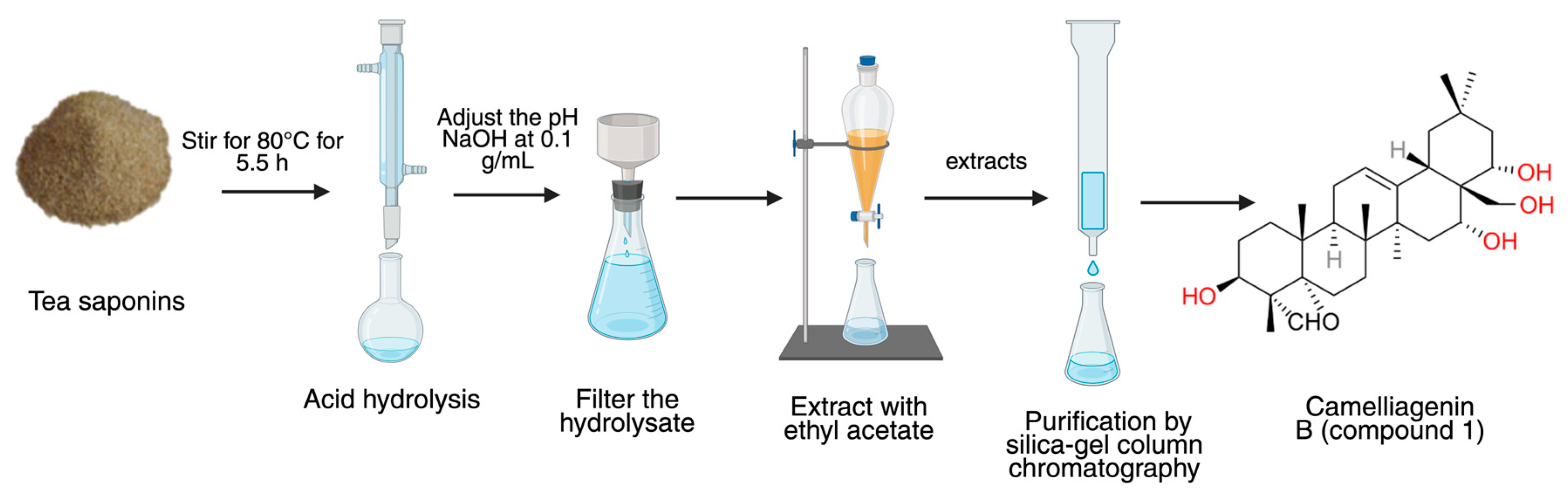
2.2. Preparation, Separation, and Purification of the Camelliagenin B (1)
2.3. Biotransformation of Camelliagenin B (1)
3. Results and Discussion
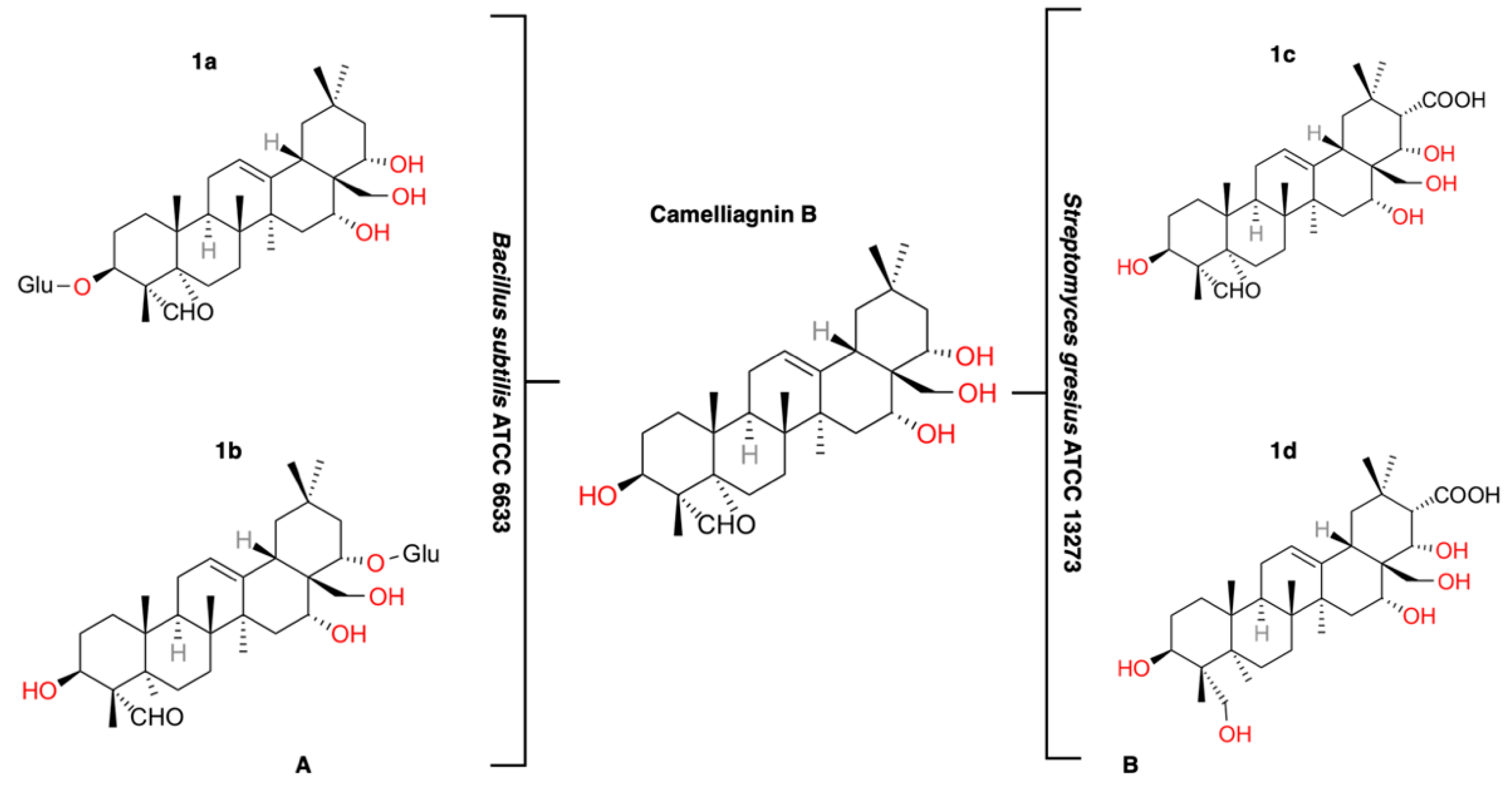
3.1. Identification of Compounds Biotransformed by B. Subtilis ATCC 6633
3.2. Identification of Compounds Biotransformed by S. Gresius ATCC 13273
3.3. Identification of Compounds Biotransformed by B. Megaterium CGMCC 1.1741
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, P.; Shen, J.; Wei, J.; Chen, Y. A Critical Review of the Bioactive Ingredients and Biological Functions of Camellia Oleifera Oil. Curr. Res. Food Sci. 2024, 8, 100753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Han, Y.Y.; Bao, G.H.; Ling, T.J.; Zhang, L.; Gao, L.P.; Xia, T. A New Saponin from Tea Seed Pomace (Camellia Oleifera Abel) and Its Protective Effect on PC12 Cells. Molecules 2012, 17, 11721–11728. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huo, R.; Cui, C.; Gao, Q.; Zong, J.; Wang, Y.; Sun, Y.; Hou, R. Anticancer Activity and Mechanism of Total Saponins from the Residual Seed Cake of: Camellia Oleifera Abel. in Hepatoma-22 Tumor-Bearing Mice. Food Funct. 2019, 10, 2480–2490. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Nie, S.P.; Huang, D.F.; Li, C.; Xie, M.Y.; Wan, Y. Antimicrobial Activity of Saponin-Rich Fraction from Camellia Oleifera Cake and Its Effect on Cell Viability of Mouse Macrophage RAW 264.7. J. Sci. Food Agric. 2012, 92, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yang, Q.; Fang, F.; Li, Y. The Camelliagenin from Defatted Seeds of Camellia Oleifera as Antibiotic Substitute to Treat Chicken against Infection of Escherichia Coli and Staphylococcus Aureus. BMC Vet. Res. 2015, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Sawada, N.; Murakami, T. The Structures of Camelliagenin A, B, and C Obtained from Camellia japonica L. Tetrahedron Lett. 1967, 8, 597–601. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Q.; Hua, X.; Yang, R. Highly Efficient Isolation and Purification of High-Purity Tea Saponins from Industrial Camellia Oil Production by Porous Polymeric Adsorbents. J. Sci. Food Agric. 2023, 103, 7006–7020. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.L.; He, Y. Tea Saponins: Effective Natural Surfactants Beneficial for Soil Remediation, from Preparation to Application. RSC Adv. 2018, 8, 24312–24321. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Karima, G.; Khan, M.Z.; Shin, J.H.; Kim, J.D. Therapeutic Effects of Saponins for the Prevention and Treatment of Cancer by Ameliorating Inflammation and Angiogenesis and Inducing Antioxidant and Apoptotic Effects in Human Cells. Int. J. Mol. Sci. 2022, 23, 10665. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Garcia-Perez, P.; Cassani, L.; Chamorro, F.; Cao, H.; Barba, F.J.; Simal-Gandara, J.; Prieto, M.A. Camellia japonica: A Phytochemical Perspective and Current Applications Facing Its Industrial Exploitation. Food Chem. X 2022, 13, 100258. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Ghosh, A.; Bhattacharya, M. Natural Anti-Inflammatory Terpenoids in Camellia japonica Leaf and Probable Biosynthesis Pathways of the Metabolome. Bull. Natl. Res. Cent. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Nakamura, S.; Muraoka, O.; Yoshikawa, M. New Biofunctional Effects of Oleanane-Type Triterpene Saponins. J. Nat. Med. 2023, 77, 644. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.M.; Sousa, C. A Review on the Biological Activity of Camellia Species. Molecules 2021, 26, 2178. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, Z.; Yan, X.; Xie, J.; Yu, Q.; Chen, Y. Extraction Optimization of Tea Saponins from Camellia Oleifera Seed Meal with Deep Eutectic Solvents: Composition Identification and Properties Evaluation. Food Chem. 2023, 427, 136681. [Google Scholar] [CrossRef] [PubMed]
- Pingping, S.; Xuewa, J.; Jingling, Z.; Jiayi, W.; Richa, R.; Guolong, L.I.; Haixia, G.E.; Weiwei, W.; Boyang, Y.U.; Jian, Z.; et al. Isolation and Microbial Transformation of Tea Sapogenin from Seed Pomace of Camellia oleifera with Anti-Inflammatory Effects. Chin. J. Nat. Med. 2024, 22, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive Phenolic Compounds: Production and Extraction by Solid-State Fermentation. A Review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Na, C.S.; Yoo, S.A.; Seo, S.H.; Son, H.S. Biotransformation of Major Ginsenosides in Ginsenoside Model Culture by Lactic Acid Bacteria. J. Ginseng Res. 2017, 41, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Szczygiełda, M.; Prochaska, K. Downstream Separation and Purification of Bio-Based Alpha-Ketoglutaric Acid from Post-Fermentation Broth Using a Multi-Stage Membrane Process. Process Biochem. 2020, 96, 38–48. [Google Scholar] [CrossRef]
- Qu, X.; Raza, S.H.A.; Zhao, Y.; Deng, J.; Ma, J.; Wang, J.; Alkhorayef, N.; Alkhalil, S.S.; Pant, S.D.; Lei, H.; et al. Effect of Tea Saponins on Rumen Microbiota and Rumen Function in Qinchuan Beef Cattle. Microorganisms 2023, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Yin, L.; Yao, X.; Zhong, Y.; Gui, J.; Lu, F.; Zhang, F.; Zhang, J. Effects of Fermentation on the Hemolytic Activity and Degradation of Camellia Oleifera Saponins by Lactobacillus Crustorum and Bacillus Subtilis. FEMS Microbiol. Lett. 2018, 365, fny014. [Google Scholar] [CrossRef] [PubMed]
- Exploiting the Aglycon Promiscuity of Glycosyltransferase Bs-YjiC from Bacillus Subtilis and Its Application in Synthesis of Glycosides. Available online: https://www.researchgate.net/publication/315322132_Exploiting_the_aglycon_promiscuity_of_glycosyltransferase_Bs-YjiC_from_Bacillus_subtilis_and_its_application_in_synthesis_of_glycosides (accessed on 12 June 2025).
- Shin, K.-C.; Oh, D.-K. Biotransformation of Platycosides, Saponins from Balloon Flower Root, into Bioactive Deglycosylated Platycosides. Antioxidants 2023, 12, 327. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Lv, M.; Hu, J.; Huang, K.; Xu, H. Glycosylation and Activities of Natural Products. Mini Rev. Med. Chem. 2016, 16, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Ding, H.-Y.; Wang, T.-Y.; Zhang, Y.-R.; Chang, T.-S. Glycosylation of Ganoderic Acid G by Bacillus Glycosyltransferases. Int. J. Mol. Sci. 2021, 22, 9744. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Qumsani, A.T. The Contribution of Microorganisms to Sustainable Development: Towards a Green Future through Synthetic Biology and Systems Biology. J. Umm Al-Qura Univ. Appl. Sci. 2024, 2, 1–17. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Mohamed, T.A.; ElShamy, A.I.; Mohamed, A.-E.-H.H.; Mahalel, U.A.; Reda, E.H.; Shaheen, A.M.; Tawfik, W.A.; Shahat, A.A.; Shams, K.A.; et al. Microbial Biotransformation as a Tool for Drug Development Based on Natural Products from Mevalonic Acid Pathway: A Review. J. Adv. Res. 2015, 6, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Gao, L.; Sun, H.; Zhao, X.-Y.; Gao, Z.; Liu, J.; Guo, W. Advancements in Enzymatic Reaction-Mediated Microbial Transformation. Heliyon 2024, 10, e38187. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.F.; Carneiro, C.N.; de Sousa, C.B.D.C.; Gomez, F.J.V.; Espino, M.; Boiteux, J.; de los Fernández, M.A.; Silva, M.F.; de Dias, F.S. Sustainable Extraction Bioactive Compounds Procedures in Medicinal Plants Based on the Principles of Green Analytical Chemistry: A Review. Microchem. J. 2022, 175, 107184. [Google Scholar] [CrossRef]
- Shen, P.; Wang, W.; Xu, S.; Du, Z.; Wang, W.; Yu, B.; Zhang, J. Biotransformation of Erythrodiol for New Food Supplements with Anti-Inflammatory Properties. J. Agric. Food Chem. 2020, 68, 5910–5916. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Shen, P.; Zhou, J.; Ge, H.; Raj, R.; Wang, W.; Yu, B.; Zhang, J. Microbial Transformation and Inhibitory Effect Assessment of Uvaol Derivates against LPS and HMGB1 Induced NO Production in RAW264.7 Macrophages. Bioorganic Med. Chem. Lett. 2022, 58, 128523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shen, P.; Wang, W.; Zhou, J.; Raj, R.; Du, Z.; Xu, S.; Wang, W.; Yu, B.; Zhang, J. Derivatization of Soyasapogenol A through Microbial Transformation for Potential Anti-Inflammatory Food Supplements. J. Agric. Food Chem. 2021, 69, 6791–6798. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Zhou, J.; Jiang, X.; Ge, H.; Wang, W.; Yu, B.; Zhang, J. Microbial-Catalyzed Baeyer-Villiger Oxidation for 3,4-Seco-Triterpenoids as Potential HMGB1 Inhibitors. ACS Omega 2022, 7, 18745–18751. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shen, P.; Zhou, X.; Fei, Y.; Wang, W.; Raj, R.; Du, Z.; Ge, H.; Wang, W.; Xu, S.; et al. A Versatile Tailoring Tool for Pentacyclic Triterpenes of Penicillium Griseofulvum CICC 40293. Phytochem. Lett. 2021, 44, 195–201. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, J.; Zhu, Y.; Wang, W.; Yu, B.; Wang, W. Microbial Transformation of Glycyrrhetinic Acid Derivatives by Bacillus Subtilis ATCC 6633 and Bacillus Megaterium CGMCC 1.1741. Bioorganic Med. Chem. 2020, 28, 115465. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Zong, J.; Sun, Y.; Zhang, L.; Ho, C.-T.; Wan, X.; Hou, R. Triterpenoid Saponins from the Genus Camellia: Structures, Biological Activities, and Molecular Simulation for Structure-Activity Relationship. Food Funct. Rev. 2018, 9, 3069–3091. [Google Scholar] [CrossRef] [PubMed]
- Quang, T.H.; Ngan, N.T.T.; Minh, C.V.; Kiem, P.V.; Nhiem, N.X.; Tai, B.H.; Thao, N.P.; Tung, N.H.; Song, S.B.; Kim, Y.H. Anti-Inflammatory Triterpenoid Saponins from the Stem Bark of Kalopanax pictus. J. Nat. Prod. 2011, 74, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tong, Z.; Wang, C.; Li, X.; Liang, G. Oleanolic Acid Exerts Neuroprotective Effects in Subarachnoid Hemorrhage Rats through SIRT1-Mediated HMGB1 Deacetylation. Eur. J. Pharmacol. 2021, 893, 173811. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, P.; Jiang, X.; Zhu, Y.; Ye, J.; Raj, R.; Xu, S.; Wang, W.; Yu, B.; Zhang, J. Microbial Transformation of Maslinic Acid for Potential Food Supplements against Sterile Inflammation. ACS Food Sci. Technol. 2023, 3, 808–815. [Google Scholar] [CrossRef]
- Jatczak, K.; Grynkiewicz, G. Triterpene Sapogenins with Oleanene Skeleton: Chemotypes and Biological Activities. Acta Biochim. Pol. 2014, 61, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Ko, J.; Kim, E.; Kim, J.H.; Park, J.G.; Sung, N.Y.; Kim, H.G.; Yang, S.; Rho, H.S.; Hong, Y.D.; et al. 21-O-Angeloyltheasapogenol E3, a Novel Triterpenoid Saponin from the Seeds of Tea Plants, Inhibits Macrophage-Mediated Inflammatory Responses in a NF-κB-Dependent Manner. Mediat. Inflamm. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, I.; Hori, K.; Motozawa, T.; Murakami, T.; Yoshikawa, M. Structures of New Acylated Oleanene-Type Triterpene Oligoglycosides, Theasaponins E1 and E2, from the Seeds of Tea Plant, Camellia sinensis (L.) O. Kuntze. Chem. Pharm. Bull. 1998, 46, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, M.R.; Mitaine-Offer, A.C.; Miyamoto, T.; Tanaka, C.; Pouységu, L.; Quideau, S.; Porcar, C.R.; Lacaille-Dubois, M.A. Oleanane-Type Glycosides from Pittosporum tenuifolium “Variegatum” and P. Tenuifolium “Gold Star”. Phytochemistry 2017, 140, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Liu, G.D.; Zhang, B.B.; Wang, S.S.; Ma, R.; Zhong, B.S.; He, B.Q.; Liang, Y.; Wu, F.H. A New Triterpenoid Saponin from Clinopodium Chinense (Benth.) O. Kuntze. Nat. Prod. Res. 2016, 30, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Bonesi, M.; Deguin, B.; Loizzo, M.R.; Menichini, F.; Conforti, F.; Tillequin, F.; Menichini, F. Cytotoxic Activity and Inhibitory Effect on Nitric Oxide Production of Triterpene Saponins from the Roots of Physospermum Verticillatum (Waldst & Kit) (Apiaceae). Bioorganic Med. Chem. 2009, 17, 4542–4547. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Kim, S.G.; Nam, J.H.; Park, K.K.; Chung, W.Y.; Kim, W.B.; Lee, K.T.; Won, J.H.; Choi, J.W.; Park, H.J. Isolation of Saponins with the Inhibitory Effect on Nitric Oxide, Prostaglandin E2 and Tumor Necrosis Factor-Alpha Production from Pleurospermum Kamtschaticum. Biol. Pharm. Bull. 2005, 28, 1668–1671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.D.; Jiang, L.L.; Li, H.Y.; Yan, P.F.; Zhang, Y.L. Chemical Components and Pharmacological Activities of Terpene Natural Products from the Genus Paeonia. Molecules 2016, 21, 1362. [Google Scholar] [CrossRef] [PubMed]
- Kiss, F.M.; Schmitz, D.; Zapp, J.; Dier, T.K.F.; Volmer, D.A.; Bernhardt, R. Comparison of CYP106A1 and CYP106A2 from Bacillus Megaterium - Identification of a Novel 11-Oxidase Activity. Appl. Microbiol. Biotechnol. 2015, 99, 8495–8514. [Google Scholar] [CrossRef] [PubMed]
- Brill, E.; Hannemann, F.; Zapp, J.; Brüning, G.; Jauch, J.; Bernhardt, R. A New Cytochrome P450 System from Bacillus Megaterium DSM319 for the Hydroxylation of 11-Keto-β-Boswellic Acid (KBA). Appl. Microbiol. Biotechnol. 2014, 98, 1701–1717. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.; Gaillet, B.; Garnier, A. Oleic Acid Based Experimental Evolution of Bacillus Megaterium Yielding an Enhanced P450 BM3 Variant. BMC Biotechnol. 2022, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, P.K.; Keshavan, N.; Nguyen, H.Q.; Peterson, J.A.; González, J.E.; Haines, D.C. Bacillus Megaterium CYP102A1 Oxidation of Acyl Homoserine Lactones and Acyl Homoserines. Biochemistry 2007, 46, 14429–14437. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.; Marasini, B.P.; Pradhan, S.P.; Shrestha, R.K.; Shrestha, S.; Regmi, K.P.; Pandey, B.P. Biotransformation of Daidzein, Genistein, and Naringenin by Streptomyces Species Isolated from High-Altitude Soil of Nepal. Int. J. Microbiol. 2021, 2021, 9948738. [Google Scholar] [CrossRef] [PubMed]
- Rimal, H.; Subedi, P.; Kim, K.H.; Park, H.; Lee, J.H.; Oh, T.J. Characterization of CYP125A13, the First Steroid C-27 Monooxygenase from Streptomyces peucetius. J. Microbiol. Biotechnol. 2020, 30, 1750–1759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosłoniec, K.Z.; Wilbrink, M.H.; Capyk, J.K. Cytochrome P450 125 (CYP125) Catalyses C26-Hydroxylation to Initiate Sterol Side-Chain Degradation in Rhodococcus jostii RHA1. Mol. Microbiol. 2009, 74, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Xu, K.; Wang, J.N.; Li, C. Novel Catalytic Glycosylation of Glycyrrhetinic Acid by UDP-Glycosyltransferases from Bacillus Subtilis. Eng. J. 2020, 162, 107723. [Google Scholar] [CrossRef]
- Shibuya, M.; Nishimura, K.; Yasuyama, N.; Ebizuka, Y. Identification and characterization of glycosyltransferases involved in the biosynthesis of soyasaponin I in Glycine max. FEBS Lett. 2010, 584, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Wang, G.Z.; Zhang, H.Y. Sterol Biosynthesis and Prokaryotes-to-Eukaryotes Evolution. Biochem. Biophys. Res. Commun. 2007, 363, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.M.; Hu, D.; Gao, H.; Kushiro, T.; Awakawa, T.; Chen, G.D.; Wang, C.X.; Abe, I.; Yao, X.S. Biosynthesis of Helvolic Acid and Identification of an Unusual C-4-Demethylation Process Distinct from Sterol Biosynthesis. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Banta, A.B.; Wei, J.H.; Kiemle, D.J.; Feng, J.; Giner, J.L.; Welander, P.V. C-4 Sterol Demethylation Enzymes Distinguish Bacterial and Eukaryotic Sterol Synthesis. Proc. Natl. Acad. Sci. USA 2018, 115, 5884–5889. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. In Silico Prediction of Steroids and Triterpenoids as Potential Regulators of Lipid Metabolism. Mar. Drugs 2021, 19, 650. [Google Scholar] [CrossRef] [PubMed]
- Wickramaratne, S.; Ji, S.; Mukherjee, S.; Su, Y.; Pence, M.G.; Lior-Hoffmann, L.; Fu, I.; Broyde, S.; Guengerich, F.P.; Distefano, M.; et al. Bypass of DNA-Protein Cross-Links Conjugated to the 7-Deazaguanine Position of DNA by Translesion Synthesis Polymerases. J. Biol. Chem. 2016, 291, 23589. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, W.; Wang, P.; Li, Y.; Gu, P.; Gao, J. Biotransformation of Baicalin and Glycyrrhizic Acid Using Immobilized Fe3O4@Chitosan@β-Glucuronidase. 3 Biotech 2025, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yin, X.; Kou, C.; Thimmappa, R.; Hua, X.; Xue, Z. Classification, Biosynthesis, and Biological Functions of Triterpene Esters in Plants. Plant Commun. 2024, 5, 100845. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hong, S.; Chen, H.; Yin, Y.; Wang, C. Production of Helvolic Acid in Metarhizium Contributes to Fungal Infection of Insects by Bacteriostatic Inhibition of the Host Cuticular Microbiomes. Microbiol. Spectr. 2022, 10, e0262022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shen, P.; Wang, J.; Jiang, X.; Wang, W.; Raj, R.; Ge, H.; Wang, W.; Yu, B.; Zhang, J. Microbial Transformation of Pentacyclic Triterpenes for Anti-Inflammatory Agents on the HMGB1 Stimulated RAW 264.7 Cells by Streptomyces Olivaceus CICC 23628. Bioorganic Med. Chem. 2021, 52, 116494. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and Functional Diversity of Saponins, Biosynthetic Intermediates and Semi-Synthetic Derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, P.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.-C. Molecular Signaling of Ginsenosides Rb1, Rg1, and Rg3 and Their Mode of Actions. J. Ginseng Res. 2018, 42, 123–132. [Google Scholar] [CrossRef] [PubMed]
- New Approaches to the Structural Modification of Olean-Type Pentacylic Triterpenes via Microbial Oxidation and Glycosylation. Available online: https://www.researchgate.net/publication/251498556_New_approaches_to_the_structural_modification_of_olean-type_pentacylic_triterpenes_via_microbial_oxidation_and_glycosylation (accessed on 19 June 2025).
- Rustamova, N.; Huang, G.; Isokov, M.; Movlanov, J.; Farid, R.; Buston, I.; Xiang, H.; Davranov, K.; Yili, A. Modification of Natural Compounds through Biotransformation Process by Microorganisms and Their Pharmacological Properties. Fitoterapia 2024, 179, 106227. [Google Scholar] [CrossRef] [PubMed]
- Cancellieri, M.C.; Nobbio, C.; Gatti, F.G.; Brenna, E.; Parmeggiani, F. Applications of Biocatalytic CC Bond Reductions in the Synthesis of Flavours and Fragrances. J. Biotechnol. 2024, 390, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Shanu-Wilson, J.; Evans, L.; Wrigley, S.; Steele, J.; Atherton, J.; Boer, J. Biotransformation: Impact and Application of Metabolism in Drug Discovery. ACS Med. Chem. Lett. 2020, 11, 2087–2107. [Google Scholar] [CrossRef] [PubMed]


| Potato Dextrose (PD) | Soybean Meal (SM) | ||
|---|---|---|---|
| Peeled potatoes | 200 g | Glucose | 20 g |
| Glucose | 20 g | Yeast powder | 5 g |
| KH2PO4 | 3 g | NaCl | 5 g |
| MgSO4.7H2O | 1.5 g | K2HPO4 | 5 g |
| Distilled water | 1 L | Soybean meal | 5 g |
| Distilled water | 1 L | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, R.; Zhang, J.; Meng, Y.; Jiang, X.; Wang, W.; Zhang, J.; Yu, B. Assisted Isolation of Camelliagenin B from Camellia oliefera Seed Cake Meal and Microbial Transformation by Bacillus subtilis ATCC 6633, Bacillus megaterium CGMCC 1.1741, and Streptomyces gresius ATCC 13273. Fermentation 2025, 11, 407. https://doi.org/10.3390/fermentation11070407
Raj R, Zhang J, Meng Y, Jiang X, Wang W, Zhang J, Yu B. Assisted Isolation of Camelliagenin B from Camellia oliefera Seed Cake Meal and Microbial Transformation by Bacillus subtilis ATCC 6633, Bacillus megaterium CGMCC 1.1741, and Streptomyces gresius ATCC 13273. Fermentation. 2025; 11(7):407. https://doi.org/10.3390/fermentation11070407
Chicago/Turabian StyleRaj, Richa, Jingling Zhang, Yanyan Meng, Xuewa Jiang, Wei Wang, Jian Zhang, and Boyang Yu. 2025. "Assisted Isolation of Camelliagenin B from Camellia oliefera Seed Cake Meal and Microbial Transformation by Bacillus subtilis ATCC 6633, Bacillus megaterium CGMCC 1.1741, and Streptomyces gresius ATCC 13273" Fermentation 11, no. 7: 407. https://doi.org/10.3390/fermentation11070407
APA StyleRaj, R., Zhang, J., Meng, Y., Jiang, X., Wang, W., Zhang, J., & Yu, B. (2025). Assisted Isolation of Camelliagenin B from Camellia oliefera Seed Cake Meal and Microbial Transformation by Bacillus subtilis ATCC 6633, Bacillus megaterium CGMCC 1.1741, and Streptomyces gresius ATCC 13273. Fermentation, 11(7), 407. https://doi.org/10.3390/fermentation11070407





