2. Understanding Extracellular Electron Transfer
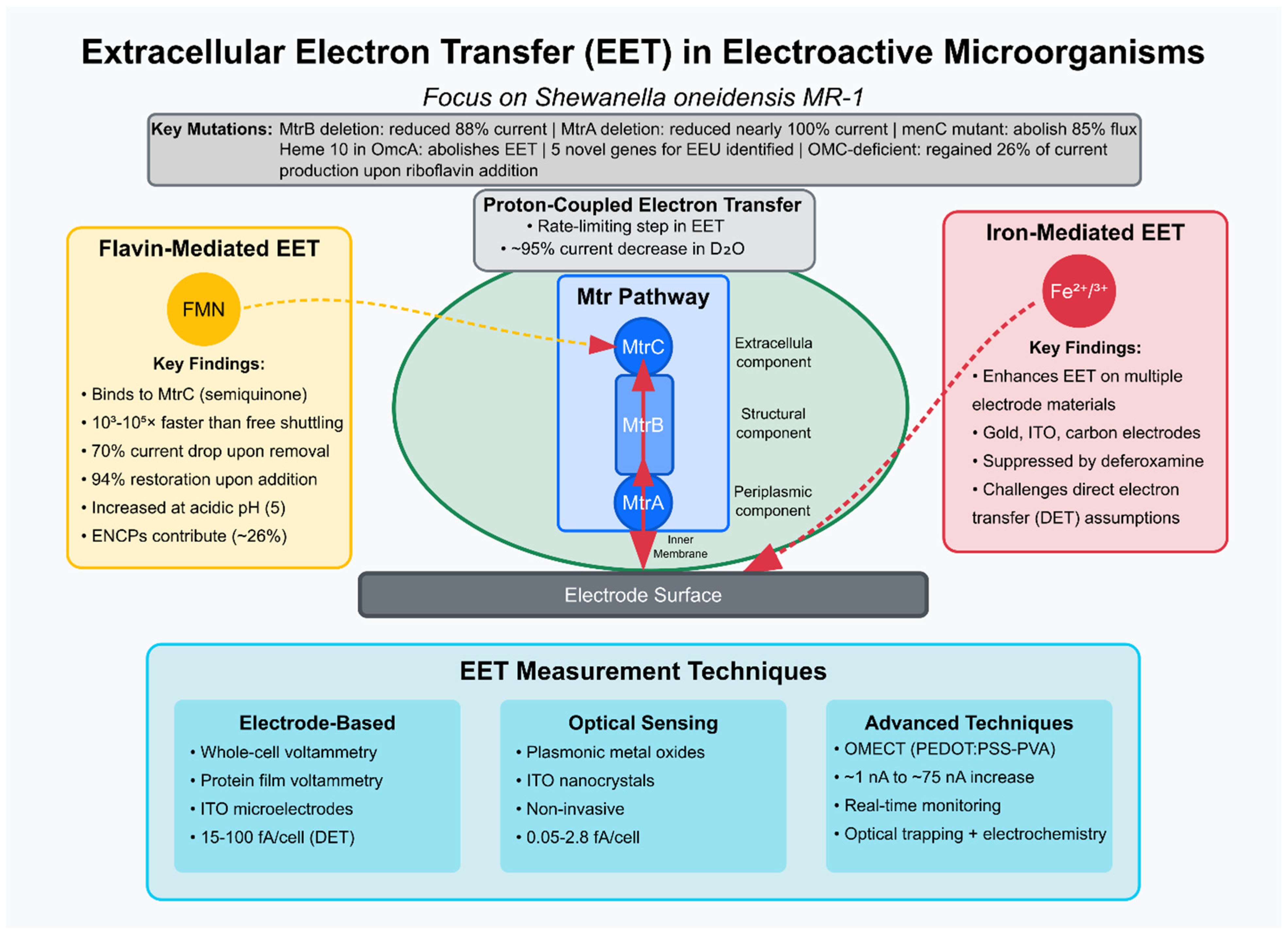
Several studies focused on understanding how EET happens in various electroactive microorganisms. Flavin-mediated EET has been a focal point for mechanistic studies in S. oneidensis MR-1.
Biffinger et al. (2008) [
6] found that flavin secretion in
Shewanella increases at acidic pH (5), enhancing the MFC power output via mediated electron transfer (MET), providing experimental support for the concept of a soluble mediator pathway in
Shewanella EET and identifies riboflavin as a key molecule involved in this process. It moves beyond hypothetical mechanisms by detecting and characterizing secreted redox-active compounds and linking their presence and concentration to the organism’s ability to transfer electrons extracellularly. This contributes to understanding the “indirect (mediated)” mechanism alongside potential direct contact mechanisms.
Marsili et al. (2008) [
7] confirmed that
Shewanella secretes riboflavin and FMN, mediating EET to carbon electrodes, with a 70% current drop upon flavin removal and 94% restoration upon reintroduction, highlighting their dual role in soluble and surface-bound transfer. This study provided evidence that secreted flavins are key players in
Shewanella’s EET, acting as soluble mediators that shuttle electrons and interact with external surfaces, thereby introducing a flavin-mediated shuttling and surface-binding mechanism into our understanding of
Shewanella’s interaction with extracellular electron acceptors. It highlights that the process involves not just direct contact or hypothetical structures but also diffusible, surface-active molecules.
Hartshorne et al. (2009) [
8] characterized the MtrCAB complex as a modular electron conduit using spectropotentiometry and protein film voltammetry, with MtrA, MtrB, and MtrC facilitating periplasmic, structural, and extracellular electron transfer, respectively. This study significantly contributes to the knowledge of the EET mechanism in
Shewanella by identifying, characterizing, and demonstrating the function of the MtrCAB protein complex as a dedicated conduit for moving electrons across the outer membrane.
The Mtr pathway’s role in
Shewanella EET has been extensively investigated. Ross et al. (2011) [
9] demonstrated the Mtr pathway’s reversibility for electrosynthesis, showing that fumarate addition to graphite electrodes at −0.36 V vs. SHE increased electron uptake, with MtrB and MtrA deletions reducing the current by 88% and nearly 100%, respectively. A surprising and significant finding was the requirement for CymA and the menaquinone pool for the majority of inward electron flux. Deletion of the gene encoding the quinone-linked cytochrome CymA eliminated 85% of the electron flux, and disruption of menaquinone biosynthesis (
menC mutant) had a similar negative effect (5% of the wild-type activity). This showed that electrons primarily flow from outer membrane cytochromes (like MtrA)
into the menaquinone pool located in the cytoplasmic membrane (CM) and then back to periplasmic FccA rather than solely through direct periplasmic protein interactions like MtrA:FccA. The data provides new evidence supporting the existence of distinct CymA respiratory units in vivo, specifically CymA:MtrA and CymA:FccA. While soluble redox mediators, like riboflavin, are known to enhance
outward EET, the study found that riboflavin could only partially restore electron transfer in mutants lacking key Mtr pathway components (like MtrB, CymA, or menaquinone biosynthesis).
Okamoto et al. (2013) [
10] used whole-cell voltammetry to reveal that flavin mononucleotide (FMN) binds to the outer-membrane cytochrome MtrC, forming a semiquinone intermediate that accelerates EET rates by 10
3–10
5 times compared to free FMN shuttling, suggesting a highly efficient flavin-binding strategy. This study significantly contributes to the knowledge of the extracellular electron transport (EET) mechanism in
Shewanella by proposing and providing evidence for an alternative mechanism involving bound flavins and one-electron transfer, which contrasts with the previously proposed diffusion-based shuttling mechanism involving free flavins and two-electron transfer. Moving beyond the conceptual model of flavin shuttling, this study proposes a more detailed, energetically favorable, and kinetically efficient mechanism, where flavin molecules are bound to specific outer membrane cytochromes (MtrC and OmcA) and mediate EET via a one-electron transfer pathway involving a stabilized semiquinone intermediate, a process tightly coupled with the cell’s metabolic activity and serving as a means of regulating electron output.
Gross and El-Naggar (2015) [
11] demonstrated early advancements in single-cell EET measurement and developed an innovative approach integrating optical trapping with electrochemical detection to study
S. oneidensis MR-1. Using an indium tin oxide (ITO) microelectrode as the anode, they measured respiration currents ranging from 15 to 100 fA per cell without exogenous electron shuttles, relying solely on direct electron transfer (DET). Mutant strains lacking outer membrane cytochromes exhibited no detectable current, underscoring the critical role of these proteins in EET. This study advances our knowledge of
Shewanella EET by providing a validated method for measuring single-cell activity, quantifying individual cell rates, reinforcing the essential role of outer membrane cytochromes at this level, and, most importantly, revealing the potential heterogeneity in EET capability within a population, setting the stage for more detailed mechanistic studies on how specific cellular components and environmental factors influence EET at the individual cell level.
While previous studies have focused on the pathways and kinetics of electron flow, Okamoto et al. (2017) [
12] highlight the often-overlooked aspect of counter-cation (proton) dynamics. The study explored proton transport’s role in EET, showing that non-covalently bound flavins in the flavocytochrome complex act as primary shuttles, with proton-coupled electron transfer as a rate-limiting step. A ~95% current decrease in deuterium water (D
2O) and a twofold higher kinetic isotope effect in mtrC/omcA mutants underscored proton transport’s significance. This study significantly advances our understanding of
Shewanella EET by identifying proton transport, particularly within the flavin-bound OM cytochrome complex, and potentially the removal of protons from the periplasm as a critical rate-limiting factor. It demonstrates that the flavin cofactor plays a crucial role in mediating this proton transport and reveals that, under these experimental conditions, EET is decoupled from PMF-driven ATP synthesis and instead supports substrate-level phosphorylation. These findings introduce the crucial concept of charge balancing and cation transport as central components in the kinetics and bioenergetics of EET in
Shewanella.
Exploring alternative pathways beyond the typically emphasized c-type cytochromes, Yang et al. (2017) [
13] studied riboflavin’s role in
Shewanella decolorationis S12 using graphite plate electrodes, showing that an OMC-deficient mutant regained 26% of wild-type current production upon riboflavin addition, suggesting that extracellular non-cytochrome c proteins (ENCPs) like Fe-S cluster-containing proteins contribute to flavin-mediated EET. In essence, while confirming the established role of OMCs in direct EET, this study significantly expands our knowledge by revealing a functional, OMC-independent, mediator-facilitated EET pathway in
Shewanella that relies on extracellular non-cytochrome c proteins, potentially including Fe–S cluster-containing proteins, present in the biofilm EPS.
The crucial role of proton transport and the properties of non-covalently bound cofactors in limiting and accelerating the rate of EET mediated by outer membrane c-type cytochromes (Cyts) was demonstrated by Tokunou et al. (2019) [
14]. Protonation of the nitrogen atom at position 5 (N5) of the isoalloxazine ring is essential for electron outflow acceleration as a bound non-covalent cofactor of cytochromes. EET mediated by cytochromes was accelerated at a rate dependent on pKa (N5). The EET rate primarily decreased in response to the addition of deuterated water (D
2O). At the same time, a low concentration of D
2O (4%) had little impact on the electron free-energy difference of the heme and non-covalent bound cofactors, strongly suggesting that the protonation of N5 limits the rate of EET. This study identified and mechanistically characterized the critical role of coupled proton transport, specifically linked to the protonation state of the N5 atom in bound flavin/quinone cofactors, as a key determinant and rate-limiting factor in the acceleration of EET mediated by outer membrane cytochromes.
Direct electron transfer assumptions were challenged by Oram and Jeuken (2016) [
15] by showing that Fe
2+/Fe
3+ mediation enhances EET on gold, ITO, and carbon electrodes suppressed by deferoxamine, suggesting iron’s significant role. They re-evaluated previously accepted interpretations, proposing an alternative, iron-mediated pathway for certain observed electrochemical signals, unveiling evidence for a previously underestimated iron-mediated EET pathway in
S. oneidensis MR-1, particularly on negatively charged and some other electrode materials, and highlighting that signals previously attributed solely to MtrC/OmcA-mediated DET might, in fact, be due to this iron shuttle. This was a critical re-evaluation of past studies and provided new insights into the complex interplay of different EET mechanisms utilized by
Shewanella depending on the specific environmental interface (
Figure 1).
An organic microbial electrochemical transistor (OMECT) with PEDOT:PSS-PVA electrodes was developed by Méhes et al. (2020) [
16] to monitor EET in
S. oneidensis MR-1, achieving a rapid increase in current from ~1 nA to ~75 nA and offering real-time insights into microbial electroactivity. Bacteria are attached to the gate of the transistor by a chronoamperometric method, and the successful attachment is confirmed by fluorescence microscopy. Monitoring EET with the OMECT configuration was achieved due to the inherent amplification of the transistor, revealing fast-time responses to lactate. The OMECT device provides a highly sensitive method to monitor EET from a small number of bacteria in confined spaces. This new capability allowed for the observation of previously difficult-to-measure phenomena, specifically revealing an unexpectedly fast temporal response of attached
S. oneidensis MR-1 to lactate. This technological advancement opens new avenues for studying the fundamental dynamics of EET in microenvironments, including potential applications in understanding microbial interactions in complex systems like the human microbiome.
Five novel genes critical for extracellular electron uptake (EEU), distinct from the Mtr pathway, were identified by Rowe et al. (2021) [
17] using genome-wide knockout screens. The authors employed a focus on the less-understood process of extracellular electron uptake (EEU), moving beyond the known electron donation pathways to specifically identify and characterize genes critical for electron uptake. It highlights that uptake is a distinct process from donation, provides candidates for the molecular bridge connecting cathodic electron transfer to aerobic respiration, offers initial functional clues for these novel genes, and suggests this pathway is widespread among electroactive bacteria.
A novel method for quantifying EET from planktonic cells was introduced and validated by Graham et al. (2022) [
18]. Plasmonic metal oxide and tin-doped indium oxide (ITO) nanocrystals as optical sensors quantified single-cell EET in planktonic
Shewanella cultures at 0.05–2.8 fA/cell, providing a new, quantitative, non-destructive optical method using plasmonic nanocrystals that enables the measurement and understanding of EET from planktonic
Shewanella populations. This method allowed researchers to directly measure how factors like metabolic state, genotype, and the expression level of a key EET gene (
mtrC) impact electron transfer rates in suspended cells. This capability expands the tools available for fundamental EET studies and screening electroactive microbes or engineered strains.
To investigate the molecular determinants of EET in the same species, Louro et al. (2023) [
19] mutated heme ligands in the outer membrane cytochrome OmcA. Using a pyrolytic graphite edge (PGE) electrode, they found that while most mutations retained EET capability, the modification of heme 10 abolished the current generation, highlighting its essential role in electrode interaction. This study provides molecular-level insights into how the structure and specific hemes of the OmcA protein influence its interaction with and electron transfer to various extracellular acceptors, emphasizing the critical role of the binding interface. This complements broader studies on EET pathways and gene identification by focusing on the detailed functional mechanisms of a core EET protein, as can be seen on the scheme presented in
Figure 2.
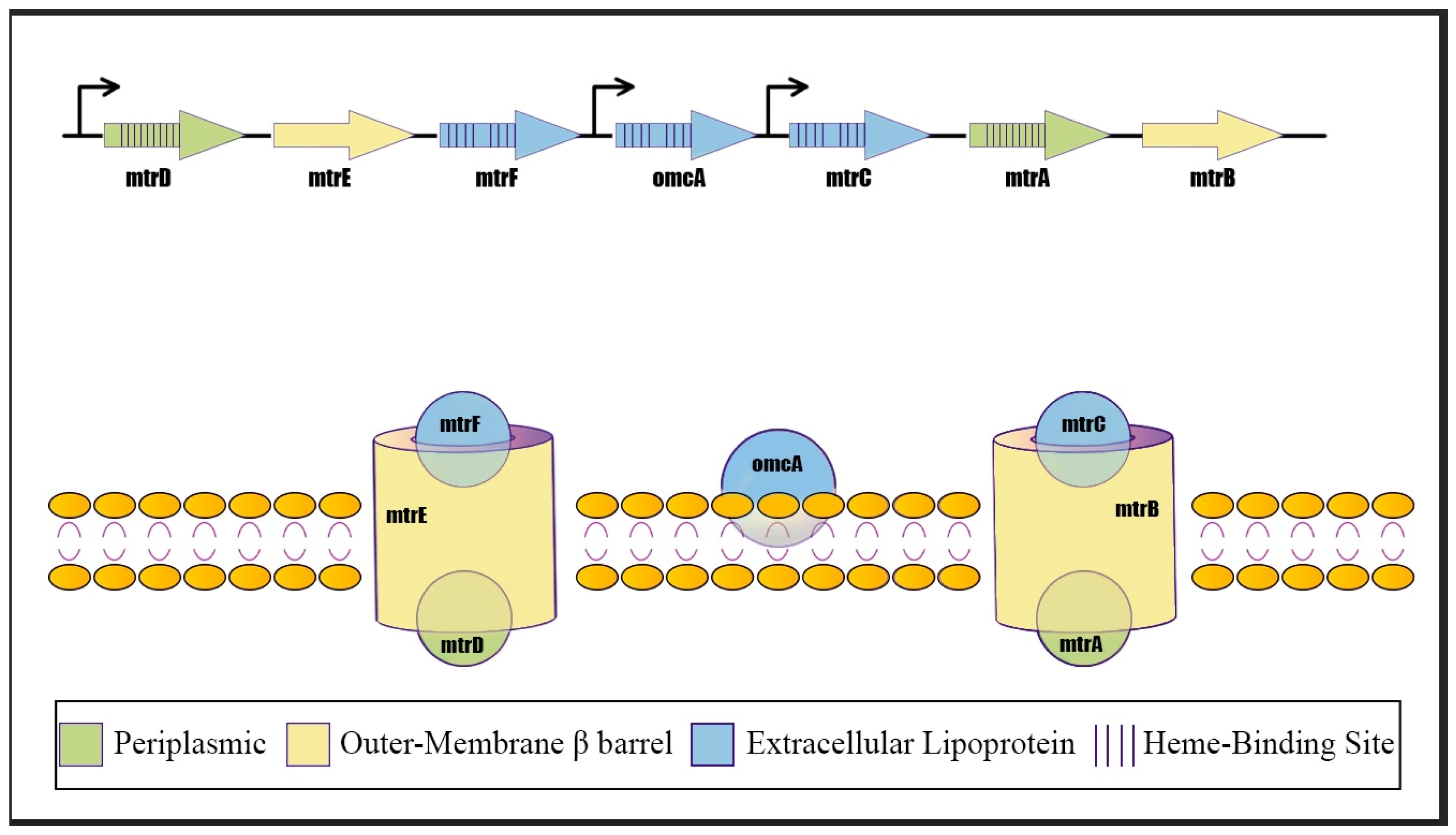
Summarizing Shewanella EET mechanisms findings, porin–cytochrome complexes (Mtr Pathway) are used to transfer electrons directly across the cell membrane and outer surface. The first indication came from genetic studies identifying a transposon insertion in mtrB, which encodes an outer membrane β-barrel protein required for Fe (III) and Mn (IV) reduction. The same genetic locus contains mtrA, encoding a decaheme c-type cytochrome, and mtrC (also known as OmcB in the early literature), encoding outer membrane decaheme c-type cytochrome lipoproteins that are surface-exposed. These proteins are essential for reducing iron and manganese minerals. A third outer membrane multiheme c-type cytochrome, MtrF, is encoded upstream of the omcA locus and can facilitate iron reduction, accounting for residual activity in omcA/mtrC double mutants.
In electron shuttling on Shewanella, an indirect EET mechanism, the redox-active small molecules facilitate electron transfer to substrates distant from the cell surface. Shewanella was proposed to have this ability early on. Experiments using alginate beads or nanoporous glass beads to sequester Fe (III) oxide showed that Shewanella strains could reduce significant amounts of iron distant from the cell. The identity of one electron shuttle produced by Shewanella was determined to be flavins. Flavin mononucleotide (FMN) is the primary flavin found in culture supernatants, along with riboflavin (B2). Adding flavins to cells, reducing electrodes or Fe (III) oxides, enhances the reduction rate.
While initial observations suggested
S. oneidensis produces conductive protein filaments similar to those hypothesized for
Geobacter, these structures were later determined to be extensions of the outer membrane. These extensions contain membrane-embedded porin–cytochrome components (like MtrCAB) and soluble periplasmic proteins. Mobility of outer membrane multiheme cytochromes MtrC and OmcA has been observed in both the outer membrane and these extensions. This integrates electron transfer through Mtr conduits, interactions between complexes, and the mobility of these components within the outer membrane structure [
20].
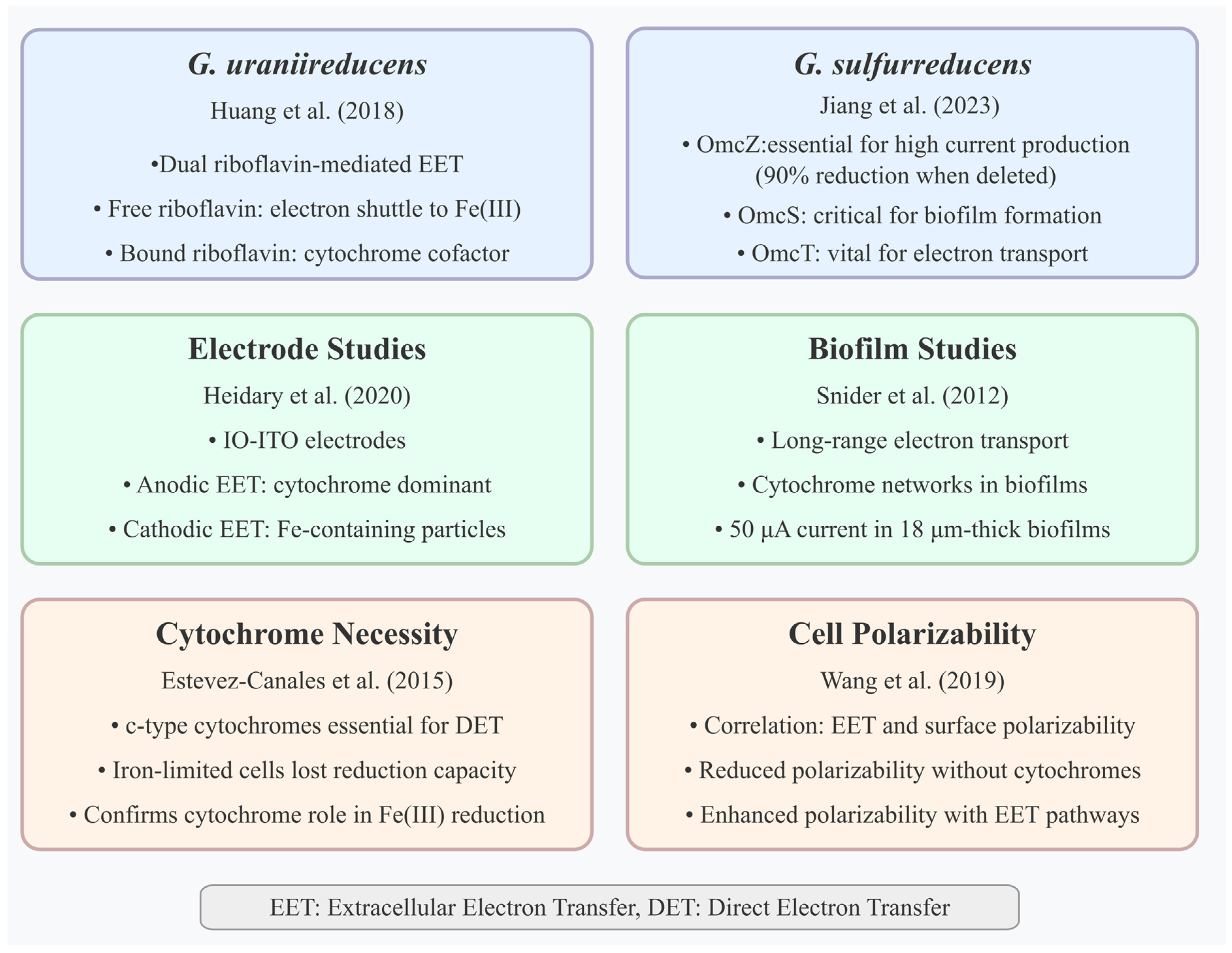
Geobacter species have also been extensively studied for the EET mechanisms, which are represented in
Figure 3. Snider et al. (2012) [
21] demonstrated long-range electron transport in
G. sulfurreducens biofilms via cytochrome networks on microelectrode arrays, with a redox gradient driving 50 μA current in 18 μm thick biofilms. The study showed experimental evidence supporting the multistep electron hopping model driven by a redox gradient and challenging the alternative metallic-like conductivity model. In addition, it highlights the involvement of a diverse set of redox cofactors, differentiating those involved in catalytic vs. noncatalytic electron transport.
Estevez-Canales et al. (2015) [
22] provided direct experimental evidence that c-type cytochromes are essential for their capacity for extracellular electron transfer, confirming c-type cytochromes’ necessity for DET in
G. sulfurreducens, as iron-limited cells with depleted cytochromes lost Fe(III) and electrode reduction capacity. Previous studies, primarily using gene deletion techniques, had implicated specific periplasmic and outer-surface c-type cytochromes in EET, but even deleting multiple cytochrome genes often left some residual EET capacity. This study’s approach, which resulted in a much broader and more severe depletion of cytochromes, provides stronger evidence for the absolute necessity of the cytochrome network for EET function.
Huang et al. (2018) [
23] identified dual riboflavin-mediated EET modes in
Geobacter uraniireducens on ITO electrodes, with free riboflavin shuttling electrons to Fe(III) oxides and bound riboflavin acting as a cytochrome cofactor for electrodes, increasing the redox peak potential. This study broadens the knowledge of EET in
Geobacter species by demonstrating that, in addition to previously recognized mechanisms like direct contact via cytochromes or conductive pili, flavin-mediated EET is important and can operate via different modes, free shuttling for insoluble electron acceptors and acting as bound cofactors with cytochromes for electrode respiration. This highlights the adaptability of
Geobacter EET strategies based on the environmental context and the properties of the terminal electron acceptor.
Wang et al. (2019) [
24] used microfluidic dielectrophoresis to show a strong correlation between bacterial EET and surface polarizability. Their analysis was made on surface polarizabilities for wild-type strains and cytochrome-deletion mutants of two model EET microbes,
G. sulfurreducens and
S. oneidensis, and for
Escherichia coli strains heterologously expressing
S. oneidensis EET pathways in various growth conditions. Their results showed that cell polarizability is diminished in response to deletions of crucial outer-membrane cytochromes and enhanced due to additions of EET pathways. This study significantly contributes to the understanding of the EET mechanism in
Geobacter, primarily
G. sulfurreducens, by establishing a direct correlation between a physical property—cell surface polarizability—and the organism’s EET capacity. This correlation is explicitly linked to the presence and activity of the c-type outer-membrane cytochromes known to be crucial for
Geobacter’s electron transfer processes.
Heidary et al. (2020) [
25] used inverse-opal indium tin oxide (IO-ITO) electrodes and multiple spectroscopies to show cytochrome dominance in anodic EET, contrasted by Fe-containing particles in cathodic mode, suggesting the possible existence of a nonheme, iron-involving EET process in cathodic mode. The study challenges the assumption that c-type cytochromes are the primary means of EET in
G. sulfurreducens for all electron acceptors and reaction directions. It provides compelling evidence that while cytochromes are crucial for anodic respiration, their role is diminished in cathodic respiration (fumarate reduction), which appears to involve a potentially nonheme, iron-mediated electron transfer pathway facilitated by secreted or released soluble Fe species and possibly iron oxide nanoparticles formed on the cell surface. This reveals the versatility and distinct mechanisms employed by
Geobacter depending on the electrochemical environment and the terminal electron acceptor.
Jiang et al. (2023) [
26] mapped the roles of PilA-N, OmcE, OmcS, OmcT, and OmcZ in
G. sulfurreducens using MFC gene deletion mutants, finding that OmcZ is essential for high current production (90% reduction when deleted), OmcS is critical for early biofilm formation, and OmcT is vital for electron transport. The study moves beyond a general understanding of protein involvement by systematically quantifying and comparing the specific contributions of PilA-N, OmcE, OmcS, OmcT, and OmcZ across Fe (III) reduction, anode respiration, and DIET, revealing their varied and context-dependent roles and identifying previously unknown contributors to the critical DIET process.
Compared to Shewanella, Geobacter employs distinct strategies for EET. While both genera use porin–cytochrome complexes to transfer electrons across the outer membrane, the proteins involved share no homology between the two systems. Furthermore, Geobacter exhibits a much broader diversity of these porin–cytochrome conduits. Geobacter also primarily relies on direct electron transfer via contact, in contrast to Shewanella, which extensively uses secreted electron shuttles.
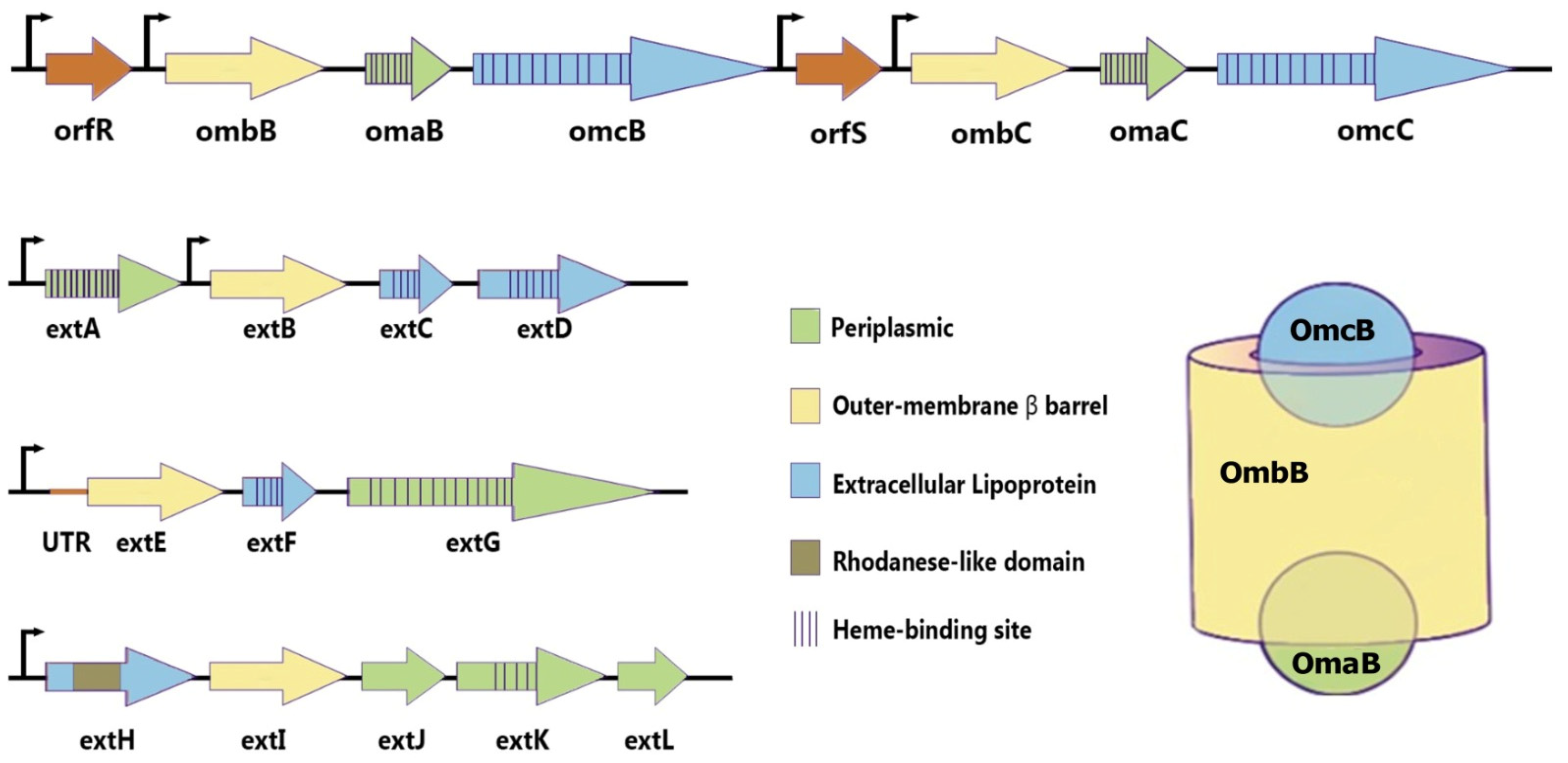
Geobacter employs porin–cytochrome complexes to move electrons across its outer membrane. The first such complex identified in G. sulfurreducens is based on the OmcB protein. Genetic and biochemical evidence suggests a transmembrane complex analogous to Shewanella’s MtrCAB, composed of the dodecaheme lipoprotein cytochrome OmcB, a small octaheme cytochrome OmbB, and a hypothetical β-barrel-forming protein OmaB. Intact complexes consistent with OmcB–OmbB–OmaB units have been purified and shown to catalyze transmembrane electron transfer when inserted into liposomes. Deletion of genes OmcB, OmbB, or OmaB causes defects in soluble Fe (III) reduction. G. sulfurreducens encodes multiple unrelated porin–cytochrome conduits, suggesting different complexes may be used for specific electron acceptors. Other identified putative conduits include ExtABCD, ExtEFG, and ExtHIJKL. Transcription levels alone do not fully explain these preferences; for example, the omcB-omcC operon is expressed ten times higher during electrode growth than extABCD, yet ExtABCD is essential for this process. One hypothesis is that specific porin–cytochrome complexes are required to interface with different types of extracellular appendages or proteins. Compared to the MtrCAB system in Shewanella, the exterior-facing components in Geobacter complexes like OmcB are often replaced by nonhomologous lipoprotein multiheme cytochromes in different Geobacter species, indicating a high degree of diversity and evolution in these components
In contrast to
Shewanella,
Geobacter strains have generally not been shown to secrete abundant soluble extracellular compounds (>20 nM) that act as electron shuttles to facilitate the reduction of insoluble electron acceptors distant from the cell surface. Experiments, where cells were separated from insoluble Fe (III) oxides using semipermeable membranes or porous beads, showed that
Geobacter required direct contact with the substrate surface or the addition of exogenous electron shuttles, like AQDS, to achieve significant reduction [
27,
28,
29,
30,
31].
Geobacter is well known for producing microbial nanowires, which are thought to facilitate direct electron transfer to insoluble acceptors or other cells. Initial studies linked these conductive filaments to a protein based on the PilA-N pilin subunit. However, it is now understood that
G. sulfurreducens also produces multiheme cytochrome filaments that have similar dimensions and properties to what were previously thought to be only pilin-based nanowires. The identified
G. sulfurreducens type IV pilus (composed of PilA-N and PilA-C subunits) is nonconductive, leading to the hypothesis that pili might play a secretory or regulatory role in cytochrome production rather than being the primary conductive element themselves. Three structures of cytochrome nanowires from
G. sulfurreducens have been determined: OmcE, OmcS, and OmcZ. OmcE and OmcS are distinct in size and morphology but share a conserved four-heme motif and a unique feature, where a heme in one subunit is coordinated by a histidine from the adjacent subunit, bringing hemes into close proximity for rapid electron transfer. OmcE and OmcS are linked to the reduction of Fe (III) and Mn (IV) oxides. OmcZ forms a different structure and heme packing arrangement compared to OmcE and OmcS. It is essential for the formation of conductive electrode biofilms and appears necessary for long-distance conductivity during electrode growth. OmcZ has a uniquely solvent-exposed heme, which may facilitate electron exchange with surfaces. The function of these different nanowires and their relationship with pili and other cytochromes is complex, as mutations in one component can affect the production or localization of others. How electrons are specifically transferred into and out of these cytochrome filaments is still not well understood [
20].
Beyond the well-studied
Shewanella and
Geobacter species, many diverse microorganisms have been identified with extracellular electron transfer (EET) capabilities. For instance, Lai et al. (2020) [
32] explored
Pseudomonas putida and its mediator-based EET to transition metal complexes. Their findings demonstrated that the EET pathway was blocked entirely when cytochrome c reductase activity was inhibited using antimycin A. While the possibility that cytochrome c and the periplasmic subunit of cytochrome c oxidase contribute electrons to metal complexes cannot be ruled out, the results establish cytochrome c reductase as a crucial component of the EET mechanism in
P. putida [
32].
Further insights into
Pseudomonas species are derived from Saunders et al. (2020) [
33], who revealed that phenazines mediate efficient EET in
Pseudomonas aeruginosa biofilms through interactions with extracellular DNA (eDNA). Their study retained pyocyanin (PYO) and phenazine carboxamide in the biofilm matrix due to their binding with eDNA. Interestingly, different phenazines could exchange electrons with or without the presence of DNA, suggesting that eDNA can facilitate direct redox reactions. In vivo, eDNA within the biofilm supported rapid electron transfer between redox-active intercalators, establishing that PYO:eDNA interactions enable a highly efficient redox cycle, surpassing the rate at which PYO diffuses from the biofilm [
33].
In thermophilic environments, Gavrilov et al. (2021) [
34] identified four novel multiheme cytochromes—OmhA, SmhA, SmhB, and SmhC—in
Carboxydothermus ferrireducens. These cytochromes enable EET to Fe(III) oxides and graphite felt anodes with current densities ranging from 55 to 68 mA/m
2. These findings reveal unique high-temperature EET pathways in monoderm bacteria, expanding our understanding of bioelectrochemical system (BES) diversity [
34].
Additional organisms exhibit alternative EET strategies. Xiao et al. (2017) [
35] showed that extracellular polymeric substances (EPS) in
Bacillus sp. WS-XY1 and
Pichia stipitis function as transient electron shuttles. Notably, removing EPS increased EET rates by 40–90%, indicating that EPS may act as a diffusion barrier under certain conditions [
35]. Meanwhile, Cereda et al. (2014) demonstrated direct EET in
Synechocystis sp. PCC6803, achieving a 0.4 mA/cm
2 photocurrent from Photosystem II water splitting on carbon cloth electrodes, highlighting the role of phototrophic bacteria in EET [
36].
Ouboter et al. (2022) [
37] confirmed methane-dependent EET in
Candidatus Methanoperedens in methanotrophic and archaeal systems, producing 274 mA/m
2 on carbon cloth electrodes. Metatranscriptomic analysis linked this activity to the expression of multiheme cytochromes [
37]. Similarly, using ITO electrodes, Tanaka et al. (2018) [
38] provided the first evidence of EET in the methanotroph
Methylococcus capsulatus (Bath). Here, outer membrane cytochrome (OMC) expression was upregulated under copper deficiency, resulting in a 5.4-fold increase in current density, with the MCA0421 gene identified as essential for electron transport [
38].
Electrochemical activity has also been detected in subsurface microbes. Jangir et al. (2016) observed EET in
Delftia and
Azonexus species, which produced currents of approximately 400 nA and 325 nA, respectively, on carbon cloth electrodes [
39]. Zhang et al. (2020) further expanded the list of EET-capable organisms by demonstrating mediator-based EET in
Capnocytophaga ochracea on ITO electrodes, with riboflavin and menadione enhancing electron transfer in a cell density-dependent manner [
40].
Faustino et al. (2021) [
41] characterized the EET mechanism in the thermophilic, Gram-positive bacterium
Thermincola ferriacetica. Using a pyrolytic graphite electrode (PGE) anode, they identified three multiheme cytochromes—ImdcA, PdcA, and CwcA—as key players in transporting electrons across the bacterium’s thick peptidoglycan layer. This system generated higher currents (0.42 mA) than the model organism
G. sulfurreducens (0.25 mA), underscoring its potential in high-performance BES applications [
41].
Considering sulfate-reducing bacteria (SRB), Deng and Okamoto 2017 [
42] showed the capability of a strain isolated from marine sediments,
Desulfovibrio ferrophilus IS5, to respire using extracellular solids by direct electron uptake via outer membrane cytochromes, which are widely conserved in various sediment sulfur-species-respiring bacteria. Liang et al. (2021) [
43] evaluated the electron uptake from pure Fe(0) and stainless steel. They indicated that, in contrast to previous speculation in the literature,
Desulfovibrio xerophilous and
Desulfopila corrodens cannot directly extract electrons from solid-phase electron-donating surfaces. However, Deng and Okamoto (2023) [
44] studied in vivo electrochemistry to show that outer-membrane cytochromes (OMCs) directly mediated extracellular electron transfer (EET) in
D. ferrophilus IS5.
Recent studies have significantly expanded the known diversity of microorganisms capable of EET beyond the extensively characterized
Shewanella and
Geobacter species. Notably,
Pseudomonas putida relies on cytochrome c reductase for mediator-based EET to transition metal complexes, while
P. aeruginosa biofilms utilize phenazine-mediated electron transfer facilitated by interactions with extracellular DNA, establishing a highly efficient redox cycle [
32,
45]. Thermophilic bacteria such as
C. ferrireducens express unique multiheme cytochromes enabling EET to Fe(III) oxides, underscoring novel high-temperature pathways in monoderm bacteria [
34]. Additional organisms, including
Bacillus sp. and
P. stipitis, employ extracellular polymeric substances as transient shuttles, though these can impede EET under certain conditions [
7,
45,
46]. Phototrophic bacteria, like
Synechocystis sp. PCC6803, directly transfer electrons derived from photosynthetic water splitting, demonstrating EET potential in cyanobacteria. Furthermore, archaeal and methanotrophic systems, exemplified by
C. Methanoperedens and
M. capsulatus, exhibit EET capacities linked to multiheme cytochrome expression, influenced by environmental factors such as copper availability. Subsurface microbes, including
Delftia, Azonexus, and
C. ochracea, rely on mediator-based EET, modulated by cell density and redox-active compounds [
38]. Thermophilic Gram-positive bacteria, like
T. ferriacetica, possess robust EET mechanisms facilitated by multiheme cytochromes spanning thick peptidoglycan layers, achieving current outputs surpassing traditional model organisms. In sulfate-reducing bacteria, strain-specific differences are evident; while
D. ferrophilus IS5 can respire using extracellular solids via outer membrane cytochromes, related species lack this capacity, challenging earlier generalizations [
34,
47]. Collectively, these findings reveal a remarkable functional and ecological diversity in microbial EET pathways, broadening the scope for bioelectrochemical applications in energy generation, bioremediation, and environmental microbiology.
3. Enhancing EET with Different Electrode Materials
The electronic acceptor material is crucial for microbial attachment and the EET process. These materials should have high conductivity, surface area, water affinity, and chemical stability. Electrode materials have been extensively investigated to improve EET.
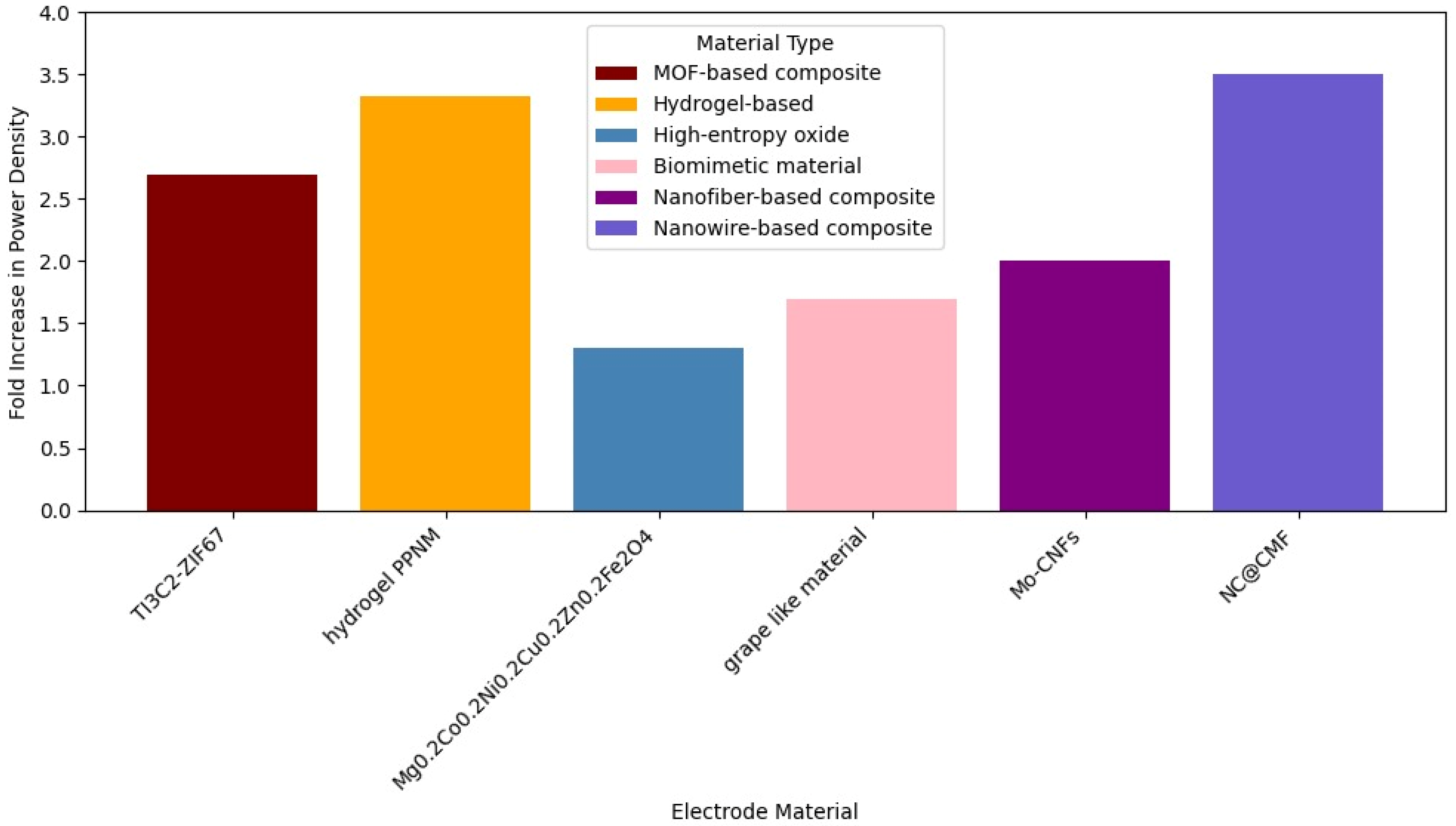
Yang et al. (2022) [
48] used Ti3C2-ZIF67 as an electrode material in an MFC with mixed culture and improved power density by 2.7 times and charge transfer resistance by 2 times. The high-throughput sequencing technology revealed that the composite possessed good colonization of microorganisms and high biodiversity, with the microbial community playing an efficient synergistic role in facilitating electricity generation. The electrode shows significantly better hydrophilicity. Hydrophilic surfaces improve microbial adhesion and promote interfacial electron transfer by facilitating the affinity between flavin and OM c-Cyts. The Ti3C2-ZIF67 composite electrode exhibits significantly lower charge transfer resistance (Rct) compared to its individual components and carbon felt. A lower Rct facilitates the transfer of electrons from the electrolyte or microorganism to the electrode. The coupling of MOF and MXene improves charge transfer and reduces resistance.
In 2024 [
49], a composite hydrogel called PPNM, through an N-MXene non-adhesive modified hydrogel anode, was investigated in mixed culture MFCs, providing a 3.32-fold increase in power density. Microbial community analysis revealed the presence of more electrochemically active species on the PPNM anode. PPNM hydrogel enhances EET by providing a highly conductive, large-surface-area, porous, and biocompatible material that facilitates microbial attachment and growth, promotes both direct and mediated electron transfer mechanisms, and reduces charge transfer resistance and anodic polarization, ultimately leading to significantly improved power generation in MFCs.
Another study from 2024 [
50] investigated the use of spinel-type high-entropy oxide as an anode material (Mg
0.2Co
0.2Ni
0.2Cu
0.2Zn
0.2)Fe
2O
4. Compared to Fe
2O
3, the power density improved by 1.3 times. The high-entropy oxide material MCNCZFO enhances EET by increasing the material’s intrinsic disorder and conductivity, leading to lower charge transfer resistance and a shift towards faster capacitance-controlled kinetics. Its structure offers enhanced specific capacitance and a favorable surface for increased microbial colonization and adhesion. This environment promotes the enrichment of key electrogenic bacteria, like
Geobacter, and facilitates diverse direct and mediated electron transfer pathways, ultimately resulting in superior bioelectrocatalytic activity and reduced anodic polarization.
In 2025 [
51], a novel biomimetic grape-like material with biocompatibility, PNTs/ZIF-8/ZIF-67@NC, which was a composite structure designed to resemble grape clusters at the nanoscale and created by growing carbonized ZIF-8/ZIF-67 particles on carbonized polypyrrole nanotubes, was investigated as an anode on mixed culture MFCs. High-throughput sequencing revealed that PNTs/ZIF-8/ZIF-67@NC could promote the acclimation of electroactive microorganisms within the microbial community, thereby enhancing the electricity generation performance of the MFCs. Compared to ZIF-8@NC, there was a 1.7-fold increase in power density. The combination of the synergistic effects of enhanced surface area, conductivity, catalytic activity, and biocompatibility significantly improved the rate and efficiency of EET by providing abundant attachment sites for microorganisms, facilitating electron transport pathways, and enriching electroactive bacteria. This led to superior performance in MFCs, achieving a maximum power density of 5.07 W/m
2.
Wu et al. (2023) [
52] developed Mo-doped carbon nanofibers (Mo-CNFs) via electrospinning, anchoring Mo
2C nanoparticles to enhance microbial colonization and electrocatalytic activity in MFCs and doubling the power density to 1287.38 mW/m
2 compared to unmodified anodes (649.69 mW/m
2). The modification of electrode interfaces with Mo
2C considerably impacts the improvement in the electron transfer rate between bacteria and the electrode. Specifically, Mo-doped carbon nanowires transformed the interfacial electrochemical reaction from a surface-controlled process into a diffusion-controlled process, promoting a rapid electrochemical reaction on the electrode surface. Mo-CNF II exhibited the lowest interfacial electron transfer impedance (Rct) in both flavin solution and bacterial suspension experiments compared to undoped CNFs.
Liu et al. (2024) [
53] introduced N-doped carbon nanowire-modified macroporous carbon foam (NC@CMF), which improved bacterial adhesion and direct EET through pyrrolic N species, yielding a 3.5-fold increase in power density (5.32 W/m
3). The macroporous structure ensures mass transport and diffusion during long-term operation, allowing acetate (fuel) to easily diffuse into the biofilm and metabolic waste to be removed. This helps maintain the long-term stability and high activity of mature biofilms, which is confirmed by CLSM images showing a higher proportion of live bacteria compared to carbon cloth (CC). Also, the highly conductive carbon nanowires act as artificial conductive wires, building abundant physical contact sites between the biofilms and the anode surface, as well as connecting different bacteria, thus accelerating interspecies electron transfer and abiotic–biotic extracellular electron transfer (EET). These nanowires can penetrate the biofilm. The active sites generated by graphitized N and pyrrolic N atoms can accelerate the EET process. These N species can optimize the adsorption energy of hemin or flavin on their surfaces, shorten the electron transfer distance, and facilitate electron transfer at the interface. Pyrrolic N can specifically reduce the binding energy and electron transfer distance of bacterial outer membrane hemin.
The key strategies to improve EET using new electrode materials in MFCs, according to the studies previously mentioned, are as follows: creating materials with high surface area, porosity, and conductivity using techniques like electrospinning and carbonization and incorporating conductive nanomaterials (CNFs, MXene, PPy, and PEDOT:PSS); enhancing biocompatibility and promoting microbial adhesion and dense, active biofilm formation through surface modification, the incorporation of specific materials (MOFs, hydrogels, and N-doping), and designing favorable structures (nanofibers, nanowires, hierarchical pores, and high entropy); designing hierarchical or macroporous structures to ensure efficient mass transport within the biofilm and prevent blockage during long-term operation; and promoting specific microbial communities and synergistic activity among bacteria for enhanced electricity generation.
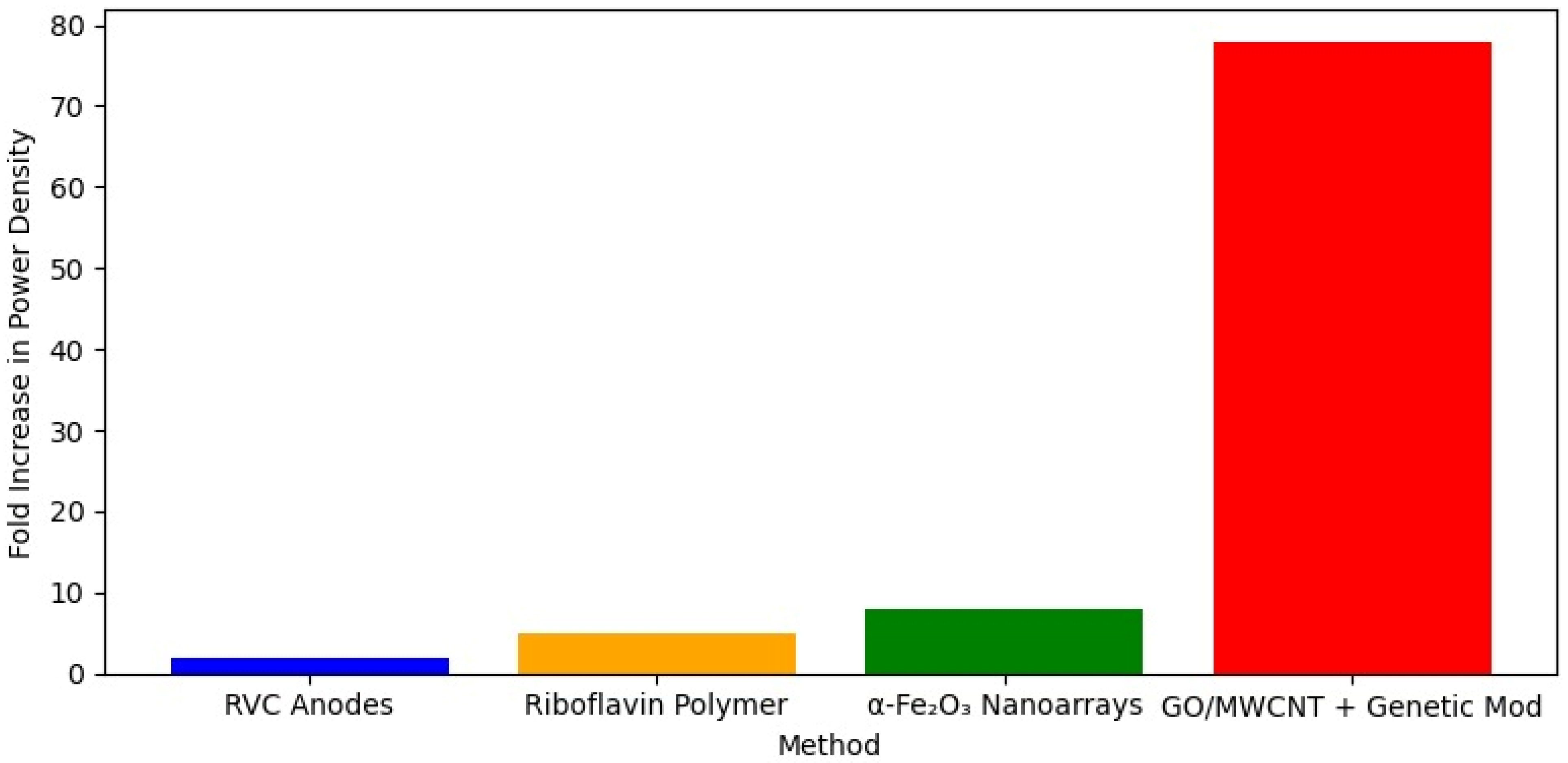
In
Shewanella species, Wang et al. (2022) [
54] explored EET mechanisms in Shewanella loihica PV-4 using reticulated vitreous carbon (RVC) anodes. By varying electrode potentials, they observed that lower potentials favored mediated electron transfer (MET) via flavins. In comparison, higher potentials (optimized at 0.24 V) enhanced DET through outer membrane cytochromes (OMCs), boosting biofilm formation and achieving a maximum current density of 673 mA/m
2. Such a result represents an 11-fold enhancement in current density and a 1.5-fold enhancement in power density. Zou et al. (2019) [
55] further enhanced EET in
S. putrefaciens CN32 by electropolymerizing riboflavin onto carbon cloth electrodes, creating a stable redox-active interface that increased the power density in microbial fuel cells (MFCs) by 4.3-fold (707 mW/m
2) and the cathodic current for fumarate reduction by 3.7 times (0.78 A/m
2). Additionally, He et al. (2024) [
56] improved EET in the same species using carbon cloth electrodes modified with vertically aligned α-Fe
2O
3 nanoarrays, achieving an 8-fold increase in power density (816 mW/m
2) through enhanced microbial adhesion and Fe-cytochrome interactions, supported by upregulated OMC and iron transport gene expression. Zhao et al. (2023) [
57] took a synthetic biology approach with
S. oneidensis MR-1, combining genetic modifications to boost riboflavin production (837.74 μM) and MtrC expression with a spider-web-like graphene oxide and multiwalled carbon nanotube hybrid biofilm, resulting in a remarkable 77.83-fold increase in power density (3736 mW/m
2).
Figure 4 shows a comparison in MFC power density for different electrode materials in
Shewanella spp.
Beyond
Shewanella, other microorganisms and electrode materials have shown promise in enhancing EET. Meanwhile, Beuth et al. (2020) [
58] showed that
Geobacter biofilms exploit conductive copper sulfide (CuS) networks on copper electrodes, achieving a 237% increase in current density (1.59 mA/cm
2) and a 460% improvement with pre-deposited CuS, highlighting the scalability of CuS-modified electrodes.
Li et al. (2024) [
59] functionalized carbon felt with semiconducting single-walled carbon nanotubes (s-SWCNTs) to enhance EET in
Clostridium ljungdahlii biofilms, boosting acetate production by 180% in microbial electrosynthesis. Jiang et al. (2014) [
60] utilized biogenic iron sulfide (FeS) nanoparticles with
S. loihica PV-4, forming a conductive network that increased the current from ~9 pA to ~500 pA, while Jiang et al. (2018) [
61] leveraged iron oxide nanoparticles (hematite and magnetite) to enhance reductive dechlorination via Fe
2+/Fe
3+ redox cycling in anaerobic systems.
Innovative electrode designs have further refined EET monitoring and performance. Roullier et al. (2023) [
62] explored polypyrrole (PPy)-coated graphite electrodes with cyanobacteria, noting a sixfold photocurrent increase in
Synechocystis sp. PCC6803 due to enhanced surface area and reduced resistance, though
Synechococcus elongatus PCC7942 showed no significant improvement, emphasizing strain-specific responses.
Biointerface engineering offers another innovative approach to optimize EET. Zhang et al. (2024) [
63] incorporated a cationic conjugated polymer (PFP) into
S. oneidensis MR-1 membranes on carbon paper electrodes, improving adhesion, viability, and electron transport, with a 1.38-fold increase in current density (31.8 μA/cm
2) and an 18-fold boost in cathodic current for fumarate reduction, alongside halved charge transfer resistance (41.8 Ω). Liu et al. (2019) [
64] coated
Shewanella xiamenensis cells with polydopamine (PDA) on carbon felt electrodes, enhancing direct electron transfer (DET) via cytochrome–electrode interactions and mediated electron transfer (MET) via flavin adsorption, yielding a 5.1-fold current density increase (142.7 µA/cm
2) and a 52% resistance reduction. These biohybrid strategies highlight how surface modifications can strengthen microbial-electrode interfaces, amplifying EET efficiency.
Collectively, these studies underscore the remarkable potential of integrating advanced electrode materials, nanostructures, and biointerface engineering to enhance EET across diverse microbial systems. From conductive sulfide networks and carbon nanotube composites to polymer-coated biofilms and biogenic nanoparticles, each strategy contributes to improved current generation, metabolic activity, and electrochemical performance. Importantly, the varied strain-specific and system-dependent responses highlight the need for tailored approaches when optimizing bioelectrochemical interfaces. As research continues to expand the toolbox of materials and methods, these innovations pave the way for more efficient, scalable, and sustainable applications in energy production, bioremediation, and microbial electrosynthesis.
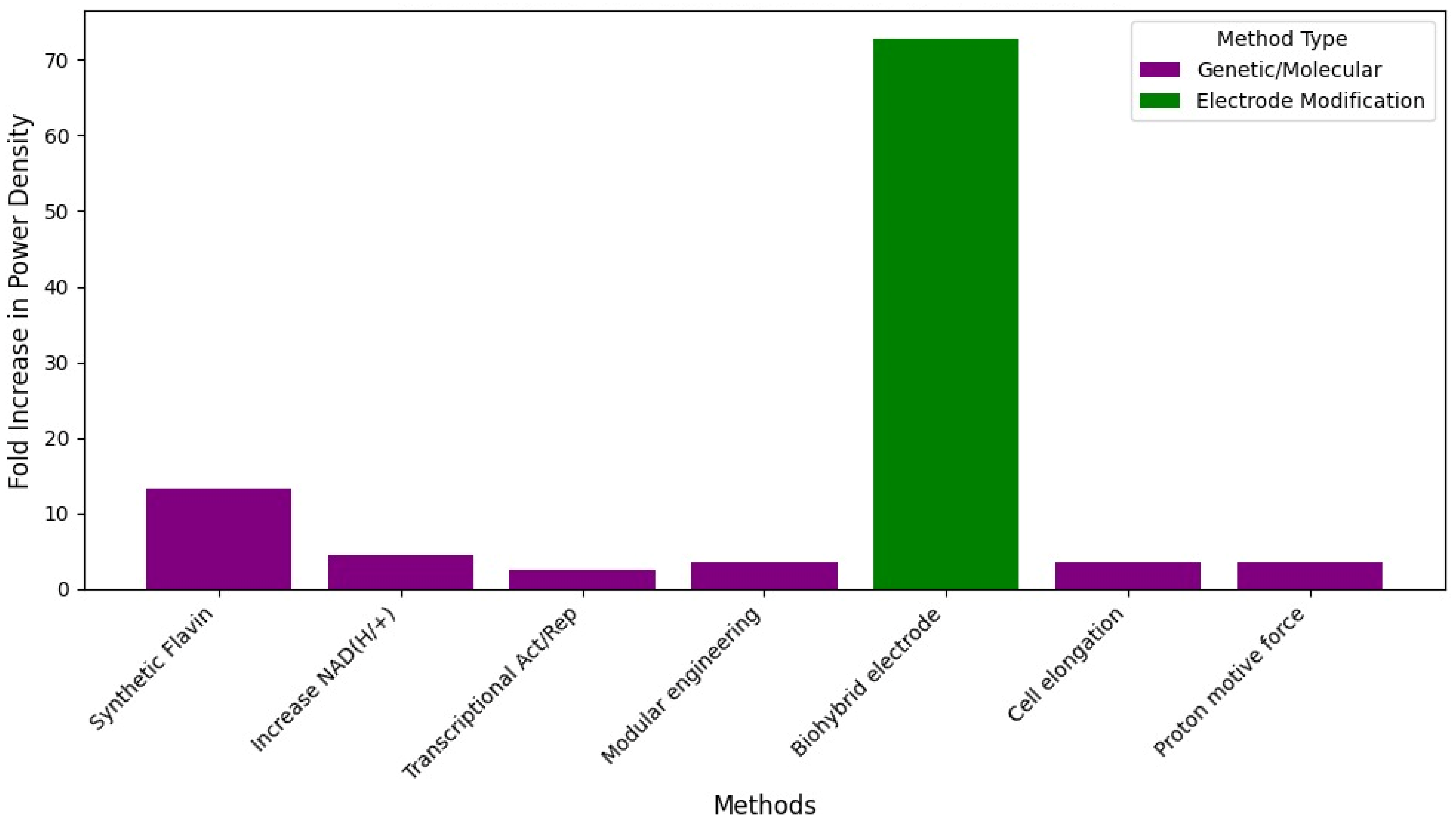
5. Enhancing Extracellular Electron Transfer Through Genetic Modification in Shewanella
Extracellular electron transfer (EET) is a critical process in microbial bioelectrochemical systems (BESs), enabling microorganisms to interface with electrodes for applications ranging from energy production to bioremediation and biosynthesis. While electrode material advancements have significantly improved EET efficiency, genetic engineering offers a complementary approach by directly modulating microbial physiology and electron transfer pathways.
In
S. oneidensis MR-1, a model organism for EET, genetic modifications have targeted specific electron transfer components. The low secretion of flavins in
S. oneidensis MR-1 limits its efficiency in extracellular electron transfer (EET) in bioelectrochemical systems (BESs). To overcome this limitation, in [
68], a synthetic flavin biosynthesis pathway from Bacillus subtilis was heterologously expressed in S. oneidensis MR-1. This modification enhanced the bidirectional EET rate, increasing the maximum power output in microbial fuel cells by ~13.2 times (233.0 mW/m
2) and the inward current by ~15.5 times (255.3 μA/cm
2). The synthetic flavin biosynthesis pathway, derived from
B. subtilis, was introduced into
S. oneidensis MR-1. This pathway synthesizes flavins from guanosine 5′-triphosphate (GTP) and D-ribulose 5′-phosphate (R5P) using enzymes encoded by the
ribADEHC genes. When this pathway was successfully constructed and functionalized in
S. oneidensis, it resulted in a significant increase in secreted flavin concentration. The strain bearing the entire flavin biosynthesis gene cluster (
ribADEHC) produced about 25.7 times higher total flavins (26.15 ± 0.40 μM) compared to the wild-type
S. oneidensis (0.98 ± 0.03 μM). The synthetic flavin pathway genetically engineered into
S. oneidensis MR-1 improved EET primarily by dramatically increasing the endogenous production and secretion of flavins. This higher flavin concentration enhanced electron transfer by accelerating the diffusion in shuttle-mediated EET and potentially increasing the binding of flavins as cofactors to OM c-cytochromes in direct contact-based EET. Additionally, the engineered strain exhibited increased biofilm formation and higher electroactivity per cell, all contributing to the observed, significantly improved bidirectional EET rates and enhanced performance in bioelectrochemical systems like MFCs.
In 2018 [
69], the overexpression of five genes involved in NAD
+ biosynthesis in
S. oneidensis increased intracellular NAD(H/+) levels, resulting in a 4.4-fold higher power density (162.8 mW/m
2) in microbial fuel cells. Additionally, the Coulombic efficiency increased 1.5-fold, and the current per cell rose 4.5-fold. This improvement was attributed to the enhancement of the intracellular electron pool, which boosted extracellular electron transfer (EET) and improved the bacterium’s bioelectrochemical performance. In contrast to previous strategies that often focused on enhancing the electron transfer steps occurring between bacteria and the electrode (e.g., increasing flavin shuttles or c-cytochrome systems), this study demonstrates that increasing the size of the intracellular electron pool itself by enhancing NAD+ biosynthesis is a valuable strategy for improving EET. The main reason for the EET enhancement was the increase in the total intracellular NAD(H/+) level, not significant changes in flavin biosynthesis or key EET pathway components like the MtrCAB complex.
Transcriptional logic gates to control mtrC, mtrA, and cymA expression achieve a 12.5-fold increase in Fe(III) reduction rates. Transcriptional logic gates and Ribosome Binding Site (RBS) optimization (aided by computational design) improve EET by providing programmable and predictable control over the electron transfer flux. They enable researchers to tune the level of EET activity by switching it on in response to a signal (the Buffer gate) and switching it off (the NOT gate). RBS optimization specifically enhances this control by maximizing the dynamic range of the response, making the “on” and “off” states more distinct and controllable. The Fe(III) reduction assay served as the quantifiable output to measure the effectiveness of these engineered controls on EET activity [
70].
Tefft and TerAvest (2019) [
71] engineered inward EET using native Mtr proteins to transfer electrons from an electrode to the inner membrane quinone pool, added a light-driven proton pump (proteorhodopsin) to generate proton-motive force to drive the reverse functioning of NADH dehydrogenases, used the reduction of acetoin to 2,3-butanediol via a heterologous butanediol dehydrogenase (Bdh) as an electron sink to track NADH generation, and demonstrated a generalizable platform to use cathodes to drive NADH-dependent reductions in S. oneidensis MR-1, increasing the cathodic current from −5 to −18 µA for acetoin reduction.
The periplasmic cytochrome c network was optimized by deleting fccA, napB, and tsdB—genes that impair EET when overproduced—and overexpressing cctA, resulting in a 3.62-fold increase in the power density (436.5 mW/m
2) on a carbon cloth anode. The genetic engineering targeting the periplasmic c-type cytochrome (c-Cyt) network involves manipulating the expression levels of specific c-Cyts to optimize electron flow. The study improves EET by specifically deleting detrimental electron sinks (FccA, NapB, and TsdB) and overproducing a key electron carrier (CctA). This optimization streamlines electron transfer from intracellular sources to the outer membrane Mtr system, leading to significantly enhanced bioelectricity generation. The study also noted that the addition of exogenous flavins could further enhance the EET of the optimized strain, which is an additive effect to genetic manipulation [
72].
In 2022 [
73], transcriptional activation and repression resulted in a 2.5-fold increase in power density, while modular engineering to redirect electron flux into the electron transfer chain led to a 3.5-fold increase. Additionally, activation of the cell division gene (minCDE) and repression of the cytotoxic gene (SO_3166) were carried out simultaneously, resulting in a 2.5-fold increase in power density and a 2.9-fold improvement in the azo dye degradation rate. This tool improves extracellular electron transfer (EET) by providing the ability to perform simultaneous, multiplexed genetic regulation. Improving EET efficiency is complex and requires regulating a wide variety of cellular activities controlled by a large number of genes, where some genes need to be upregulated and others downregulated. Previously, achieving this required combining monofunctional tools (either activation or repression), which was labor-intensive and time-consuming. The CRISPR/dCas9-RpoD system overcomes this limitation by allowing both types of regulation concurrently.
Ding et al. (2023) [
74] employed a modular approach, broadening the electron pool (SO1522 lactate transporter), boosting NADH regeneration (gapA and mdh), and promoting electron release (ndh), achieving a 3.5-fold power density increase (270.0 mW/m
2) and a 62% rise in NADH levels. The modular engineering strategy involves the genetic manipulation (specifically overexpression and assembly) of key genes related to the intracellular electron flux pathway. This strategy is designed to redirect electron flux directly from the electron donor to the electron-transfer chain. The strategy breaks down the process into three key modules and targets specific genes within them, broadening the sources of the intracellular electron pool (accelerating substrate uptake), enhancing intracellular NADH regeneration (strengthening the central carbon metabolism), and promoting electron release from intracellular electron pools (enhancing NADH oxidation). In essence, the modular engineering strategy, by systematically targeting and manipulating genes involved in substrate uptake, NADH regeneration, and NADH oxidation, acts as a tool to optimize the flow of electrons within
S. oneidensis from the initial substrate to the extracellular electron transfer pathway, thereby significantly enhancing the overall EET rate and bioelectricity generation.
Transcriptome analysis was used to identify and modify five genes (overexpressing ahpC and ccpA and inactivating putB, pubA, and tonB), yielding a 1.97-fold power density increase (651.78 mW/m
2) by reducing reactive oxygen species and enhancing heme availability. Transcriptome analysis served as a crucial discovery tool to identify candidate genes involved in cellular responses associated with enhanced flavin-mediated EET. The subsequent inverse engineering (overexpression and inactivation) of these specifically identified genes (
ahpC,
ccpA,
pubA,
putB, and
tonB) is what physically improved EET, likely by reducing reactive oxygen species damage and promoting heme accumulation [
75].
Cellular length was increased via divisome downregulation and division inhibitor overexpression, improving DET through greater cytochrome abundance and biofilm adhesion, yielding a 3.41-fold power density increase (248.0 mW/m
2). The engineering of cell elongation in
S. oneidensis MR-1 is achieved by inhibiting cell division, either by blocking the translation of division proteins using anti-sense RNAs or by expressing division inhibitors. This programmed cell elongation improves EET by synergistically boosting intracellular electron generation (specifically NADH oxidation and the quinone pool), enhancing direct electron transfer via increased c-Cyts and facilitated flavin binding, and promoting the formation of more adhesive, thicker, and conductive electroactive biofilms. The integration of QS-based dynamic regulation further optimizes this process by ensuring cell growth is not severely compromised while achieving substantial cell elongation, leading to superior EET performance [
59].
In 2025 [
76], the heterologous expression of microbial rhodopsin cR-1 in
S. oneidensis enhanced the proton motive force (PMF), improving extracellular electron transfer (EET). The recombinant strain exhibited a 3.49-fold higher power density (0.87 W/m
2) compared to the wild-type strain. In essence, the light-driven proton pump acts as a supplementary energy generator, utilizing light to boost the cell’s PMF. This extra energy fuels lactate metabolism to produce more intracellular electrons (NADH), which are then more efficiently transferred to the extracellular environment via the EET pathway, resulting in significantly enhanced bioelectricity generation.
Zhao et al. (2023) [
57] took a synthetic biology approach with
S. oneidensis MR-1, combining genetic modifications to boost riboflavin production (837.74 μM) and MtrC expression with a spider-web-like graphene oxide and multiwalled carbon nanotube hybrid biofilm, resulting in a remarkable 77.83-fold increase in power density (3736 mW/m
2). While cell elongation significantly improved biofilm formation and, subsequently, EET, the source also notes a limitation: although the biofilm was denser, the increase in power was “not as high as expected”. Impedance analysis showed that the elongated strain still had a relatively high charge–transfer resistance. This suggested that while elongation helped build a better structure, further steps were needed to optimize conductivity and reduce overpotential, which led to the development of the hybrid biofilm incorporating conductive materials. In summary, the tool of engineering cell elongation using sulA overexpression improves EET primarily by dramatically increasing the ability of
Shewanella cells to form a denser, thicker, and more adhesive electroactive biofilm on the electrode surface, which facilitates electron transfer to the electrode.
These diverse strategies highlight the versatility of genetic engineering in enhancing both outward and inward EET in
Shewanella.
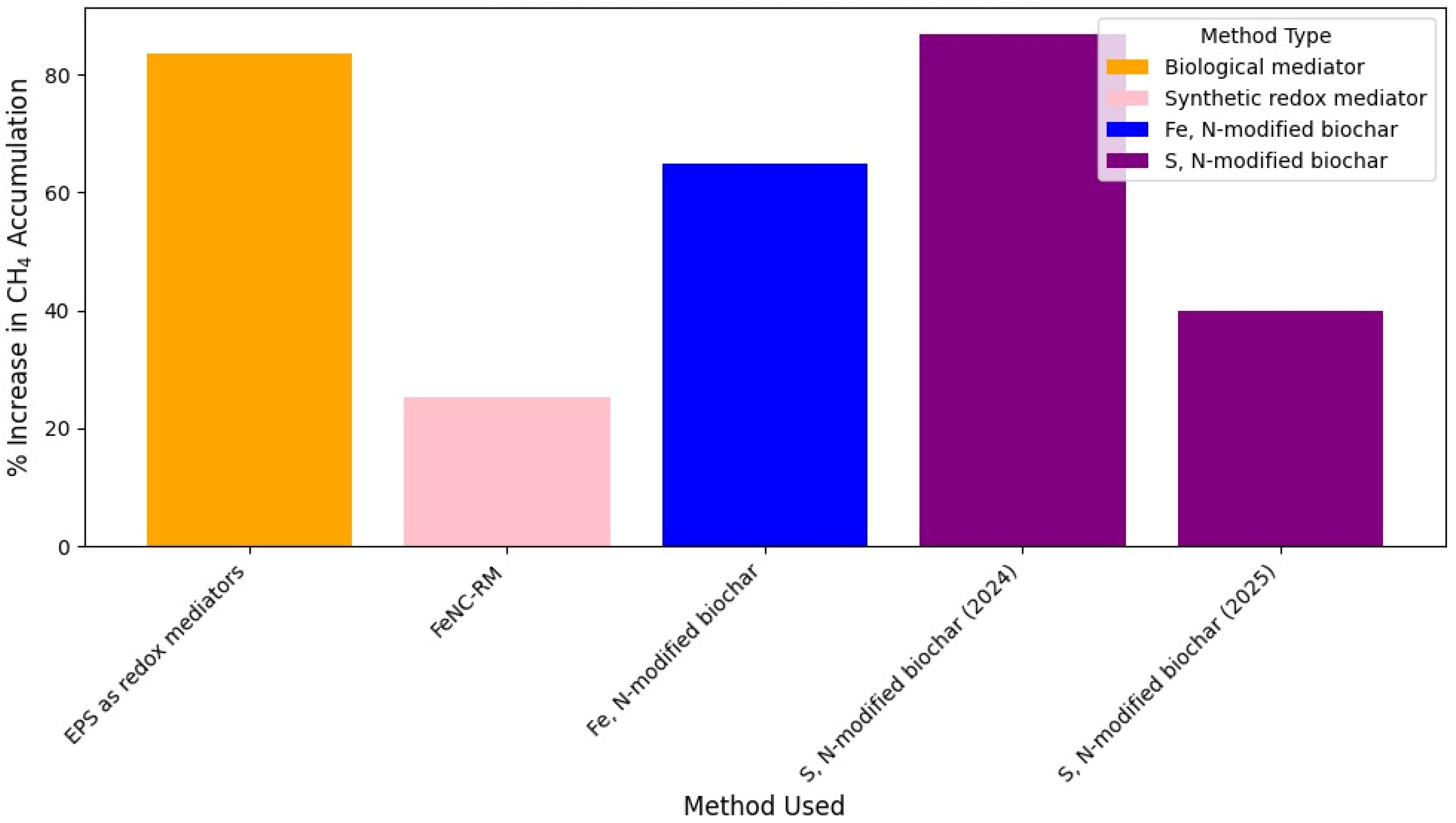
Figure 5 shows the effectiveness of different anode materials to improve EET in mixed culture MFCs, whereas
Figure 6 summarizes the effects of electrode materials obtained with
S. oneidensis cultures.
Figure 7 summarizes the relative efficacy of different strategies to enhance extracellular electron transfer (EET) in
S. oneidensis.
Several genetic engineering tools and strategies have been employed to improve EET in S. oneidensis MR-1. Important conclusions can be drawn.
EET is a Multifaceted Process with Multiple Bottlenecks: The diverse range of genetic targets indicates that EET efficiency in Shewanella is not limited by a single factor but involves multiple interconnected cellular processes. Different tools target different aspects: improving intracellular electron generation and flux by enhancing substrate uptake, NADH regeneration, and NADH oxidation; optimizing the periplasmic electron transfer network by adjusting the composition and abundance of specific c-type cytochromes; boosting energy availability (ATP/PMF) to potentially fuel the metabolic pathways and electron transfer components; enhancing biofilm formation and cell–electrode interaction by modifying cell morphology or surface properties; increasing the availability of electron shuttles like flavins; and directly regulating the expression of core EET pathway components like the Mtr system and CymA.
Different Genetic Strategies Can Lead to Significant Improvements: Despite targeting distinct bottlenecks, various genetic modifications have demonstrated notable improvements in EET performance [
74,
76,
77,
78].
Simply Increasing Total Component Abundance is Not Always Effective as Balance and Specificity Matter: It is not enough to increase the overall levels of certain EET components. The specific type and relative abundance are crucial. Overexpressing the ccmFGH operon to increase total c-type cytochrome content surprisingly
impairs bioelectricity generation. Specific periplasmic cytochromes (FccA, NapB, and TsdB) negatively influence EET when in excess, while others (like CctA) have positive impacts; optimizing the composition by deleting inhibitory ones and overexpressing beneficial ones is key [
13,
76,
79].
Combining Different Genetic Modifications or Strategies Often Leads to Enhanced Performance: Many studies show that assembling multiple targeted genetic changes can yield additive or synergistic improvements [
9,
68,
80,
81].
Understanding Underlying Mechanisms is Crucial for Rational Design: Successful engineering relies heavily on investigating the specific physiological and molecular impacts of genetic changes [
13,
72,
79].
Genetic Tools Allow for Programmable Control, Not Just Maximal Enhancement: Tools like CRISPR/dCas9-RpoD and transcriptional logic gates demonstrate the ability to precisely tune gene expression (upregulation, downregulation, and simultaneous control) and link it to predictable EET activity (turn-on and turn-off behavior). This moves beyond simply maximizing output to enabling more complex, dynamic control over EET flux for potential applications like biosensing or computing [
70].
In conclusion, the genetic engineering tools applied to Shewanella reveal that improving EET is a complex endeavor requiring strategies that address various cellular bottlenecks. Success often comes from understanding the specific roles and interactions of genes and proteins, optimizing the balance of components rather than just increasing total levels, combining complementary genetic modifications, and leveraging precise regulatory systems for programmable control over electron transfer pathways.
6. Enhancing Extracellular Electron Transfer Through Genetic Modification in Geobacter
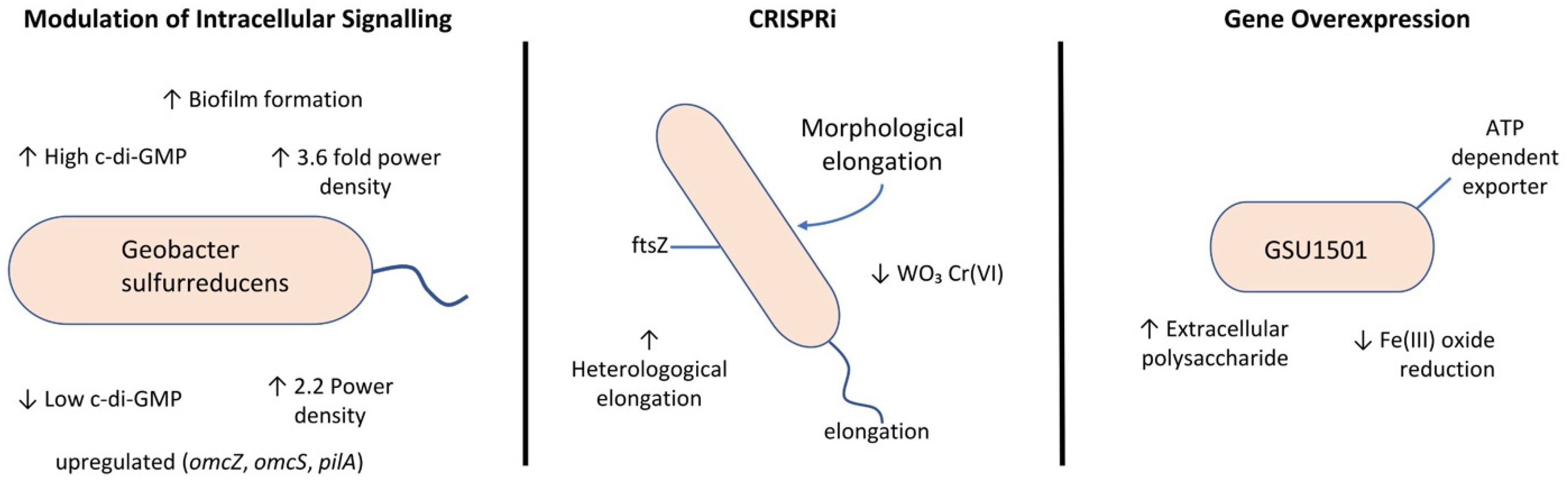
One promising field for EET enhancement involves modulating intracellular signaling pathways. Hu et al. (2024) [
82] demonstrated this in
G. sulfurreducens by manipulating cyclic di-GMP (c-di-GMP) levels using a carbon cloth anode. The study proposes a regulatory model where low and high c-di-GMP levels both enhance EET in
G. sulfurreducens biofilms, but they do so through different mechanisms and impacts on the biofilm structure. A low level reduces biofilm thickness but upregulates extracellular electron carriers (like OmcZ, OmcS, OmcE, and PilA) to improve efficiency, boosting the power density by 2.2-fold, with OmcZ playing a decisive role in electricity generation. A high level promotes thick biofilm formation and upregulates periplasmic cytochromes (the PpcA family) to enhance electron transfer within single cells, increasing the power density by 3.6-fold. This highlights a complex, differential regulatory network governed by c-di-GMP in
G. sulfurreducens EET.
In 2023, a new genetic editing tool, specifically the CRISPR interference (CRISPRi) system, was constructed in
G. sulfurreducens. This tool does not directly improve EET itself but rather provides a means for the efficient and precise control of gene expression, enabling the study and manipulation of gene functions, including those related to EET. A clustered regularly interspaced short palindromic repeats interference (CRISPRi) system was constructed in
G. sulfurreducens to achieve the repression of an essential gene-aroK and the morphogenic genes ftsZ and mreB [
83]. Finally, applying the engineered strain to the reduction of tungsten trioxide (WO3), methyl orange (MO), and Cr(VI), it was found that morphological elongation through ftsZ repression amplified the extracellular electron transfer proficiency of
G. sulfurreducens and facilitated its contaminant transformation efficiency. The authors suggest that this improvement in EET due to elongation may be caused by several factors: an increased surface area of the bacteria after elongation, an increased amount of multiheme c-type cytochromes on the outer membrane, allowing for increased electron transfer to extracellular acceptors, and the facilitation of bacterial interconnectivity and aggregate formation, which was observed in elongated strains during Cr(VI) reduction, potentially enhancing contact with the electron acceptor. In contrast, cell rounding caused by mreB repression using the CRISPRi system did not show a significant improvement in EET ability and marginally diminished the Cr(VI) reduction capacity.
Extracellular polysaccharide, a sticky component surrounding microbes, plays an important role in EET. In 2020, ref. [
84] researchers constructed a mutant strain (PCA-1501) of
G. sulfurreducens PCA by overexpressing the gene GSU1501. GSU1501 is part of the ATP-dependent exporter in the polysaccharide biosynthesis gene operon and is involved in the synthesis and export of polysaccharides in
G. sulfurreducens. The experimental results showed that the overexpression of GSU1501 increased extracellular polysaccharide secretion by 25.5%, which promoted the formation of biofilms with higher thickness and viability, as well as the content of extracellular c-type cytochromes. Compared with the control strain, the mutant showed a higher capacity of Fe(III) oxide reduction and current generation (increased by 20.4% and 22.2%, respectively). This study improved EET in
G. sulfurreducens primarily by significantly increasing extracellular polysaccharide production, which, in turn, led to the formation of a thicker, more viable biofilm and anchored more extracellular c-type cytochromes, particularly OmcZ.
Ueki et al. (2018) [
85] engineered the
G. sulfurreducens strain ACL with ATP-dependent citrate lyase for autotrophic growth on cathodes. This was achieved by introducing genes (aclA and aclB) encoding an ATP-dependent citrate lyase from
Chlorobium limicola into the chromosome of
G. sulfurreducens. This enzyme is necessary to complete a reverse TCA cycle, enabling the synthesis of biosynthetic precursors from carbon dioxide. This genetic modification, enabling autotrophic growth using carbon dioxide as the sole carbon source, fundamentally improves the ability of
Geobacter to perform cathode-based EET, which is the uptake of electrons from an insoluble extracellular source, such as an electrode. The introduction of the ATP-dependent citrate lyase acts as a tool by enabling autotrophic growth, which, in turn, allows
G. sulfurreducens to overcome limitations in utilizing cathodes as electron donors, resulting in significantly improved growth and electron uptake efficiency on these electrodes. This makes the strain ACL a valuable model organism for studying the mechanisms of electron transfer from extracellular donors.
Several genetic engineering tools and strategies have been employed to improve EET in Geobacter. Important conclusions can be drawn.
EET enhancement is multifaceted: no single method universally outperforms others across all conditions. Biofilm structure, morphology, genetic regulation, and metabolic capabilities each impact different aspects of EET. Fine control over intracellular signaling (e.g., c-di-GMP) offers powerful, dual-mode improvements but requires precise dynamic regulation to balance biofilm structure and EET efficiency [
82]. CRISPRi provides precision and versatility, but EET outcomes are highly gene- and context-dependent. Morphological modifications can improve or hinder EET based on how they alter cell surface area, cytochrome expression, and cell–cell connectivity [
83]. Biofilm matrix engineering via polysaccharide overexpression enhances EET through structural stability and cytochrome retention but risks reduced conductivity if overproduced. Metabolic engineering for autotrophy (ACL strain) represents a strategic platform for expanding EET applications to cathode-based and CO
2-fixation systems, though it introduces complexity and niche specificity [
85]. Future strategies might integrate multi-factorial approaches, for example, combining CRISPRi to optimize cytochrome expression with c-di-GMP modulation for tailored biofilm structures while ensuring metabolic versatility for diverse EET platforms. However, trade-offs between growth, biofilm conductivity, energy efficiency, and system scalability remain critical constraints that must be balanced through systems biology-guided optimization.
Figure 8 shows the main alternatives for enhancing extracellular electron transfer through genetic modification in
Geobacter.
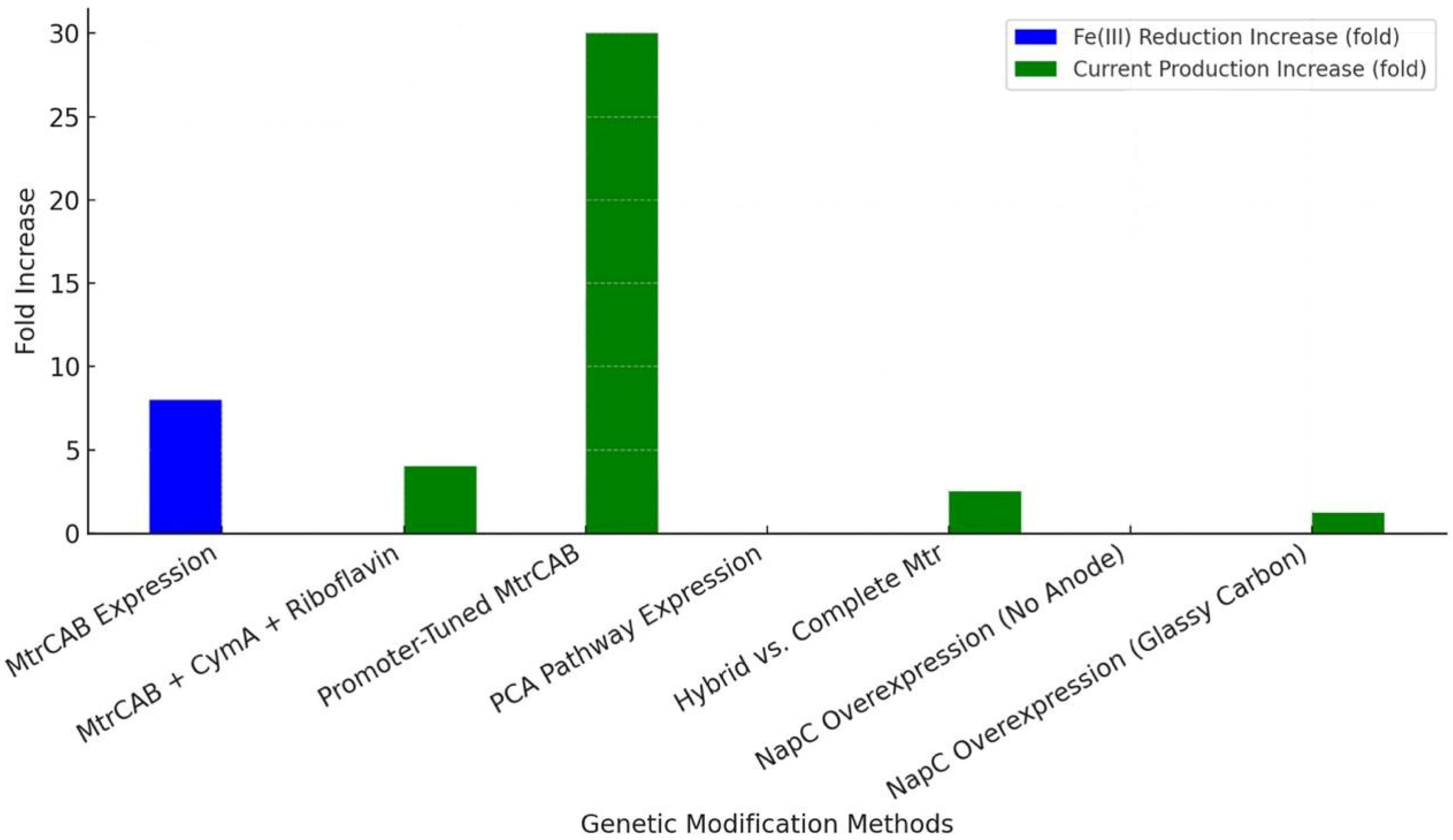
Genetic engineering has also enabled non-electrogenic bacteria, like
E. coli, to perform EET by introducing heterologous electron transfer pathways. Jensen et al. (2010) [
86] engineered
E.
coli with the
S. oneidensis MtrCAB conduit, achieving an 8-fold increase in Fe(III) citrate reduction and a 4-fold increase in Fe
2O
3 reduction without an anode or exogenous shuttle. Similarly, Jensen et al. (2016) [
87] improved this system by coexpressing CymA with MtrCAB on a carbon felt anode, using riboflavin as a shuttle, resulting in a ~4-fold increase in current production and improved Fe(III) reduction rates. Goldbeck et al. (2012) [
88] further optimized MtrCAB expression in E. coli through promoter tuning, achieving a 30-fold increase in current per colony-forming unit by balancing cytochrome synthesis and cellular stress. Feng et al. (2020) [
89] took a different approach, expressing the
P. aeruginosa phenazine-1-carboxylic acid (PCA) pathway in E. coli with a carbon felt anode, enabling PCA to act as a self-secreted mediator that enhanced fumarate reduction to succinate. Mouhib et al. (2021) [
90] compared hybrid and complete Mtr pathways in E. coli, finding that the entire Mtr system with periplasmic shuttle STC increased current production 2-3-fold on graphite felt electrodes. Bennett et al. (2022, 2023) [
91,
92] modulated E. coli’s trans-plasma membrane electron transport system by overexpressing NapC, altering Fe(III) reduction rates and enhancing iron-catalyzed polymerization kinetics without an anode (2022) and achieving a 1.21-fold increase in the polymerization rate with glassy carbon electrodes (2023).
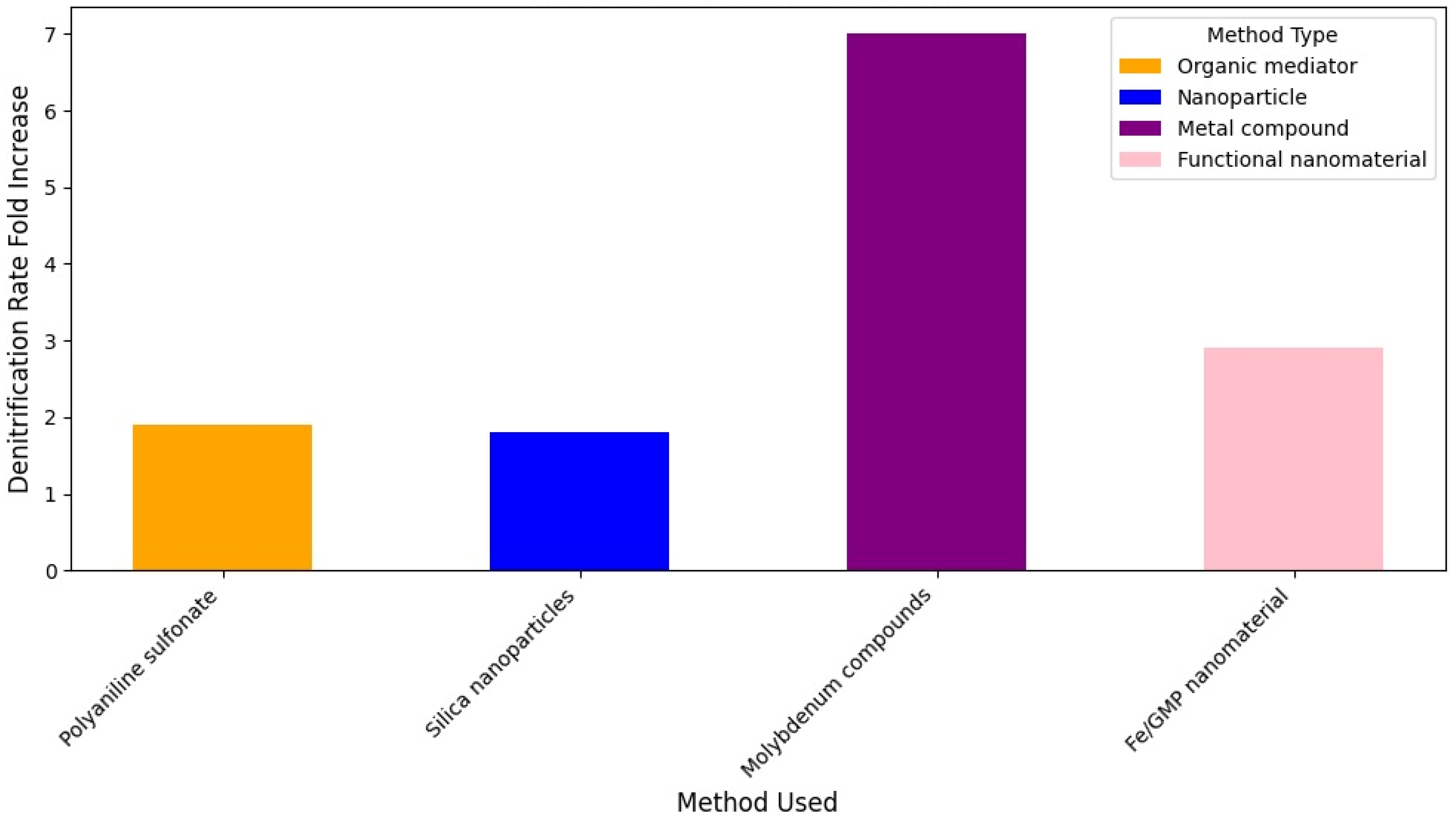
Figure 9 shows a power density increase comparison between genetic modification methods to improve EET in
E. coli.
Other bacteria have also been considered in genetic EET enhancement studies. Gu et al. (2023) [
93] rewired
Lactococcus lactis MG1363’s respiratory pathway by blocking menaquinone biosynthesis and promoting ACNQ-mediated electron transfer, improving ferricyanide reduction on glassy carbon electrodes, with adaptive laboratory evolution further boosting efficiency via menA mutations.