Abstract
Mannoproteins are structural components of the yeast cell wall exhibiting extensive functionality applicable to the food, feed, and medical industries. They are characterized mostly by immunostimulatory, prebiotic, antimicrobial, antibiofilm, antioxidant, and emulsifying properties. The bioactive properties of mannoproteins underscore their significance in functional food production, therapy, and animal husbandry. This review critically examines the literature on yeast mannoproteins, focusing on their chemical characteristics, biological activity, and potential applications. Considering gaps in the literature data regarding detailed chemical characterization and mechanisms of action of mannoproteins, future research should aim at precise structural analysis, particularly of mannoproteins derived from nonconventional yeast, to uncover new potential industrial and health applications.
1. Introduction
Yeast cells are surrounded by a cell wall, which constitutes 15 to 30% of the cell’s dry mass. The components of the yeast cell wall include mannoproteins, beta-glucans, and chitin, as schematically illustrated in Figure 1. These polymers form distinct layers that not only protect the cell from physical and chemical stresses but also provide it with structural support. Their inherent bioactive properties have promising applications in the food, feed, and medical industries. Beta-glucans and chitin ensure the rigidity and structural stability of the cell wall [1,2]. In contrast, mannoproteins, which are located in the outer layer, are primarily responsible for the adhesive properties of yeast cells to various surfaces—including living cells—and act as a molecular sieve for chemical substances [3,4].
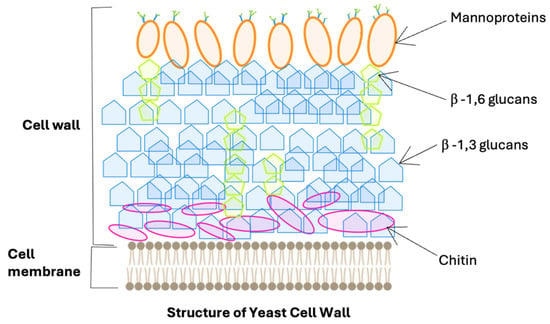
Figure 1.
The structure of the yeast cell wall.
Mannoproteins isolated from the yeast cell wall exhibit a wide range of interesting properties, including immunostimulatory effects, the ability to adsorb mycotoxins, support for wound healing processes, and antioxidant and emulsifying actions [5,6,7,8,9,10,11]. Additionally, they possess prebiotic properties while demonstrating antimicrobial activity against pathogenic bacteria, contributing to the positive modulation of gut microbiota [12,13]. Mannan, a component of mannoproteins, is utilized by lactic acid bacteria in fermentation processes, leading to the production of short-chain fatty acids such as butyric acid, which positively affects gut health [14]. A graphical summary of the examples of the main functional properties of mannoproteins is presented in Figure 2.
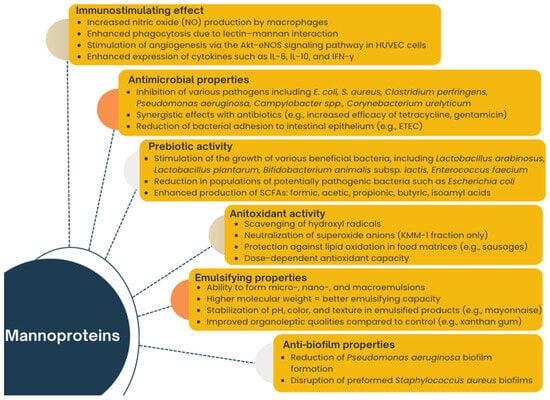
Figure 2.
Multifunctional roles of yeast mannoproteins.
The composition and localization of the individual components constituting the yeast cell wall are influenced by growth conditions, the cell cycle, and the growth phase, which may vary among different yeast species [1,13,15]. Each yeast species is characterized by a distinct proportion of cell wall polymers and the chemical structure of these components, directly impacting their functional properties. Precisely elucidating these structural and chemical attributes is essential to fully understand and harness their bioactivities. Only then will it be possible to further refine isolation methods and optimize yeast cultivation conditions to maximize the synthesis yield and efficacy of these preparations.
Yeast of the genus Saccharomyces, considered conventional yeast, face significant challenges due to their poor tolerance to environmental stress and limited ability to utilize diverse carbon sources [16]. Additionally, the exclusive use of these yeast in fermentation processes restricts the sensory characteristics of the products, thereby complicating the adaptation to diverse consumer preferences [17]. The scientific literature extensively describes these yeast, including their cell wall mannoproteins, in contrast to other yeast species referred to as nonconventional ones.
The group of nonconventional yeast includes other species known as non-Saccharomyces. These yeast have been observed to easily adapt to adverse environmental conditions [18,19,20,21], exhibiting tolerance to high temperatures and low pH, as well as the ability to metabolize atypical carbon sources. They demonstrate the capacity for the biosynthesis of products with desirable properties, such as polyalcohols, enzymes, proteins, ethanol, squalene, and lycopene [22,23,24,25,26]. Currently, the group of non-conventional yeast utilized on an industrial scale includes, for example, Debaromyces hansenii, Kluyveromyces marxianus, Cyberlidnera jadinii (formerly Candida utilis), Yarrowia lipolytica, and Pichia kudriavzevii. It is worth noting that 17 species of nonconventional yeast are listed under the Qualified Presumption of Safety (QPS) status in the European Union [27,28,29,30]. Characterization of mannoprotein properties isolated from non-conventional yeast cells is of particular interest, because they may potentially exhibit even more intriguing properties compared to those obtained from S. cerevisiae.
This review was conducted as a narrative synthesis based on literature identified through structured searches in Google Scholar and PubMed. The search strategy involved combinations of keywords such as “yeast mannoproteins,” “yeast cell wall,” “bioactivity,” “nonconventional yeast,” “prebiotic,” “antioxidant,” “antimicrobial,” and “emulsifying properties.” Publications were selected based on relevance to the topic, scientific quality (peer-reviewed journals), and language (English). Approximately 180 articles were initially screened, of which about 100 were selected and critically evaluated. The primary focus was directed on non-Saccharomyces yeast; however, due to the limited availability of data on these species, relevant findings concerning Saccharomyces cerevisiae were also included for comparative purposes.
Previous reviews on yeast mannoproteins have primarily focused on conventional species, particularly Saccharomyces cerevisiae, discussing their chemical structure, extraction techniques, and industrial uses. While some publications have addressed selected functional aspects—such as sensory contributions in winemaking or animal feed supplementation—comparative evaluations involving both conventional and nonconventional yeast species (e.g., Yarrowia lipolytica, Debaryomyces hansenii, Kluyveromyces marxianus) remain limited. Moreover, there is a lack of integrated analyses linking the molecular structure of mannoproteins with their biological activity, particularly in lesser-studied non-Saccharomyces species. This review aims to provide a critical synthesis and structured overview of the existing literature on yeast-derived mannoproteins, with particular emphasis on their chemical structure, biological activity, and potential applications in the food, feed, and pharmaceutical industries. Special attention is given to nonconventional yeast species, whose mannoprotein-related properties remain insufficiently explored. This review also identifies key knowledge gaps—particularly in the context of structure–function relationships—and outlines directions for future research.
2. Characteristics of Mannoproteins
Mannoproteins are highly glycosylated proteins that can be classified into two groups based on the type of glycosylation: N-glycosylated and O-glycosylated. A schematic overview of N-linked and O-linked glycosylation types is presented in Figure 3.
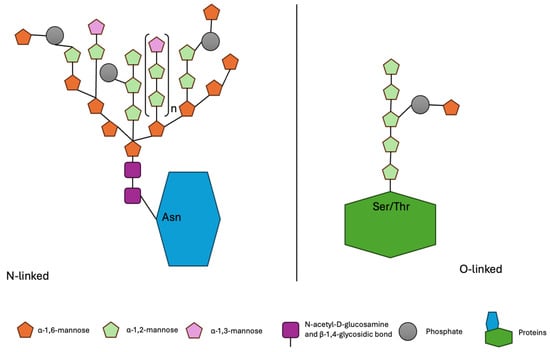
Figure 3.
Schematic representation of N-linked and O-linked glycosylation in yeast mannoproteins.
N-glycosylated mannoproteins consist of approximately 10% protein and about 90% carbohydrates, with the carbohydrate portion comprising between 50 and 200 mannose units. The main mannose chain is built through α-1,6-glycosidic bonds, with short, branched side chains containing α-1,2- and α-1,3-glycosidic linkages. The carbohydrate chain is attached to the asparagine residue of the protein via an N-glycosidic bond. Furthermore, two N-acetylglucosamine molecules, linked by a β-1,4 glycosidic bond, serve as a bridge between the carbohydrate main chain and the protein. O-glycosylated mannoproteins have a higher protein content, around 50%, and possess a short and unbranched carbohydrate chain (up to five mannose units). An α-1,2-glycosidic bond exists between the first two mannose molecules, followed by α-1,3-glycosidic bonds between subsequent mannose residues. The branching of the chain occurs through an α-1,6-glycosidic bond.
Some mannose residue chains can undergo phosphorylation, forming mannose-6-phosphate. Phosphorylation of mannose residues is an example of a cellular response to oxidative stress. This process contributes to the negative surface charge of the cell, which plays a significant role in protecting yeast cells. It helps maintain a more rigid cell wall structure and enhances hydrophilic properties, thereby increasing oxidative stress tolerance [31,32,33,34,35,36,37].
Proteins comprising the yeast cell wall (commonly referred to as cell wall proteins, or CWPs) can associate with the polysaccharide layer either covalently or non-covalently. They are classified into three groups based on the type of bonds, the first two of which are 1. PIR-CWPs: connected by bonds sensitive to alkaline compounds, and 2. GPI-CWPs: linked via an interaction with other cell wall proteins. This classification reflects the diversity in the nature of the bonds that integrate these proteins into the cell wall structure [1,8,38,39].
The first group includes PIR-CWPs (with internal repeats). These proteins are connected to β-1,3-glucan by bonds sensitive to mild bases. PIR-CWPs appear to be evenly distributed in the inner layer of the cell wall skeleton. In the analyzed yeast cell walls, it has been determined that PIR-CWPs are present in the cell walls of yeast, except for Schizosaccharomyces pombe. The number of genes encoding these proteins varies depending on the yeast species. The physiological role of PIR-CWPs is not entirely understood. They are not essential for the proper functioning of the cell, but their damage or complete removal from the cell wall disrupts its osmotic stability, increases sensitivity to harmful environmental factors, and causes changes in cell morphology [38,39,40].
The next group is the GPI-CWPs. These proteins are linked to β-1,6-glucan via a glycosylphosphatidylinositol (GPI) anchor at the C-terminus. They are further distinguished by three additional features: the presence of a serine/threonine-rich region, a hydrophobic tail, and an N-terminal secretion signal peptide [41]. About 60–70 types of GPI-CWPs have been identified in Saccharomyces cerevisiae cells. About 40 of them are attached to the yeast cell membrane, where they perform specific functions, while the rest are covalently linked to β-1,6-glucan [3]. The best-known GPI-CWP is Sag1, which is involved in agglutination during mating [3]. Some GPI-CWPs retain enzymatic activity, while others serve primarily structural roles [1]. Their presence on the cell wall can impart hydrophobic and antigenic properties to the yeast surface. They are involved in the biosynthesis and remodeling of the cell wall and may be responsible for adhesive properties and virulence. GPI proteins isolated from S. cerevisiae cells include flocculins, adhesins, and proteins designated as CWPs. The latter term refers to proteins that are likely to have a non-enzymatic function. These typically small proteins are enriched in threonine and serine residues, which indicates a high degree of O-glycosylation. Examples of such proteins include Cwp1p, Ssr1p, Tir1p, Tip1p, Ccw12p, and Sed1p [42].
The final group of proteins includes those that are linked to other proteins by non-covalent bonds or disulfide bridges, such as Bgl2p. These proteins are classified as secretory proteins with an N-terminal signal peptide and are likely to undergo mannosylation [38]. The presence of proteins connected by disulfide bridges has been confirmed using reducing agents (e.g., 2-mercaptoethanol). These glycoproteins form a protective barrier around the yeast cell wall, preventing the entry of external glycosylhydrolases [1].
The structural features of mannoproteins—including their overall structure, total charge, and charge distribution—may vary considerably among yeast species. For instance, mannoproteins in the cell wall of Schizosaccharomyces pombe typically feature linkages between β-1,3-glucan and pyruvylated galactose residues [43]. In Pichia pastoris, these are diester bonds with mannose phosphate. In contrast, Kluyveromyces lactis and S. pombe lack mannose phosphate in their cell wall composition [44].
Consequently, the diverse structure and chemical composition of mannoproteins—which vary by yeast species—may play a crucial role in determining their functional properties. They facilitate interactions with pathogenic bacterial cells through mannose-specific fimbriae located on bacterial cells. These specific fimbriae can bind to mannans present in the yeast cell wall, potentially limiting the ability of bacterial cells to interact with intestinal epithelial cells. Furthermore, mannan can be degraded by the periplasmic enzyme α-mannosidase into mannose residues, which can serve as a prebiotic substance for lactic acid bacteria [4].
3. Functional Properties of Mannoproteins and Mannoprotein-Rich Preparations
3.1. Anti-Biofilm Properties
Mannoproteins exhibit antimicrobial properties that include the inhibition of bacterial biofilm formation—a significant challenge in various industries, such as food production. Mannoproteins derived from extracellular structures are responsible for inhibiting the formation and dispersion of bacterial biofilms [41]. Table 1 presents a summary of the anti-biofilm properties of yeast mannoproteins as evidenced in the literature. A reduction in biofilm formation by 12–87% was observed when 10% mannoproteins from Saccharomyces cerevisiae were added to the culture medium [42]. In another study, mannoproteins (at concentrations ranging from 2% to 10%) derived from S. cerevisiae 102 were shown to notably inhibit the biofilm formation of Staphylococcus aureus ATCC 29213, with the highest inhibition (63.4%) observed at a 4% concentration [43]. The pathogenic bacteria Staphylococcus aureus ATCC 29213 were used as a model organism. Other studies evaluated the anti-biofilm effect of the cell-free supernatant (CFS) from Saccharomyces cerevisiae cultures against Listeria monocytogenes. The CFS was found to significantly inhibit biofilm formation, with inhibition rates ranging from 52.6% to 79.5% [44]. Despite the lack of precise characterization of the supernatant’s composition, it is presumed to contain mannoproteins released from the cells. A mannoprotein preparation derived from the cell wall of Saccharomyces cerevisiae BY, when added to bacterial cultures at a concentration of 200 mg/mL, was observed to exert inhibitory effects on the formation of Pseudomonas aeruginosa (51.8%) and Staphylococcus aureus (19.7%) biofilms [45].

Table 1.
Antibiofilm properties of selected preparations isolated from yeast.
3.2. Antimicrobial Properties
A summary of the available literature data indicating the antimicrobial effect of yeast mannoprotein preparations is presented in Table 2. For instance, one study observed an antimicrobial effect against Pseudomonas aeruginosa using a biosurfactant obtained from S. cerevisiae (exact composition not provided) [45]. In another investigation, a pure biosurfactant consisting of mannoproteins isolated from S. cerevisiae demonstrated a more pronounced inhibitory effect—evidenced by an inhibition zone diameter of 18 mm—against Corynebacterium urelyticum compared to a partially purified extract [46]. Mannoproteins isolated from the cell wall of S. cerevisiae yeast by the thermal–alkaline method demonstrated inhibitory capacity against E. coli (37%) at a preparation concentration of 5% (m/v) and against B. subtilis (80%) at a concentration of 3% (m/v) [49]. A preparation of mannoproteins derived from the cell wall of the yeast S. cerevisiae BY demonstrated inhibitory capacity against the bacteria P. aeruginosa and S. aureus, with zones of inhibition observed at mannoprotein concentrations ranging from 50 to 200 mg/mL [47]. Mannoproteins isolated from the cell biomass of the yeast Saccharomyces cerevisiae ATCC 7090 demonstrated a dose-dependent reduction in the growth of the bacteria P. aeruginosa ATCC 27853, P. mirabilis ATCC 27853, and S. Enteritidis ATCC 13076, with inhibition rates of approximately 77–95%. In the case of mannoproteins isolated from the yeast Metschnikowia reukaufii WLP 4650, the greatest inhibition of the growth of Staphylococcus aureus ATCC 25923 and Escherichia coli ATCC 25922 was observed in cultures containing 6% of the preparation (93.6% and 91% inhibition, respectively). Conversely, a mannoprotein preparation derived from the biomass of the yeast Wickerhamomyces anomalus CCY 38-1-13 demonstrated a reduction in the growth of P. aeruginosa ATCC 29212 (84.4%) when the preparation was added at a dose of 6%, and of E. coli ATCC 25922 (45.5–70.5%) depending on the preparation dose in the range of 2 to 6% [48].

Table 2.
Antimicrobial properties of selected preparations isolated from yeast.
Specifically, YCW preparations decrease enterotoxigenic Escherichia coli (ETEC) adhesion to intestinal villi, as confirmed by scanning electron microscopy, which revealed that ETEC cells adhere to the yeast cell wall rather than to the host tissue [52]. Furthermore, all examined YCW preparations inhibited the growth of the pathogen Clostridium perfringens by reducing its growth rate and maximum growth level while prolonging the lag phase. The extent of these effects was both time- and dose-dependent [53]. In vivo, studies on female White Leghorn chickens have shown that supplementation with a YCW preparation (0.5 g/kg feed) for 21 days reduced the abundance of Salmonella in chicken feces (2.60 log CFU/g) compared to the control group (3.99 log CFU/g). Similar positive outcomes have been observed in pigs. In these studies, incorporating yeast cell wall-derived mannan into the diet at 800 mg/kg feed was associated with a 16.8% increase in the height of the small intestinal villi and a 21.5% increase in average daily feed intake when assessed using 16S rRNA gene sequencing. Although these changes did not significantly impact overall animal growth performance, piglets supplemented with mannan exhibited a reduced number of Campylobacter bacteria in the lumen of the large intestine [54,55].
It has been observed that the synergistic effects of a mannoprotein preparation in conjunction with certain antibiotics are present. The combination of mannoprotein preparation isolated from the cell wall of S. cerevisiae BY yeast and antibiotics (tetracycline, gentamicin, ampicillin, and ciprofloxacin) (1:1) was investigated at a concentration of 1 mg/mL. The results demonstrated a reinforcement of the inhibition zones by 26, 29, 33, and 36% against P. aeruginosa, while the reinforcement was 20, 33, 40, and 14%, respectively, in the case of S. aureus [47].
3.3. Prebiotic Activity
The qualitative and quantitative composition of the human gut microbiota plays a crucial role in maintaining proper health. Dysbiosis in the microbiome has been linked to a number of diseases, including inflammatory bowel disease, multiple sclerosis, diabetes (type 1 and 2), allergies, asthma, autism, and cancer. It is noteworthy that both the qualitative and quantitative composition of the microbiome depend on genetic predispositions, diet, lifestyle, antibiotics, and other factors. Consequently, there is no single representative composition for the human gut microbiome; its profile varies considerably among individuals and even within the same individual over time [56,57,58]. The most prevalent bacterial families in a healthy individual (i.e., one who is not afflicted by any diseases) include Bacteroidaceae, Clostridiaceae, Prevotellaceae, Eubacteriaceae, Ruminococcaceae, Bifidobateriaceae, Enterobacteriaceae, Saccharomycetaceae, and Methanobacteriacea [57]. The composition of the gut microbiota can be influenced by a multitude of external factors. One such factor is mannoproteins, which possess prebiotic properties and stimulate the growth of selected lactic acid bacteria (LAB) and bifidobacteria, as well as probiotic bacteria. Moreover, mannoproteins enhance the survivability of these beneficial bacteria in the gastrointestinal environment, promoting their successful colonization of the intestines. Furthermore, mannan, a component of mannoproteins, is broken down into mannose residues by the α-mannosidase enzyme, which undergoes fermentation by the gastrointestinal microflora, resulting in the production of short-chain fatty acids. Additionally, mannan enhances the expression of genes associated with the electron transport chain and ATP synthesis in mitochondria, supplying energy to intestinal cells and thereby contributing to the maintenance of proper intestinal morphology and function [14].
Table 3 presents data collected based on an analysis of literature regarding the prebiotic activity of mannoprotein preparations isolated from the yeast cell wall. Three fractions of α-mannan isolated from Kluyveromyces marxianus yeast demonstrated a stronger stimulation of bacteria belonging to the Lactobacillus paracasei ssp. tolerans ZY-1 genus compared to the control group (lack of carbon source in the culture medium) and inulin. It is noteworthy that both the bacterium and the mannan fractions were isolated from Tibetan kefir, which may have contributed to the observed stronger stimulation compared to other tested bacteria in this trial. Depending on the fraction of α-mannan, a greater increase in selected Lactobacillus bacteria was observed compared to the control group (lack of carbon source in the culture medium) or inulin. Notably, since both the bacterium and the α-mannan fractions were isolated from Tibetan kefir, the observed stimulation might be partly attributed to their shared origin. Depending on the specific α-mannan fraction, researchers observed an increased abundance of selected Lactobacillus bacteria relative to the control or inulin. In parallel, the addition of these α-mannan fractions resulted in a higher population of Bacteroides ovatus and Phascolarctobacterium faecium, while E. coli numbers decreased, as determined by 16S rRNA gene analysis [13]. Mannoproteins isolated from the cellular biomass of Saccharomyces cerevisiae ATCC 7090 yeast demonstrated a stimulatory effect on the growth of bacteria. In particular, the growth of Lactobacillus arabinosus ATCC 8014 increased by approximately 159% and that of Bifidobacterium animalis subsp. lactis B12 by roughly 135% compared to a control group receiving a 1–2% addition of the mannoprotein preparation. In the case of mannoproteins isolated from Metschnikowia reukaufii WLP 4650 yeast, a concentration of 2% mannoproteins was observed to stimulate the growth of Lactobacillus arabinosus ATCC 8014 by approximately 214%. In contrast, the mannoprotein preparation derived from the yeast biomass of Wickerhamomyces anomalus CCY 38-1-13 stimulated the growth of Lactobacillus arabinosus ATCC 8014 by approx. 140–160% depending on the preparation dosage [50]. In other studies investigating the impact of yeast cell wall preparations rich in mannoproteins (see Table 3) on male Cobb broilers, a higher level of Lactobacillus (7.80 log CFU/g) and Bifidobacterium (8.60 log CFU/g) was observed in the cecal contents compared to the control group (7.50 and 8.37 log CFU/g, respectively). A higher level of Bifidobacterium (8.60 log CFU/g) bacteria was observed in the cecal contents compared to the control group (7.50 and 8.37 log CFU/g, respectively) [59].

Table 3.
Prebiotic properties of preparations rich in mannoproteins isolated from yeast.
In the studies conducted in artificial intestinal juice, extracts of mannoproteins isolated from S. cerevisiae, obtained by the thermal method, were used. These were then subjected to ultrafiltration and purification by chromatography using concanavalin A. The preparations stimulated growth and influenced the greater survivability of five strains of lactic acid bacteria. Notably, the extracts improved the viability of strains such as Enterococcus faecium, Lactobacillus plantarum, and Lactobacillus salivarius [60].
The impact of yeast S. cerevisiae cell wall extract on the concentration of short-chain fatty acids in the cecal digesta of male broilers was investigated in experimental models. The study demonstrated that broilers supplemented with the yeast cell wall extract exhibited higher concentrations of various SCFAs—namely formic, acetic, propionic, isoamyl, and butyric acids—compared to the control group. Specifically, the SCFA concentrations in the supplemented group were 1.05, 76.92, 30.08, 1.02, and 18.73 mmol/g, respectively, versus 0.73, 57.81, 21.66, 0.86, and 11.71 mmol/g in the control group [61]. With regard to α-mannan isolated from yeast, which is consumed by humans, the results indicated that it may facilitate the growth of intestinal bacteria, including Bacteroides spp. [14,62].
3.4. Immunostymulating Effect
Mannoproteins serve as inducers of humoral and cellular immunity in both humans and animals. They can activate the effectors of human innate immunity, such as macrophages and natural killer (NK) cells, and induce the secretion of lactoferrin. Moreover, mannoproteins interact with lectins, which are mannose-binding proteins that participate in the opsonization process and facilitate immunophagocytosis [63]. This binding enhances phagocytosis and lectin interaction, ultimately activating the complement system and initiating innate immune responses [64]. This constitutes a mechanism of non-specific immunity [63]. In addition, mannan regulates the immune response by stimulating macrophages to produce nitric oxide and promoting their mitosis [65]. Table 4 presents data from a literature review on the immunostimulatory properties of preparations rich in mannoproteins derived from yeast cell walls.

Table 4.
Immunostimulatory properties of selected preparations isolated from yeast.
Beagle dogs receiving a diet supplemented with mannoproteins from S. cerevisiae (400 mg/kg) exhibited significant modulation of both specific and non-specific immune responses. Neutrophils from these animals, when stimulated by bacterial lipopolysaccharides (LPSs), produced increased amounts of hydrogen peroxide (H2O2) compared to those in the control group. Ref. [66] demonstrated in their study that the addition of mannoproteins to the diet of Beagle dogs significantly affected the specific and non-specific immune responses of these animals. In another study, mannoproteins isolated from the cell wall of mutant strain S. cerevisiae K48L3 were shown to stimulate nitric oxide-induced angiogenesis and activate the Akt-eNOS signaling pathway in human umbilical vein endothelial cells (HUVECs) ex vivo [67]. These findings further confirmed the immunostimulatory properties of mannan on ovine rumen epithelial cells (ORECs). The impact of mannan (at a dose of 50 μg/mL) on the gastrointestinal environment of sheep was investigated in vitro by analyzing the expression of SBD-1 protein (a peptide secreted by rumen epithelial cells with antimicrobial activity) and further signaling pathways stimulated by ORECs. Mannan was found to significantly increase the expression of SBD-1 protein (5.8-fold compared to the control group), thereby confirming the immunostimulatory properties of mannan on ORECs [68]. In broiler chickens challenged with bacterial LPSs from E. coli, supplementation with yeast cell wall preparations resulted in a notable modulation of cytokine expression in blood serum [69]. A yeast cell wall preparation from Pichia guilliermondii (at doses of 0.1% or 0.2%) was employed in the diet of broilers infected with coccidia, resulting in an enhancement in the immunity of the broilers. This was evidenced by a reduction in the number of regulatory T cells (Tregs) and an increase in the level of interferon γ (IFN-γ) in the intestine [70]. A study by Ref. [71] demonstrated that the inclusion of S. cerevisiae yeast cell wall at a concentration of 1000 mg/kg in the diet of Ross 308 broilers led to improvements in feed conversion ratio, a reduction in the number of E. coli and Salmonella cells in the gut content, and an enhancement of the humoral immune memory response of broilers to Newcastle disease virus.
The effects of three yeast species, namely Cyberlindnera jadinii, Blastobotrys adeninivorans, and Wickehamomyces anomalus, on the mitigation of intestinal inflammation were investigated in Atlantic salmon. The research findings indicated that the autolysate of W. anomalus was effective in alleviating intestinal inflammation, whereas the other two strains demonstrated only limited effects. The efficacy of the yeast preparations was found to depend on the type and production method employed [73]. The replacement of up to 40% of dietary protein with yeast biomass from Candida utilis LYCC 7549 was found to positively influence intestinal transit time and stool structure in pigs. This indicates that deactivated yeast cells of C. utilis enhance digestive system function and promote the growth of probiotic bacteria [72].
3.5. Antioxidant Activity
Mannan also exhibits antioxidant properties (Table 5), which contribute to the maintenance of optimal functioning in animal organisms by enhancing the activity of antioxidant enzymes, including superoxide dismutase, catalase, and glutathione peroxidase. Moreover, the modified mannans exhibited an enhanced capacity to protect lipids from oxidation compared to their unmodified counterpart [74,75]. The inhibitory effect of mannans extracted from yeast cell walls and subjected to chemical modifications on lipid peroxidation and their capacity to scavenge hydroxyl radicals were investigated. The modifications yielded phosphorylated mannan (P-M), sulfated mannan (S-M), carboxymethylated mannan (CM-M), carboxymethylated–phosphorylated mannan (CMP-M), and carboxymethylated–sulfated mannan (CMS-M). The scavenging ability of hydroxyl radicals was observed to be 15% higher in the case of P-M and CMP-M compared to unmodified mannan. Moreover, the antioxidant capacity against lipids was found to be higher than that of unmodified mannan [75]. Mannoproteins extracted from the cell wall of Saccharomyces cerevisiae have also demonstrated practical antioxidant applications. For instance, their incorporation into pork sausages delayed the oxidation rate of lipids, irrespective of the percentage of pork fat replaced by the mannoprotein preparation [76]. Furthermore, the extract from S. cerevisiae MYN04 demonstrated DPPH free radical scavenging activity at a level of 51.8% (with a preparation addition of 0.5 mL/mL) [77]. Studies utilizing the unconventional yeast strain Kluyveromyces marxianus, from which four mannan fractions differing in composition and molecular weight were isolated (refer to Table 5), demonstrated that all fractions possessed antioxidant activity and neutralized hydroxyl free radicals in a dose-dependent manner. Notably, only the KMM-1 fraction also showed the ability to neutralize superoxide ions [63].

Table 5.
Antioxidant properties of selected preparations isolated from yeast.
It is crucial to emphasize that the antioxidant activity of mannoproteins containing protein fragments may also be associated with the reducing potential of the aromatic amino acid tryptophan [78]. A series of studies conducted on broilers, whose diets were supplemented with a cell wall-based supplement (at doses of 0.5, 1.0, and 1.5 g/kg), demonstrated an increase in the levels of reduced glutathione and glutathione reductase in the intestine. These findings corroborate the notion that yeast cell wall components possess the capacity to regulate glutathione pathways, which are of paramount importance for antioxidant defense [79].
3.6. Emulsifying Properties
Yeast-derived glycoproteins are distinguished by their non-toxicity, low production cost, and biodegradability, rendering them an appropriate choice for use as emulsifying and stabilizing agents. This property is attributed to their amphiphilic structure, in which proteins are bonded to hydrophilic mannose polymers. Furthermore, these glycoproteins display beneficial emulsifying properties in vitro, which are influenced by pH levels and the concentrations of selected salts used in the food industry [80,81]. Under appropriate conditions—such as during homogenization—they can form microemulsions, nanoemulsions, and macroemulsions. Furthermore, the emulsifying performance of these glycoproteins is closely tied to their molecular weight; higher molecular weights, which reflect an increased protein content in mannoprotein preparations, correlate with improved emulsifying capacity [82]. Table 6 presents a review of the literature on the emulsifying properties of mannoproteins and yeast extracts.

Table 6.
Emulsifying properties of selected preparations isolated from yeast.
Mannoproteins isolated from the cell wall of S. cerevisiae demonstrated a significant effect on maintaining emulsion stability after seven days of storage (at mannoprotein concentrations of 6% and 8%) compared to the control group (without mannoprotein addition) [83]. In other studies, using extracts from S. cerevisiae MYN04 (containing 27.1% carbohydrates and 72.9% protein), an emulsification index of 80% was achieved in emulsions of wheat germ oil, corn oil, and olive oil after 24 h of storage (with an extract addition of 0.5 mg/mL) [77]. The phase of the emulsion was maintained after 48 h of storage in emulsions at pH 3, where an extract from Saccharomyces cerevisiae EC 1118, isolated using physical and Sur Lies methods (25 mg/7.5 mL), was used. The extracts contained sugars ranging from 564.4 to 980.5 mg/g dry weight, with mannose accounting for 69.4–93.5% and protein content ranging from 10.6 to 48.3 mg/g dry weight (variability depending on the isolation method). Furthermore, it was demonstrated that the extracts were more efficacious in maintaining the emulsion phase at lower pH levels [85]. A mannoprotein preparation derived from the cell wall of Kluyveromyces marxianus IBRC-M 30114 (at concentrations of 1.25% and 1.5%) was found to result in enhanced emulsion stability, a more negative zeta potential, smaller oil droplets, and an increased emulsion viscosity [84]. Emulsion stability at a level of 76% of the emulsion phase after 90 days of storage at 4 °C was observed in the presence of mannoprotein preparations isolated from K. marxianus FII 510700 (FRR 1586) with an addition of 0.12 g/10 mL [86]. Furthermore, emulsions (4:6) with the addition of mannoprotein preparations from S. pastorianus, isolated using subcritical fluid at high temperature, demonstrated emulsifying capacity and stability at 72% after 30 days of storage [87].
Mannoproteins isolated from the cell wall of S. cerevisiae have been demonstrated to enhance the network structure and textural properties of pork sausages without compromising the sensory characteristics of the final product. The replacement of animal fats in food products with emulsions containing mannoproteins can beneficially alter the lipid profile of the product by reducing the total fat content, as well as the saturated and trans-fat fractions, while increasing the polyunsaturated fatty acids present in vegetable oils (e.g., canola oil, sunflower oil, olive oil) [76]. Mannoprotein preparations derived from the cell wall of Saccharomyces uvarum, which are produced as a by-product of beer production, were employed as a substitute for xanthan gum (an emulsifier) in the production of mayonnaise. Mayonnaise samples formulated with mannoprotein additions (0.6, 0.8, or 1.0 g/100 g) maintained stable pH values after 28 days of refrigerated storage and exhibited superior stability compared to control samples containing xanthan gum. In addition, these mayonnaise samples displayed a bright color with reduced yellowing during storage, and they received high scores in organoleptic evaluation [80].
4. Conclusions
This review article examines the potential applications of mannoproteins isolated from the yeast cell wall, considering their diverse functional properties. These include prebiotic, immunostimulatory, antimicrobial, antibiofilm, emulsifying, and antioxidant activities. A critical aspect discussed is the synergistic effect observed when mannoproteins are combined with antibiotics, a strategy that may allow for reduced drug dosages without sacrificing therapeutic efficacy. These findings have significant implications for both animal husbandry and therapeutic prospects in humans. The absence of precise dose–response data—together with incomplete chemical characterization—limits our understanding of the mechanisms underlying their biological effects. Many studies do not include detailed structural analysis of these compounds, hindering the ability to effectively correlate their functionality with specific chemical features. Future research efforts should prioritize comprehensive structural and chemical analyses of mannoproteins to establish a robust correlation between their functional properties and molecular composition. Moreover, while mannoproteins from Saccharomyces cerevisiae have been extensively studied, mannoproteins derived from nonconventional yeast remain largely unexplored, representing a valuable avenue for future investigation. It is therefore of interest to continue research in this area, which may provide new insights into the potential applications of these glycoproteins in the food, medical, and cosmetic industries, as well as in animal and human nutrition and prophylaxis.
Nonetheless, certain research gaps remain unaddressed. These include the lack of harmonized methodologies for mannoprotein isolation and quantification, insufficient structure–activity data, and limited exploration of strain-specific biofunctional properties. Future studies should aim to fill these gaps by applying advanced analytical techniques, conducting comparative assessments across yeast species, and integrating functional assays with molecular profiling. Such efforts will be essential to expand the scientific foundation for the targeted application of mannoproteins in health and industry.
Author Contributions
Study conception and design and literature collection—A.B.-W. and P.C. Review of the literature and literature data collection and analysis—P.C. First draft of the manuscript—P.C. Deep revision and correction—A.B.-W. Supervision—A.B.-W. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Orlean, P. Architecture and Biosynthesis of the Saccharomyces cerevisiae Cell Wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Zheng, F.; Niu, C.; Liu, C.; Li, Q.; Sun, J. Cell Wall Polysaccharides: Before and after Autolysis of Brewer’s Yeast. World J. Microbiol. Biotechnol. 2018, 34, 137. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of Cell Wall Structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Schiavone, M.; Déjean, S.; Sieczkowski, N.; Castex, M.; Dague, E.; François, J.M. Integration of Biochemical, Biophysical and Transcriptomics Data for Investigating the Structural and Nanomechanical Properties of the Yeast Cell Wall. Front. Microbiol. 2017, 8, 1806. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Du, C.G.; Guo, Y.Q.; Zhao, Y.F.; Aorigele, C.; Wang, C.J.; Simujide, H.; Aqima, W.; Zhang, X.Y. Antibacterial Spectrum of Four Compounds from Yeasts in Koumiss. Pol. J. Vet. Sci. 2021, 24, 167–173. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Wu, X.; Algharib, S.A.; Gong, F.; Hu, J.; Luo, W.; Zhou, M.; Pan, Y.; Yan, Y.Y.; et al. Structure, Preparation, Modification, and Bioactivities of β-Glucan and Mannan from Yeast Cell Wall: A Review. Int. J. Biol. Macromol. 2021, 173, 445–456. [Google Scholar] [CrossRef]
- Valáriková, J.; Čížová, A.; Račková, L.; Bystrický, S. Anti-Staphylococcal Activity of Quaternized Mannan from the Yeast Candida albicans. Carbohydr. Polym. 2020, 240, 116288. [Google Scholar] [CrossRef]
- Borovikova, D.; Teparić, R.; Mrša, V.; Rapoport, A. Anhydrobiosis in Yeast: Cell Wall Mannoproteins Are Important for Yeast Saccharomyces cerevisiae Resistance to Dehydration. Yeast 2016, 33, 347–353. [Google Scholar] [CrossRef]
- Sima, P.; Vannucci, L.; Vetvicka, V. β-Glucans and Cholesterol (Review). Int. J. Mol. Med. 2018, 41, 1799–1808. [Google Scholar] [CrossRef]
- Korolenko, T.A.; Bgatova, N.P.; Ovsyukova, M.V.; Shintyapina, A.; Vetvicka, V. Hypolipidemic Effects of β-Glucans, Mannans, and Fucoidans: Mechanism of Action and Their Prospects for Clinical Application. Molecules 2020, 25, 1819. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Vetvickova Jana Anti-Infectious and Anti-Tumor Activities of β-Glucans. Anticancer. Res. 2020, 40, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Baumgärtner, S.; James, J.; Ellison, A. The Supplementation of a Prebiotic Improves the Microbial Community in the Gut and the Skin of Atlantic Salmon (Salmo salar). Aquac. Rep. 2022, 25, 101204. [Google Scholar] [CrossRef]
- Tang, N.; Wang, X.; Yang, R.; Liu, Z.; Liu, Y.; Tian, J.; Xiao, L.; Li, W. Extraction, Isolation, Structural Characterization and Prebiotic Activity of Cell Wall Polysaccharide from Kluyveromyces marxianus. Carbohydr. Polym. 2022, 289, 119457. [Google Scholar] [CrossRef]
- Abbott, D.W.; Martens, E.C.; Gilbert, H.J.; Cuskin, F.; Lowe, E.C. Coevolution of Yeast Mannan Digestion: Convergence of the Civilized Human Diet, Distal Gut Microbiome, and Host Immunity. Gut Microbes 2015, 6, 334–339. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Błażejak, S.; Kieliszek, M.; Pobiega, K.; Falana, K.; Janowicz, M. Modification of the Cell Wall Structure of Saccharomyces cerevisiae Strains during Cultivation on Waste Potato Juice Water and Glycerol towards Biosynthesis of Functional Polysaccharides. J. Biotechnol. 2018, 281, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thorwall, S.; Schwartz, C.; Chartron, J.W.; Wheeldon, I. Stress-Tolerant Non-Conventional Microbes Enable next-Generation Chemical Biosynthesis. Nat. Chem. Biol. 2020, 16, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Verstrepen, K.J. Taming Wild Yeast: Potential of Conventional and Nonconventional Yeasts in Industrial Fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef]
- Kosel, J.; Čadež, N.; Raspor, P. Factors Affecting Volatile Phenol Production During Fermentations with Pure and Mixed Cultures of Dekkera bruxellensis and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2014, 52, 35–45. [Google Scholar]
- Kosel, J.; Raspor, P.; Cadež, N. Maximum Residue Limit of Fungicides Inhibits the Viability and Growth of Desirable Non-Saccharomyces Wine Yeasts. Aust. J. Grape Wine Res. 2019, 25, 43–52. [Google Scholar] [CrossRef]
- Kosel, J.; Cadež, N.; Schuller, D.; Carreto, L.; Franco-Duarte, R.; Raspor, P. The Influence of Dekkera bruxellensis on the Transcriptome of Saccharomyces cerevisiae and on the Aromatic Profile of Synthetic Wine Must. FEMS Yeast Res. 2017, 17, fox018. [Google Scholar] [CrossRef]
- Zupan, J.; Avbelj, M.; Butinar, B.; Kosel, J.; Šergan, M.; Raspor, P. Monitoring of Quorum-Sensing Molecules during Minifermentation Studies in Wine Yeast. J. Agric. Food Chem. 2013, 61, 2496–2505. [Google Scholar] [CrossRef] [PubMed]
- Kręgiel, D.; Pawlikowska, E.; Antolak, H. Non-Conventional Yeasts in Fermentation Processes: Potentialities and Limitations. Old. Yeasts New Quest. 2017, 87. [Google Scholar] [CrossRef]
- Binati, R.L.; Salvetti, E.; Bzducha-Wróbel, A.; Bašinskienė, L.; Cizeikienė, D.; Bolzonella, D.; Felis, G.E. Non-Conventional Yeasts for Food and Additives Production in a Circular Economy Perspective. FEMS Yeast Res. 2021, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Gao, H.; Qian, X.; Jiang, Y.; Zhou, J.; Dong, W.; Xin, F.; Zhang, W.; Jiang, M. Biotechnological Applications of the Non-Conventional Yeast Meyerozyma guilliermondii. Biotechnol. Adv. 2021, 46, 107674. [Google Scholar] [CrossRef]
- Patra, P.; Das, M.; Kundu, P.; Ghosh, A. Recent Advances in Systems and Synthetic Biology Approaches for Developing Novel Cell-Factories in Non-Conventional Yeasts. Biotechnol. Adv. 2021, 47, 107695. [Google Scholar] [CrossRef]
- Chu, Y.; Li, M.; Jin, J.; Dong, X.; Xu, K.; Jin, L.; Qiao, Y.; Ji, H. Advances in the Application of the Non-Conventional Yeast Pichia kudriavzevii in Food and Biotechnology Industries. J. Fungi 2023, 9, 170. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Update of the List of QPS-Recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA 13: Suitability of Taxonomic Units Notified to EFSA until September 2020. EFSA J. 2021, 19, e06377. [Google Scholar] [CrossRef] [PubMed]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Guidance on the Characterisation of Microorganisms Used as Feed Additives or as Production Organisms. EFSA J. 2018, 16, e05206. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Girones, R.; Koutsoumanis, K.; Herman, L.; Lindqvist, R.; Nørrung, B.; et al. Update of the List of QPS-Recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA 5: Suitability of Taxonomic Units Notified to EFSA until September 2016. EFSA J. 2017, 15, e04663. [Google Scholar] [CrossRef]
- Deckers, M.; Deforce, D.; Fraiture, M.A.; Roosens, N.H.C. Genetically Modified Micro-Organisms for Industrial Food Enzyme Production: An Overview. Foods 2020, 9, 326. [Google Scholar] [CrossRef]
- Zhang, W.; Ballou, C.E. Saccharomyces kluyveri Cell Wall Mannoprotein. Structures of the O- and N-Linked Carbohydrate Components. J. Biol. Chem. 1981, 256, 10073–10079. [Google Scholar] [CrossRef]
- Van Rinsum, J.; Klis, F.M.; Ende, H. Van Den Cell Wall Glucomannoproteins of Saccharomyces cerevisiae Mnn9. Yeast 1991, 7, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Roemer, T.; Bussey, H. Yeast Kre1p Is a Cell Surface O-Glycoprotein. Mol. Gen. Genet. MGG 1995, 249, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Young, M.; Davies, M.J.; Bailey, D.; Gradwell, M.J.; Smestad-Paulsen, B.; Wold, J.K.; Barnes, R.M.R.; Hounsell, E.F. Characterization of Oligosaccharides from an Antigenic Mannan of Saccharomyces cerevisiae. Glycoconj. J. 1998, 15, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Jigami, Y.; Odani, T. Mannosylphosphate Transfer to Yeast Mannan. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1999, 1426, 335–345. [Google Scholar] [CrossRef]
- Lesage, G.; Bussey, H. Cell Wall Assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef]
- Bastos, R.; Coelho, E.; Coimbra, M.A. Modifications of Saccharomyces pastorianus Cell Wall Polysaccharides with Brewing Process. Carbohydr. Polym. 2015, 124, 322–330. [Google Scholar] [CrossRef]
- Klis, F.M.; Boorsma, A.; De Groot, P.W.J. Cell Wall Construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef]
- Teparić, R.; Mrša, V. Proteins Involved in Building, Maintaining and Remodeling of Yeast Cell Walls. Curr. Genet. 2013, 59, 171–185. [Google Scholar] [CrossRef]
- Klis, F.M.; De Groot, P.; Hellingwerf, K. Molecular Organization of the Cell Wall of Candida albicans. Med. Mycol. Suppl. 2001, 39, 1–8. [Google Scholar] [CrossRef]
- Hamada, K.; Fukuchi, S.; Arisawa, M.; Baba, M.; Kitada, K. Screening for Glycosylphosphatidylinositol (GPI)-Dependent Cell Wall Proteins in Saccharomyces cerevisiae. Mol. General. Genet. 1998, 258, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.Y.; De Groot, P.W.J.; Dekker, H.L.; De Jong, L.; Klis, F.M.; De Koster, C.G. Comprehensive Proteomic Analysis of Saccharomyces cerevisiae Cell Walls: Identification of Proteins Covalently Attached via Glycosylphosphatidylinositol Remnants or Mild Alkali-Sensitive Linkages. J. Biol. Chem. 2005, 280, 20894–20901. [Google Scholar] [CrossRef]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Cell Wall Polysaccharides Released during the Alcoholic Fermentation by Schizosaccharomyces pombe and S. japonicus: Quantification and Characterization. Food Microbiol. 2017, 61, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Marx, H.; Sauer, M.; Resina, D.; Vai, M.; Porro, D.; Valero, F.; Ferrer, P.; Mattanovich, D. Cloning, Disruption and Protein Secretory Phenotype of the GAS1 Homologue of Pichia pastoris. FEMS Microbiol. Lett. 2006, 264, 40–47. [Google Scholar] [CrossRef]
- Walencka, E.; Wieckowska-Szakiel, M.; Rozalska, S.; Sadowska, B.; Rozalska, B. A Surface-Active Agent from Saccharomyces cerevisiae Influences Staphylococcal Adhesion and Biofilm Development. Z. Naturforschung Sect. C J. Biosci. 2007, 62, 433–438. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yu, H.H.; Song, Y.J.; Park, Y.J.; Lee, N.K.; Paik, H.D. Anti-Biofilm Effect of the Cell-Free Supernatant of Probiotic Saccharomyces cerevisiae against Listeria monocytogenes. Food Control 2021, 121, 107667. [Google Scholar] [CrossRef]
- Rasheed, H.G.; Haydar, N.H. Purification, characterization and evaluation of biological activity of mannoprotein produced from Saccharomyces cerevisiae by. Iraqi J. Agric. Sci. 2023, 54, 347–359. [Google Scholar] [CrossRef]
- Bzducha Wróbel, A.; Farkaš, P.; Chraniuk, P.; Popielarz, D.; Synowiec, A.; Pobiega, K.; Janowicz, M. Antimicrobial and Prebiotic Activity of Mannoproteins Isolated from Conventional and Nonconventional Yeast Species-the Study on Selected Microorganisms. World J. Microbiol. Biotechnol. 2022, 38, 256, Erratum in World J. Microbiol. Biotechnol. 2023, 39, 92. https://doi.org/10.1007/S11274-023-03528-0. [Google Scholar] [CrossRef]
- Kuntsova, M.; Meledina, T.; Davydenko, S.; Manshin, D.; Andreeva, A. Obtaining Yeast Mannoproteins with Antimicrobial Properties. Funct. Foods Health Dis. 2023, 13, 437–447. [Google Scholar] [CrossRef]
- Ali, L.H.; Ali, W.S. Production and Antibacterial Activity of Biosurfactant from Saccharomyces cerevisiae. J. Phys. Conf. Ser. 2019, 1234, 012080. [Google Scholar] [CrossRef]
- Mahmood Nibras Nazar Effect of Biosurfactants Purified from Saccharomyces cerevisiae Against Corynebacterium Urealyticum. Available online: https://www.proquest.com/docview/2030154838?pq-origsite=gscholar&fromopenview=true (accessed on 27 January 2023).
- Trevisi, P.; Priori, D.; Gandolfi, G.; Colombo, M.; Coloretti, F.; Goossens, T.; Bosi, P. In Vitro Test on the Ability of a Yeast Cell Wall Based Product to Inhibit the Escherichia coli F4ac Adhesion on the Brush Border of Porcine Intestinal Villi. J. Anim. Sci. 2012, 90, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Santovito, E.; Greco, D.; Marquis, V.; Raspoet, R.; D’Ascanio, V.; Logrieco, A.F.; Avantaggiato, G. Antimicrobial Activity of Yeast Cell Wall Products Against Clostridium Perfringens. Foodborne Pathog. Dis. 2019, 16, 638–647. [Google Scholar] [CrossRef]
- Rahimi, S.; Kathariou, S.; Fletcher, O.; Grimes, J.L. Effect of a Direct-Fed Microbial and Prebiotic on Performance and Intestinal Histomorophology of Turkey Poults Challenged with Salmonella and Campylobacter. Poult. Sci. 2019, 98, 6572–6578. [Google Scholar] [CrossRef]
- Fouhse, J.M.; Dawson, K.; Graugnard, D.; Dyck, M.; Willing, B.P. Dietary Supplementation of Weaned Piglets with a Yeast-Derived Mannan-Rich Fraction Modulates Cecal Microbial Profiles, Jejunal Morphology and Gene Expression. Animal 2019, 13, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current Understanding of the Human Microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Liu, N.; Wang, J.; Liu, Z.; Wang, Y.; Wang, J. Effect of Supplemental Yeast Cell Walls on Growth Performance, Gut Mucosal Glutathione Pathway, Proteolytic Enzymes and Transporters in Growing Broiler Chickens. J. Anim. Sci. 2018, 96, 1330–1337. [Google Scholar] [CrossRef]
- Ganan, M.; Carrascosa, A.V.; De Pascual-Teresa, S.; Martinez-Rodriguez, A.J. Effect of Mannoproteins on the Growth, Gastrointestinal Viability, and Adherence to Caco-2 Cells of Lactic Acid Bacteria. J. Food Sci. 2012, 77, M176–M180. [Google Scholar] [CrossRef]
- Xue, G.D.; Wu, S.B.; Choct, M.; Swick, R.A. Effects of Yeast Cell Wall on Growth Performance, Immune Responses and Intestinal Short Chain Fatty Acid Concentrations of Broilers in an Experimental Necrotic Enteritis Model. Anim. Nutr. 2017, 3, 399–405. [Google Scholar] [CrossRef]
- Cuskin, F.; Lowe, E.C.; Temple, M.J.; Zhu, Y.; Cameron, E.A.; Pudlo, N.A.; Porter, N.T.; Urs, K.; Thompson, A.J.; Cartmell, A.; et al. Human Gut Bacteroidetes Can Utilize Yeast Mannan through a Selfish Mechanism. Nature 2015, 517, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Galinari, É.; Almeida-Lima, J.; Macedo, G.R.; Mantovani, H.C.; Rocha, H.A.O. Antioxidant, Antiproliferative, and Immunostimulatory Effects of Cell Wall α-d-Mannan Fractions from Kluyveromyces marxianus. Int. J. Biol. Macromol. 2018, 109, 837–846. [Google Scholar] [CrossRef]
- Liu, L.; Dang, Y. Antimicrobial Activity of Mannose Binding Lectin in Grass Carp (Ctenopharyngodon idella) In Vivo and In Vitro. Fish. Shellfish. Immunol. 2020, 98, 25–33. [Google Scholar] [CrossRef]
- Pontón, J.; Omaetxebarría, M.J.; Elguezabal, N.; Alvarez, M.; Moragues, M.D. Immunoreactivity of the Fungal Cell Wall. Med. Mycol. 2001, 39, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kroll, F.S.A.; Putarov, T.C.; Zaine, L.; Venturini, K.S.; Aoki, C.G.; Santos, J.P.F.; Pedrinelli, V.; Vendramini, T.H.A.; Brunetto, M.A.; Carciofi, A.C. Active Fractions of Mannoproteins Derived from Yeast Cell Wall Stimulate Innate and Acquired Immunity of Adult and Elderly Dogs. Anim. Feed. Sci. Technol. 2020, 261, 114392. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Lee, S.M.; Chang, H.I.; Ha, C.H. Mannoproteins from Saccharomyces cerevisiae Stimulate Angiogenesis by Promoting the Akt-ENOS Signaling Pathway in Endothelial Cells. Biochem. Biophys. Res. Commun. 2019, 519, 767–772. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, M.; Cao, G.F.; Yang, Y.F. Saccharomyces cerevisiae Mannan Induces Sheep Beta-Defensin-1 Expression via Dectin-2-Syk-P38 Pathways in Ovine Ruminal Epithelial Cells. Vet. Res. 2019, 50, 8. [Google Scholar] [CrossRef]
- Alizadeh, M.; Rodriguez-Lecompte, J.C.; Rogiewicz, A.; Patterson, R.; Slominski, B.A. Effect of Yeast-Derived Products and Distillers Dried Grains with Solubles (DDGS) on Growth Performance, Gut Morphology, and Gene Expression of Pattern Recognition Receptors and Cytokines in Broiler Chickens. Poult. Sci. 2016, 95, 507–517. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Sifri, M.; Selvaraj, R.K. Effect of Yeast Cell Product Supplementation on Broiler Cecal Microflora Species and Immune Responses during an Experimental Coccidial Infection. Poult. Sci. 2013, 92, 1195–1201. [Google Scholar] [CrossRef]
- Ghosh, T.K.; Haldar, S.; Bedford, M.R.; Muthusami, N.; Samanta, I. Assessment of Yeast Cell Wall as Replacements for Antibiotic Growth Promoters in Broiler Diets: Effects on Performance, Intestinal Histo-Morphology and Humoral Immune Responses. J. Anim. Physiol. Anim. Nutr. 2012, 96, 275–284. [Google Scholar] [CrossRef]
- Cruz, A.; Håkenåsen, I.M.; Skugor, A.; Mydland, L.T.; Åkesson, C.P.; Hellestveit, S.S.; Sørby, R.; Press, C.M.L.; Øverland, M. Candida Utilis Yeast as a Protein Source for Weaned Piglets: Effects on Growth Performance and Digestive Function. Livest. Sci. 2019, 226, 31–39. [Google Scholar] [CrossRef]
- Agboola, J.; Schiavone, M.; Øverland, M.; Morales-Lange, B.; Lagos, L.; Arntzen, M.; Lapeña, D.; Eijsink, V.; Horn, S.; Mydland, L.; et al. Impact of Down-Stream Processing on Functional Properties of Yeasts in Diets of Atlantic Salmon (Salmo salar): Implications for Gut Health. Sci Rep. 2021, 11, 4496. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Huang, G.; Zhao, F.; Zhou, L.; Huang, S.; Li, H. The Antioxidant Activities of Six (1 → 3)-β-d-Glucan Derivatives Prepared from Yeast Cell Wall. Int. J. Biol. Macromol. 2017, 98, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, G. The Derivatization and Antioxidant Activities of Yeast Mannan. Int. J. Biol. Macromol. 2018, 107, 755–761. [Google Scholar] [CrossRef]
- Zhong, L.; Guo, X.; Xue, H.; Qiao, Y.; Mao, D.; Ye, X.; Cui, Z.; Li, Z.; Hu, G.; Huang, Y. Quality Characteristics of Reduced-Fat Emulsified Sausages Made with Yeast Mannoprotein Enzymatically Prepared with a β-1,6-Glucanase. Foods 2023, 12, 2486. [Google Scholar] [CrossRef]
- Elsaygh, Y.A.; Gouda, M.K.; Elbahloul, Y.; Hakim, M.A.; El Halfawy, N.M. Production and Structural Characterization of Eco-Friendly Bioemulsifier SC04 from Saccharomyces cerevisiae Strain MYN04 with Potential Applications. Microb. Cell Fact. 2023, 22, 176. [Google Scholar] [CrossRef]
- Jaehrig, S.C.; Rohn, S.; Kroh, L.W.; Fleischer, L.G.; Kurz, T. In Vitro Potential Antioxidant Activity of (1→3),(1→6)-β-d-Glucan and Protein Fractions from Saccharomyces cerevisiae Cell Walls. J. Agric. Food Chem. 2007, 55, 4710–4716. [Google Scholar] [CrossRef]
- Liu, N.; Wang, J.Q.; Jia, S.C.; Chen, Y.K.; Wang, J.P. Effect of Yeast Cell Wall on the Growth Performance and Gut Health of Broilers Challenged with Aflatoxin B1 and Necrotic Enteritis. Poult. Sci. 2018, 97, 477–484. [Google Scholar] [CrossRef]
- Da Silva Araújo, V.B.; de Melo, A.N.F.; Costa, A.G.; Castro-Gomez, R.H.; Madruga, M.S.; de Souza, E.L.; Magnani, M. Followed Extraction of β-Glucan and Mannoprotein from Spent Brewer’s Yeast (Saccharomyces uvarum) and Application of the Obtained Mannoprotein as a Stabilizer in Mayonnaise. Innov. Food Sci. Emerg. Technol. 2014, 23, 164–170. [Google Scholar] [CrossRef]
- de Melo, A.N.F.; de Souza, E.L.; da Silva Araujo, V.B.; Magnani, M. Stability, Nutritional and Sensory Characteristics of French Salad Dressing Made with Mannoprotein from Spent Brewer’s Yeast. LWT—Food Sci. Technol. 2015, 62, 771–774. [Google Scholar] [CrossRef]
- Li, J.; Karboune, S. Characterization of the Composition and the Techno-Functional Properties of Mannoproteins from Saccharomyces cerevisiae Yeast Cell Walls. Food Chem. 2019, 297, 124867. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Xia, C.; Liu, L.; Tang, L.; Wang, J.; Xu, C.; Wang, J.; Zhang, L.; Ye, X.; Huang, Y.; et al. Structural Characterization and Emulsifier Property of Yeast Mannoprotein Enzymatically Prepared with a β-1,6-Glucanase. LWT 2022, 168, 113898. [Google Scholar] [CrossRef]
- Hajhosseini, A.; Doroud, D.; Sharifan, A.; Eftekhari, Z. Stress Response and Characterization of Oil-in-Water Emulsions Stabilized with Kluyveromyces marxianus Mannoprotein. J. Food Sci. 2021, 86, 454–462. [Google Scholar] [CrossRef] [PubMed]
- De Iseppi, A.; Curioni, A.; Marangon, M.; Vincenzi, S.; Kantureeva, G.; Lomolino, G. Characterization and Emulsifying Properties of Extracts Obtained by Physical and Enzymatic Methods from an Oenological Yeast Strain. J. Sci. Food Agric. 2019, 99, 5702–5710. [Google Scholar] [CrossRef]
- Lukondeh, T.; Ashbolt, N.J.; Rogers, P.L. Evaluation of Kluyveromyces marxianus FII 510700 Grown on a Lactose-Based Medium as a Source of a Natural Bioemulsifier. J. Ind. Microbiol. Biotechnol. 2003, 30, 715–720. [Google Scholar] [CrossRef]
- Reis, S.F.; Fernandes, P.A.R.; Martins, V.J.; Gonçalves, S.; Ferreira, L.P.; Gaspar, V.M.; Figueira, D.; Castelo-Branco, D.; Mano, J.F.; Coimbra, M.A.; et al. Brewer’s Spent Yeast Cell Wall Polysaccharides as Vegan and Clean Label Additives for Mayonnaise Formulation. Molecules 2023, 28, 3540. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).