Impact of Spontaneous Fermentation on the Physicochemical and Sensory Qualities of Cacao
Abstract
1. Introduction
2. Materials and Methods
| Title-abs-key | Title-abs-key | Title-abs-key | Publication year | |||
| Genotypes of cacao | Fermentation of cacao | Fermentation methods | >2014 | |||
| OR | AND | OR | AND | OR | AND | AND |
| Cacao genotypes | Cacao fermentation | Fermentation process | <2025 |
| Title-abs-key | Title-abs-key | Publication year | ||
| Spontaneous fermentation of cacao | Cacao bean quality | >2014 | ||
| OR | AND | OR | AND | AND |
| Natural fermentation of cacao | Quality of cacao beans | <2025 |
| Title-abs-key | Title-abs-key | Publication year | ||
| Fermentation methods of cacao | Turning time | >2014 | ||
| OR | AND | OR | AND | AND |
| Cacao fermentation techniques | Cacao beans | <2025 |
Cacao Fermentation Methods Globally
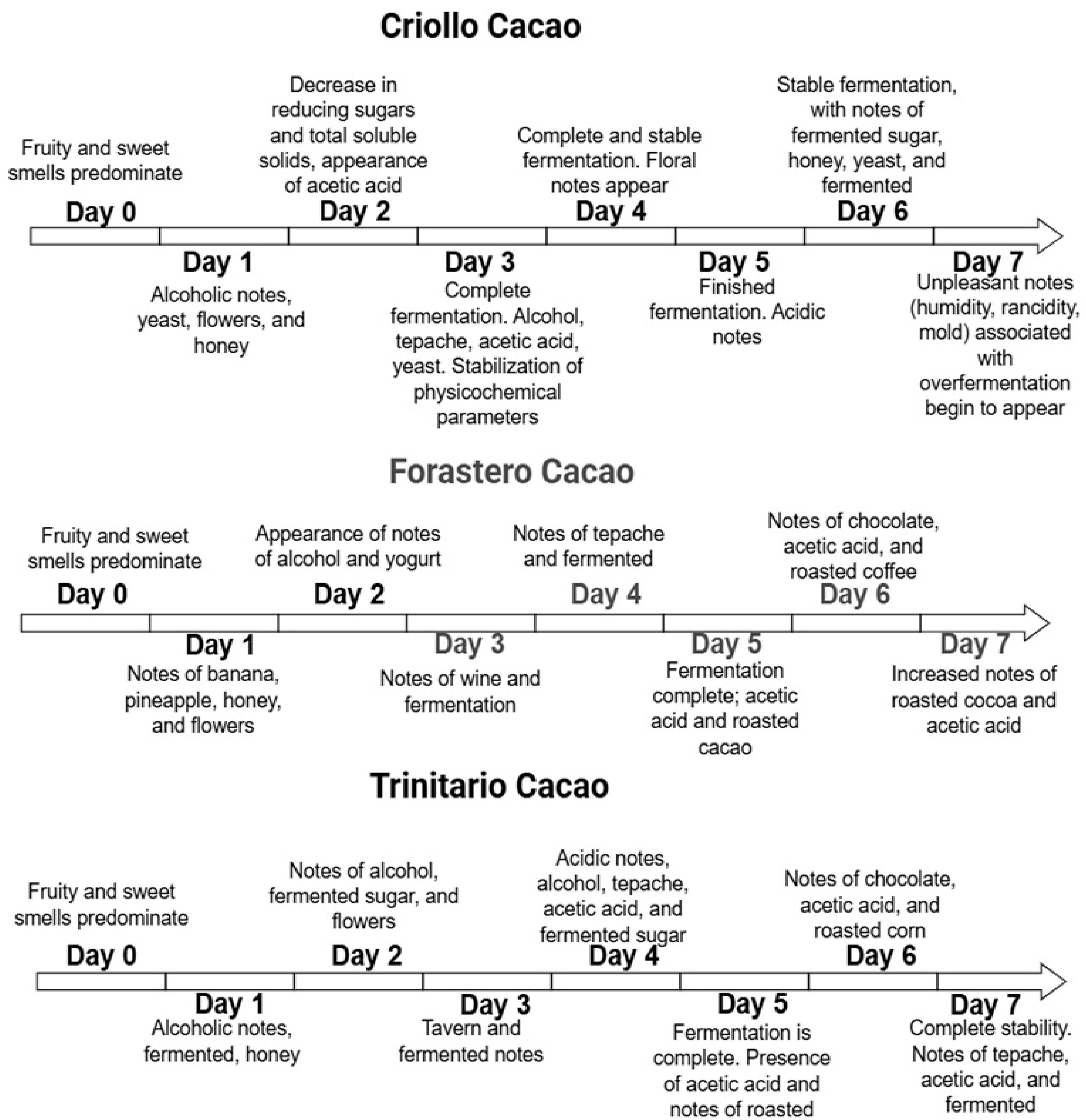
3. Results and Discussion
3.1. Factors Associated with Cacao Fermentation Influence the Final Bean Quality
3.2. Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, H.; Fei, T.; Liu, X.; Lin, X.; Wang, L. Oat (Avena sativa L.) Fermented by GRAS-Grade Microorganisms: From Improvement of the Quality Properity and Health Benefits, Safety Assessment to Potential Industrial Applications. Trends Food Sci. Technol. 2025, 160, 105020. [Google Scholar] [CrossRef]
- Liu, X.; Ma, J.; Fan, G. Microbiological Safety and Quality of Fermented Products. Foods 2023, 12, 2204. [Google Scholar] [CrossRef]
- Abbaspour, N. Fermentation’s Pivotal Role in Shaping the Future of Plant-Based Foods: An Integrative Review of Fermentation Processes and Their Impact on Sensory and Health Benefits. Appl. Food Res. 2024, 4, 100468. [Google Scholar] [CrossRef]
- Amato, K.R.; Mallott, E.K.; Maia, P.D.; Sardaro, M.L.S. Predigestion as an Evolutionary Impetus for Human Use of Fermented Food. Curr. Anthropol. 2021, 62, S207–S219. [Google Scholar] [CrossRef]
- Tangyu, M.; Fritz, M.; Tan, J.P.; Ye, L.; Bolten, C.J.; Bogicevic, B.; Wittmann, C. Flavour by Design: Food-Grade Lactic Acid Bacteria Improve the Volatile Aroma Spectrum of Oat Milk, Sunflower Seed Milk, Pea Milk, and Faba Milk towards Improved Flavour and Sensory Perception. Microb. Cell Fact. 2023, 22, 133. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, X.; Hao, L.; Zhang, G.; Jin, Z.; Li, C.; Yang, Y.; Rao, J.; Chen, B. Traditional Fermented Soybean Products: Processing, Flavor Formation, Nutritional and Biological Activities. Crit. Rev. Food Sci. Nutr. 2022, 62, 1971–1989. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Islam, F.; Ateeq, H.; Asghar, A.; Shah, Y.A.; Ofoedu, C.E.; Chacha, J.S. Nutritional Health Perspective of Natto: A Critical Review. Biochem. Res. Int. 2022, 2022, 5863887. [Google Scholar] [CrossRef]
- Hachmeister, K.A.; Fung, D.Y.C. Tempeh: A Mold-Modified Indigenous Fermented Food Made from Soybeans and/or Cereal Grains. Crit. Rev. Microbiol. 1993, 19, 137–188. [Google Scholar] [CrossRef]
- Terefe, Z.K.; Omwamba, M.N.; Nduko, J.M. Effect of Solid State Fermentation on Proximate Composition, Antinutritional Factors and In Vitro Protein Digestibility of Maize Flour. Food Sci. Nutr. 2021, 9, 6343–6352. [Google Scholar] [CrossRef]
- Soro-Yao, A.A.; Brou, K.; Amani, G.; Thonart, P.; Djè, K.M. The Use of Lactic Acid Bacteria Starter Cultures during the Processing of Fermented Cereal-Based Foods in West Africa: A Review. Trop. Life Sci. Res. 2014, 25, 81–100. [Google Scholar]
- Schwan, R.F.; Wheals, A.E. The Microbiology of Cocoa Fermentation and its Role in Chocolate Quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Arulmari, R.; Visvanathan, R. Effect of Fermentation Methods and Turning Interval on the Quality of Cocoa Beans (Theobroma cacao). Agric. Res. 2024, 13, 586–598. [Google Scholar] [CrossRef]
- Awaliyah, F.; Langkong, J.; Syarifuddin, A. An Overview: The Effect of Fermentation and Roasting Methods on Cocoa Quality. IOP Conf. Ser. Earth Environ. Sci. 2023, 1230, 012160. [Google Scholar] [CrossRef]
- Kongor, J.E.; Hinneh, M.; Van de Walle, D.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors Influencing Quality Variation in Cocoa (Theobroma cacao) Bean Flavour Profile—A Review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Nascimento, L.L.; Pereira, M.S.; de Almeida, L.S.; da Silveira Ferreira, L.; de Moura Pita, B.L.; de Souza, C.O.; Ribeiro, C.D.F.; Fricks, A.T. Innovation in Cocoa Fermentation: Evidence from Patent Documents and Scientific Articles. Fermentation 2024, 10, 251. [Google Scholar] [CrossRef]
- Rahardjo, Y.P.; Rahardja, S.; Samsudin; Saidah; Dalapati, A.; Amalia, A.F.; Purwaningsih, H.; Syamsu, K. A Literature Review on Cocoa Fermentation Techniques to Shorten Fermentation Time. IOP Conf. Ser. Earth Environ. Sci. 2022, 974, 012111. [Google Scholar] [CrossRef]
- Junior, G.C.A.C.; do Espírito-Santo, J.C.A.; Ferreira, N.R.; Marques-Da-Silva, S.H.; Oliveira, G.; Vasconcelos, S.; de Fátima Oliveira de Almeida, S.; Silva, L.R.C.; Gobira, R.M.; de Figueiredo, H.M.; et al. Isolamento e Identificação de Leveduras Durante a Fermentação Natural de Cacau Em Uma Região Altamente Produtora No Norte Do Brasil. Sci. Plena 2020, 16. [Google Scholar] [CrossRef]
- De Vuyst, L.; Weckx, S. The Cocoa Bean Fermentation Process: From Ecosystem Analysis to Starter Culture Development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Functional Role of Yeasts, Lactic Acid Bacteria and Acetic Acid Bacteria in Cocoa Fermentation Processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor Formation and Character in Cocoa and Chocolate: A Critical Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Miller, K.B.; Rank, C.; Stuart, D.A. Impact of Fermentation, Drying, Roasting, and Dutch Processing on Epicatechin and Catechin Content of Cacao Beans and Cocoa Ingredients. J. Agric. Food Chem. 2010, 58, 10518–10527. [Google Scholar] [CrossRef] [PubMed]
- Endang, S.; Jumiono, A. Faktor-Faktor Pasca Panen Yang Memengaruhi Mutu Kakao. Jurnal Ilmiah Pangan Halal 2020, 2, 73–78. [Google Scholar]
- Velásquez-Reyes, D.; Rodríguez-Campos, J.; Avendaño-Arrazate, C.; Gschaedler, A.; Alcázar-Valle, M.; Lugo-Cervantes, E. Forastero and Criollo Cocoa Beans, Differences on the Profile of Volatile and Non-Volatile Compounds in the Process from Fermentation to Liquor. Heliyon 2023, 9, e15129. [Google Scholar] [CrossRef] [PubMed]
- Daymond, A.; Mendez, D.G.; Hadley, P.; Bastide, P. A Global Review of Cocoa Farming Systems; International Cocoa Organization (ICCO): Abidjan, Côte d’Ivoire, 2021. [Google Scholar]
- Saltini, R.; Akkerman, R.; Frosch, S. Optimizing Chocolate Production through Traceability: A Review of the Influence of Farming Practices on Cocoa Bean Quality. Food Control. 2013, 29, 167–187. [Google Scholar] [CrossRef]
- Campos, S.d.M.; Martínez-Burgos, W.J.; Dos Reis, A.G.; Ocán-Torres, D.Y.; Dos Santos Costa, G.; Rosas Vega, F.; Alvarez Badel, B.; Coronado, L.S.; Serra, J.L.; Soccol, C.R. The Role of Microbial Dynamics, Sensorial Compounds, and Producing Regions in Cocoa Fermentation. Microbiol. Res. 2025, 16, 75. [Google Scholar] [CrossRef]
- Ferrari, A.; Vinderola, G.; Weill, R. Alimentos Fermentados: Microbiología, Nutrición, Salud y Cultura; The Danone Institute: Palaiseau, France, 2020. [Google Scholar]
- Samagaci, L.; Ouattara, H.; Niamké, S.; Lemaire, M. Pichia Kudrazevii and Candida Nitrativorans Are the Most Well-Adapted and Relevant Yeast Species Fermenting Cocoa in Agneby-Tiassa, a Local Ivorian Cocoa Producing Region. Food Res. Int. 2016, 89, 773–780. [Google Scholar] [CrossRef]
- Rottiers, H.; Tzompa Sosa, D.A.; De Winne, A.; Ruales, J.; De Clippeleer, J.; De Leersnyder, I.; De Wever, J.; Everaert, H.; Messens, K.; Dewettinck, K. Dynamics of Volatile Compounds and Flavor Precursors during Spontaneous Fermentation of Fine Flavor Trinitario Cocoa Beans. Eur. Food Res. Technol. 2019, 245, 1917–1937. [Google Scholar] [CrossRef]
- Niikoi Kotey, R.; Asomaning Odoom, D.; Kumah, P.; Oppong Akowuah, J.; Donkor, E.F.; Kwatei Quartey, E.; Kofi Sam, E.; Owusu-Kwarteng, J.; Gyasi Santo, K.; Kwami-Adala, F.; et al. Effects of Fermentation Periods and Drying Methods on Postharvest Quality of Cocoa (Theobroma cacao) Beans in Ghana. J. Food Qual. 2022. [Google Scholar] [CrossRef]
- Crafack, M.; Keul, H.; Eskildsen, C.E.; Petersen, M.A.; Saerens, S.; Blennow, A.; Skovmand-Larsen, M.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Impact of Starter Cultures and Fermentation Techniques on the Volatile Aroma and Sensory Profile of Chocolate. Food Res. Int. 2014, 63, 306–316. [Google Scholar] [CrossRef]
- Figueroa-Hernández, C.; Mota-Gutierrez, J.; Ferrocino, I.; Hernández-Estrada, Z.J.; González-Ríos, O.; Cocolin, L.; Suárez-Quiroz, M.L. The Challenges and Perspectives of the Selection of Starter Cultures for Fermented Cocoa Beans. Int. J. Food Microbiol. 2019, 301, 41–50. [Google Scholar] [CrossRef]
- Assa, A.; Rosniati; Yunus, M.R. Effects of Cocoa Clones and Fermentation Times on Physical and Chemical Characteristics of Cocoa Beans (Theobroma cacao L.). IOP Conf. Ser. Mater. Sci. Eng. 2019, 528, 012079. [Google Scholar] [CrossRef]
- Dewandari, K.T.; Rahmawati, R.; Munarso, S.J. The Effect of Techniques and Fermentation Time on Cocoa Beans Quality (Theobroma cacao L.). IOP Conf. Ser. Earth Environ. Sci. 2021, 653, 012046. [Google Scholar] [CrossRef]
- Fang, Y.; Li, R.; Chu, Z.; Zhu, K.; Gu, F.; Zhang, Y. Chemical and Flavor Profile Changes of Cocoa Beans (Theobroma cacao L.) During Primary Fermentation. Food Sci. Nutr. 2020, 8, 4121–4133. [Google Scholar] [CrossRef]
- Hamdouche, Y.; Meile, J.C.; Lebrun, M.; Guehi, T.; Boulanger, R.; Teyssier, C.; Montet, D. Impact of Turning, Pod Storage and Fermentation Time on Microbial Ecology and Volatile Composition of Cocoa Beans. Food Res. Int. 2019, 119, 477–491. [Google Scholar] [CrossRef]
- Schwan, R.F.; Fleet, G.H. Cocoa and Coffee Fermentations; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Barišić, V.; Kopjar, M.; Jozinović, A.; Flanjak, I.; Ačkar, Đ.; Miličević, B.; Šubarić, D.; Jokić, S.; Babić, J. The Chemistry behind Chocolate Production. Molecules 2019, 24, 3163. [Google Scholar] [CrossRef]
- Deus, V.L.; Bispo, E.S.; Franca, A.S.; Gloria, M.B.A. Understanding Amino Acids and Bioactive Amines Changes during On-Farm Cocoa Fermentation. J. Food Compos. Anal. 2021, 97, 103776. [Google Scholar] [CrossRef]
- Pallares Pallares, A.; Estupiñán A, M.R.; Perea Villamil, J.A.; López Giraldo, L.J. Impacto de La Fermentación y Secado Sobre El Contenido de Polifenoles y Capacidad Antioxidante Del Clon de Cacao CCN-51. Revista ION 2017, 29, 7–21. [Google Scholar] [CrossRef]
- Velásquez, C.L.; Eduardo, R.S.; Edith, C.C. Efecto de La Frecuencia de Volteo y El Tiempo de Fermentación Sobre Las Propiedades de Calidad y Actividad Antioxidante de Los Granos de Cacao (Theobroma cacao); Universidad Nacional de Colombia: Bogotá, Colombia, 2016. [Google Scholar]
- García-Rincón, P.; Núñez-Ramírez, J.; Bahamón-Monje, A. Physicochemical and Sensory Characteristics of Fermented Almonds of National Cacao (Theobroma cacao L.) with Addition of Probiotics in the Amazonic Research Center, Cimaz Macagual (Caquetá, Colombia). Ingeniería y Competitividad 2021, 23, e21210885. [Google Scholar] [CrossRef]
- Liendo, R.J. Revista de la Facultad de Agronomía; Universidad del Zulia: Zulia, Venezuela, 2015; Volume 31, pp. 41–62. [Google Scholar]
- Sara, D. Estudio Fenotípico de Las Levaduras Presentes En La Fermentación de Los Granos de Cacao Forastero Proveniente de La Localidad de Caño Rico, Estado Miranda, Venezuela. EDUCAB 2023, 14, 80–90. [Google Scholar]
- Ruíz Muñoz Efecto de La Temperatura de Fermentación Sobre La Calidad Física y Organoléptica Del Grano de Cacao (Theobroma cacao L.); Universidad Nacional de San Martin-TAarapoto: Tarapoto, Peru, 2019.
- Gutierrez Paredes, R.R.; Gonzalez Fuentes, G. Evaluación de La Aplicación de Tecnologías Para La Fermentación y Secado Del Cacao (Theobroma cacao L.) Tipo CCN-51 y Criollo. Revista Amazónica De Ciencias Básicas Y Aplicadas 2022, 1, e156. [Google Scholar] [CrossRef]
- Ipanaqué, W.; Castillo, J.; Robles, H.; Belupú, I. Desarrollo e Implementación de Un Prototipo de Acero Inoxidable Para Evaluar El Proceso de Fermentación de Granos de Cacao. In Proceedings of the International Symposium on Cocoa Research (ISCR), Lima, Peru, 13–17 November 2017. [Google Scholar]
- Albertini, B.; Schoubben, A.; Guarnaccia, D.; Pinelli, F.; Della Vecchia, M.; Ricci, M.; Di Renzo, G.C.; Blasi, P. Effect of Fermentation and Drying on Cocoa Polyphenols. J. Agric. Food Chem. 2015, 63, 9948–9953. [Google Scholar] [CrossRef] [PubMed]
- Teneda Llerena, W.F. Mejoramiento Del Proceso de Fermentación Del Cacao (Theobroma cacao L.) Variedad Nacional y Variedad CCN51; Universidad Nacional de Andalucia: Andalusia, Spain, 2016. [Google Scholar]
- Santos, D.S.; Rezende, R.P.; dos Santos, T.F.; de Lima Silva Marques, E.; Ferreira, A.C.R.; de Cerqueira e Silva, A.B.; Romano, C.C.; da Cruz Santos, D.W.; Dias, J.C.T.; Tavares Bisneto, J.D. Fermentation in Fine Cocoa Type Scavina: Change in Standard Quality as the Effect of Use of Starters Yeast in Fermentation. Food Chem. 2020, 328, 127110. [Google Scholar] [CrossRef] [PubMed]
- Galvez Del Cid, D.I. Efectos Del Secado En La Fermentación y Características Del Cacao (Theobroma Cacao L.) de Almendra Blanca Var. Carmelo; Escuela Agrícola Panamericana, Zamorano: Tegucigalpa, Honduras, 2019. [Google Scholar]
- Streule, S.; André, A.; Freimüller Leischtfeld, S.; Chatelain, K.; Gillich, E.; Chetschik, I.; Miescher Schwenninger, S. Influences of Depulping, Pod Storage and Fermentation Time on Fermentation Dynamics and Quality of Ghanaian Cocoa. Foods 2024, 13, 2590. [Google Scholar] [CrossRef]
- Koné, M.K.; Guéhi, S.T.; Durand, N.; Ban-Koffi, L.; Berthiot, L.; Tachon, A.F.; Brou, K.; Boulanger, R.; Montet, D. Contribution of Predominant Yeasts to the Occurrence of Aroma Compounds during Cocoa Bean Fermentation. Food Res. Int. 2016, 89, 910–917. [Google Scholar] [CrossRef]
- Koné, K.M.; Assi-Clair, B.J.; Kouassi, A.D.D.; Yao, A.K.; Ban-Koffi, L.; Durand, N.; Lebrun, M.; Maraval, I.; Bonlanger, R.; Guehi, T.S. Pod Storage Time and Spontaneous Fermentation Treatments and Their Impact on the Generation of Cocoa Flavour Precursor Compounds. Int. J. Food Sci. Technol. 2021, 56, 2516–2529. [Google Scholar] [CrossRef]
- Maïmouna Kouamé, L. Cocoa Fermentation from Agnéby-Tiassa: Biochemical Study of Microflora. Am. J. BioScience 2015, 3, 203–211. [Google Scholar] [CrossRef]
- Kouassi, A.D.D.; Koné, K.M.; Assi-Clair, B.J.; Lebrun, M.; Maraval, I.; Boulanger, R.; Fontana, A.; Guehi, T.S. Effect of Spontaneous Fermentation Location on the Fingerprint of Volatile Compound Precursors of Cocoa and the Sensory Perceptions of the End-Chocolate. J. Food Sci. Technol. 2022, 59, 4466–4478. [Google Scholar] [CrossRef]
- Oussou, K.F.; Guclu, G.; Kelebek, H.; Selli, S. Elucidating the Contribution of Microorganisms to the Spontaneous Fermentation and the Quality of Ivorian Cacao (Theobroma cacao) Beans: The Quality of Ivorian Cacao (Theobroma cacao) Beans. Qual. Assur. Saf. Crop. Foods 2022, 14, 23–35. [Google Scholar] [CrossRef]
- Koff, O.; Samagaci, L.; Goualie, B.; Niamke, S. Diversity of Yeasts Involved in Cocoa Fermentation of Six Major Cocoa-Producing Regions in Ivory Coast. Eur. Sci. J. ESJ 2017, 13, 496. [Google Scholar] [CrossRef][Green Version]
- Visintin, S.; Alessandria, V.; Valente, A.; Dolci, P.; Cocolin, L. Molecular Identification and Physiological Characterization of Yeasts, Lactic Acid Bacteria and Acetic Acid Bacteria Isolated from Heap and Box Cocoa Bean Fermentations in West Africa. Int. J. Food Microbiol. 2016, 216, 69–78. [Google Scholar] [CrossRef]
- Ale, M.O.; Akintade, A.A.; Orungbemi, O.O. Effects of Fermentation Techniques on the Quality of Cocoa Beans. Int. J. Biol. Life Agric. Sci. 2018, 12, 251–254. [Google Scholar]
- Lin, Y.C.; Choong, Y.M. Characterization and Yield of Crude Cocoa Butter Extracted from Taiwanese Cocoa Beans under Different Fermentation Degree and Roasting Conditions. J. Food Nutr. Res. 2022, 10, 151–157. [Google Scholar] [CrossRef]
- Tunjung-Sari, A.B.; Firmanto, H.; Wahyudi, T. Small-Scale Fermentation of Cocoa Beans and on-Process Monitoring. Pelita Perkebunan 2021, 37, 76–84. [Google Scholar] [CrossRef]
- Sari, A.B.T.; Fahrurrozi; Marwati, T.; Djaafar, T.F.; Hatmi, R.U.; Purwaningsih; Wanita, Y.P.; Lisdiyanti, P.; Perwitasari, U.; Juanssilfero, A.B.; et al. Chemical Composition and Sensory Profiles of Fermented Cocoa Beans Obtained from Various Regions of Indonesia. Int. J. Food Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Apriyanto, M. Microbia and Biochemistry of Cocoa Bean During Fermentation. Asian J. Dairy Food Res. 2016, 35, 160–163. [Google Scholar] [CrossRef]
- Yen, D.T.K.; Ha, N.V.H. Effects of Maturity Stages and Fermentation of Cocoa Beans on Total Phenolic Contents and Antioxidant Capacities in Raw Cocoa Powder. Vietnam. J. Biotechnol. 2018, 14, 743–752. [Google Scholar] [CrossRef]
- Dang, Y.K.T.; Nguyen, H.V.H. Effects of Maturity at Harvest and Fermentation Conditions on Bioactive Compounds of Cocoa Beans. Plant Foods Hum. Nutr. 2019, 74, 54–60. [Google Scholar] [CrossRef]
- Binh, P.T.; Tru, N.V.; Dung, V.T.; Thoa, N.T.; Thao, P. V Bacteria in Wooden Box Fermentation of Cocoa in Daklak, Vietnam. Asian J. Dairy Food Res 2017, 5, 00176. [Google Scholar]
- Castillo Ramos, J.M. Diseño de Un Fermentador Orientado a Mejorar El Proceso de Fermentación Del Cacao Criollo Blanco de Piura; Universidad de Piura: Piura, Peru, 2019. [Google Scholar]
- Ordoñez-Araque, R.H.; Landines-Vera, E.F.; Urresto-Villegas, J.C.; Caicedo-Jaramillo, C.F. Microorganisms during Cocoa Fermentation: Systematic Review. Food Raw Mater. 2020, 8, 155–162. [Google Scholar] [CrossRef]
- Sarbu, I.; Csutak, O. The Microbiology of Cocoa Fermentation. In Caffeinated and Cocoa Based Beverages: Volume 8. The Science of Beverages; Woodhead Publishing: Sawston, UK, 2019; pp. 423–446. [Google Scholar] [CrossRef]
- Ruiz-Santiago, F.L.; Márquez-Rocha, F.J.; García-Alamilla, P.; Carrera-Lanestosa, A.; Ramírez-López, C.; Ocaranza-Sánchez, E.; Jiménez-Rodríguez, D.J. Physicochemical and Biochemical Changes in Cocoa during the Fermentation Step. Fermentation 2024, 10, 405. [Google Scholar] [CrossRef]
- Criollo-Nuñez, J.; Sandoval-Aldana, A.P.; Bolívar, G.; Ramírez Toro, C.; Criollo-Nuñez, J.; Sandoval-Aldana, A.P.; Bolívar, G.; Ramírez Toro, C. Selection of Promising Yeasts for Starter Culture Development Promoter of Cocoa Fermentation. Ingeniería y Competitividad 2024, 26. [Google Scholar] [CrossRef]
- Gutiérrez-Ríos, H.G.; Suárez-Quiroz, M.L.; Hernández-Estrada, Z.J.; Castellanos-Onorio, O.P.; Alonso-Villegas, R.; Rayas-Duarte, P.; Cano-Sarmiento, C.; Figueroa-Hernández, C.Y.; González-Rios, O. Yeasts as Producers of Flavor Precursors during Cocoa Bean Fermentation and Their Relevance as Starter Cultures: A Review. Fermentation 2022, 8, 331. [Google Scholar] [CrossRef]
- Calvo, A.M.; Botina, B.L.; García, M.C.; Cardona, W.A.; Montenegro, A.C.; Criollo, J. Dynamics of Cocoa Fermentation and Its Effect on Quality. Sci. Rep. 2021, 11, 16746. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products—An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef]
- Guehi, T.S.; Dadie, A.T.; Koffi, K.P.B.; Dabonne, S.; Ban-Koffi, L.; Kedjebo, K.D.; Nemlin, G.J. Performance of Different Fermentation Methods and the Effect of Their Duration on the Quality of Raw Cocoa Beans. Int. J. Food Sci. Technol. 2010, 45, 2508–2514. [Google Scholar] [CrossRef]
- Kononenko, S.Y.; Myronenko, L.S.; Timchenko, V.K. Comparison and Selection of Fermentation Method That Would Be Optimal for Obtaining High Quality Cocoa Beans. ХІМІЯ, БІО-І ФАРМТЕХНОЛОГІЇ, ЕКОЛОГІЯ ТА ЕКОНОМІКА В ХАРЧОВІЙ, КОСМЕТИЧНІЙ ТА ФАРМАЦЕВТИЧНІЙ ПРОМИСЛОВОСТІ. 2023. Available online: https://repository.kpi.kharkov.ua/items/714b9aff-b748-47c7-9574-0dabe0feb4c7 (accessed on 25 January 2025).
- Srikanth, V.; Rajesh, G.K.; Kothakota, A. Physio-Chemical and Microbial Changes in Cocoa Beans during Different Methods of Fermentation. Indian J. Ecol. 2019, 46, 154–159. [Google Scholar]
- Calderon, D.; Tejedor, W.; Melgar, O.; Franco, A. Effect of the Type of Fermenter on the Cocoa Fermentation Process and Quality of the Cacao Beans. In Proceedings of the 2022 8th International Engineering, Sciences and Technology Conference (IESTEC), IEEE, Panama City, Panama, 19–21 October 2022; pp. 413–419. [Google Scholar]
- Ghisolfi, R.; Bandini, F.; Vaccari, F.; Bellotti, G.; Bortolini, C.; Patrone, V.; Puglisi, E.; Morelli, L. Bacterial and Fungal Communities Are Specifically Modulated by the Cocoa Bean Fermentation Method. Foods 2023, 12, 2024. [Google Scholar] [CrossRef]
- Velásquez Reyes, D.; Gschaedler, A.; Kirchmayr, M.; Atevendaño Arraza, C.; Rodríguez Campos, J.; Calva Estrada, S.d.J.; Lugo Cervantes, E. Cocoa Bean Turning as a Method for Redirecting the Aroma Compound Profile in Artisanal Cocoa Fermentation. Heliyon 2021, 7, e07694. [Google Scholar] [CrossRef]
- Camu, N.; González, A.; De Winter, T.; Van Schoor, A.; De Bruyne, K.; Vandamme, P.; Takrama, J.S.; Addo, S.K.; De Vuyst, L. Influence of Turning and Environmental Contamination on the Dynamics of Populations of Lactic Acid and Acetic Acid Bacteria Involved in Spontaneous Cocoa Bean Heap Fermentation in Ghana. Appl. Environ. Microbiol. 2008, 74, 86–98. [Google Scholar] [CrossRef]
- Ganeswari, I.; Bariah, K.S.; Amizi, M.A.; Sim, K.Y. Effects of Different Fermentation Approaches on the Microbiological and Physicochemical Changes during Cocoa Bean Fermentation. Int. Food Res. J. 2015, 22, 70–75. [Google Scholar]
- Cempaka, L.; Aliwarga, L.; Purwo, S.; Kresnowati, M.T.A.P. Dynamics of Cocoa Bean Pulp Degradation during Cocoa Bean Fermentation: Effects of Yeast Starter Culture Addition. J. Math. Fundam. Sci. 2014, 46, 14–25. [Google Scholar] [CrossRef]
- María Cardona Velásquez, L.; Rodríguez-Sandoval, E.; Marleny, E.; Chamorro, C. Diagnóstico de Las Prácticas de Beneficio Del Cacao En El Departamento de Arauca. Rev. Lasallista Investig. 2016, 13, 94–104. [Google Scholar] [CrossRef]
- Mbonomo, R.B.; Medap, A.Z.; Brecht, J.K.; Eyame, G. A Study of the Combined Effect of Post-Harvest Fermentation, Turning and Drying of Cocoa (Theobroma cacao L.) on Beans Quality. J. Multidiscipl. Eng. Sci. Technol. (JMEST) 2016, 3, 5023–5027. [Google Scholar]
- Rojas, K.; Hernández Aguirre, C.; Mencía Guevara, A. Transformaciones Bioquímicas Del Cacao (Theobroma cacao L.) Durante Un Proceso de Fermentación Controlada. Agron. Costarric. 2021, 45, 53–65. [Google Scholar] [CrossRef]
- Escobar-Cruz, F.; Vázquez-Ovando, A. Influencia Del Tiempo de Fermentación Sobre Las Características Sensoriales Del Cacao Cultivado En Soconusco, Chiapas, México. IBCIENCIAS 2024, 7, 1–9. [Google Scholar]
- Ackah, E.; Dompey, E. Effects of Fermentation and Drying Durations on the Quality of Cocoa (Theobroma cacao L.) Beans during the Rainy Season in the Juaboso District of the Western-North Region, Ghana. Bull. Natl. Res. Cent. 2021, 45, 175. [Google Scholar] [CrossRef]
- López-Hernández, M.d.P.; Melo-Martinez, S.E.; Criollo-Núñez, J. Effect of the Maturity Stage, Genotype, and Geographical Location on the Physicochemical Characteristics of the Cocoa Bean during Fermentation. Ingeniería Y Competitividad 2023, 25. [Google Scholar] [CrossRef]
- Anita Sari, I.; Murti, R.H.M.; Misnawi, M.; Putra, E.T.S.; Susilo, A.W. Sensory Profiles of Cocoa Genotypes in Indonesia. Biodiversitas J. Biol. Divers. 2022, 23. [Google Scholar] [CrossRef]
- Putri, D.N.; De Steur, H.; Juvinal, J.G.; Gellynck, X.; Schouteten, J.J. Sensory Attributes of Fine Flavor Cocoa Beans and Chocolate: A Systematic Literature Review. J. Food Sci. 2024, 89, 1917–1943. [Google Scholar] [CrossRef]
- Dewi, P.M.S.; Damat, D.; Ekawati, I.; Siskawardani, D.D.; Raba, A.; Wiryono, B.; Munsif, F.; Utomo, B. A Short Review: Changes in the Physical-Chemical Properties of Cacao Beans During the Fermentation Process. BIO Web Conf. 2024, 104, 00032. [Google Scholar] [CrossRef]
- Santander Muñoz, M.; Rodríguez Cortina, J.; Vaillant, F.E.; Escobar Parra, S. An Overview of the Physical and Biochemical Transformation of Cocoa Seeds to Beans and to Chocolate: Flavor Formation. Crit. Rev. Food Sci. Nutr. 2020, 60, 1593–1613. [Google Scholar] [CrossRef] [PubMed]
- Farghal, H.H.; Mansour, S.T.; Khattab, S.; Zhao, C.; Farag, M.A. A Comprehensive Insight on Modern Green Analyses for Quality Control Determination and Processing Monitoring in Coffee and Cocoa Seeds. Food Chem. 2022, 394, 133529. [Google Scholar] [CrossRef] [PubMed]
- Peláez, P.P.; Guerra, S.; Contreras, D. Cambios En La Características Físicas y Químicas de Granos de Cacao (Theobroma cacao) Fermentados Con Transferencia Manual y Semi-Mecanizada, Entre Las Cajas de Fermentación. Sci. Agropecu. 2016, 7, 111–119. [Google Scholar] [CrossRef]
- Lares Amaiz, M.; Pérez Sira, E.; Álvarez Fernández, C.; Perozo González, J.; El Khori, S. Cambios de Las Propiedades Físico-Químicas y Perfil de Ácidos Grasos En Cacao de Chuao, Durante El Beneficio. Agron. Trop. 2013, 63, 37–47. [Google Scholar]
- Husna, A.; Saputra, A.; Harini, N.; Wahyudi, V.; Anggriani, R.; Manshur, H.; Elianarni, D. Physical and Flavor Qualities of Cocoa Beans Affected by Different Box Fermenter Capacity, Fermentation Length, and Microbial Cultures. BIO Web Conf. 2024, 143, 03005. [Google Scholar] [CrossRef]


| Region | Country | Genotype | Fermentation Method | References |
|---|---|---|---|---|
| America | Colombia | CCN-51 Cacao | Wooden boxes | [40] |
| CCN-51 Cacao | Wooden boxes | [41] | ||
| CCN-51, LUKE-40, ICS 95 | Wooden boxes | [42] | ||
| Venezuela | Criollo cacao | Wooden boxes | [43] | |
| Forastero cacao | Plastic basket | [44] | ||
| Peru | Cacao beans from the CCN-51 clone | Wooden boxes | [45] | |
| Criollo cacao and CCN-51 cacao | Wooden boxes, sacks | [46] | ||
| Organic cacao | Wooden boxes | [47] | ||
| Ecuador | National cacao (top) | Wooden boxes | [48] | |
| CCN51 and National | Rotary fermenter and horizontal wood fermenter | [49] | ||
| Brazil | Fine cacao type Scavina | Wooden boxes | [50] | |
| Forastero cacao | Wooden boxes | [39] | ||
| Honduras | Carmelo variety cacao (criollo cacao) | Wooden boxes | [51] | |
| Mexico | CCN51 and National | Rotary fermenter and horizontal boxes | ||
| Cacao clones H12, H13, RIM 44, RIM 88, RIM 105, Carmelo, Criollo INIFAP, and Lagarto | Wooden boxes | [49] | ||
| Criollo and Forastero cacao | Wooden boxes | [23] | ||
| Africa | Ghana | Hybrid cacao and the Amazon | Pile | [30] |
| Unspecified | Pile | [52] | ||
| Côte d’Ivoire | Mixed varieties | Wooden boxes, pile | [53] | |
| Unspecified | Wooden boxes | [54] | ||
| Forastero, Trinitario, and Criollo | Pile | [55] | ||
| “Mercedes” (Amelonado × Trinitario | Plastic boxes | [56] | ||
| Unspecified | Wooden and plastic boxes, Pila. | [57] | ||
| Mixed genotypes (Forastero, Trinitario, Criollo) | Pile | [58] | ||
| Hybrid Forastero | Pile, wooden boxes | [59] | ||
| Nigeria | Unspecified | Pile | [60] | |
| Asia | China | Taiwanese cacao | Wooden boxes | [61] |
| India | Mixed F1 progeny varieties | Wooden boxes, bamboo basket, pile | [12] | |
| Indonesia | Unspecified | Wooden boxes | [62] | |
| Forastero/Trinitario Criollo | Bamboo baskets, wooden boxes | [63] | ||
| Unspecified | Wooden boxes | [64] | ||
| Vietnam | TD3 genotype | Wooden boxes | [65] | |
| TD3 genotype | Wooden boxes | [66] | ||
| Forastero and Trinitario | Wooden boxes | [67] |
| Microorganism | Action |
|---|---|
| Yeasts (Saccharomyces cerevisiae, Candida, Kluyveromyces). | Yeasts ferment the sugars present in the cacao mucilage, producing ethanol, which is subsequently utilized by acetic acid bacteria, along with the generation of heat and carbon dioxide (CO2). |
| Lactic acid bacteria (Lactobacillus, Pediococcus) Maximum exponential growth occurs between 24 and 36 h. | The presence of oxygen promotes an increase in lactic acid bacteria, which convert glucose into lactic acid, mannitol, glycerol, ethanol, and other aromatic compounds within the cacao seed. |
| Acetic acid bacteria (Acetobacter, Gluconobacter) Maximum exponential growth occurs between 24 and 36 h. | Acetic acid bacteria oxidize ethanol into water and acetic acid. This organic acid penetrates the seed and triggers biochemical transformations of molecules associated with flavor and aroma. |
| Characteristics | Criollo | Trinitario | Forastero |
|---|---|---|---|
| General | Mainly grown in Latin America and is valued for its high quality. | Mainly grown in Latin America and tropical regions. It is a hybrid that combines the quality of the Criollo variety with the robustness of the Forastero. | Mainly grown in West Africa and South America. Of lower quality compared to Criollo and Trinitario. |
| Aromatic profile | Fine and delicate aroma (it includes compounds such as 2-methylpropanal, phenylacetaldehyde, 2,3,5-trimethylpyrazine, and others that contribute to its distinctive aroma). | Balanced and varied aroma. (It combines floral, fruity and nutty notes. Pyrazines and aldehydes contribute to its aroma.) | Less complex aromatic profile, aroma with strong and bitter notes. |
| Taste | Soft, sweet taste, with less bitterness, accompanied by fruity, vinous and citrus notes, with a slightly bitter touch. | Balanced flavor, combining the softness of the Criollo with the intensity of the Forastero. It is fruity, with notes of nuttiness, vinous and aromatic, and presents a balance between bitterness and sweetness. | Strong flavor, highly astringent and bitter, with less sweetness, with fruity and earthy notes. |
| Use in chocolate | Preferred for fine chocolates. | Medium- to high-quality chocolate, it is widely used in the industry for its versatility. | Used in commercial and lower quality chocolates. Due to its intensity, it is useful for mixing. |
| Production | Lower production due to susceptibility to disease and lower yield, more expensive. | Intermediate production. It is less susceptible to diseases compared to Criollo, but more delicate than Forastero. | Higher production, more accessible globally. It is known for its high performance and resistance to diseases. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintana-Fuentes, L.F.; García-Jerez, A.; Rodríguez-Negrette, A.C.; Hoyos-Merlano, N.T.; Alvis-Bermúdez, A. Impact of Spontaneous Fermentation on the Physicochemical and Sensory Qualities of Cacao. Fermentation 2025, 11, 377. https://doi.org/10.3390/fermentation11070377
Quintana-Fuentes LF, García-Jerez A, Rodríguez-Negrette AC, Hoyos-Merlano NT, Alvis-Bermúdez A. Impact of Spontaneous Fermentation on the Physicochemical and Sensory Qualities of Cacao. Fermentation. 2025; 11(7):377. https://doi.org/10.3390/fermentation11070377
Chicago/Turabian StyleQuintana-Fuentes, Lucas Fernando, Alberto García-Jerez, Ana Carolina Rodríguez-Negrette, Nurys Tatiana Hoyos-Merlano, and Armando Alvis-Bermúdez. 2025. "Impact of Spontaneous Fermentation on the Physicochemical and Sensory Qualities of Cacao" Fermentation 11, no. 7: 377. https://doi.org/10.3390/fermentation11070377
APA StyleQuintana-Fuentes, L. F., García-Jerez, A., Rodríguez-Negrette, A. C., Hoyos-Merlano, N. T., & Alvis-Bermúdez, A. (2025). Impact of Spontaneous Fermentation on the Physicochemical and Sensory Qualities of Cacao. Fermentation, 11(7), 377. https://doi.org/10.3390/fermentation11070377






