Characterization and Multi-Omics Basis of Biofilm Formation by Lactiplantibacillus plantarum
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Culture Conditions, and Biofilm Formation
2.2. Crystal Violet Assay
2.3. Scanning Electron Microscopy Analysis
2.4. Confocal Laser Scanning Microscopy Analysis
2.5. Cell Viability Assessment During Environmental Stresses
2.6. Transcriptomic and Proteomic Analyses
2.7. Statistical Analysis
3. Results
3.1. Biofilm Formation by L. plantarum
3.2. Scanning Electron Microscopy Images of the Biofilm Matrix
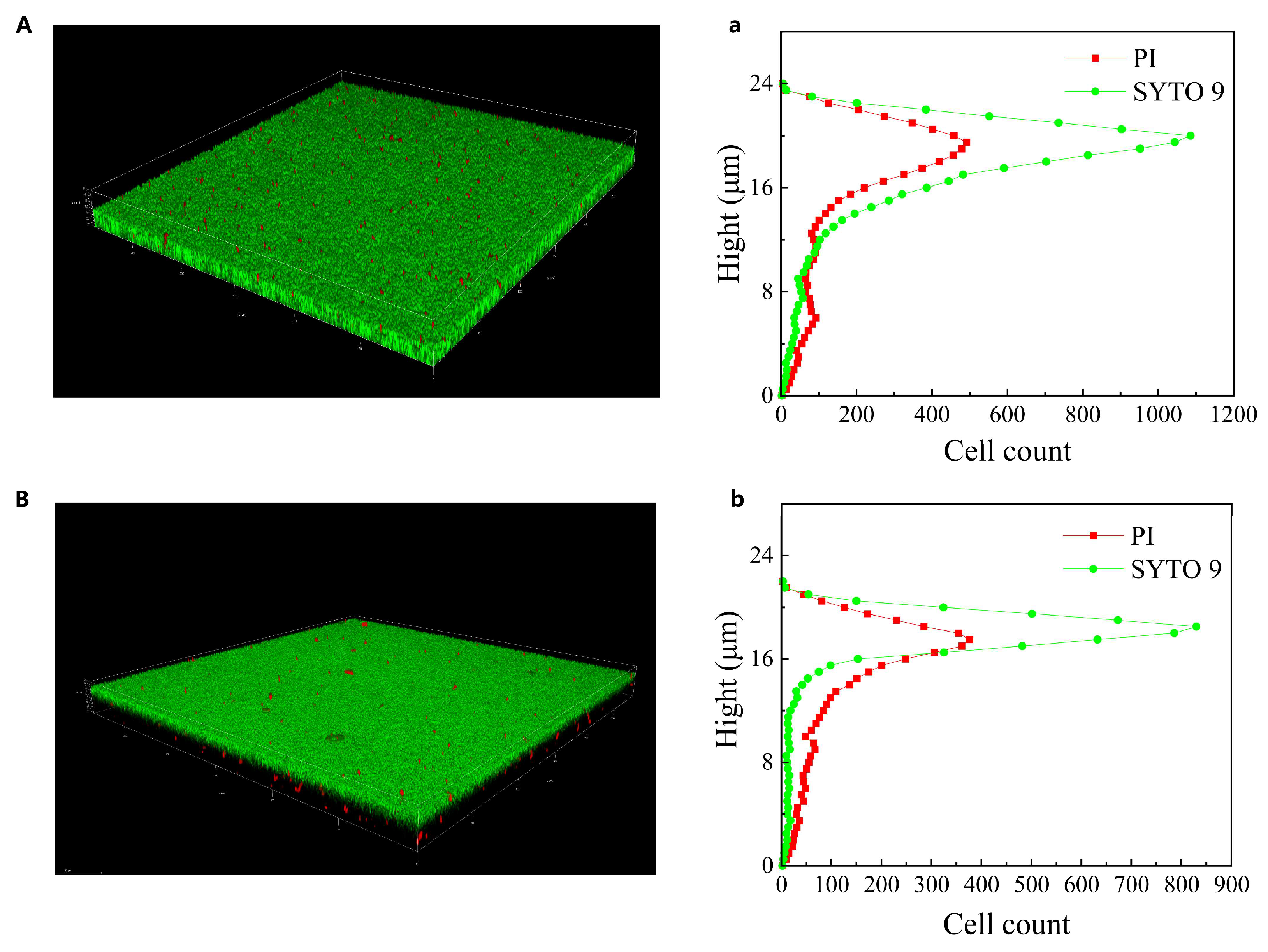
3.3. Three-Dimensional Architecture and Cell Distribution of L. plantarum Biofilm
3.4. Effect of Biofilm Formation on Cell Viability Under Environmental Stress
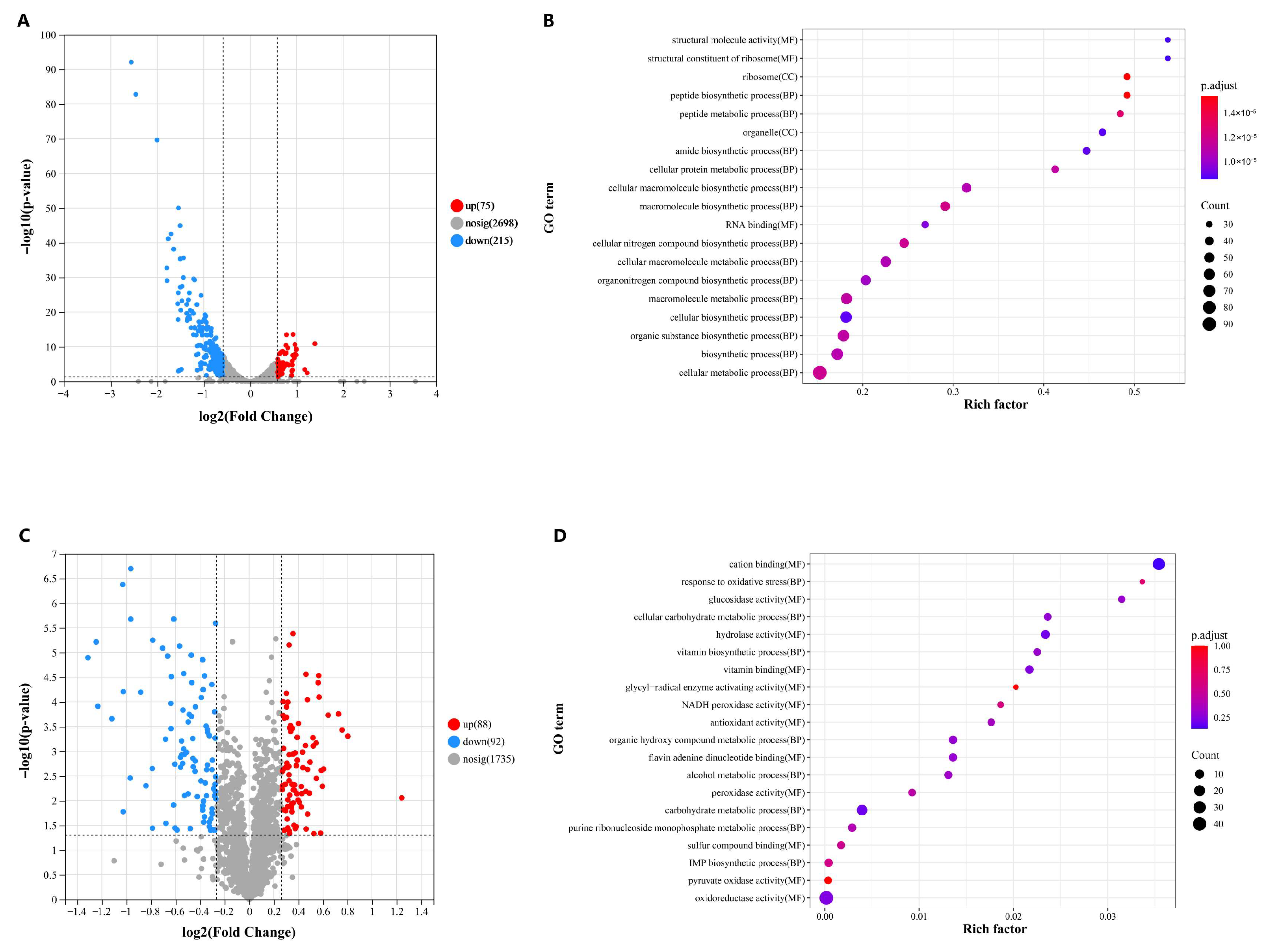
3.5. Transcriptomic and Proteomic Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brück, H.L.; Delvigne, F.; Dhulster, P.; Jacques, P.; Coutte, F. Molecular strategies for adapting 168 biosurfactant production to biofilm cultivation mode. Bioresour. Technol. 2019, 293, 122090. [Google Scholar] [CrossRef] [PubMed]
- Merino, L.; Procura, F.; Trejo, F.M.; Bueno, D.J.; Golowczyc, M.A. Biofilm formation by Salmonella sp. in the poultry industry, Detection, control and eradication strategies. Food Res. Int. 2019, 119, 530–540. [Google Scholar]
- Hu, X.W.; Chen, K.; Lai, X.K.; Ji, S.P.; Kaiser, K. Effects of Fe (III) on biofilm and its extracellular polymeric substances (EPS) in fixed bed biofilm reactors. Water Sci. Technol. 2016, 73, 2060–2066. [Google Scholar] [CrossRef]
- Da Silva, D.P.; Schofield, M.C.; Parsek, M.R.; Tseng, B.S. An update on the sociomicrobiology of quorum sensing in gram-negative biofilm development. Pathogens 2017, 6, 51. [Google Scholar] [CrossRef]
- Tseng, B.S.; Majerczyk, C.D.; da Silva, D.P.; Chandler, J.R.; Greenberg, E.P.; Parsek, M.R. Quorum sensinginfluences biofilm development and matrix production. J. Bacteriol. 2016, 198, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadidi, A.; Navarro, J.; Goodman, S.D.; Bailey, M.T.; Besner, G.E. Lactobacillus reuteri in its biofilm state improves protection from experimental necrotizing enterocolitis. Nutrients 2021, 13, 918. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S. Studies on formation, control and application of biofilm formed by food related microorganisms. Biosci. Biotechnol. Biochem. 2015, 79, 1050–1056. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Nguyen, T.T.; Nguyen, T.T.U.; Hoang, Q.K.; Nguyen, H.T. Improve the viability and extracellular polymeric substances bioactivity of VAL6 using the environmental adaptation. Food Bioprod. Process. 2022, 131, 149–155. [Google Scholar] [CrossRef]
- Berlanga, M.; Guerrero, R. Living together in biofilms: The microbial cell factory and its biotechnological implications. Microb. Cell Factories 2016, 15, 165. [Google Scholar] [CrossRef]
- Assaf, J.C.; El Khoury, A.; Chokr, A.; Louka, N.; Atoui, A. A novel method for elimination of aflatoxin M1 in milk using GG biofilm. Int. J. Dairy Technol. 2019, 72, 248–256. [Google Scholar] [CrossRef]
- Pannella, G.; Lombardi, S.J.; Coppola, F.; Vergalito, F.; Iorizzo, M.; Succi, M.; Tremonte, P.; Iannini, C.; Sorrentino, E.; Coppola, R. Effect of biofilm formation by on the malolactic fermentation in model wine. Foods 2020, 9, 797. [Google Scholar] [CrossRef]
- Hu, M.X.; Li, J.N.; Guo, Q.; Zhu, Y.Q.; Niu, H.M. Probiotics biofilm-integrated electrospun nanofiber membranes: A new starter culture for fermented milk production. J. Agric. Food Chem. 2019, 67, 3198–3208. [Google Scholar] [CrossRef]
- Shokri, D.; Khorasgani, M.R.; Mohkam, M.; Fatemi, S.M.; Ghasemi, Y.; Taheri-Kafrani, A. The inhibition effect of Lactobacilli against growth and biofilm formation of Pseudomonas aeruginosa. Probiotics Antimicrob. Proteins 2018, 10, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.D.F.; Smid, E.J.; Abee, T.; Groot, M.N.N. Characterisation of biofilms formed by WCFS1 and food spoilage isolates. Int. J. Food Microbiol. 2015, 207, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Speranza, B.; Sinigaglia, M.; Corbo, M.R. Non starter lactic acid bacteria biofilms: A means to control the growth of Listeria monocytogenes in soft cheese. Food Control 2009, 20, 1063–1067. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Yao, S.J.; Hao, L.Y.; Zhou, R.Q.; Jin, Y.; Huang, J.; Wu, C.D. Formation of biofilm by Tetragenococcus halophilus benefited stress tolerance and anti-biofilm activity against S. aureus and S. Typhimurium. Front. Microbiol. 2022, 13, 819302. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zhang, L.; Xu, Y. Effects of Tetragenococcus halophilus and Candida versatilis on the production of aroma-active and umami-taste compounds during soy sauce fermentation. J. Sci. Food Agric. 2020, 100, 2782–2790. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, L.; Li, J.S.; Jin, Y.; Zou, J.P.; Huang, J.; Zhou, R.Q.; Huang, M.Q.; Wu, C.D. Cell surface properties and transcriptomic analysis of cross protection provided between heat adaptation and acid stress in Tetragenococcus halophilus. Food Res. Int. 2021, 140, 110005. [Google Scholar] [CrossRef]
- Desai, S.; Sanghrajka, K.; Gajjar, D. High adhesion and increased cell death contribute to strong biofilm formation in Klebsiella pneumoniae. Pathogens 2019, 8, 277. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Liu, L.C.; Chen, L.; Teng, J.; Zhu, X.P.; Zhao, J.M.; Wang, Q. Spatial and seasonal variations in biofilm formation on microplastics in coastal waters. Sci. Total Environ. 2021, 770, 145303. [Google Scholar] [CrossRef] [PubMed]
- Terraf, M.C.L.; Juárez, T.M.S.; Nader-Macías, M.E.F.; Silva, C. Screening of biofilm formation by beneficial vaginal lactobacilli and influence of culture media components. J. Appl. Microbiol. 2012, 113, 1517–1529. [Google Scholar] [CrossRef]
- Lin, Q.R.; Liu, M.W.; Ni, H.; Hao, Y.; Yu, Y.Q.; Chen, Y.R.; Wu, Q.; Shen, Y.; Zhang, L.; Lyu, M.; et al. High-degree polymerizate IMOs of dextranase hydrolysates enhance Lactobacillus acid metabolism: Based on growth, and metabolomic and transcriptomic analyses. LWT 2023, 187, 115345. [Google Scholar] [CrossRef]
- Slížová, M.; Nemcová, R.; Mad’ar, M.; Jana, H.; Soňa, G.; Miroslav, P.; Juraj, P. Analysis of biofilm formation by intestinal lactobacilli. Can. J. Microbiol. 2015, 61, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Chen, H.Z.; Liao, Q.; Nizami, A.; Xia, A.; Huang, Y.; Zhu, X.Q.; Zhu, X. Effects of operational parameters on biofilm formation of mixed bacteria for hydrogen fermentation. Sustainability 2020, 12, 8863. [Google Scholar] [CrossRef]
- Yang, C.; Huang, B.H.; Lin, J.H.; Yang, Q.J.; Guo, Y.Q.; Liu, D.W.; Sun, B.L. Isolation and screening of high biofilm producing lactic acid bacteria, and exploration of its effects on the microbial hazard in corn straw silage. J. Hazard. Mater. 2024, 480, 136009. [Google Scholar] [CrossRef]
- Muruzovic, M.Z.; Mladenovic, K.G.; Comic, L.R. In vitro evaluation of resistance to environmental stress by planktonic and biofilm form of lactic acid bacteria isolated from traditionally made cheese from Serbia. Food Biosci. 2018, 23, 54–59. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Geesey, G.G.; Wigglesworth-Cooksey, B.; Cooksey, K.E. Influence of calcium and other cations on surface adhesion of bacteria and diatoms: A review. Biofouling 2000, 15, 195–205. [Google Scholar] [CrossRef]
- Heistad, A.; Scott, T.; Skaarer, A.M.; Seidu, R.; Hanssen, J.F.; Stenström, T.A. Virus removal by unsaturated wastewater filtration: Effects of biofilm accumulation and hydrophobicity. Water Sci. Technol. 2009, 60, 399–407. [Google Scholar] [CrossRef]
- Volle, C.B.; Ferguson, M.A.; Aidala, K.E.; Spain, E.M.; Núñez, M.E. Spring constants and adhesive properties of native bacterial biofilm cells measured by atomic force microscopy. Colloids Surf. B-Biointerfaces 2008, 67, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Senda, S.; Nomura, N.; Tokuda, H.; Uchiyama, H. Biofilm formation by lactic acid bacteria and resistance to environmental stress. J. Biosci. Bioeng. 2008, 106, 381–386. [Google Scholar] [CrossRef]
- Rezaei, Z.; Khanzadi, S.; Salari, A. A survey on biofilm formation of Lactobacillus rhamnosus (PTCC 1637) and Lactobacillus plantarum (PTCC 1745) as a survival strategy of probiotics against antibiotic in vitro and yogurt. J. Food Process. Preserv. 2022, 46, e15991. [Google Scholar] [CrossRef]
- Wang, M.M.; Liu, Q.H.; Kang, X.X.; Zhu, Z.B.; Yang, H.; Xi, X.Y.; Zhang, X.; Du, Y.; Guo, M.Z.; Tang, D.Q.; et al. Glycogen metabolism impairment via single gene mutation in the glgBXCAP operon alters the survival rate of Escherichia coli under various environmental stresses. Front. Microbiol. 2020, 11, 588099. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef]
- Booth, S.C.; Workentine, M.L.; Wen, J.; Shaykhutdinov, R.; Vogel, H.J.; Ceri, H.; Turner, R.J.; Weljie, A.M. Differences in metabolism between the biofilm and planktonic response to metal stress. J. Proteome Res. 2011, 10, 3190–3199. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, K.; Liu, Q.; Diao, X.; Ma, W.; Liu, G. Transcriptomics analysis of the mechanism behind Lactobacillus rhamnosus Gr18 biofilm formation in an Mn2+-deficient environment. LWT 2024, 200, 116163. [Google Scholar] [CrossRef]
- Xu, H.; Wu, L.; Pan, D.; Zeng, X.; Cai, Z.; Guo, Y.; Wang, W.; Wu, Z. Adhesion characteristics and dual transcriptomic and proteomic analysis of Lactobacillus reuteri SH23 upon gastrointestinal fluid stress. J. Proteome Res. 2021, 20, 2447–2457. [Google Scholar] [CrossRef]
- Mikkelsen, H.; Sivaneson, M.; Filloux, A. Key two-component regulatory systems that control biofilm formation in Pseudomonas aeruginosa. Environ. Microbiol. 2011, 13, 1666–1681. [Google Scholar] [CrossRef]
- Pöntinen, A.; Lindström, M.; Skurnik, M.; Korkeala, H. Screening of the two-component-system histidine kinases of Listeria monocytogenes EGD-e. LiaS is needed for growth under heat, acid, alkali, osmotic, ethanol and oxidative stresses. Food Microbiol. 2017, 65, 36–43. [Google Scholar] [CrossRef]
- Zhang, F.X.; Gao, J.Y.; Wang, B.N.; Huo, D.X.; Wang, Z.X.; Zhang, J.C.; Shao, Y.Y. Whole-genome sequencing reveals the mechanisms for evolution of streptomycin resistance in Lactobacillus plantarum. J. Dairy Sci. 2018, 101, 2867–2874. [Google Scholar] [CrossRef] [PubMed]
- Heptinstall, S.; Archibald, A.R.; Baddiley, J. Teichoic acids and membrane function in bacteria. Nature 1970, 225, 519. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.J.; Zhou, R.Q.; Jin, Y.; Huang, J.; Qin, J.F.; Wu, C.D. Formation of biofilm changed the responses of Tetragenococcus halophilus to ethanol stress revealed by transcriptomic and proteomic analyses. Food Res. Int. 2022, 161, 111817. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.L.; Zhao, J.W.; Yan, J.L.; Liu, S.L.; Zhao, K. Comparative transcriptomic analysis of Lactiplantibacillus plantarum RS66CD biofilm in high-salt conditions and planktonic cells. Peerj 2020, 8, e9639. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Zhao, D.; Zhou, R.; Zheng, J.; Wu, C. Characterization and Multi-Omics Basis of Biofilm Formation by Lactiplantibacillus plantarum. Fermentation 2025, 11, 400. https://doi.org/10.3390/fermentation11070400
Ma R, Zhao D, Zhou R, Zheng J, Wu C. Characterization and Multi-Omics Basis of Biofilm Formation by Lactiplantibacillus plantarum. Fermentation. 2025; 11(7):400. https://doi.org/10.3390/fermentation11070400
Chicago/Turabian StyleMa, Ruitang, Dong Zhao, Rongqing Zhou, Jia Zheng, and Chongde Wu. 2025. "Characterization and Multi-Omics Basis of Biofilm Formation by Lactiplantibacillus plantarum" Fermentation 11, no. 7: 400. https://doi.org/10.3390/fermentation11070400
APA StyleMa, R., Zhao, D., Zhou, R., Zheng, J., & Wu, C. (2025). Characterization and Multi-Omics Basis of Biofilm Formation by Lactiplantibacillus plantarum. Fermentation, 11(7), 400. https://doi.org/10.3390/fermentation11070400






